Abstract
Introduction:
A previous paper used latent class analysis to assign individuals to 1 of 4 adolescent/young adult smoking trajectory classes and then established an association between maternal smoking before, during, and after pregnancy and these classes. In this paper, we examine one possible pathway for this relationship: that maternal smoking during pregnancy may set off a behavioral trajectory which increases the likelihood of problem behaviors generally, of which smoking is one manifestation.
Methods:
We used the Behavior Problems Index measure from age 8 through age 12 as a potential mediator. We used a path analysis modeling approach within a multinomial logistic regression (using Mplus) to estimate direct and indirect effects (via behavioral problems) between maternal smoking pattern and child trajectory class.
Results:
We found small but statistically significant indirect effects via behavioral problems from maternal smoking to child smoking trajectory for membership in all 3 smoking classes, relative to the nonsmoking trajectory, indicating partial mediation. Mediated effects were associated with maternal smoking after pregnancy, no statistically significant mediated effects were found for smoking before or during pregnancy.
Conclusions:
The results provided no evidence that the effects of maternal smoking during pregnancy on child smoking trajectory are mediated by problem behavior. Effects from smoking after birth to child smoking trajectory appear to be partially mediated by problem behavior, supporting a behavioral rather than physiological effect of smoking during pregnancy but not ruling out more complex physiological pathways.
Introduction
It is well established that children of smokers are at greater risk of smoking than are children of nonsmokers (Borland & Rudolph, 1975; Buka, Shenassa, & Niaura, 2003; Gottleib, 1982; Newman & Ward, 1989) and that children of mothers who smoke during pregnancy (SDP) are particularly at risk (Kandel, Wu, & Davies, 1994; Hellstrom-Lindahl & Nordberg, 2002; Weden & Miles, 2011).
Maternal SDP has been found to be associated with the child’s age of smoking initiation, current smoking status, smoking level, nicotine dependence, and progression to daily smoking (Cornelius, Leech, Goldschmidt, & Day, 2000, 2005; Griesler, Kandel, & Davies, 1998; Kandel et al., 1994; Lawlor et al., 2005; Munafò, Wileyto, Murphy, & Collins, 2006; O’Callaghan et al., 2006, 2009; Roberts et al., 2005; Weden & Miles, 2011).
Several mechanisms for these relationships between smoking during pregnancy and smoking outcome of the child have been hypothesized, which may be classified as either direct or indirect. The first potential mechanism for the link is the physiological route, which hypothesizes a direct relationship—nicotine passes the placental blood barrier and affects the developing fetus. The fetus is thereby sensitized to nicotine, predisposing the child to smoke (Kandel et al., 1994; Kandel & Udry, 1999). The second hypothesized mechanism is indirect—that of mediation through problem behavior. Exposure to the constituents of tobacco smoke adversely affects the fetus in a number of ways, including neurological effects (Fried, Watkinson, & Gray, 1998), and is associated with behavioral problems and antisocial behavior (Wakschlag, Pickett, Cook, Benowitz, & Leventhal, 2002). Smoking and continued smoking have been found to be associated with a range of problem behaviors (Wakschlag et al., 2003). To the extent that parental smoking influences a child’s social and cognitive development, it may also set in motion a behavioral trajectory which makes initiation and progression of smoking more likely. According to this hypothesis, smoking in adolescence may be a manifestation of these problems (Benowitz, 2001), and therefore, problem behavior measures in general might be expected to mediate the relationship between SDP and youth smoking. Exposure to parental smoking after the child’s birth may also influence children’s smoking through the social influences of norms and behavior modeling, or they may operate through physiological mechanisms (i.e., secondhand smoke in the home causing sensitization to nicotine; Conrad, Flay, & Hill, 1992; Klerman, 2004; Niaura et al., 2001).
Finally, there may be more distal factors further “upstream” (McKinlay, 1979; World Health Organization, 2002) from the parent’s smoking (i.e., family or neighborhood socioeconomic status [SES]) that are associated with both the youth’s and the parent’s smoking. These “upstream” factors may operate directly, as would be the case for community smoking norms, or they may operate indirectly, as might be the case for family factors such as the parenting approach that are also associated with SES (Hill, Hawkins, Catalano, Abbott, & Guo, 2005; Lynch & Bonnie, 1994).
