Abstract
STUDY QUESTION
Do exogenous male hormonal contraceptives that suppress intratesticular testosterone and spermatogenesis interfere with the blood–testis barrier integrity in men?
SUMMARY ANSWER
When spermatogenesis was suppressed by testosterone alone or combined with levonorgestrel (LNG) treatment in men, the structural appearance of Sertoli cell tight junctions remained intact in the human testis.
WHAT IS ALREADY KNOWN
Testosterone promotes the integrity of the blood–testis barrier. Intratesticular androgen deprivation induced by exogenous testosterone plus a progestin to suppress spermatogenesis in a contraceptive regimen may disturb the structural and functional integrity of the blood–testis barrier.
STUDY DESIGN, SIZE AND DURATION
Testicular biopsies were obtained from a sub-study of a randomized clinical trial of 36 healthy Chinese men who were treated for 18 weeks and followed for at least a 12-week recovery period.
PARTICIPANTS/MATERIAL, SETTING, METHODS
Healthy Chinese male volunteers (27–48 years) were randomized to two treatment groups (n = 18/group) for 18 weeks: (1) testosterone undecanoate (TU) 1000 mg i.m. injection followed by a 500 mg injection every 6 weeks and (2) TU + LNG 250 μg orally daily. Blood samples were obtained from all participants before and during treatment and at the end of the recovery phase. Open testicular biopsies for this study were obtained from four men before treatment and from four men in each of the TU and TU + LNG groups at 2 and 9 weeks of treatment. The presence of antisperm antibodies was checked in the archived serum samples of the subjects at baseline, during treatment and at the end of the recovery period. Stored testicular biopsy samples from cynomolgus monkeys treated with either sub-cutaneous testosterone or placebo for 12 weeks were used for additional protein expression studies.
MAIN RESULTS AND ROLE OF THE CHANCE
Expression of blood–testis barrier associated proteins quantified by immunohistochemistry (claudin 3, claudin 11, junctional adhesion molecule-A, zonula occludens-1) remained unchanged despite a significant decrease in the numbers of pachytene spermatocytes and round spermatids in the seminiferous tubules at 9 weeks in the TU + LNG group. This was confirmed by immunoblots showing a lack of quantitative change in these tight junction proteins in monkeys after testosterone treatment. There were no increases in serum antisperm antibodies in the volunteers during the study.
LIMITATIONS/REASONS FOR CAUTION
The duration of the study was short and the long-term effects of male hormonal contraceptive treatments on the integrity of the blood–testis barrier remain to be determined.
WIDER IMPLICATIONS OF THE FINDINGS
This study supports the safety of male hormonal contraceptive treatment and does not corroborate the previous findings of disturbed immunological integrity of the blood–testis barrier from animal studies such as androgen receptor knockout mice and exogenous hormonal treatment in rats.
STUDY FUNDING/COMPETING INTEREST
The study was supported by grants from the Contraceptive Research and Development Program and the Mellon Foundation (MFG-02-64, MFG-03-67), Endocrine, Metabolism and Nutrition Training Grant (T32 DK007571), the Clinical and Translational Science Institute at Los Angeles Biomedical and Harbor-UCLA Medical Center (UL1RR033176 and UL1TR000124) and the Los Angeles Biomedical Research Institute Summer High School Student Program.
Keywords: blood–testis barrier, Sertoli cell tight junctions, male contraception
Introduction
The blood–testis barrier, formed by tight junctions, basal ectoplasmic specializations, gap junctions and apical desmosome-like junctions, separates the seminiferous tubule into the basal and adluminal compartments. Tight junctions provide a barrier to restrict the diffusion of molecules through the paracellular pathway (Mitic and Anderson, 1998; Stevenson and Keon, 1998), to create a boundary between compartments with different protein and lipid compositions, and to maintain the epithelial and endothelial polarity (Rodriguez-Boulan and Nelson, 1989). The blood–testis barrier also forms an immunological barrier excluding the entry of immunoglobulins and lymphocytes into the adluminal compartment and preventing germ cell components crossing the barrier to elicit an immunological response in the body (Setchell et al., 1969; Morita et al., 1999). Tight junctions in the testis are not assembled until early puberty (15 days after birth) in the rat (Cheng and Mruk, 2002) and they are established when the spermatogonia proliferate to produce primary spermatocytes in men (Furuya et al., 1978). The blood–testis barrier is dynamic and is located close to the basal lamina of the seminiferous tubules. As the migrating spermatocytes traverse the blood–testis barrier, old tight junctions are dissembled and new tight junctions are formed behind them allowing spermatocyte migration without unsealing the blood–testis barrier (Cheng and Mruk, 2002). This process is regulated by testosterone at the hormone-sensitive stage of the seminiferous epithelium cycle (,Siu et al., 2005; Cheng and Mruk, 2009).
