Antibodies to Staphylococcus aureus capsular polysaccharides (CP) and poly-N-acetyl glucosamine (PNAG) antigens interfere in protection. Active immunization of mice failed to overcome interference. Natural nonprotective antibodies to PNAG in normal human serum may prevent effective vaccination against S. aureus CP antigens.
Abstract
Background. Vaccines against Streptococcus pneumoniae, Neisseria meningitidis, and Hemophilus influenzae type b induce functional opsonic or bactericidal antibodies to surface capsular polysaccharides (CP). Targeting the comparable Staphylococcus aureus CP seems logical, but to date such efforts have failed in human trials. Studies using immunization-induced animal antibodies have documented interference in opsonic and protective activities of antibodies to CP by antibodies to another S. aureus cell surface polysaccharide, poly-N-acetyl glucosamine (PNAG). Here we evaluated whether natural antibody to PNAG in normal human serum (NHS) had a similar deleterious effect.
Methods. Functional and/or protective activities of antibody to S. aureus CP and PNAG antigens in patients with bacteremia, in mice immunized with combinations of CP and PNAG conjugate vaccines, and in serum samples of healthy subjects with natural antibody to PNAG, to which immunization-induced animal antibodies to CP antigens were added, were evaluated.
Results. Antibodies to PNAG and CP that mutually interfered with opsonic killing of S. aureus were detected in 9 of 15 bacteremic patients. Active immunization of mice with combinations of PNAG and CP conjugate antigens always induced antibodies that interfered with each other's functional activity. Non-opsonic natural antibodies to PNAG found in NHS interfered with the functional and protective activities of immunization-induced antibody to CP antigens during experimental infection with S. aureus.
Conclusions. Both immunization-induced animal antibodies and natural antibodies to PNAG in NHS interfere with the protective activities of immunization-induced antibody to S. aureus CP5 and CP8 antigens, representing potential barriers to successful use of CP-specific vaccines.
Staphylococcus aureus is arguably one of the most important human pathogens [1–3]. Treatment is complicated by the explosion of methicillin-resistant and other antibiotic-resistant strains [3–5]. A protective vaccine could significantly reduce the morbidity, mortality, and costs associated with S. aureus infections [6, 7]. Based on analogies with successful vaccines to other bacterial pathogens [8], including Streptococcus pneumoniae [9], Neisseria meningitidis [10], Hemophilus influenzae type b [11], and Salmonella enterica serovar Typhi [12], S. aureus capsular polysaccharides (CP) should be excellent components of a vaccine. Conjugated S. aureus CP antigens [13] have been used to engender adaptive immunity in humans, yet all clinical trials targeting these antigens have, to date, failed [13, 14]. One major issue regarding vaccine development for S. aureus is a lack of any significant understanding as to what constitutes high-level immune resistance in humans to these infections [15], preventing research and clinical trials directed at inducing known and specific immune effectors.
There are other potential explanations for the failure of previous S. aureus CP vaccines. Elsewhere [16] we found that when immunization-induced antibodies to 2 S. aureus surface polysaccharide antigens, either poly-N-acetyl glucosamine (PNAG) or the CP type 5 or type 8 (CP5 or CP8), antigens were combined, instead of the expected additive or synergistic effects on bacterial killing and animal protection, an interference between these effectors resulted, which neutralized the individual functional efficacies. These findings led us to evaluate whether interference by natural human antibodies would inhibit the opsonic and protective properties of immunization-induced antibodies to PNAG or CP antigens.
METHODS
Serum samples from hospitalized patients were obtained under protocols approved by the University Hospital Freiburg (Freiburg, Germany). Serum samples from healthy subjects were obtained from 15 volunteers who gave informed consent for drawing blood. A pool of normal human serum (NHS) was purchased from GeneTex. All animal studies were conducted under a protocol approved by the Harvard Medical Area institutional animal care and use committee (Boston, MA). A P value of <.05 was considered to be statistically significant.