In support of the mediation hypothesis, some studies have also related maternal smoking during pregnancy to poor health and behavioral outcomes. These poor child outcomes range from poor fetal development and low birth weight to decreased cognitive ability and lower early school performance (Little, Preacher, Selig, & Card, 2007); for review see Floyd, Rimer, Giovino, Mullen, and Sullivan (1993). They also include influences later in the child’s development, such as increases in behavior problems which may be specific to externalizing problems such as conduct problems, oppositional defiance, and attention deficit/hyperactivity problems (for review see Humes, Jones, & Ramirez, 2010; Shrestha & Heisler, 2011). Neurotoxic effects of tobacco have been demonstrated through animal research and are hypothesized to occur through reduction of fetal blood flow and the associated hypoxic effects, as well as teratological effects on the development of the fetal nervous system (see reviews by Shea & Steiner, 2008; Humes et al., 2010).
However, a further possibility is that the role of SDP on adolescent smoking trajectories may operate through the mother’s smoking behavior in the child’s adolescence. Mothers who SDP are very likely to continue smoking through the child’s early life and adolescence (Floyd et al., 1993). Studies suggest that this type of exposure to parental smoking may solidify a child’s values and beliefs about smoking and demonstrate prosmoking norms (for review see Chassin, Presson, Rose, & Sherman, 1998; Darling & Cumsille, 2003). Although there is debate about the relative strength of parents’ influence on their children’s smoking behaviors (Chassin, Presson, Sherman, Montello, & McGrew, 1986), studies have generally observed that mothers (as opposed to fathers) have the strongest influence during adolescence (Avenevoli & Merikangas, 2003).
The cognitive and developmental problems associated with SDP briefly reviewed above, combined with the large body of research on cognitive and developmental origins of youth problem behaviors (see Smith, Leve, & Chamberlain, 2011) substantiate how the effects of SDP might be mediated by child and youth behavior problems. Only two studies, to our knowledge, have explored whether the effect of SDP operates through childhood behavior problems to influence tobacco use during adolescence (Cornelius et al., 2005; Griesler et al., 1998). Both studies observe a positive relationship between SDP and regular smoking among adolescents that is mediated by childhood behavior problems. In this preliminary research, the findings have held after controlling for SES and concurrent smoking by the mother (Griesler et al., 1998). It is not clear whether these findings will also hold in larger datasets with data collected over longer time periods, which allows more extensive analyses of confounders and potential methodological bias. Further research could also illuminate how SDP might influence the trajectories and progression of youth smoking.
In the research presented in this paper, we extend the work of Weden and Miles (2011) to include a test of the mediation through problem behavior hypothesis. In their work, Weden and Miles used multinomial logistic regression to examine whether patterns of maternal smoking (before, during, and after pregnancy) were associated with smoking patterns in their offspring, finding that maternal smoking during pregnancy was associated with an increased likelihood of youth smoking. In this paper, we examine the potential for measures of problem behavior to mediate those relationships.
Methods
Data come from the Children and Young Adults of the National Longitudinal Survey of Youth 1979 cohort (NLSY79-CYA), a public-use panel survey of all offspring of women in a population-representative cohort (NLSY79) commissioned by the U.S. Bureau of Labor Statistics (2010; Children and Youth of the National Longitudinal Survey of Youth 1979 Cohort, http://www.bls.gov/nls/nlsy79ch.htm). The NLSY79-CYA employs a biennial, cohort-sequential design in which all children born to NLSY79 women by 1986 have been followed, as well as all subsequent children born after 1986. The NLSY79-CYA thus includes multiple birth cohorts and multiple children-per-mother—children represent the unit of analysis, and one mother may appear in the dataset more than one time. We select respondents aged 14–25 years observed at any of the biennial surveys between 1994 and 2006 (i.e., birth cohorts 1970–1992). The NLSY79-CYA yearly completion rates range from 83.0% to 88.4% (Center for Human Resource Research, 2009). By 2006, 6,643 youth aged 14 years and older were eligible for the NLSY79-CYA and had been located for at least one interview between 1994 and 2006. From this sample, 6,349 youth responded to questions about cigarette smoking at least once.
The youth smoking trajectory was assessed by asking respondents if they had smoked cigarettes in the past 30 days (SPTD) at each biennial survey wave. A latent class analysis approach (described fully in Weden & Miles, 2011) was then employed to categorize each individual into one of four trajectory classes: early start, early experiment, late start, and nonsmokers.
Early start (12.6% of the sample) begins to smoke at a relatively young age and continues into young adulthood. Their rates of SPTD increase rapidly from 30% to 90% between age 14 and 16 and remain high at each subsequent age through young adulthood (87% at age 25).
Early experiment smokers (2.6% of the sample) are likely to have smoked in the past 30 days at younger ages, but then the rates of SPTD drop back to 30% by age 21 and remain at an average of 35% through age 25, suggesting early initiation but then quitting during early adulthood.
Late start smokers (19.1% of the sample) report almost no SPTD prior to age 16, but then have dramatically increasing rates (climbing from essentially zero to 69% over age 16–19), with continued increases to age 25, when 90% report SPTD.
