Abstract
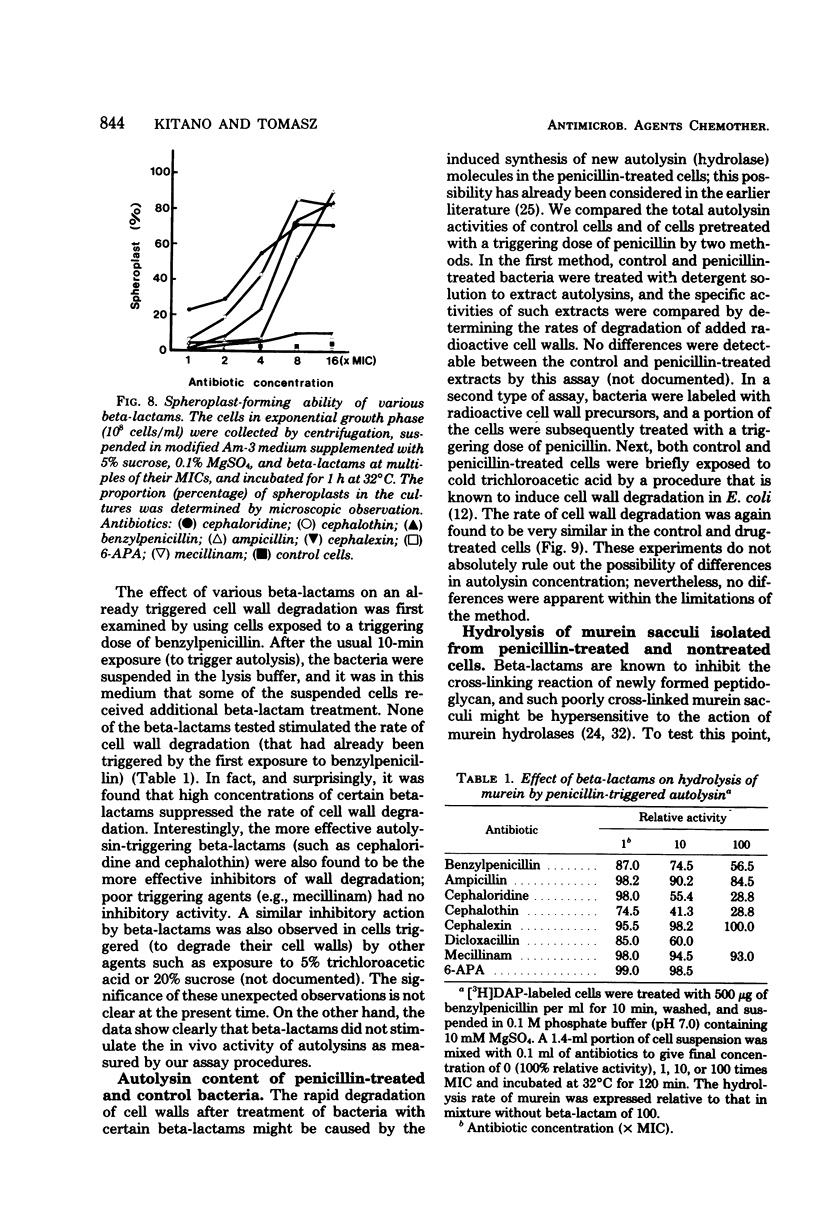
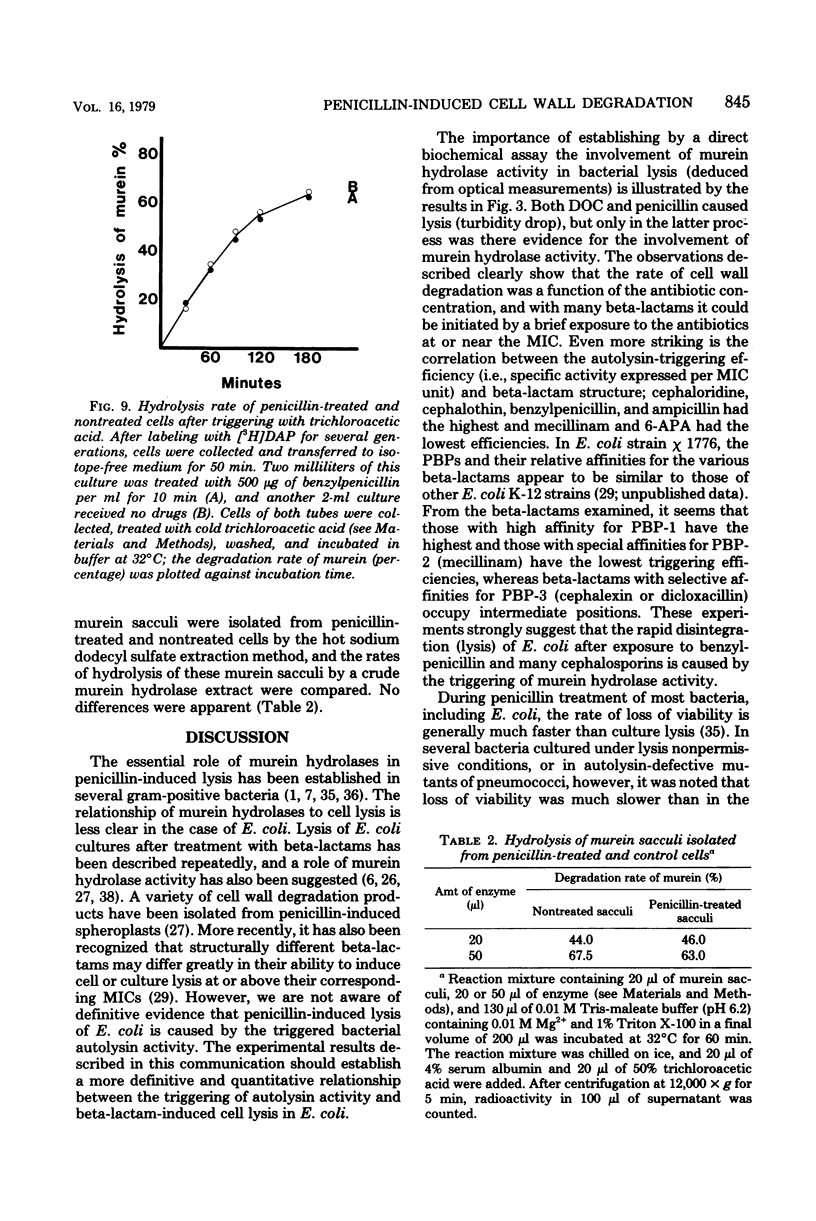
A biochemical method was developed to quantitatively compare the effectiveness of beta-lactams in triggering murein degradation (autolysin activity) in Escherichia coli. Bacteria prelabeled in their cell walls with radioactive diaminopimelic acid in growth medium were exposed for 10 min to the antibiotics at the appropriate minimal growth inhibitory concentrations and at multiples of these values, and the rate of cell wall degradation was followed during subsequent penicillin-binding protein (PBP)-1 were the most effective triggers of autolytic wall degradation; beta-lactams selective for PBP-2 were the poorest; and antibiotics preferentially binding to PBP-3 showed intermediate activities. The relative effectiveness of beta-lactams in autolysin triggering was found to parallel the effectiveness of the same drugs in causing rapid loss of viability, culture lysis, and spheroplast formation. Autolysin triggering was suppressed by inhibitors of protein and ribonucleic acid biosynthesis but not by inhibitors of deoxyribonucleic acid synthesis. The beta-lactam-induced cell wall degradation did not seem to involve a direct stimulation of enzyme activity or synthesis of new enzyme molecules, and murein sacculi isolated from cells that had been preexposed to a triggering dose of beta-lactam treatment exhibited the same sensitivity to crude, homologous autolysins as sacculi prepared from untreated control bacteria. On the basis of these observations, mechanisms are considered for the triggering of E. coli autolysins and for the role of autolytic activity in bacterial spheroplast formation, lysis, and death.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Yoneda Y., Yamane K., Maruo B. Pleiotropic phenomena in autolytic enzyme(s) content, flagellation, and simultaneous hyperproduction of extracellular alpha-amylase and protease in a Bacillus subtilis mutant. J Bacteriol. 1975 Oct;124(1):459–469. doi: 10.1128/jb.124.1.459-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Biological containment and cloning vector transmissibility. J Infect Dis. 1978 May;137(5):668–675. doi: 10.1093/infdis/137.5.668. [DOI] [PubMed] [Google Scholar]
