The treatment of hepatitis C virus infection has rapidly evolved after the approval and use of new protease inhibitor antiviral agents. This change affects the evaluation process, monitoring, and adverse event management. Treatments have become both more complex and more efficacious.
Abstract
Recent advances in the treatment of hepatitis C virus infection (HCV) have led to high rates of viral cure. However, the use of newly approved protease inhibitors with activity against HCV still requires careful patient selection, counseling, and decision making before initiation of treatment. Laboratory work-up, staging of liver disease, and careful review of comorbid conditions is mandatory. Patients with cirrhosis may require treatment regimens that differ from those without cirrhosis. Because pegylated interferon alfa and ribavirin remain a key part of the treatment regimen, absolute and relative contraindications to their use must be considered. Management of common adverse events including anemia and rash must be embraced by the healthcare provider.
The approval of 2 new direct-acting agents (DAAs) for hepatitis C virus (HCV) infection in 2011 ushered in a new era of antiviral therapy. Healthcare providers must now determine candidacy for treatment, and initiate complex treatment plans for those patients newly diagnosed with HCV infection using treatment algorithms and decision trees that vary from those previously used. The treatment remains difficult for patients and their care providers, and the selection of appropriate candidates requires careful thought, evaluation, and discussion and counseling for each individual patient. In this review, we will examine issues related to the selection of patients for treatment intervention with current therapies, the evaluation and testing that is necessary to arrive at appropriate treatment decisions, and the management of common issues that arise in the context of DAA-based therapy.
THERAPEUTIC APPROACH
Selection of Patients for Treatment
The selection of patients for treatment is complex and represents one of the greatest challenges to the clinician. The decision to treat is linked to both individual patient issues and system issues. We know that the literature is rife with studies describing the proportion of patients who are not selected for HCV treatment. Using the Veterans Affairs (VA) HCV Clinical Case Registry, Kramer and colleagues reported the proportion of those who received treatment with pegylated interferon and ribavirin among 99 166 patients with HCV viremia. Only 11.6% received treatment, and 6.4% completed treatment. Contraindications to treatment were documented in 57.2% of patients. Absolute contraindications were history of depression, prior organ transplant (renal, heart, lung), autoimmune hepatitis, severe hypertension, severe heart failure, significant coronary artery disease, poorly controlled diabetes, or severe chronic obstructive pulmonary disease. Depression was the most commonly cited (16.3%). Relative contraindications included use of drugs or alcohol (29.7%), human immunodeficiency virus (HIV; 6.3%), chronic renal disease (2.3%), decompensated cirrhosis (4.7%), liver transplant (0.4%), or uncontrolled psychiatric disease (5.3%) [1]. Interestingly, Cecil reported a treatment rate of 43% in a single VA hospital and suggested that low rates of treatment intervention in the VA may reflect cost-related limitations blamed on medical issues rather than true contraindications to treatment [2]. Other potential barriers to a positive decision regarding treatment include patient non-adherence, patient fears and misunderstandings, stigmatization, lack of financial resources, transportation or other logistical concerns, and communication difficulties [3].
Development of an ongoing therapeutic relationship is probably the most important aspect of treatment initiation and future treatment success. In general, patients should be seen by the clinician for several visits before a decision regarding treatment candidacy is reached. During this time, many potential treatment confounders can be addressed. For those with underlying cardiac disease, a stress test can be used to determine whether active angina is present. Stable coronary artery disease after revascularization or stenting does not preclude treatment. Similarly, history of depression does not prevent treatment. Active depression with suicidal ideation does require intervention before treatment is initiated. Patients with a long history of nonadherence or missed scheduled appointments should be told that they must come to clinic on a regular basis if they desire treatment. It is counterproductive to use poor compliance as an excuse if the patient is not given an opportunity to correct this conception. The negative impact of alcohol use on treatment success remains controversial, although heavy use can interfere with treatment compliance. However, recent data suggests that history of active alcohol use before treatment initiation plays little or no role in likelihood of achieving sustained virologic response (SVR) if the patient is compliant with the treatment regimen [4]. Of course, alcohol can cause liver damage leading to cirrhosis independent of treatment, and use should be strongly discouraged in all patients with underlying liver disease. Presence of active autoimmune immune processes that could flare with treatment that includes interferon should exclude those patients from HCV therapy. Similarly, any evidence of decompensated liver disease excludes the patient from therapy by clinicians who are not functioning in the setting of an active liver transplant center.