Nonsmokers (67% of the sample) report no, or very low, SPTD at every age. The mean rate of SPTD over age 14–25 was 2% across all years.
To describe maternal smoking patterns before, during, and after the pregnancy and birth of the respondent, we used several measures from the NLSY. Mother ever smoked daily is a dichotomous indicator for any maternal report of “daily” smoking in the NLSY79 substance use history supplements taken in 1992, 1994, and 1998. Mother SDP is a categorical indicator for mother’s reported cigarette consumption (“did not smoke”, “less than 1 pack per day”, or a combination of “1–2 packs per day” and “2 or more packs per day”) from the NLSY79 birth history taken within 1 year of birth for this study’s sample. Because of notable item nonresponse (n = 1,237), an identical retrospective question in the 2004 NLSY79-CYA was used to estimate reliability across the two assessments and fill nonresponses. (For respondents in which maternal smoking was assessed in both the birth history and the 2004 retrospective question, we observed high agreement with a kappa statistic of 0.93 and only 33 cases of nonagreement. For cases with disagreement between the measures, we used the earlier birth history assessment. It should be noted that the NLSY did not distinguish between a response of “no” and a missing response in the 2004 maternal survey unless the respondent volunteered a “no” answer.). Mother’s smoking history distinguishes the full pattern of prepregnancy, prenatal, and postnatal exposures. It addresses the timing of initiation and cessation of daily smoking (reported and updated in the three NLSY79 substance use supplements) in relationship to youth’s date of birth. We classify mothers into one of six maternal smoking patterns. The six exposure categories were never smoked daily or during pregnancy (never -smoker, 45.2%); quit daily before birth of child and no SDP (pre birth smoker, 7.4%); no SDP pregnancy but relapse to daily smoking (post birth smoker, 10.0%); no SDP, relapse, but then quit daily smoking (post birth former smoker, 6.7%); smoked any cigarettes during pregnancy and smoked daily, but quit after birth (former smoker who SDP, 6.7%); and smoked any cigarettes during pregnancy and smoked daily after birth (continuous smoker, 24.2%).
The Behavior Problems Index (BPI) used in the NLSY79-CYA is based on the Child Behavior Check List (Achenbach, 1991) and comprises 25 items in five subscales. Subscales are antisocial (e.g., “cheats or tells lies”), anxious/depressed (e.g., “feels worthless or inferior”), headstrong (e.g., “argues too much”), hyperactive (e.g., “difficulty concentrating/paying attention”), and peer problems (e.g., “not liked by other children”). We used assessments of BPI from age 8 through 12, and where more than one assessment had been taken, the mean across assessments was used. The total scale score was used for all analyses (although we also carried out sensitivity analyses using subscales). Responses were made on a 3-point scale, with high scores indicating fewer problems. Coefficient alphas for the scale were consistent and high, ranging from .90 to .91.
Control variables entail youth sociodemographics (age at baseline, first smoking assessment; sex; and race/ethnicity), maternal sociodemographics (age at the child’s birth, and educational attainment and marital status when the child was age 14), and maternal behavioral indicators of her proclivity for health and/or risk behaviors (child breastfed, prenatal care, and a score of the mother’s endorsement in 1980 of 21 delinquency behaviors comprising the NLSY79-modified Self-Reported Delinquency Interview).
Statistical Procedures
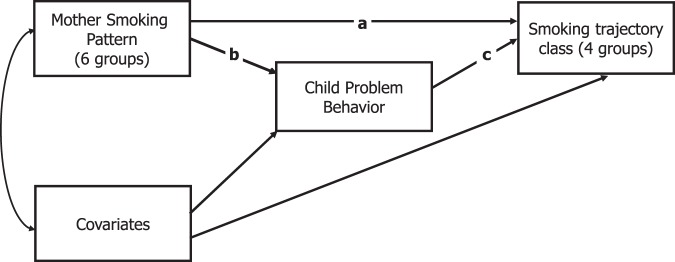
All analyses were carried out with Mplus v.6.0 (Muthén & Muthén, 2010), using robust maximum likelihood estimation and incorporated sample weights and cluster-corrected SEs to account for multiple children in each household. Full information estimation maximum likelihood estimation was employed, which provides unbiased estimates in the presence of data which are missing at random or missing completely at random. The analysis presented in Weden and Miles (2011) was replicated using Mplus—in this model, smoking trajectory class was regressed on the maternal smoking pattern along with control variables (referred to as the direct effects model). A model was then estimated in which the behavioral problems index was included as a mediator of the relationship between the maternal smoking pattern and the youth smoking trajectories (the mediation model). Covariances between control variables and smoking history variables were estimated, and both behavioral problems index and maternal smoking pattern were regressed on control variables. A path diagram representation of the model is shown in Figure 1; straight single-headed arrows represent regression paths, the curved two-headed arrow represents a covariance. In both models for the youth smoking trajectory outcome, the class non smoker was used as the base category. This meant that we report the odds ratio (OR) for being an early start, late start, or early experiment smoker relative to nonsmoker. We assessed intergenerational transmission by considering the OR of these youth smoking trajectories (relative to nonsmoker) for mothers who did and did not smoke. The mother’s smoking pattern was dummy coded, with never-smoker used as the reference category. Thus, we estimated the OR of a youth smoking pattern (i.e., early start, late start, or early experiment) versus nonsmoking for youth whose mothers smoked (i.e., prebirth smoker, postbirth smoker, postbirth former smoker, former smoker who SDP, or continuous smoker) compared with youth whose mothers never smoked.