Tight junction associated integral membrane proteins present in the blood–testis barrier include occludin, claudin and junctional adhesion molecules (JAM), while zonula occludens-1 (ZO-1) is a linker protein coupling the transmembrane tight junction proteins to the cytoskeleton. Occludin/ZO-1, claudin/ZO-1, JAM-A/ZO-1 and JAM-B/ZO-1 complexes are expressed in the blood–testis barrier (Cheng and Mruk, 2002, 2009; Siu et al., 2005). Occludin is present in tight junctions of the mouse and rat but not in human or guinea pig Sertoli cells (Moroi et al., 1998). The blood–testis barrier in young adult occludin-knockout mice is intact and these mice are fertile probably due to temporary compensation by other tight junction proteins. When these knockout mice age, they become infertile, with atrophic seminiferous tubules devoid of germ cells, suggesting that the compensatory increase in other proteins cannot sustain spermatogenesis in adults (,Saitou et al., 2000; Takehashi et al., 2007). Claudins are the principal proteins responsible for constructing seal-forming elements in tight junctions (,Tsukita and Furuse, 1999, 2000; Saitou et al., 2000). At least 24 claudins have been identified in tight junctions in different tissues (Mitic et al., 2000). Claudins 1, 3, 4, 5, 7, 8 and 11 are found in the testis (Cheng and Mruk, 2002). Claudin 11 expression in the testis appears to be limited to Sertoli cells (Cheng and Mruk, 2002) and has a crucial role in the assembly of tight junctions: mice lacking claudin 11 are sterile (Gow et al., 1999). However, testicular biopsies in men with defective spermatogenesis have an increased expression of claudin 11 in their testes; the cause of this increase is not clear (Nah et al., 2011). Claudin 3 is associated with newly formed tight junctions in the mouse testis (Meng et al., 2005). ZO-1 expression becomes progressively restricted to the site of developing tight junctions between Days 7 and 14 in the rat and mouse correlating with the assembly of the blood–testis barrier (Cheng and Mruk, 2002, 2009).
Testosterone promotes the integrity of the blood–testis barrier in vivo (Meng et al., 2005; Wang et al., 2006) and in vitro by enhancing the recycling of internalized proteins to the cell surface and relocating these proteins to reassemble and seal the barrier (Chung and Cheng, 2001; Kaitu'u-Lino et al., 2007; Yan et al., 2008). In rats, testicular androgen deprivation induced by testosterone plus estradiol implants results in germ cell loss but the blood–testis barrier integrity is maintained by an increase in tight junction proteins (Xia et al., 2005). However, another study showed that gonadotrophin releasing hormone (GnRH) antagonist treatment of adult rats to suppress serum LH and FSH, intratesticular testosterone and spermatogenesis induces seminiferous tubule permeability to biotin, loss of occludin and redistribution of claudin 11; this was partially reversed by treatment with human chorionic gonadotrophin to increase intratesticular testosterone (McCabe et al., 2010). Sertoli cell-specific ablation of the androgen receptor in mice results in decreased expression of claudin 3 and increased permeability of the blood–testis barrier (Meng et al., 2005), and is associated with the presence of antibodies against germ cell antigens in serum (Meng et al., 2011). These latter two rodent models suggest that the depletion of testosterone action on Sertoli cells disrupts tight junctional components and function, and raise the question of whether exogenous male hormonal contraceptives that suppress intratesticular testosterone interfere with the blood–testis barrier integrity in men, resulting in increased permeability, loss of immunological privilege of germ cells and leakage of germ cells components into the circulation.
To investigate whether decreased intratesticular testosterone induced by hormonal male contraceptive treatment disrupts the blood–testis barrier integrity in human testes, we examined testicular biopsy samples from men treated with either testosterone alone or in combination with levonorgestrel (LNG) in a male hormonal contraceptive clinical trial (Wang et al., 2007). Our data on the immunohistochemical localization of the tight junction proteins in human testis biopsy samples were complemented by immunoblots of these proteins in monkey testicular samples obtained after treatment with testosterone. We also measured serum antisperm antibodies before, during and after treatment in the human volunteers to investigate whether the blood–testis barrier is compromised, allowing sperm cell components to escape into the circulation to induce an immunological response.