Evaluation and Treatment Selection Process
Laboratory Evaluation
There are multiple parameters that require evaluation before commitment to treatment intervention and to rule out other causes of liver disease. All patients should undergo testing for HCV viral load, HCV genotype and subtype, HIV antibody, hepatitis B virus status (hepatitis B surface antigen, hepatitis B surface antibody), hepatitis A virus antibody, a hepatic profile (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, bilirubin with fractionation, albumin), complete blood cell count with differential, platelet count, renal profile (creatinine, calculated creatinine clearance), thyrotropin, autoimmune markers (antinuclear antibody, ASMA, AMA), ceruloplasmin, α1-antitrypsin level, and iron saturation. Determination of the IL28B gene polymorphism genotype, which predicts likelihood of spontaneous clearance or treatment response, has become a valuable clinical tool in predicting which patients might have a shorter versus longer course of therapy as well as those that may have spontaneous clearance after acute HCV infection. For example, a patient who is reluctant to commit to 48 weeks of therapy may be encouraged to initiate therapy if the IL28B CC genotype is present. The role of each of these parameters in the treatment decision process is described in Table 1.
Table 1.
Laboratory Tests Used in the Treatment Decision Process for Hepatitis C Virus Infection
| Test | Purpose |
|---|---|
| HCV viral load | Predicts treatment response; does not predict disease severity |
| HCV genotype/subtype | Predicts treatment response; critical to choose correct treatment regimen |
| Hepatitis B surface antigen | Positive result indicates HBV coinfection |
| Hepatitis B surface antibody | Demonstrates protection against HBV and indicates need for vaccination |
| Hepatitis A virus antibody | Demonstrates protection against hepatitis A and indicates need for vaccination |
| Hepatic profile | ALT and AST indicate degree of liver injury present; bilirubin and alkaline phosphatase suggest presence of cholestatic liver processes |
| Complete blood cell count with differential | Provides baseline data before treatment with marrow-suppressive agents |
| Renal profile | Creatinine and creatinine clearance needed to determine treatment candidacy and need for adjustment of dose of some medications |
| Thyrotropin | Marker of thyroid disease that may need to be addressed before or during HCV therapy |
| Autoimmune markers ANA, ASMA (anti-actin antibody), AMA | May indicate presence of underlying comorbid processes that can affect liver; titers >1:80 suggest need to evaluate liver biopsy before treatment initiation |
| α1-Antitrypsin | Protein made by liver; low levels may indicate presence of 1 or 2 alleles for gene polymorphism associated with chronic liver injury |
| Iron saturation | Iron/total iron–binding capacity; levels >50% may suggest presence of genetic hemochromatosis |
| Ceruloplasmin | Copper transport protein; low levels may indicate presence of Wilson's disease (rare) |
| IL28B genotype | Predicts response to HCV treatment |
Abbreviations: ALT, alanine aminotransferase; AMA, antimitochondrial antibody; ANA, antinuclear antibody; ASMA, anti-smooth muscle antibody; AST, aspartate aminotransferase; HBV, hepatitis B virus; HCV, hepatitis C virus.
Liver Disease Staging
Liver biopsy remains an important modality in the classification and management of liver disease in those with HCV infection. The liver biopsy provides information regarding degree of hepatic fibrosis, the amount of inflammation present and may provide information regarding the presence of other liver diseases and processes including hepatic steatosis, evidence of prior alcohol injury, autoimmune disorders, and other infiltrating processes. Many experts believe that the importance of liver biopsy may be decreasing as treatments become more effective, but key treatment decisions still revolve around the presence or absence of cirrhosis when using first-generation DAAs like telaprevir and boceprevir. If a patient is cirrhotic, both the Food and Drug Administration and most clinician experts believe that longer therapy maximizes treatment response. Despite this, overall rates of SVR are lower among those with cirrhosis. Finally, there are significant implications to the presence of advanced fibrosis/cirrhosis that go beyond treatment prognosis and treatment duration, namely the implementation of surveillance for hepatocellular carcinoma and esophageal varices. Therefore, it is incumbent on the healthcare provider to obtain information regarding fibrosis stage.