Figure 1.
Path diagram representation of mediation model showing direct effect of maternal smoking pattern (Path a) and indirect effects via problem behavior (Paths b and c).
Three paths are labeled in Figure 1. Path a represents the set of direct effects of child smoking trajectories regressed on maternal smoking pattern. Path b represents the regression of the Child Problem Behavior Index on maternal smoking pattern and comprises five regression estimates (six smoking patterns, dummy coded, with nonsmoking as the reference). Path c represents the regression of smoking trajectory on child problem behavior (three estimates, with nonsmoker as the reference category). The indirect effects of smoking behavior are represented by the products of the regression parameters represented by paths b and c. The SEs of the product of the estimates are calculated using the delta method (MacKinnon, 2008). Two multivariate tests were carried out to examine the direct and indirect parameters; first, we tested the null hypothesis that all direct effects (from maternal smoking patterns to trajectory group) were equal to zero; second, we tested the null hypothesis that the total indirect effect was equal to zero.
Results
Cases were excluded where maternal data were missing or control variables were missing, giving a final sample size of 5,027. Because siblings were included, children were clustered within 2,619 mothers in the sample. Table 1 shows the number of people, and weighted percentage for each maternal smoking pattern, in each youth trajectory class.
Table 1.
Cross-tabulation of Maternal Smoking Categorization and Individual Smoking Trajectory Class
| Nonsmoker | Quit before pregnancy | Not SDP, then relapse | Not SDP, relapse, then quit | SDP, quit after pregnancy | SDP, continue after pregnancy | Total | ||
| Early start | N | 202 | 33 | 83 | 48 | 42 | 225 | 633 |
| % | 8.1 | 10.9 | 16.1 | 13.3 | 14.3 | 21.2 | 12.6 | |
| Early experiment | N | 48 | 9 | 17 | 9 | 8 | 36 | 127 |
| % | 1.9 | 3.0 | 3.3 | 2.5 | 2.7 | 3.4 | 2.5 | |
| Late start | N | 385 | 37 | 133 | 95 | 70 | 238 | 958 |
| % | 15.5 | 12.2 | 25.7 | 26.2 | 23.8 | 22.4 | 19.1 | |
| Never smoke | N | 1,854 | 224 | 284 | 210 | 174 | 563 | 3,309 |
| % | 74.5 | 73.9 | 54.9 | 58.0 | 59.2 | 53.0 | 65.8 | |
| Total | N | 2,489 | 303 | 517 | 362 | 294 | 1,062 | 5,027 |
| % | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Note. SDP = smoke during pregnancy.
Table 2 presents the findings from the two models described above, with Model 1 having only direct effects from maternal smoking pattern to childsmoking trajectory class and Model 2 adds behavioral problems as a mediator in the model.
Table 2.