Materials and Methods
Subjects
Healthy Chinese male volunteers (ages ranging from 27 to 48 years) were recruited from a factory near the Nanjing center. The volunteers had no significant medical history, and all had normal physical examination at enrollment. None of the subjects was undernourished. They had normal baseline hematology, blood biochemistry, urinalysis and fasting lipid profiles and three consecutive semen analyses collected at 3-week intervals that were within the WHO reference range. The study details have been described previously (Wang et al., 2007).
Study design
After a pretreatment observation phase of 4 weeks, the eligible subjects were randomized to two treatment groups (n = 18 per group) for 18 weeks: (1) testosterone undecanoate (TU) (TU 1000 mg i.m. injection of TU followed by TU 500 mg i.m. injection every 6 weeks and (2) TU + LNG 250 μg orally daily. The treatment phase was followed by a recovery phase of at least 12 weeks until the sperm concentration returned to the reference range (>20 million/ml). Detailed data on semen characteristics and hormone assays of these volunteers have been reported previously (Wang et al., 2007).
Testicular biopsy and tissue preparation
Open testicular biopsies were performed in four men before treatment (control) and four men from each of the TU and TU + LNG groups at 2 and 9 weeks during treatment. A testicular biopsy was taken only once in a given volunteer from one testis. Each specimen was fixed in Bouin's solution overnight and embedded in paraffin, and sections were prepared at a thickness of 5 μm for immunohistochemistry (Wang et al., 2007).
Immunohistochemistry and immunofluorescence
Testicular biopsy sections were prepared as previously described in our laboratory (Wang et al., 2007). Immunohistochemistry was used to detect VASA as a viable germ cell marker for locating and counting pachytene spermatocytes and round spermatids (Wang et al., 2007). Immunohistochemistry for tight junction proteins was performed with rabbit anti-claudin 3, rabbit anti-claudin 11, rabbit anti-JAM-A, rabbit anti-occludin and mouse anti-ZO-1 antibodies (all antibodies obtained from Zymed laboratories, Invitrogen Corporation, Carlsbad, CA, USA) used at a dilution of 1:50 for immunohistochemistry or immunofluorescence. Antigen retrieval was performed in sodium citrate buffer (pH 6) for claudin 3 immunofluorescence staining. An Alexa Flor 488 goat anti-rabbit antibody (Invitrogen Corporation) was used for ZO-1 staining and Alexa Flor 594 goat anti-rabbit antibody (Invitrogen Corporation) was used for all other blood–testis barrier components, as the secondary antibody for immunofluorescence staining.
The immunoexpression of claudin 3, claudin 11, JAM-A and ZO-1 in the seminiferous tubules was quantified by measuring optical densities of protein expression using immunohistochemistry in the seminiferous tubules from the testicular biopsy sections. Ten tubules were randomly selected from the testicular biopsy sections for each subject. Because sections were from a testicular biopsy, some areas showed damage of the tubules due to the testicular biopsy procedure and others had artifacts. All incomplete or damaged tubules were excluded from the assessment. The quantification of protein expression was performed by one observer (N.I.) measuring the optical density of immunostaining intensity on images taken from the testicular sections using a computer software program (Image-pro Plus 6.0, Media Cybernetics, Inc., Bethesda, MD, USA) as described previously (Ferrini et al., 2001).
Immunoblots of monkey testicular tissues
To assess the expression of tight junction proteins quantitatively, we performed Western-blot analysis on archived frozen testicular tissue from monkeys. These tissues were from adult cynomolgus monkeys randomly assigned (n = 8 per group) to two treatment groups for 12 weeks followed by an 8-week recovery period: (1) the control group: received two empty SILASTIC implants (Dow Corning, Midland, MI, USA) on Day 1 and (2) the testosterone group: received two testosterone implants (T, 5.5 cm, inner diameter 0.33 and outer diameter 0.46 cm) on Day 1. Testicular biopsies were performed on five monkeys from each group. Testicular samples were obtained before the treatment and at 3, 8, 28 and 84 days during the treatment phase (Lue et al., 2006). Western blotting was performed on testicular tissues as described previously (Jia et al., 2007). We used dilution of 1:500 for each antibody, claudin 3, claudin 11 and JAM-A (Invitrogen, Carlsbad, CA, USA). Band intensities were determined using Quantity One software from Bio-Rad (Hercules, CA, USA).