There is significant interest in the use of noninvasive markers of fibrosis. This may be accomplished by either biochemical biomarker panels, or by use of transient elastography. Data on biomarker panels, such as HCV FibroSURE, FIB-4, and APRI, are mixed in terms of predictive capacity. In a large study of 2060 patients, the sensitivity for detection of cirrhosis was 63%, with a specificity of 85%. The misclassification rate was 34% compared with liver biopsy [5]. There is also significant interlaboratory variability in the results, especially in predicting the presence of F4 (cirrhosis) disease [6]. Of course, liver biopsy is also subject to sampling error and comparisons of noninvasive test methods to inadequate biopsies must be considered when interpreting comparative studies.
Transient elastography is widely used in Europe to stage liver fibrosis by determining the “stiffness” of the liver after interrogation with sound waves. The procedure has high predictive capacity for cirrhosis, but can be confounded by presence of fatty infiltrates or inflammatory cells. Ziol et al reported 84%–86% sensitivity when separating those with F0–F3 disease from those with F4 disease [7]. Magnetic resonance elastography has also been employed with a high degree of correlation to liver biopsy yielding areas under the curve >91.8% in differentiating cirrhosis from other disease stages [8].
In a practical sense, the decision to obtain a liver biopsy rather than perform a noninvasive marker test is highly linked to the comfort of the clinician in both using the data derived from the study and the way that the value of the assessment is conveyed to the patient. In the author's experience, many clinicians suggest that patients do not want to undergo a biopsy, but this is really an imprint of the healthcare provider's beliefs, not the view of the patient. Shire et al reported that 85% of 179 patients who underwent liver biopsy would be willing to have another biopsy performed [9]. It is important to ensure that if a patient is subjected to the risk and cost of a liver biopsy, that (1) the biopsy should be performed with a ≥16-gauge needle; (2) a ≥2-cm sample be obtained; (3) a cutting needle should be used, particularly if advanced fibrosis is suspected; and (4) the pathologic findings be interpreted by someone with special training in interpreting liver tissue [10, 11].
Decisions Regarding Treatment Medications
The first generation of DAAs have 2 important characteristics. First, they MUST be used in combination with pegylated interferon and ribavirin. Failure to do so ensures treatment failure due to selection of drug resistant variants that exist at the time of drug initiation. Second, both boceprevir and telaprevir are targeted against genotype 1 HCV virus. There are very limited data that suggest an incremental treatment benefit for use of telaprevir in patients with genotype 2 HCV but not those with genotype 3 [12]. Therefore, once a patient is selected as a candidate for therapy, the next question revolves around genotype. Most experts argue that all treatment-naive patients with genotype 1 should be offered triple-drug therapy with a DAA, pegylated interferon, and ribavirin. Some argue that pretreatment knowledge of IL28B genotype classification can help determine whether a DAA should be employed. Although patients with IL28B genotype CC have a high rate of response to pegylated interferon and ribavirin therapy, most will require 48 weeks of treatment. Many of these patients would be eligible for shorter treatment if boceprevir or telaprevir are included in the treatment regimen. Therefore, although longer treatment might be efficacious and cost-effective, it exposes patients to increased risks associated with longer term exposure to interferon alfa and ribavirin. Overall, 50%–65% of treatment-naive patients given triple-drug regimens will be eligible for shorter, response-guided therapy lasting 24–28 weeks.
The choice of which DAA to use is primarily dependent on drug availability and clinician comfort with that agent. Although no head-to-head trials have been performed, a recent meta-regression analysis failed to find an overall response difference between these choices [13]. There are clear differences in regimen (lead-in phase for boceprevir with longer DAA drug exposure) and side effect profiles (rash for telaprevir; dysgeusia for boceprevir). In addition, the stopping rules vary between agents, including cutoff values for treatment futility and the treatment intervals at which stopping and decision rules are obtained. These are clearly delineated in the product insert, and it behooves the treater to understand and apply these rules.
Common Management Issues
Anemia
Drug-associated anemia has represented a long-standing issue with regard to HCV treatment using pegylated interferon and ribavirin. Ribavirin is phosphorylated in erythrocytes, which leads to trapping and accumulation of metabolites, inducing hemolysis that leads to anemia [14]. This process is exacerbated by the marrow-suppressive properties of alfa interferons. Addition of both boceprevir and telaprevir contribute to even more significant anemia, presumably owing to increased direct suppression of erythropoiesis [15, 16]. Although the approved treatment regimens differ significantly in terms of the duration of drug exposure (12 weeks for telaprevir vs 24–44 weeks for boceprevir), a meta-regression failed to find statistically meaningful differences in rates of anemia between the 2 DAAs [13].