RESULTS
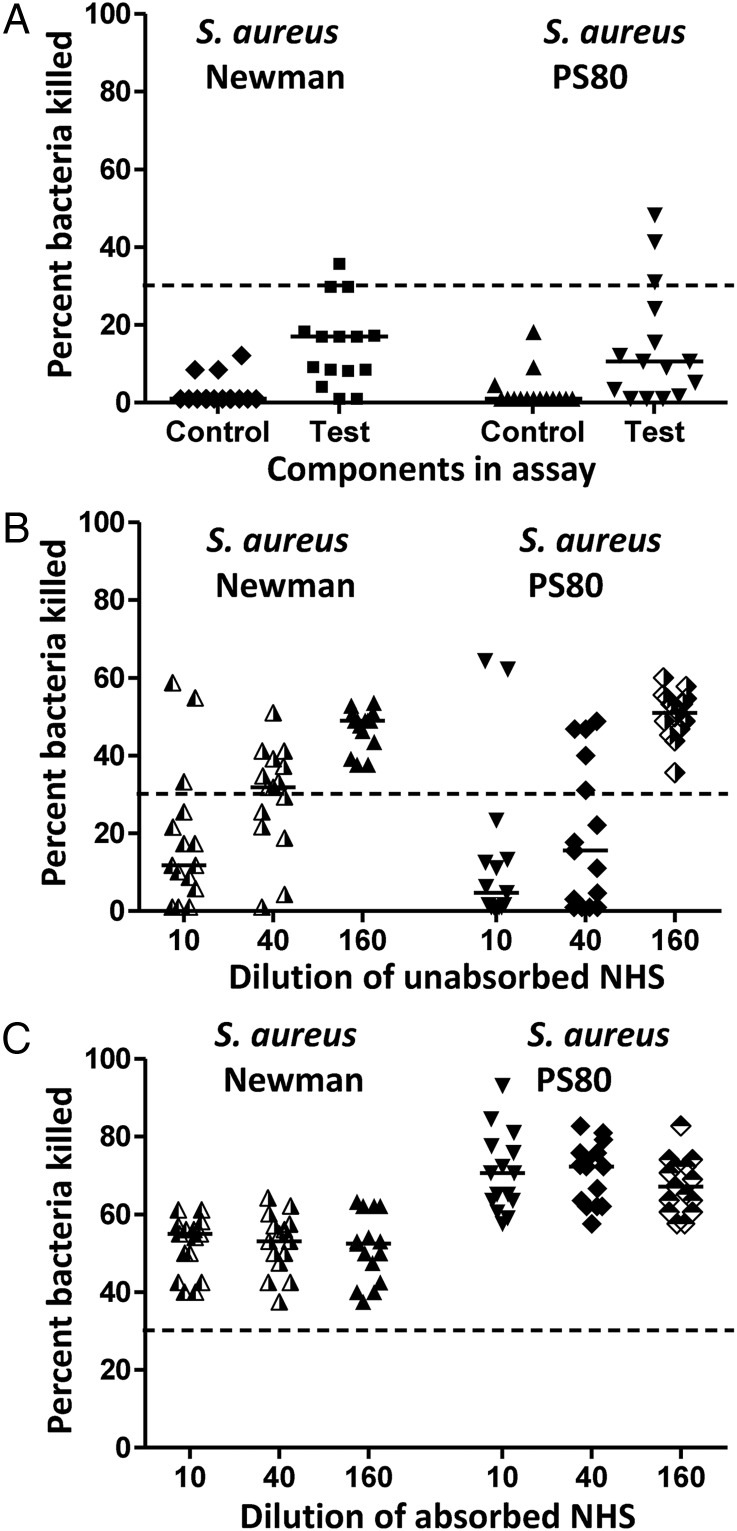
Functional opsonophagocytic killing activity (OPKA) of antibodies to S. aureus CP and PNAG antigens was evaluated in serum samples from 15 German patients with S. aureus bacteremia (Table 1) originating from skin and soft tissue infections, endocarditis, osteomyelitis, or pneumonia. Six patients had OPKA levels in unabsorbed serum of >30%, which is considered to be significant because serum OPKA levels of ≤30% do not protect against experimental S. aureus infection [16, 17]. These 6 serum samples had a range of killing of 40%–68% (Figure 1A). After absorbing these serum samples with cells of either the CP-negative S. aureus Δcap8 strain or PNAG-negative Δica strain to leave behind only the antibodies to CP or PNAG antigens, respectively, all of the patient's serum samples were able to mediate OPK of S. aureus, with 35%–80% killing (Figure 1B). However, when the serum samples were absorbed with a double mutant of S. aureus, Δcap8 + Δica, which leaves antibodies to both PNAG and CP, 9 of 15 serum samples had killing of only 1%–35% (patients 7–15; Table 1; Figure 1B). One patient (No. 2) had an increase in OPK levels in all absorbed samples compared with the unabsorbed serum, suggesting the presence of an absorbable, interfering factor in this sample, whereas another patient (No. 8) had OPK levels of 49% in unabsorbed serum but evidence of interference after absorption of the samples. Overall, in 9 of 15 patient's serum samples we found evidence for low OPKA levels that increased significantly when antibodies to either PNAG or CP antigens were removed.
Table 1.