Results of Fitting Mediation Models From Maternal Smoking Status to Offspring Smoking Trajectory Classa
| Model 1: Direct effects | Model 2: Mediation | ||||||||
| OR (95% CIs) | p | OR (95% CIs) | p | OR (95% CIs) | p | ||||
| No mediator | Direct effect | Indirect effect | |||||||
| Early start | |||||||||
| Quit before pregnancy | 1.62 (0.98, 2.66) | .058 | 1.67 (1.02, 2.52) | .037 | 0.98 (0.94, 1.03) | .416 | |||
| Not SDP, then relapse | 2.07 (1.44, 2.99) | <.001 | 1.92 (1.32, 2.62) | <.001 | 1.09 (1.03, 1.14) | .002 | |||
| Not SDP, relapse, then quit | 1.86 (1.14, 3.03) | .013 | 1.78 (1.08, 2.70) | .020 | 1.05 (0.99, 1.1) | .093 | |||
| SDP, quit after pregnancy | 2.12 (1.29, 3.48) | .003 | 2.02 (1.24, 3.05) | .003 | 1.07 (0.99, 1.15) | .075 | |||
| SDP, continue after pregnancy | 2.75 (2.03, 3.73) | <.001 | 2.54 (1.87, 3.28) | <.001 | 1.10 (1.05, 1.15) | <.001 | |||
| Early experiment | |||||||||
| Quit before pregnancy | 3.01 (1.21, 7.50) | .018 | .008 | 0.98 (0.94, 1.02) | .424 | ||||
| Not SDP, then relapse | 2.46 (1.23, 4.91) | .011 | 3.11 (1.25, 7.71) | .016 | 1.08 (1.00, 1.15) | .043 | |||
| Not SDP, relapse, then quit | 1.08 (0.40, 2.88) | .883 | 2.29 (1.15, 4.57) | .935 | 1.04 (0.99, 1.10) | .147 | |||
| SDP, quit after pregnancy | 2.45 (0.93, 6.43) | .069 | 2.33 (0.90, 6.06) | .081 | 1.06 (0.98, 1.15) | .146 | |||
| SDP, continue after pregnancy | 2.12 (1.14, 3.94) | .017 | 1.98 (1.08, 3.65) | .026 | 1.09 (1.01, 1.17) | .023 | |||
| Late start | |||||||||
| Quit before pregnancy | 1.06 (0.67, 1.68) | .810 | 1.07 (0.68, 1.70) | .769 | 0.99 (0.97, 1.01) | .430 | |||
| Not SDP, then relapse | 1.66 (1.23, 2.25) | .001 | 1.61 (1.19, 2.19) | .002 | 1.04 (1.01, 1.08) | .017 | |||
| Not SDP, relapse, then quit | 1.83 (1.27, 2.62) | .001 | 1.79 (1.24, 2.58) | .002 | 1.02 (0.99, 1.05) | .118 | |||
| SDP, quit after pregnancy | 1.97 (1.31, 2.95) | .001 | 1.93 (1.28, 2.9) | .001 | 1.03 (0.99, 1.07) | .102 | |||
| SDP, continue after pregnancy | 1.66 (1.28, 2.16) | <.001 | 1.60 (1.23, 2.09) | <.001 | 1.05 (1.01, 1.08) | .005 | |||
Note. OR = odds ratio; SDP = smoke during pregnancy.
aEstimates are ORs for prediction of being in that class, rather than nonsmoking class, comparing maternal smoking status with the no maternal smoking group.
In Model 1, all children of mothers who smoked, regardless of whether this was before, during, or after pregnancy, were found to be less likely to be in the nonsmoker class than any other class, rather than one of the three smoking classes (indicated by an OR greater than 1), although this effect does not reach statistical significance in all cases.
In Model 2, when problem behavior was introduced as a mediator, all direct effects of maternal smoking pattern on youth smoking trajectory remained similar in magnitude, and all direct effects, which were statistically significant in Model 1, remained statistically significant in Model 2. This indicates that there was not complete mediation by problem behavior—that is, if maternal smoking behavior influenced child smoking behavior, problem behavior was not the only mechanism. We carried out multivariate Wald tests to test the null hypothesis that the direct effects (labeled c in Figure 1) were equal to zero and the total indirect effects (the paths labeled a and b in Figure 1) were equal to zero. For the direct effects, this gave χ 2 (15) = 59, p < .001; for the indirect effects χ 2(7) = 23, p = .002, indicating that both the direct and indirect effects are statistically significant.
Of more interest are those relationships where the indirect effect is statistically significant. We observed a statistically significant indirect association between having a mother who was a postbirth smoker and belonging to each of the respective youth smoking classes, in specific early start (OR = 1.09), early experiment (OR = 1.08), and late start (OR = 1.04). Similarly, we observed a statistically significant indirect association between having a mother who was a continuous smoker and belonging to each of the respective youth smoking classes, in specific early start (OR = 1.10), early experiment (OR = 1.09), and late start (OR = 1.05). In addition, the indirect association between having a mother who was a former smoker who SDP and belonging to the early start smoking approaches statistical significance (OR = 1.07, p = .075).
The magnitude of the indirect effects that achieve statistical significance ranges from 1.04 to 1.09; in comparison, the magnitude of the direct effects range from 1.6 to 3.1. Hence, the mediation effects are small, relative to the total effect.