Detection of antisperm antibodies
The indirect immunobead test was used to detect antisperm antibodies (IgA and IgG) in heat-inactivated (56°C for 30 min) human serum samples (diluted 1:5 with buffer) according to standardized method (World Health Organization, 2010). Briefly, donor spermatozoa from healthy volunteers were washed and mixed with serum incubated at 37°C for 1h. The sperm suspension was washed twice and immunobeads (Irvine Scientific, Santa Ana, CA, USA) were added and mixed on a glass slide. The percentage of motile spermatozoa with two or more immunobeads attached was counted. The test is regarded as clinically significant if 50% or more of motile spermatozoa are coated with beads.
Statistical analyses
ANOVA with Tukey's multiple comparison correction was used to compare the difference in the number of VASA-positive spermatocytes and round spermatids at baseline, and at 2 and 9 weeks of treatment with testosterone alone or testosterone plus LNG. Two-way ANOVA was used to compare the mean optical density of blood–testis barrier junctional proteins between the subjects in each treatment group and between the treatments. Differences were considered significant when P < 0.05.
Results
There were no significant changes in the mean serum total testosterone and free testosterone concentrations between baseline and 9 weeks of treatment with TU alone or the TU + LNG group, as previously reported (Wang et al., 2007). After 9 weeks of TU ± LNG treatment, all the subjects had marked suppression of both gonadotrophins to undetectable levels (<0.2 IU/l) and the mean sperm concentration was suppressed from 74.4 ± 28.1 to 39.9 ± 16.4 million/ml and from 66.3 ± 29.6 to 16.0 ± 13.5 million/ml in the TU and TU + LNG groups, respectively. At the end of the treatment period (Week 18), 4 out of 10 subjects in the TU group reached azoospermia or sperm concentrations ≤1 million/ml, whereas in the TU plus LNG group, 6 out of 7 subjects reached azoospermia and the other 1 reached a sperm concentration ≤1 million/ml.
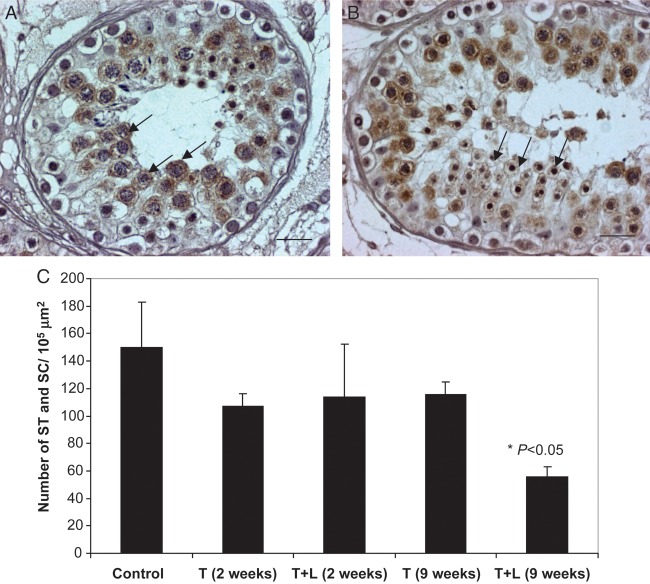
To quantify the effect of TU ± LNG treatment on testicular germ cell loss in these men after 9 weeks treatment, we performed immunohistochemistry of VASA, a male germ cell-specific RNA helicase that is specifically localized in spermatocytes and round spermatids (Fig. 1A and B). While no significant decreases were observed in the number of VASA-positive germ cells at 2 weeks after TU or TU + LNG and at 9 weeks of TU alone treatment, TU + LNG treatment for 9 weeks markedly decreased the number of pachytene spermatocytes and round spermatids (P < 0.05) within the adluminal compartment (Fig. 1C). This observation prompted us to study the integrity of the tight junction proteins that formed the blood–testis barrier.
Figure 1.
(A and B) VASA immunoreactivity is present in pachytene spermatocytes (A) and round spermatids (B) in the human testis (control group). These cells are the most susceptible germ cells to apoptosis induced by exogenous administration of androgens and progestins. Magnification bar: 20 µm. (C) The mean number of pachytene spermatocyte (SC) and round spermatids (ST) per 105 μm2 in each treatment group. The number of pachytene spermatocytes and ST significantly decreased after TU + LNG treatment for 9 weeks (P < 0.05) but not in the other groups when compared with the control group.