Site of Origin of Staphylococcus aureus Bacteremia in 15 German Patients
| Patient No. | Origin of the Bacteremia |
|---|---|
| 1 | Abscess (psoas muscle) |
| 2 | Wound |
| 3 | Osteomyelitis, multiple abscesses |
| 4 | Endocarditis with pacemaker infection |
| 5 | Infected peripheral venous catheter |
| 6 | Foreign body infection (port infection) and chemotherapy |
| 7 | Foreign body infection (port infection) |
| 8 | Foreign body infection (port infection) |
| 9 | Foreign body infection (port infection) |
| 10 | Osteomyelitis |
| 11 | Foreign body infection (port infection) |
| 12 | Pleural empyema |
| 13 | Foreign body infection (port infection) |
| 14 | Foreign body infection (port infection) |
| 15 | Endocarditis |
Figure 1.
Opsonophagocytic killing activity (OPKA) of 15 human serum samples from patients with Staphylococcus aureus bacteremia. A, Serum tested without absorption. Killing of <30%, indicative of a lack of specific OPKA, was measured in all but 1 sample (patient 8) of the unabsorbed serum containing interfering antibodies. Bars indicate the means of 4 replicates per assay. B, Serum tested after absorption with S. aureus MN8 Δcap8, MN8 Δica, or MN8 Δcap8 + Δica (leaving behind, respectively, antibodies to capsular polysaccharides [CP], to poly-N-acetyl glucosamine [PNAG], or to both PNAG and CP). Stars indicate a decrease of >30% in killing, observed in 9 of 15 of the serum samples when anti-CP and anti-PNAG activities were combined together (patients 7–15), indicating the presence of interfering antibodies. Abbreviations: CP, capsular polysaccharides; PNAG, poly-N-acetyl glucosamine.
To determine whether combining deacetylated PNAG (dPNAG) conjugate vaccines [17, 18] and CP-conjugate vaccines could induce effective, noninterfering immunity if antigens were administered simultaneously, several immunization protocols were studied using dPNAG, CP5, and CP8 conjugated to tetanus toxoid (TT). These antigens were injected subcutaneously alone, mixed together, or into 2 different injection sites (Table 2).
Table 2.
Antigens Used to Immunize 9 Groups of Mice (n = 4 per group)
| Group | Antigens Injected (1 μg/dose) |
|---|---|
| Antigens injected individually | |
| 1 | dPNAG-TT |
| 2 | CP8-TT |
| 3 | CP5-TT |
| Antigens mixed together and injected | |
| 4 | dPNAG-TT + CP8-TT |
| 5 | dPNAG-TT + CP5-TT |
| 6 | dPNAG-TT + CP8-TT + CP5-TT |
| Antigens injected into separate flanks | |
| 7 | dPNAG-TT and CP8-TT |
| 8 | dPNAG-TT and CP5-TT |
| 9 | dPNAG-TT and CP8-TT + CP5-TT |
Abbreviations: CP5, capsular polysaccharide 5; CP8, capsular polysaccharide 8; dPNAG, deacetylated poly-N-acetyl glucosamine; TT, tetanus toxoid.
No natural antibodies to PNAG, CP5, or CP8 were detected in any pre-immunization mouse serum samples (Supplementary Figure 1). Immunization with dPNAG-TT alone (Supplementary Figure 2A) generated a good immune response to PNAG. A diminished immune response to PNAG was measured when mice were immunized with dPNAG-TT plus one or both of the CP5-TT or CP8-TT antigens, except when immunization was performed by injecting dPNAG-TT at one site and a combination of CP5-TT and CP8-TT at another site (Supplementary Figure 2A). After immunization by CP5-TT alone or CP8-TT alone (Supplementary Figure 2B and 2C), good immune responses to CP5 and CP8, respectively, were detected. Positive control binding curves are shown in Supplementary Figure 2D. Combining CP-TT antigens with dPNAG-TT antigens had little impact on immunogenicity and binding of CPs, as determined by enzyme-linked immunosorbent assay (ELISA).
Mice immunized with dPNAG-TT or CP8-TT alone, but not CP5-TT, had an OPKA level against the PNAG- and CP8- producing S. aureus strain PS80 of ≥50% (Figure 2A). Mice immunized with dPNAG-TT alone or CP5-TT alone, but not CP8-TT, had serum with OPKA levels of ≥50% against the PNAG- and CP5-producing S. aureus strain Newman (Figure 2B). However, when a bivalent vaccine containing dPNAG-TT plus either CP5-TT or CP8-TT was administered into either the same or separate injection sites, we could not detect OPKA against either S. aureus strains Newman or PS80 or the non–CP-producing S. aureus strain LAC (pulsed-field type USA300; Supplementary Figure 3A–3C). Similarly, when we mixed different dilutions of the antiserum raised to dPNAG-TT alone, that had OPKA level of ≥50%, with a constant concentration of the antiserum to either CP5 (Figure 2C) or CP8 (Figure 2D), we found the OPKA level of the antibodies to CP was reduced to <30%, and this interference titered out as the concentrations of antibodies to PNAG were reduced.