Discussion
In the research reported in this paper, we investigated whether the relationship between maternal smoking behavior before, during, and after pregnancy and child smoking trajectory class was partially mediated by problem behavior in middle childhood. We found some evidence for a partially mediated relationship, for two groups of children exposed to maternal smoking—those whose mothers smoked during pregnancy, and continued to smoke, and those whose mothers quit while pregnant and then resumed smoking after the birth of the child, and did not quit.
The effects from two patterns of maternal smoking to child smoking class were found to be partially mediated by problem behavior. The two groups of mothers where the partial mediation was found were those who smoked after pregnancy—specifically, the “not SDP, then relapse” group and the SDP and continue after pregnancy group. This result provides little evidence to support a simple mediation hypothesis of prenatal exposure; that is, that tobacco smoke introduces physiological change, which causes increase in problem behavior, manifesting itself as smoking. However, this does not rule out the possibility of more complex processes than we have modeled, for example, those of cumulative exposure or threshold effects (Singh-Manoux, 2005). These results might suggest a mechanism in which continued maternal smoking after pregnancy is related to problem behavior, later manifesting as smoking. This mechanism is possibly a factor, which is predictive of initiation (or perhaps reinitiation) of smoking in both mothers and their offspring. The mechanism may be a genetic predisposition to smoking or may be a feature of the shared environment, which is associated with problem behavior in children and maternal smoking, such as family or environmental stress or sand family values, beliefs, or norms. In addition, there may be interactions with other substances, for example, alcohol.
We believe that this study is the first to investigate mediators of the effect of maternal smoking during pregnancy using population-representative data and latent classification techniques. That mediation effects from smoking during pregnancy were not detected suggests that although problem behavior is associated with maternal smoking patterns, smoking in children is not a manifestation of this problem behavior.
Several limitations should be borne in mind. The smoking reports were all collected by self-report, which may be prone to bias due because respondents do not want to admit to smoking or simply because they forget. However, self-report has been shown to provide a reasonable estimate of actual smoking (Chan, 2008; Dolcini, Adler, Lee, & Bauman, 2003), and (as mentioned) the data were collected using Computer Assisted Personal Interviewing to minimize reporting bias, although some maternal smoking data were not collected until some time after birth. Further, the primary source of maternal SDP data comes from reports within 1 year of the child’s birth, addressing a limitation of long recall times discussed elsewhere (Kandel & Udry, 1999; O’Callaghan et al., 2006). Other exposure to tobacco smoke was not assessed which might occur from father or other family members smoking; such exposure may exacerbate the genetic, physiological, and social mechanisms of intergenerational transmission. We also did not account for other methods of nicotine use, such as smokeless tobacco or smoking of tobacco through means other than cigarettes (e.g., pipe, cigar). These will be likely to bias our results in the direction of the null hypothesis as children who were exposed to nicotine will have been classified incorrectly as nonsmokers.
Some issues arise with the sample. The sample was large, but even such a large sample led to relatively small numbers of individuals in, for example, the early experiment group with a sample of 127. This small sample size has limited statistical power in analyses including this group. The data on smoking are recorded biennially; therefore, our trajectories cannot capture short-term fluctuations in cigarette behavior but rather describe overarching patterns of youth smoking across adolescence and young adulthood. Finally, due to the cohort structure of the NLSY79-CYA sample, the most complete portion of data covers the period age 14–16 and thus may be less representative of smoking patterns in early adulthood than have been described in previous population representative studies The small size of the mediation effect, relative to the direct effect of maternal smoking (of any class) means that the result is vulnerable to omission of control variables that are precursors to maternal smoking, child smoking, and child behavior problems. Although we included a wide range of control variables, additional unmeasured covariates that are associated with all three variables of interest would create an artifactual mediation effect.
The results of this study provide a rigorous test of the mediation through behavior problems hypothesis and suggest that the mechanism of intergenerational transmission of smoking via SDP follows a more direct route, potentially via a physiological mechanism. However, maternal smoking after pregnancy seemed to be associated with youth smoking, at least in part, because of a mediation effect of problem behavior. This result suggests that interventions to reduce youth smoking should focus on determining and reducing family related factors, such as environmental stressors or family beliefs and norms and also that harms from postpregnancy smoking women are not limited to those from secondhand smoke.
Funding
This paper was funded by the Eunice Kennedy Shriver Institute of Child Health and Human Development, grant R03HD060673.
Declaration of Interests
The authors declare that they have no conflict of interest.
Acknowledgments
We thank Michael Pollard and Peter Brownell for comments on an earlier draft of this article.