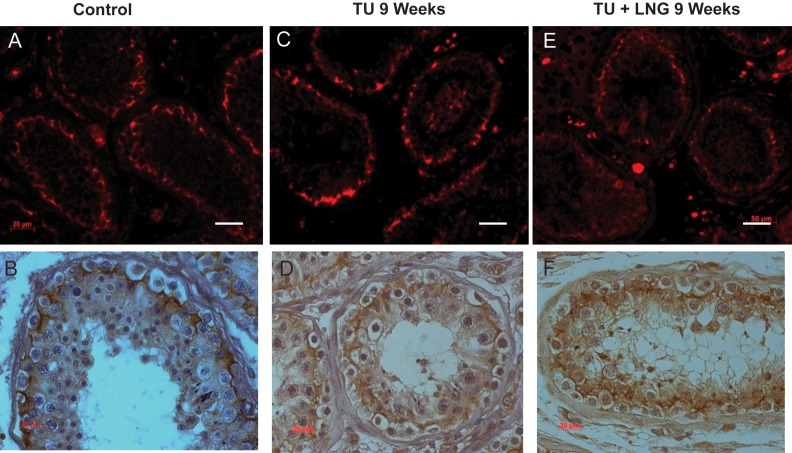
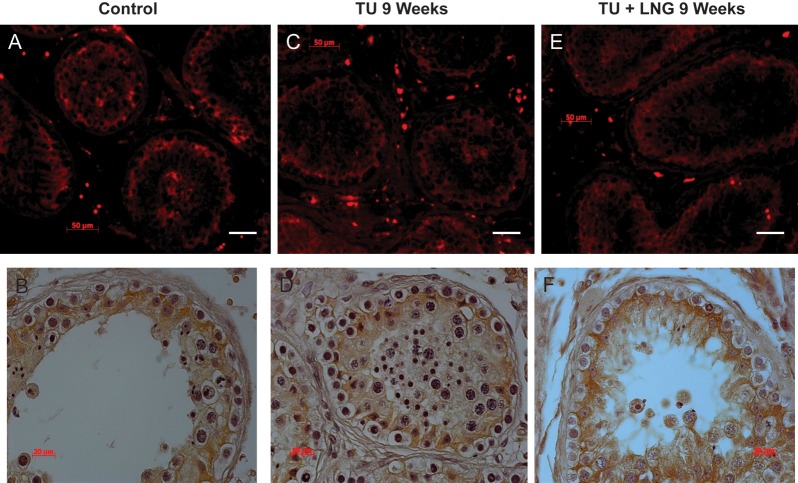
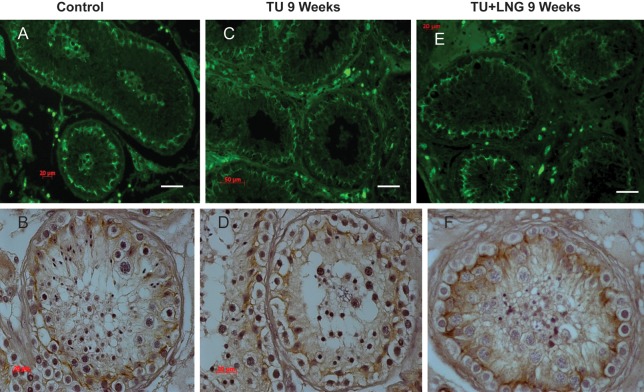
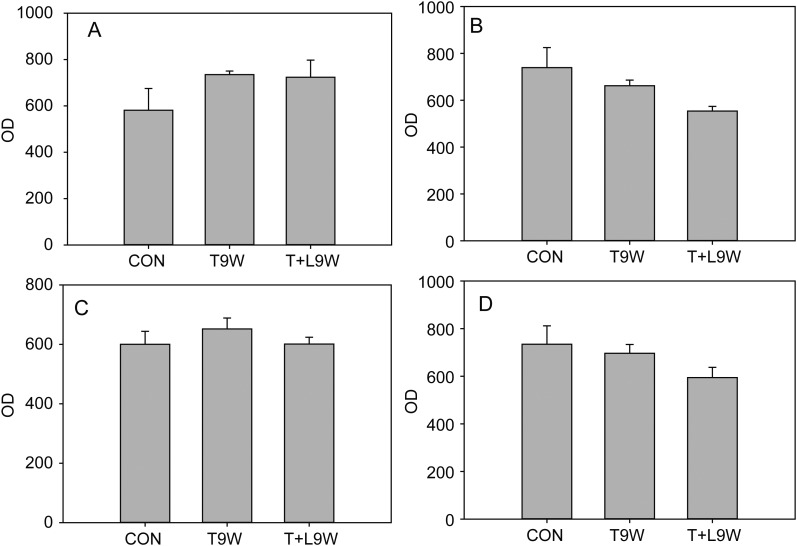
In control testicular biopsies, claudin 11 (Fig. 2), JAM-A (Fig. 3) and ZO-1 (Fig. 4) were present in human testes and localized to areas between Sertoli cells and between Sertoli cells and spermatogonia where tight junctions were expected to be present. Tight junctions formed by these proteins clearly separated the seminiferous tubule into a basal compartment and an adluminal compartment. After 2 and 9 weeks, treatment with TU or TU ± LNG, the localization and expression of claudin 11, JAM-A and ZO-1 remained unchanged (Figs 2–4, respectively). Claudin 3 (Fig. 5), a component of newly formed tight junctions, was localized between Sertoli cells adjacent to the basement membrane of the seminiferous tubules. Claudin 3 expression also exhibited no differences after TU or TU ± LNG treatment (Fig. 5). Occludin immunoexpression was not detected in tight junctions of the seminiferous tubules in human testicular biopsies before or after TU or TU + LNG treatment (data not shown). The quantification of optical densities of each tight junction protein on testicular biopsy sections using immunohistochemistry showed no significant difference in any of these proteins after 9 weeks of exogenous hormonal treatment in comparison with the baseline (Fig. 6).