Figure 2.
Opsonophagocytic killing activity in antiserum from mice immunized by antigens injected individually or in combination. A and B, Killing of Staphylococcus aureus PS80 (CP8) or S. aureus Newman (CP5), respectively, by indicated antiserum at the dilution indicated on the x-axis from mice immunized with dPNAG-TT, CP5-TT, or CP8-TT antigens alone. C and D, Killing of CP5 or CP8, respectively, by antibody to dPNAG-TT or CP injected individually or mixed together (decreasing amounts of antibody to dPNAG-TT added to constant amount of antibody to CP). Bars indicate the means of 4 replicates per assay. Controls were pre-immune serum (A and B) or tubes lacking complement (C and D). Abbreviations: CP, capsular polysaccharide; dPNAG, deacetylated poly-N-acetyl glucosamine; TT, tetanus toxoid.
We next studied whether OPKA could be measured in the antiserum to both CP and PNAG antigens by removing antibody to one or the other surface polysaccharide. Serum samples were absorbed with a PNAG-negative Δica strain or with a CP-negative Δcap strain of S. aureus (Supplementary Figure 4A–4D). After absorption, there was clear OPKA against S. aureus Newman or PS80 in serum of mice co-immunized with bivalent dPNAG-TT and CP5-TT or CP8-TT (Supplementary Figure 4A and 4B), indicating interference in the functional activity of these antibodies when both are present in the same serum. Serum absorbed with a double ica and cap mutant to leave behind antibodies to both CP and PNAG antigens had little OPKA. Removal of antibodies to PNAG in antiserum of mice immunized with the trivalent combination of dPNAG-TT, CP8-TT, and CP5-TT antigens increased the OPKA quite clearly (Supplementary Figure 4C), again confirming that functional antibodies to CP were present in the antiserum whose activity was being inhibited by the antibodies to PNAG.
In the serum of mice injected with dPNAG-TT and CP-TT antigens separately into contralateral flanks that would be drained by different lymph nodes, there was no OPKA either before or after absorptions with the different single mutants to remove either antibody to PNAG or CP antigens (Supplementary Figure 5A–5D). This failure to elicit measurable OPKA levels occurred in all 3 groups of mice immunized in separate flanks with dPNAG-TT and CP5-TT or dPNAG-TT and CP8-TT as well as in mice immunized with dPNAG-TT in one flank and a mixture of CP5-TT and CP8-TT in the contralateral flank. Thus, in spite of detecting binding antibody by ELISA to these antigens, this method of immunization did not result in induction of functional OPKA antibodies.
In serum samples from 15 healthy adult subjects with no history of significant S. aureus infection, as well as a pool of commercially available NHS, we detected immunoglobulin G antibodies to PNAG, with titers of ≥640 (Figure 3A). One NHS sample had antibodies to CP5 (Figure 3B) and 2 had antibodies to CP8 (Figure 3C), one being from the same individual with antibodies to CP5. To determine whether antibodies or other factors in NHS interfered with binding of rabbit polyclonal antibodies raised to either CP5-TT or CP8-TT to their respective antigens, we added 1:10 dilutions of the animal antiserum to 1:10 dilutions of the 15 NHS samples, subsequently diluted these samples, and measured the reactivity of the rabbit polyclonal antibodies to CP antigens. Overall, the rabbit antibodies bound well to CP5 or CP8 antigens (Figure 3D and 3E), although several of the NHS samples did produce some diminution (up to 25% decrease) of the binding of the rabbit polyclonal antibodies to CP antigens.
Figure 3.
Binding of immunoglobulin G (IgG) in normal human serum to Staphylococcus aureus antigens poly-N-acetyl glucosamine (PNAG), capsular polysaccharide 5 (CP5), and capsular polysaccharide 8 (CP8). A–C, Binding of IgG in 15 normal human serum (NHS) samples and a pool of NHS to S. aureus antigens: PNAG (0.06 µg/well), CP5 (3 µg/well) or CP8 (3 µg/well), respectively. Dashed lines indicate NHS with high level of IgG to CP5 (B) or CP8 (C). D and E, Binding of rabbit antibody raised to CP5–tetanus toxoid (TT) to CP5 or rabbit antibody raised to CP8-TT to CP8, respectively, in the presence of 1:10 dilutions of 15 NHS samples. F, Positive controls: rabbit antiserum raised to PNAG (top), rabbit antiserum raised to CP5-TT (center), or rabbit antiserum raised to CP8-TT (bottom). Abbreviations: CP, capsular polysaccharide; OD, optical density; PNAG, poly-N-acetyl glucosamine; TT, tetanus toxoid.