References
- Achenbach TM. Integrative guide to the 1991 CBCL/4-18, YSR, and TRF profiles. Burlington, VT: Department of Psychology, University of Vermont; 1991. [Google Scholar]
- Avenevoli S, Merikangas KR. Familial influences on adolescent smoking. Addiction. 2003;98:1–20. doi: 10.1046/j.1360-0443.98.s1.2.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. The nature of nicotine addiction. In: Slovic P, editor. Smoking: Risk, perception and policy. Thousand Oaks, CA: Sage; 2001. pp. 159–187. [Google Scholar]
- Borland BL, Rudolph JP. Relative effects of low SES, parental smoking, and poor scholastic performance on smoking among high school students. Social Science & Medicine. 1975;9:27–30. doi: 10.1016/0037-7856(75)90155-9. [DOI] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: A 30-year prospective study. American Journal of Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. doi:10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. (2010). Children and youth of the National Longitudinal Survey of Youth 1979 Cohort. Retrieved from http://www.bls.gov/nls/nlsy79ch.htm.
- Center for Human Resource Research. NLSY79 child & young adult data users guide. Columbus, OH: Center for Human Resource Research; 2009. [Google Scholar]
- Chan D. Why ask me? Are self-report data really that bad? In: Lance CE, Vandenberg RJ, editors. Statistical and methodological myths and urban legends: Doctrine, verity and fable in organizational and social sciences. New York, NY: Routledge; 2008. pp. 309–336. [Google Scholar]
- Chassin L, Presson CC, Rose JS, Sherman SJ. Maternal socialization of adolescent smoking: Intergenerational transmission of smoking-related beliefs. Psychology of Addictive Behaviors. 1998;12:206–216. doi: 10.1037//0012-1649.34.6.1189. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Montello D, McGrew D. Changes in peer and parent influence during adolescence: Longitudinal versus cross-sectional perspectives on smoking initiation. Developmental Psychology. 1986;22:327–334. [Google Scholar]
- Conrad KM, Flay BR, Hill D. Why children start smoking cigarettes: Predictors of onset. British Journal of Addiction. 1992;87:1711–1724. doi: 10.1111/j.1360-0443.1992.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: Is it a risk factor for early tobacco experimentation? Nicotine & Tobacco Research. 2000;2:45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study. Neurotoxicology and Teratology. 2005;27:667–676. doi: 10.1016/j.ntt.2005.05.006. doi:10.1016/j.ntt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Darling N, Cumsille P. Theory, measurement, and methods in the study of family influences on adolescent smoking. Addiction. 2003;98:21–36. doi: 10.1046/j.1360-0443.98.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Dolcini MM, Adler NE, Lee P, Bauman KE. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine & Tobacco Research. 2003;5:473–483. doi:10.1080/1462220031000118586. [PubMed] [Google Scholar]
- Floyd RL, Rimer BK, Giovino GA, Mullen PD, Sullivan SE. A review of smoking in pregnancy—Effects on pregnancy outcomes and cessation efforts. Annual Review of Public Health. 1993;14:379–411. doi: 10.1146/annurev.pu.14.050193.002115. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology. 1998;20:293–306. doi: 10.1016/s0892-0362(97)00091-3. doi:10.1016/S0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- Gottleib NH. The effects of peer and parental smoking on the smoking careers of college women: A sex-related phenomenon. Social Science & Medicine. 1982;16:595–600. doi: 10.1016/0277-9536(82)90314-8. [DOI] [PubMed] [Google Scholar]
- Griesler PC, Kandel DB, Davies M. Maternal smoking in pregnancy, child behavior problems, and adolescent smoking. Journal of Research on Adolescence. 1998;8:159–185. [Google Scholar]
- Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: A way to transfer the addiction to the next generation? Respiration. 2002;69:289–293. doi: 10.1159/000063261. doi:10.1159/000063261 [pii] [DOI] [PubMed] [Google Scholar]
- Hill KG, Hawkins JD, Catalano RF, Abbott RD, Guo J. Family influences on the risk of daily smoking initiation. Journal of Adolescent Health. 2005;37:202–210. doi: 10.1016/j.jadohealth.2004.08.014. doi:10.1016/j.jadohealth.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. Washington, DC: U.S. Census Bureau; 2010. [Google Scholar]