In the pivotal trials, the management of anemia was quite different for boceprevir and telaprevir. The boceprevir trials permitted use of erythrocyte-stimulating agents (ESAs), which were used extensively to manage anemia. Poordad et al reported that 43% of patients enrolled in both the response-guided therapy arm and the 48-week therapy arm took erythropoietin for anemia management. In contrast, only 24% of those in the pegylated interferon alfa 2b plus ribavirin control arm required ESAs [17]. In contrast, ESAs were not allowed in the phase 3 trials of telaprevir in treatment-naive patients. Instead, ribavirin dose reduction was the primary modality for management of anemia, although a handful of subjects did receive ESAs. Dose reduction guidelines followed those in the ribavirin package insert. Severe anemia was uncommon (2%–3%), but overall rates of anemia were reported as 37%–39% in the 12-week telaprevir arms [18, 19].
Subsequent analyses and additional trials shed additional light on anemia management issues. Poordad et al reported the results of a randomized trial comparing ribavirin dose reduction versus erythropoietin in patients receiving boceprevir at a recent international forum. Ribavirin dose reduction did not affect SVR rates compared with early use of erythropoietin, nor did it affect reporting of any adverse event [20]. Sulkowski et al examined the effect of ribavirin dose modification on SVR using pooled data from the 12-week treatment arms of the ADVANCE and ILLUMINATE trials [18, 19]. 50% of treatment-naive patients receiving telaprevir experienced a dose reduction of ribavirin. Dose reduction to ≤600 mg/d had no substantial effect on SVR rate [21]. These data do not suggest, however, that we can or should start treating patients with lower doses of ribavirin. Anemia has been cited as a surrogate for drug effectiveness in terms of SVR. It would be presumptive to extrapolate these finding to the assumption that less than 600 mg/d of ribavirin would be an effective starting dosage in all patients. That said, it seems that dose reduction is a safe and effective modality for initial management of all patients on triple therapy with either boceprevir or telaprevir. It may be reasonable to dose reduce ribavirin to 600 mg/d for all patients whose hemoglobin levels fall to <10 mg/dL and to avoid use of ESAs whenever possible.
Rash
The development of skin manifestations as a result of treatment is a common finding in patients treated with pegylated interferon and ribavirin. Alfa interferons are associated with both generalized rashes and local injection site irritation that occasionally progresses to dermal ulceration [22–24]. Ribavirin may also be associated with development of a drug eruption rash, although severe rashes are uncommon [25, 26]. Telaprevir is frequently associated with rashes, some severe. Only a small percentage of skin reactions attributable to telaprevir result in drug discontinuation, but overall rates of cutaneous diagnoses during treatment seem to exceed 50% of treated patients [18, 19]. Severe skin reactions, including drug rash with eosinophilia and systemic symptoms (DRESS) have been reported [27]. An example of this rash is seen in Figure 1.
Figure 1.
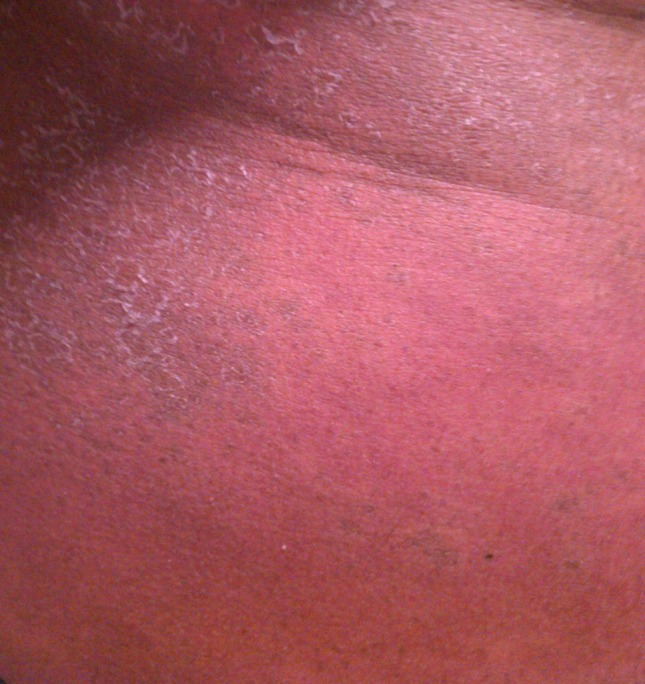
Example of rash and desquamation seen with telaprevir.