Assessment of the endogenous OPKA to S. aureus in the NHS samples found that only the 2 serum samples that had binding by ELISA to a CP antigen had OPKA levels of >30% against S. aureus Newman or PS80 (Figure 4A). To determine whether antibodies to PNAG in the NHS could interfere with the OPKA of immunization-induced rabbit antibodies to CP5 or CP8, we evaluated the effect of mixing the rabbit antiserum with either unabsorbed NHS or S. aureus Δica absorbed NHS. Thirteen of 15 unabsorbed NHS samples inhibited killing of S. aureus Newman or PS80 mediated by rabbit antibodies to CP5 or CP8 (Figure 4B); the 2 exceptions were found to correspond with NHS samples 2 and 3 (Figure 3C), which had endogenous activity to CP antigens. The maximal interference was observed at the highest concentration of NHS tested, after which the interference titered out. One NHS sample (No. 8) inhibited the OPKA mediated by antibodies to CP8 but not to CP5 (Figure 4B and 4C). The interference was removed by absorbing the NHS with S. aureus Δcap5 or S. aureus Δcap8 (Figure 4C) to remove the antibodies to PNAG. Thus, absorption of antibodies to PNAG from most NHS removes interference and restores OPKA mediated by rabbit antibodies to CP antigens.
Figure 4.
Opsonophagocytic killing activity (OPKA) of 15 normal human serum (NHS) samples. A, OPKA of Staphylococcus aureus Newman or PS80 strains. Controls lacked functional complement; test samples had all OPK components present. Symbols indicate OPK in 1 NHS sample, and bars indicate the group medians. Control rabbit antibody to CP5 or CP8 mediated 62%–70% killing at a 1:10 serum dilution. B, OPKA of S. aureus Newman or PS80 strains mediated by rabbit antibody to CP5-TT or to CP8-TT, respectively, when added to the indicated dilution of 15 unabsorbed NHS samples. Three NHS samples showed a greater decrease in OPKA of the rabbit antibody to capsular polysaccharide (CP) at a 1:40 dilution compared with that at a 1:10 dilution (1 for CP5, 2 for CP8), and 1 NHS sample had no reduction in the OPKA of rabbit antibody to CP5. Symbols indicate OPKA of rabbit immune serum mixed with 1 NHS sample at the indicated dilution, and bars indicate the group medians. Control rabbit antibody to CP5 and CP8 in the absence of added NHS mediated 60%–62% killing at a 1:10 serum dilution. Controls lacking complement had <1% killing. C, OPKA of S. aureus Newman or PS80 strains mediated by rabbit antibody to CP5-TT or CP8-TT, respectively, when added to indicated dilutions of 15 NHS samples that had been absorbed to remove natural antibody to poly-N-acetyl glucosamine. Results shown are in the presence of all OPK components. Symbol indicate activity of the rabbit antiserum in 1 NHS sample at the indicated dilution, and bars indicate the group medians. Control rabbit antibody to CP5 and CP8 in the absence of added NHS mediated 65%–70% killing at a 1:10 serum dilution. Controls lacking complement had <1% killing. Abbreviations: NHS, normal human serum; TT, tetanus toxoid.
These results were confirmed with a pool of NHS samples, wherein no OPKA level of >30% against S. aureus strains Newman (Figure 5A) or PS80 (Figure 5B) was present regardless of whether NHS samples were unabsorbed or absorbed to remove antibody to either PNAG or CP antigens. When the pooled NHS, either unabsorbed or absorbed to leave behind antibody to PNAG, was added to rabbit antibody to CP5-TT, there was a small interference (20% decrease) in OPKA, but a stronger interference of OPKA by the rabbit antibody to CP8-TT (Figure 5C and 5D). Absorption of the pooled NHS with S. aureus Newman Δcap5 or PS80 Δcap8 to remove antibodies to PNAG eliminated the interfering effect.
Figure 5.
Opsonophagocytic killing activity (OPKA) in a pool of normal human serum (NHS) samples. A and B, OPK of Staphylococcus aureus strain Newman or PS80, respectively, mediated by rabbit antibody raised to dPNAG-TT or rabbit antibody raised to CP5-TT or CP8-TT or mediated by the NHS pool. Controls had no complement. Rabbit antiserum raised to dPNAG-TT or CP-TT antigens individually had good killing. No killing (>30%) was mediated by the pool of NHS, unabsorbed or absorbed with S. aureus mutants to leave behind only antibodies to poly-N-acetyl glucosamine (PNAG; absorption with S. aureus Δica) or to capsular polysaccharide (CP; absorption with S. aureus Δcap). C and D, Effect on OPKA of the addition of indicated dilutions of pooled NHS to a constant amount of rabbit antibody to either CP5-TT or CP8-TT, respectively. NHS samples were either unabsorbed or absorbed to remove antibody to CP antigens (absorption with Δica mutant) or PNAG (absorption with Δcap mutant). Bars indicate the means of 4 replicates per assay. Controls lacking complement had killing of <1%. Abbreviations: CP, capsular polysaccharide; dPNAG, deacetylated poly-N-acetyl glucosamine; NHS, normal human serum; TT, tetanus toxoid.