Figure 2.
Claudin 11 showed no difference in expression between baseline and 9 weeks of treatment with TU or TU + LNG. Immunofluorescence (red, upper panel) and immunohistochemistry (lower panel): control (A and B); 9 weeks of treatment with TU (C and D); 9 weeks of treatment with TU + LNG (E and F). Magnification bar: 20 µm.
Figure 3.
JAM-A showed no difference in expression between baseline and 9 weeks of treatment with TU or TU + LNG. Immunofluorescence (red, upper panel) and immunohistochemistry (lower panel): control (A and B); 9 weeks of treatment with TU (C and D); 9 weeks of treatment with TU + LNG (E and F). Magnification bar: 50 µm.
Figure 4.
ZO-1 showed no difference in expression between baseline and 9 weeks of treatment with TU or TU + LNG. Immunofluorescence (green, upper panel) and immunohistochemistry (lower panel): control (A and B); 9 weeks of treatment with TU (C and D); 9 weeks of treatment with TU + LNG (E and F). Magnification bar: 50 µm.
Figure 5.
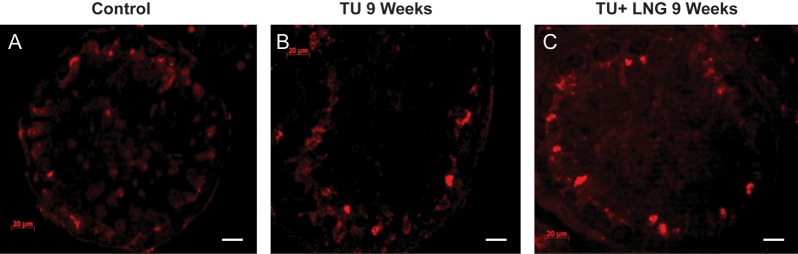
Expression of claudin-3 at baseline (A), TU treatment (B) and TU + LNG treatment (C) at 9 weeks (immunofluorescence staining red). Claudin 3 is only present in newly formed tight junctions, and thus not all the junctions are stained. Magnification bar: 20 µm.
Figure 6.
Mean optical density of tight junction proteins in men at baseline and 9 weeks after TU or TU + LNG treatment: (A) claudin 11, (B) JAM-A, (C) Zo-1 and (D) claudin3. OD, optic density; CON, control; T9W, TU treatment at 9 weeks; T + L9W, TU + LNG treatment at 9 weeks (the bar represents the standard error of mean).
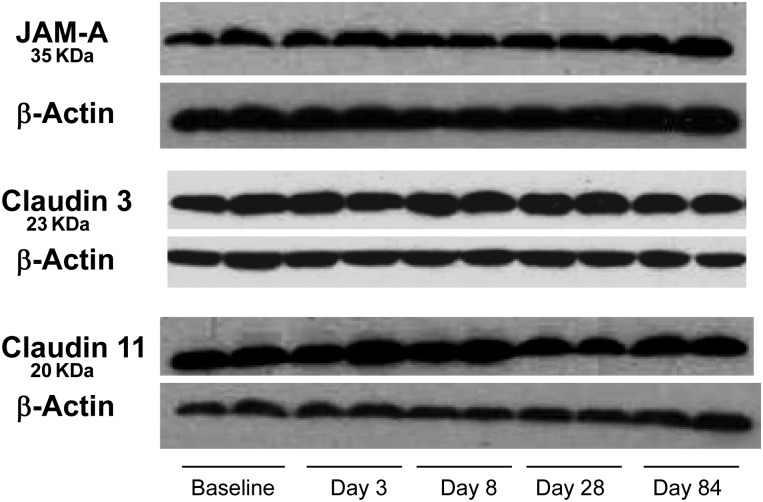
To demonstrate antibody specificity and to quantify protein expression of claudin 3, claudin 11, JAM-A, we performed Western blots using previously obtained and archived monkey testicular lysates at baseline and after T implant insertion. The immunoblots showed that the expression of claudin 3, claudin 11 and JAM-A did not change from baseline during treatment with T implants at Days 3, 8, 28 and 84 (Fig. 7).