Management of rash in the setting of HCV treatment is more art than science. Site rotation for pegylated interferon injections seems to reduce risk of local reactions. A 4-quadrant approach is recommended using abdominal sites. This permits healing resolution of local reactions with a 4-week window before an injection is administered in a particular quadrant. Skin breakdown (ulceration) requires discontinuation of therapy. Anecdotal data suggests these lesions will not heal while interferon is being administered. Both ribavirin rash and telaprevir rash can be treated symptomatically using topical steroids for milder cases. Data from the ADVANCE trial suggest that telaprevir discontinuation between week 8 and 12 has little detrimental effect on SVR rates [18]. Therefore, in this period, telaprevir should be discontinued if a severe rash (>50% of body surface area or with any sign of mucosal involvement or bulla formation) appears. Patients with DRESS must have discontinuation of all drugs and evaluation to determine whether systemic steroids are indicated. Rash whose suspected cause may be ribavirin could be treated by temporary withdrawal of ribavirin, with restart after 1–2 weeks.
Other Adverse Events
A host of other side effects and adverse events might be observed in the context of triple therapy. Boceprevir is associated with dysgeusia (bad taste in the mouth). There is no effective management for this symptom. Autoimmune disease processes may be exacerbated by pegylated interferons and generally represent contraindications to therapy (eg, rheumatoid arthritis, Crohn's disease). Psoriasis may be worsened by treatment of HCV due to the interferon immune effects. However, use of topical steroids and occasionally phototherapy does make treatment tolerable in some patients. Psychiatric risks to treatment are due to increased risk of depression in the setting of interferon usage. Mild to moderate depression can be managed by the HCV-treating physician and does not necessarily require comanagement with mental health professionals. Development of expertise with a limited number of selective serotonin reuptake inhibitors is recommended. More severe cases of depression, poorly controlled bipolar disease, and schizophrenia generally require evaluation and assistance of a psychiatrist before embarking on therapy.
CONCLUSION
The treatment of HCV in the dawn of the era of DAAs has become more, not less, complex. The healthcare provider must carefully select patients for treatment candidacy, stage their liver disease appropriately, choose a treatment regimen, and provide ongoing support throughout the treatment period. Future therapies are likely to have fewer side effects and less frequent dosing, which will result in higher rates of uptake by potential providers.
Notes
Financial support. This work was supported by the National Institutes of Health (NIDDK K24 DK070528 to K. E. S.).
Potential conflicts of interest. Author certifies no potential conflicts of interest.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–5. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Cecil B. Reply to “Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012 doi: 10.1016/j.jhep.2011.05.032. http://dx.doi.org/10.1016/j.jhep.2012.03.009 . [DOI] [PubMed] [Google Scholar]
- 3.McGowan CE, Fried MW. Barriers to hepatitis C treatment. Liver Int. 2012;32(Suppl 1):151–6. doi: 10.1111/j.1478-3231.2011.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell M, Pauly MP, Moore CD, et al. The impact of lifetime alcohol use on hepatitis C treatment outcomes in privately insured members of an integrated health care plan. Hepatology. 2012 doi: 10.1002/hep.25755. doi:10.1002/hep.25755. [published online ahead of print 5 April 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581–9. doi: 10.3748/wjg.v17.i41.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gressner OA, Beer N, Jodlowski A, Gressner AM. Impact of quality control accepted inter-laboratory variations on calculated Fibrotest/Actitest scores for the non-invasive biochemical assessment of liver fibrosis. Clin Chim Acta. 2009;409:90–5. doi: 10.1016/j.cca.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 8.Yin M, Woollard J, Wang X, et al. Quantitative assessment of hepatic fibrosis in an animal model with magnetic resonance elastography. Magn Reson Med. 2007;58:346–53. doi: 10.1002/mrm.21286. [DOI] [PubMed] [Google Scholar]