There was no interference in OPKA against either S. aureus Newman (Supplementary Figure 6A) or PS80 (Supplementary Figure 6B) when we added polyclonal rabbit antibodies to PNAG to either unabsorbed or absorbed NHS.
In experimental S. aureus skin abscesses, mice immunized intraperitoneally with polyclonal antibodies to dPNAG-TT alone or mixed together with NHS (equal volumes) had a significant reduction in the number of colony-forming units (CFUs) per abscess with S. aureus PS80 (Figure 6A) or Newman (Figure 6B), compared with mice injected with NHS alone, indicating that antibodies to PNAG were protective in the presence of NHS. Mice challenged with S. aureus PS80 after injection with antibody to CP8 had significantly reduced numbers of CFUs per abscess, whereas coinjection of antibody to CP8-TT and NHS abrogated this protection (P < .006; Figure 6C). Interestingly, but consistent with the OPKA data showing only small interference when unabsorbed pooled NHS was added to rabbit antibody to CP5-TT (Figure 5C), no interference in protective activity was observed in vivo when the rabbit antibody to CP5-TT was added to the pooled NHS (Figure 6D). As the pooled NHS had low interference of the OPKA mediated by the CP5-TT induced antibody, whereas some of the individual NHS samples tested had high interference in this setting, we pooled 6 individual NHS samples with high in vitro interference in the OPKA and tested them alone or after mixing with rabbit antibodies to CP5-TT to test for OPKA (Supplementary Figure 7A) and protection (Supplementary Figure 7B). We detected high levels of interference in OPKA with the selected NHS pool, as well as in vivo, wherein the rabbit antibodies to CP5 lost their protective efficacy when combined with these selected human serum samples. Using this selected NHS pool, we then found that the interference mediated by the natural antibodies to PNAG on the OPKA of the anti-capsule antibodies could also be removed after absorption of the NHS by a non–S. aureus PNAG-producing bacterium such as Escherichia coli (Supplementary Figure 8). Finally, the interference mediated by the natural antibodies to PNAG present in NHS was also present when a mouse model of bacteremia was used (Supplementary Figure 9) with significantly fewer CFUs in the blood of the mice immunized by rabbit antibodies to CP5 (Supplementary Figure 9A) or CP8 (Supplementary Figure 9B) than in the blood of mice immunized with NHS or a mix of NHS and anti-CP antibodies.
Figure 6.
Effect of pooled normal human serum (NHS) samples on the number of colony-forming units (CFUs) of Staphylococcus aureus per abscess detected in mice given rabbit antibody to deacetylated poly-N-acetyl glucosamine (dPNAG)–tetanus toxoid (TT) or to CP5-TT or CP8-TT. A and B, Antibody raised to dPNAG-TT without or with NHS (equal volume) significantly reduced the CFUs of S. aureus per abscess compared with mice injected with NHS. Challenge was S. aureus PS80 (A) or Newman (B). Overall analysis of variance (ANOVA); P < .0005; pairwise comparisons shown on figure. C, CFUs of S. aureus per abscess in mice injected with rabbit antibody to CP8-TT, CP8-TT plus NHS, or NHS alone. Overall ANOVA; P < .006; pairwise comparisons shown on figure. Challenge was S. aureus PS80 (106 CFUs). D, CFUs of S. aureus per abscess in mice injected with rabbit antibody to CP5-TT, CP5-TT plus NHS, or NHS alone. Overall ANOVA; P < .0001; pairwise comparisons shown on figure. Challenge was S. aureus Newman (106 CFUs). Abbreviations: CP, capsular polysaccharide; NHS, normal human serum; NS, not significant; PNAG, poly-N-acetyl glucosamine; TT, tetanus toxoid.