Figure 7.
Expression of JAM-A, claudin 3 and claudin 11 after sub-cutaneous T implant insertion (5.5 cm, inner diameter 0.33 and outer diameter 0.46 cm) in adult cynomolgus monkeys at baseline and Days 3, 8, 28 and 84 during the treatment phase. Detection of a single band on Western blot demonstrates the specificity of the antibodies.
Serum antisperm antibodies were negative at baseline, Weeks 3, 9 and 12 of treatment and at the end of recovery for 10 subjects treated with TU and 7 subjects treated with TU + LNG. One subject from the TU-treated group had IgG antisperm antibodies at baseline, which remained positive at the end of study while his IgA antibody was negative and remained negative.
Discussion
We have previously demonstrated that healthy men treated with TU alone have a markedly decreased sperm concentration, and spermatogenesis is further suppressed by treatment with TU + LNG (Wang et al., 2007). We have also shown that the suppression of sperm output as a result of intratesticular testosterone deprivation is mainly due to an increase in germ cell apoptosis (Hikim et al., 1995, 2005; Lue et al., 2000, 2006; Jia et al., 2007; Wang et al., 2007). Tight junctions formed between the Sertoli cells are a major component of the blood–testis barrier and are regulated by testosterone. Previous studies in rodent models suggest that disruption of the blood–testis barrier occurs after the depletion of intratesticular androgen or androgen action (Meng et al., 2005, 2011; McCabe et al., 2010). We studied whether depletion of intratesticular testosterone by exogenous administration of TU and LNG would affect these key tight junction proteins: claudin 11, JAM-A, ZO-1 and claudin 3 in archived human testicular tissues (Wang et al., 2007). We demonstrated that claudin 11, JAM-A, ZO-1 and claudin 3 were expressed in the human testes and localized between Sertoli cells, where tight junctions are formed between the basal and adluminal compartment of the seminiferous tubules. In the present study, treatment with TU or TU + LNG for 2 and 9 weeks did not alter the location or expression of these proteins in seminiferous tubules, despite the significant loss of spermatocytes and round spermatids. We demonstrated the presence of claudin 3 before and after TU and TU + LNG treatment in human testes but the expression of this protein did not form a contiguous perimeter around the adluminal compartment. Since claudin 3 is present in newly formed tight junctions at Stage VIII of the seminiferous tubular epithelial cycle in mice (Meng et al., 2005), our inability to demonstrate the continuous junctions by immunofluorescence may be due to the differences in the organization of the stages of spermatogenesis between men and rodents. Our results of tight junction protein expression in human testicular samples after intratesticular testosterone suppression contrasted with those of rodent models. This finding was supported by the Western blot data, which showed no quantitative changes of these proteins in monkey testes after testosterone treatment.
The difference between our findings in human or monkey and those in rodent testes could be related to species differences or differences in the study paradigms. In rats treated with a GnRH antagonist for 7 weeks, there was decreased expression of occludin and redistribution of claudin 11 within the tight junctions, but claudin 3 was not studied. GnRH antagonist treatment also increased biotin entry into the seminiferous tubules, as demonstrated only after the testes were removed from the rat but not in live animals (McCabe et al., 2010). Using the Sertoli cell-specific androgen receptor knockout mouse, Meng et al. (2005) showed that claudin 3 expression disappeared at the tight junctions resulting in increased permeability of the blood–testis barrier allowing injected biotin to access the adluminal compartment. Disruption of the immunological barrier was suggested by detectable antibodies against germ cell antigens in serum (Meng et al., 2011). Androgen action is completely abolished from birth in this knockout model, whereas intratesticular androgen levels in men treated with male hormonal contraceptive regimen were suppressed to low but not non-detectable levels (Page et al., 2007). There are also significant species differences in the function of blood–testis barrier components. Occludin knockout mice have morphologically normal tight junctions and normal fertility in young adult animals, although these animals become infertile as they age (Saitou et al., 2000; Takehashi et al., 2007); however, suppression of rat testicular occludin function causes a loss of germ cells in vivo and partial disruption of Sertoli cell tight junctions in vitro (Chung et al., 2001). Sertoli cell-specific androgen receptor ablation in mice results in decreased claudin 3 and increased permeability of the blood–testis barrier (Meng et al., 2005), but in adult rat testes when Sertoli cell androgen receptor levels are highest on Day 90, claudin 3 protein expression is lower than in Days 16–25 rat testes (Yan et al., 2008). These data suggest possible differences in blood–testis barrier regulation among species.