- 9.Shire NJ, Dorey-Stein Z, Ferra T, et al. Willingness to undergo a repeat liver biopsy among HIV/HCV coinfected and HCV-monoinfected patients. 2009 doi: 10.1097/MCG.0b013e318266fe70. Conference on Retroviruses and Opportunistic Infections (CROI) [abstract 846]. Alexandria, VA: CROI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman KE, Goodman ZD, Sullivan ST, Faris-Young S. Liver biopsy in cirrhotic patients. Am J Gastroenterol. 2007;102:789–93. doi: 10.1111/j.1572-0241.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- 11.Bejarano PA, Koehler A, Sherman KE. Second opinion pathology in liver biopsy interpretation. Am J Gastroenterol. 2001;96:3158–64. doi: 10.1111/j.1572-0241.2001.05273.x. [DOI] [PubMed] [Google Scholar]
- 12.Foster GR, Hezode C, Bronowicki JP, et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology. 2011;141:881–9. doi: 10.1053/j.gastro.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Cooper CL, Druyts E, Thorlund K, et al. Boceprevir and telaprevir for the treatment of chronic hepatitis C genotype 1 infection: an indirect comparison meta-analysis. Ther Clin Risk Manag. 2012;8:105–30. doi: 10.2147/TCRM.S29830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page T, Connor JD. The metabolism of ribavirin in erythrocytes and nucleated cells. Int J Biochem. 1990;22:379–83. doi: 10.1016/0020-711x(90)90140-x. [DOI] [PubMed] [Google Scholar]
- 15.Products FDoA. Background material for boceprevir advisory committee. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/antiviraldrugsadvisorycommittee/ucm252341.pdf . Accessed 30 April 2012. [Google Scholar]
- 16.Birnkrant D. Director, Division of Antiviral Products, FDA. 2011 Advisory Committee Briefing Document for NDA 201-017 Telaprevir. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/antiviraldrugsadvisorycommittee/UCM252561.pdf . Accessed 30 April 2012. [Google Scholar]
- 17.Limaye AR, Draganov PV, Cabrera R. Boceprevir for chronic HCV genotype 1 infection REPLY. N Engl J Med. 2011;365:176; author reply 177–8. doi: 10.1056/NEJMc1105515. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 19.Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection [published correction appears in N Engl J Med 2011;365:1551] N Engl J Med. 2011;365:1014–24. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poordad FF, Lawitz EJ, Reddy KR, et al. A randomized trial comparing ribavirin dose reduction versus erythropoietin for anemia management in previously untreated patients with chronic hepatitis C receiving boceprevir plus peginterferon/riba. 2012 EASL: The International Liver Congress 2012. Barcelona, Spain. Amsterdam: Elsevier. [Google Scholar]
- 21.Sulkowski MS, Roberts S, Afdhal N, et al. Ribavirin dose modification in treatment-naive and previously treated patients who received telaprevir combination treatment: no impact on sustained virologic response in phase 3 trials. 2012 EASL: The International Liver Congress 2012. Barcelona, Spain. Amsterdam: Elsevier. [Google Scholar]
- 22.Sickler JB, Simmons RA, Cobb DK, Sherman KE. Cutaneous necrosis associated with interferon alpha-2b. Am J Gastroenterol. 1998;93:463–4. doi: 10.1111/j.1572-0241.1998.00463.x. [DOI] [PubMed] [Google Scholar]
- 23.Sidhu-Malik NK, Kaplan AL. Multiple fixed drug eruption with interferon/ribavirin combination therapy for hepatitis C virus infection. J Drugs Dermatol. 2003;2:570–3. [PubMed] [Google Scholar]
- 24.Mistry N, Shapero J, Crawford RI. A review of adverse cutaneous drug reactions resulting from the use of interferon and ribavirin. Can J Gastroenterol. 2009;23:677–83. doi: 10.1155/2009/651952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–44. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 26.Veluru C, Atluri D, Chadalavada R, Burns E, Mullen KD. Skin rash during chronic hepatitis C therapy. Gastroenterol Hepatol (N Y) 2010;6:323–5. [PMC free article] [PubMed] [Google Scholar]
- 27.Montaudie H, Passeron T, Cardot-Leccia N, Sebbag N, Lacour JP. Drug rash with eosinophilia and systemic symptoms due to telaprevir. Dermatology. 2010;221:303–5. doi: 10.1159/000318904. [DOI] [PubMed] [Google Scholar]