DISCUSSION
For many bacterial pathogens, acquisition of bactericidal or opsonic antibodies to surface capsules correlates with long-term immunity [9, 11, 19]. However, S. aureus infections have recurrence rates of 20%–33% [4, 20, 21], usually with the same strain [20, 22], suggesting that the human immune response to infection with this pathogen often does not lead to capsule-specific protective responses. Prior results [16, 23], and results presented here, indicate that opsonic antibodies to S. aureus CP or PNAG antigens are uncommon in either NHS or convalescent serum from S. aureus–infected humans [16]. One exception has been found in serum from S. aureus–infected bacteremic patients [16]. However, within these serum samples there is a frequent occurrence of interference in OPKA between the antibodies to CP and PNAG antigens [16]. Here we demonstrated that this interference can occur not only with infection-induced antibodies to PNAG but also with natural, non-opsonic antibodies to PNAG that are present in most NHS samples by showing that adding NHS to immunization-induced rabbit antibodies to CP5 and CP8 abrogates their OPKA and protective efficacy in mouse models of skin infection and bacteremia. This finding could have been an important factor in the failures of 2 clinical trials of S. aureus CP-conjugate vaccines in hemodialysis patients [13, 24, 25] and could impact newer attempts to use the CP antigens as vaccines.
Interference was specific to natural human antibodies to PNAG, as absorption of NHS with a PNAG-producing S. aureus strain, but not a PNAG-deficient mutant strain, restored the OPKA and protective efficacy of immunization-induced antibody to CP antigens mixed with the absorbed NHS. The OPKA was also restored after absorption of the NHS with a non–S. aureus PNAG-producing bacterium such as E. coli. We did find the interference in the OPKA and protective activity of antibodies to CP5 in a pool of NHS was low and did not affect in vivo protection, but when a pool of NHS with higher interference in the CP5-specific OPKA was used, the rabbit antibodies to CP5 lost efficacy in vivo. Thus, interference between NHS antibodies to PNAG and antibodies to CP5 may be more variable than the interference between NHS antibodies to PNAG and antibodies to CP8. Although one can overcome the interference in OPKA and in vivo protection with a sufficiently high level of antibody to one of the S. aureus surface antigens in the presence of antibody to the other polysaccharide antigen [16], maintaining this high level over time following immunization may be difficult. This hypothesis is consistent with the results of a vaccine trial of CP-conjugate vaccine [13] wherein protective efficacy against S. aureus bacteremia was found at 40 weeks postvaccination but was lost at the study endpoint at 54 weeks. Although one explanation might be loss of adequate levels of antibody after 40 weeks in the immunized group, the reported mean antibody levels to the CP antigens at this point in the immunized group were quite high (>70 µg/mL), and thus a reasonable alternative explanation for the failure of this clinical trial is that the antibody to CP antigens had dropped below the efficacious level due to interference from the natural antibody to PNAG in the patient's serum.
There was no interference in the binding of mixtures of antibody to CP and PNAG antigens as determined by ELISA. We attribute this to the much higher concentration of antigen in an ELISA as compared to an OPK assay or an in vivo infection. ELISA plates are coated with 60–3000 ng of antigen per well in total, but in an OPK assay or in vivo infection, where the number of bacteria is approximately 2 × 105 CFUs/mL to perhaps a maximum of 106 CFUs/mL in vivo, respectively, the amount of surface polysaccharide present is likely in the low femtogram range [26, 27], thus 6–9 orders of magnitude lower than in the ELISA.
We attempted to engender noninterfering antibodies by injecting mice with dPNAG-TT and CP-TT antigens either combined together or into different anatomic sites that would be drained by different lymph nodes. Antibody responses were detectable by ELISA, but they were not functional in OPK assays. We were able to relieve the interferences by absorbing the serum with a mutant strain of S. aureus that left behind only antibodies to CP or PNAG antigens. The only exceptions were the mice given dPNAG-TT in one flank and CP5-TT and/or CP8-TT in other flank, wherein no OPKA was measured, even following serum absorptions. Overall, coimmunization did not redirect the antibody responses to noninterfering types.
Taken as a whole, the presence of natural, non-opsonic, nonprotective antibodies to PNAG in NHS could have several major negative consequences for human immunity to S. aureus. For example, patients with S. aureus bacteremia often make antibodies to the CP antigens [16], which could potentially be a protective immune response against reinfection, but the natural non-opsonic antibodies to PNAG present in most NHS appear to neutralize their protective efficacy in vivo. Additionally, natural antibodies to PNAG might have a deleterious effect on CP-directed active and passive vaccination strategies. Although there might be an initial period after vaccination wherein the level of antibodies to CP are high enough to overwhelm any interference from natural antibodies to PNAG, the decrease in antibody levels to the CP antigens would eventually be associated with a loss of protective activity when the interference from natural antibodies to PNAG takes effect. Notably, we found little to no natural antibodies to CP antigens in NHS, which suggests that opsonic antibody to PNAG may not suffer from the same consequence of interference in NHS as antibodies to the S. aureus CP antigens potentially do from the natural antibody to PNAG.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. D. S. designed, performed, and analyzed the animal studies and the study on the healthy subjects. A. K., C. T., and J. H. designed, performed, and analyzed the study with the German patients. D. R. performed and analyzed the study with the healthy subjects. D. S. and G. B. P. designed, analyzed, and interpreted the whole study and wrote the paper. All the authors critically revised the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants AI46706 and AI057159, a component of award U54 AI057159).