- Fontana R., Satta G., Romanzi C. A. Penicillins activate autolysins extracted from both Escherichia coli and Klebsiella pneumoniae envelopes. Antimicrob Agents Chemother. 1977 Dec;12(6):745–747. doi: 10.1128/aac.12.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Fazio M., Tomasz A. Effect of benzylpenicillin on the synthesis and structure of the cell envelope of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978 Mar;13(3):514–526. doi: 10.1128/aac.13.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. FL 1060: a new beta-lactam antibiotic with novel properties. J Clin Pathol. 1973 Jan;26(1):1–6. doi: 10.1136/jcp.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. The two sites of penicillin action in Escherichia coli. J Infect Dis. 1973 Dec;128(6):791–794. doi: 10.1093/infdis/128.6.791. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Bock-Hennig S. B., Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. doi: 10.1111/j.1432-1033.1974.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J. Mechanism of action of penicillin. J Bacteriol. 1957 Jan;73(1):144–144. doi: 10.1128/jb.73.1.144-144.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Oka T. Mode of action of penicillins in vivo and in vitro in Bacillus megaterium. Antimicrob Agents Chemother. 1976 Oct;10(4):579–591. doi: 10.1128/aac.10.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. Induction of bacterial lysis by penicillin. J Bacteriol. 1957 Jul;74(1):48–59. doi: 10.1128/jb.74.1.48-59.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARZ U., WEIDEL W. ZUM WIRKUNGSMECHANISMUS VON PENICILLIN. I. ISOLIERUNG UND CHARAKTERISIERUNG 2,6-DIAMINOPIMELINSAEURE ENTHALTENDER NIEDERMOLEKULARER PEPTIDE AUS PENICILLINSPHAEROPLASTEN VON ESCHERICHIA COLI B. Z Naturforsch B. 1965 Feb;20:147–153. [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V., Zimmermann W. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob Agents Chemother. 1977 Sep;12(3):406–409. doi: 10.1128/aac.12.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Willoughby E., Kamiryo T., Blumberg P. M., Yocum R. R. Penicillin-sensitive enzymes and penicillin-binding components in bacterial cells. Ann N Y Acad Sci. 1974 May 10;235(0):210–224. doi: 10.1111/j.1749-6632.1974.tb43267.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]