- Kandel DB, Udry JR. Prenatal effects of maternal smoking on daughters’ smoking: Nicotine or testosterone exposure? American Journal of Public Health. 1999;89:1377–1383. doi: 10.2105/ajph.89.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. American Journal of Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman LV. Protecting children: Reducing their environmental tobacco smoke exposure. Nicotine & Tobacco Research. 2004;6:S239–S252. doi: 10.1080/14622200410001669213. doi:10.1080/14622200410001669213. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, O’Callaghan MJ, Mamun AA, Williams GM, Bor W, Najman JM. Early life predictors of adolescent smoking: Findings from the Mater-University study of pregnancy and its outcomes. Paediatric and Perinatal Epidemiology. 2005;19:377–387. doi: 10.1111/j.1365-3016.2005.00674.x. doi:10.1111/j.1365-3016.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- Little TD, Preacher KJ, Selig JP, Card NA. New developments in latent variable panel analyses of longitudinal data. International Journal of Behavioral Development. 2007;31:357–365. doi:10.1177/0165025407077757. [Google Scholar]
- Lynch BS, Bonnie RJ. Growing up tobacco free: Preventing nicotine addition in children and youths. Atlanta, GA: National Academies Press; 1994. [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Mahwah, NJ: Erlbaum Psychology Press; 2008. [Google Scholar]
- McKinlay JB. A case for refocusing upstream: The political economy of illness. In: Jaco EG, editor. Patients, physicians, and illness: A sourcebook in behavioral science and health. 3rd ed. New York: Free Press; 1979. [Google Scholar]
- Munafò MR, Wileyto EP, Murphy MFG, Collins BN. Maternal smoking during late pregnancy and offspring smoking behaviour. Addictive Behaviors. 2006;31:1670–1682. doi: 10.1016/j.addbeh.2005.12.010. doi:10.1016/j.addbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén L. Mplus 6.0: Muthen and Muthen. 2010. [Google Scholar]
- Newman IM, Ward JM. The influence of parental attitude and behavior on early adolescent cigarette smoking. Journal of School Health. 1989;59:150–152. doi: 10.1111/j.1746-1561.1989.tb04688.x. [DOI] [PubMed] [Google Scholar]
- Niaura R, Bock B, Lloyd EE, Brown R, Lipsitt LP, Buka S. Maternal transmission of nicotine dependence: Psychiatric, neurocognitive and prenatal factors. American Journal on Addictions. 2001;10:16–29. doi: 10.1080/105504901750160420. [DOI] [PubMed] [Google Scholar]
- O’Callaghan FV, Al Mamun A, O’Callaghan M, Alati R, Najman JM, Williams GM, et al. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults—A birth cohort study. Australian and New Zealand Journal of Public Health. 2009;33:371–377. doi: 10.1111/j.1753-6405.2009.00410.x. doi:10.1111/j.1753-6405.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W, Alati R. Prediction of adolescent smoking from family and social risk factors at 5 years, and maternal smoking in pregnancy and at 5 and 14 years. Addiction. 2006;101:282–290. doi: 10.1111/j.1360-0443.2006.01323.x. doi:10.1111/j.1360-0443.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- Roberts KH, Munafo MR, Rodriguez D, Drury M, Murphy MFG, Neale RE, et al. Longitudinal analysis of the effect of prenatal nicotine exposure on subsequent smoking behavior of offspring. Nicotine & Tobacco Research. 2005;7:801–808. doi: 10.1080/14622200500262840. doi:10.1080/14622200500262840. [DOI] [PubMed] [Google Scholar]
- Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine & Tobacco Research. 2008;10:267–278. doi: 10.1080/14622200701825908. doi:10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- Shrestha LB, Heisler EJ. The changing demographic profile of the United States. Vol. 7. Washington, DC: Congression Research Service; 2011. [Google Scholar]
- Singh-Manoux A. Commentary: Modelling multiple pathways to explain social inequalities in health and mortality. International Journal of Epidemiology. 2005;34:638–639. doi: 10.1093/ije/dyi074. doi:10.1093/ije/dyi074. [DOI] [PubMed] [Google Scholar]
- Smith D, Leve L, Chamberlain P. Preventing internalizing and externalizing problems in girls in foster care as they enter middle school: Impact of an intervention. Prevention Science. 2011;12:1–9. doi: 10.1007/s11121-011-0211-z. doi:10.1007/s11121-011-0211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Jr, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: A review. American Journal of Public Health. 2002;92:966–974. doi: 10.2105/ajph.92.6.966. doi:10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Middlecamp MK, Walton LL, Tenzer P, Leventhal BL. Pregnant smokers who quit, pregnant smokers who don’t: Does history of problem behavior make a difference? Social Science & Medicine. 2003;56:2449–2460. doi: 10.1016/s0277-9536(02)00248-4. doi:10.1016/S0277-9536(02)00248-4. [DOI] [PubMed] [Google Scholar]
- Weden M, Miles JNV. Intergenerational relationships between the smoking patterns of a population-representative sample of US mothers and the smoking trajectories of their children. American Journal of Public Health. 2011 doi: 10.2105/AJPH.2011.300214. doi:10.2105/AJPH.2011.300214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World health report 2002: Reducing risks, promoting healthy life. Geneva, Switzerland: Author; 2002. [Google Scholar]