Potential conflicts of interest. G. B. P. is an inventor of intellectual property (PNAG vaccine and human monoclonal antibody to PNAG) that is licensed by Brigham and Women's Hospital (BWH) to Alopexx Vaccines and Alopexx Pharmaceuticals, companies in which G. B. P. owns equity. As an inventor of the intellectual property, he also has the right to receive a share of licensing-related income (royalties and fees) through BWH from Alopexx Pharmaceuticals and Alopexx Vaccines. G. B. P.'s interests were reviewed and are managed by the BWH and Partners Healthcare in accordance with their conflict of interest policies. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12:1715–23. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Edwards JR, Gaynes RP. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47:927–30. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 6.Lee BY, Ufberg PJ, Bailey RR, et al. The potential economic value of a Staphylococcus aureus vaccine for neonates. Vaccine. 2010;28:4653–60. doi: 10.1016/j.vaccine.2010.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BY, Wiringa AE, Bailey RR, Lewis GJ, Feura J, Muder RR. Staphylococcus aureus vaccine for orthopedic patients: an economic model and analysis. Vaccine. 2010;28:2465–71. doi: 10.1016/j.vaccine.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niessen LW, ten Hove A, Hilderink H, Weber M, Mulholland K, Ezzati M. Comparative impact assessment of child pneumonia interventions. Bull World Health Organ. 2009;87:472–80. doi: 10.2471/BLT.08.050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernatoniene J, Finn A. Advances in pneumococcal vaccines: advantages for infants and children. Drugs. 2005;65:229–55. doi: 10.2165/00003495-200565020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Trotter CL, McVernon J, Ramsay ME, et al. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine. 2008;26:4434–45. doi: 10.1016/j.vaccine.2008.05.073. [DOI] [PubMed] [Google Scholar]
- 11.Swingler G, Fransman D, Hussey G. Conjugate vaccines for preventing Haemophilus influenzae type b infections. Cochrane Database Syst Rev. 2009;4:CD001729. doi: 10.1002/14651858.CD001729. [DOI] [PubMed] [Google Scholar]
- 12.Lin FY, Ho VA, Khiem HB, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–9. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 13.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 14.Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:560–7. doi: 10.1093/cid/cir828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol. 2012;34:335–48. doi: 10.1007/s00281-011-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skurnik D, Merighi M, Grout M, et al. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J Clin Invest. 2010;120:3220–33. doi: 10.1172/JCI42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-β-(1–6)-glucosamine. Infect Immun. 2005;73:6752–62. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gening ML, Maira-Litran T, Kropec A, et al. Synthetic β-(1->6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect Immun. 2010;78:764–72. doi: 10.1128/IAI.01093-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granoff DM. Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine. 2009;27:B117–25. doi: 10.1016/j.vaccine.2009.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang SS, Hinrichsen VL, Datta R, et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS One. 2011;6:e24340. doi: 10.1371/journal.pone.0024340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–5. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 22.Huang SS, Diekema DJ, Warren DK, et al. Strain-relatedness of methicillin-resistant Staphylococcus aureus isolates recovered from patients with repeated infection. Clin Infect Dis. 2008;46:1241–7. doi: 10.1086/529381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fattom A, Schneerson R, Watson DC, et al. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun. 1993;61:1023–32. doi: 10.1128/iai.61.3.1023-1032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broughan J, Anderson R, Anderson AS. Strategies for and advances in the development of Staphylococcus aureus prophylactic vaccines. Exp Rev Vacc. 2011;10:695–708. doi: 10.1586/erv.11.54. [DOI] [PubMed] [Google Scholar]
- 25.Thomsen I, Dudney H, Creech CB. Searching for the holy grail of a staphylococcal vaccine. Hum Vaccin. 2010;6:1068–70. doi: 10.4161/hv.6.12.12917. [DOI] [PubMed] [Google Scholar]
- 26.Cerca N, Maira-Litran T, Jefferson KK, Grout M, Goldmann DA, Pier GB. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proc Natl Acad Sci USA. 2007;104:7528–33. doi: 10.1073/pnas.0700630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeth J, Lee JC. Antibodies to capsular polysaccharides are not protective against experimental Staphylococcus aureus endocarditis. Infect Immun. 1995;63:375–80. doi: 10.1128/iai.63.2.375-380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.