In this study, when sperm concentration was suppressed to very low levels compatible with effective contraception in men, serum antisperm antibodies were absent, suggesting that no germ cell components traverse the blood–testis barrier, supporting our anatomical demonstration of intact tight junctions. Our findings are important for male hormonal contraceptive development because the blood–testis barrier provides an immunological barrier to sequester the antigenic determinants of germ cells from the systemic circulation, on the one hand, and to exclude the entry of immunoglobulins and lymphocytes to the adluminal compartment, on the other hand, preventing autoimmune orchitis and germ cell death (Setchell et al., 1969). The usual complete recovery of spermatogenesis after withdrawal of male hormonal contraceptives (Liu et al., 2006) further supports the notion that the effect of marked suppression of intratesticular testosterone levels induced by exogenous testosterone with a progestin causes severe spermatogenesis suppression but does not disturb the blood–testis barrier in men.
Our study has some limitations. The evaluation of testicular tight junction proteins was only performed at 9 weeks and antisperm antibodies were measured at 18 weeks during treatment with TU ± LNG. The long-term effect of male hormonal contraceptive treatment on the structural and functional integrity of the blood–testis barrier remains to be determined in humans.
Authors' roles
N.I., N.A., Y.-H.L., R.S.S., Y.J. and C.W. contributed to the study design, execution, analyses, discussion, writing and review of the manuscript. S.B., A.A. and C.T. performed analyses of the samples and participated in the discussions of the results. Y.-G.C., X.-H.W., Z.-M.Z. and J.-H.S. contributed to the clinical study and the collection of the testis samples.
Funding
The study was supported by grants from the Contraceptive Research and Development Program and the Mellon Foundation (MFG-02-64, MFG-03-67), Endocrine, Metabolism and Nutrition Training Grant (T32 DK007571), the Clinical and Translational Science Institute at Los Angeles Biomedical and Harbor-UCLA Medical Center (UL1RR033176 and UL1TR000124) and the Los Angeles Biomedical Research Institute Summer High School Student Program.
Conflict of interest
None declared.
References
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–263. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung NP, Cheng CY. Is cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. doi:10.1210/en.142.5.1878. [DOI] [PubMed] [Google Scholar]
- Chung NP, Mruk D, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. doi:10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- Ferrini M, Wang C, Swerdloff RS, Sinha Hikim AP, Rajfer J, Gonzalea-Cadavid NF. Aging-related increased expression of inducible nitric oxide synthase and cytotoxicity markers in rat hypothalamic regions associated with male reproductive function. Neuroendocrinology. 2001;74:1–11. doi: 10.1159/000054665. doi:10.1159/000054665. [DOI] [PubMed] [Google Scholar]
- Furuya S, Kumamoto Y, Sugiyama S. Fine structure and development of Sertoli junctions in human testis. Arch Androl. 1978;1:211–219. doi: 10.3109/01485017808988339. [DOI] [PubMed] [Google Scholar]
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. doi:10.1016/S0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- Hikim AP, Wang C, Leung A, Swerdloff RS. Involvement of apoptosis in the induction of germ cell degeneration in adult rats after gonadotropin-releasing hormone antagonist treatment. Endocrinology. 1995;136:2770–2775. doi: 10.1210/endo.136.6.7750502. doi:10.1210/en.136.6.2770. [DOI] [PubMed] [Google Scholar]
- Hikim AP, Vera Y, Elhag RI, Lue Y, Cui YG, Pope V, Leung A, Atienza V, Wang C, Swerdloff RS. Mouse model of male germ cell apoptosis in response to a lack of hormonal stimulation. Indian J Exp Biol. 2005;43:1048–1057. [PubMed] [Google Scholar]
- Jia Y, Hikim AP, Lue YH, Swerdloff RS, Vera Y, Zhang XS, Hu ZY, Li YC, Liu YX, Wang C. Signaling pathways for germ cell death in adult cynomolgus monkeys (Macaca fascicularis) induced by mild testicular hyperthermia and exogenous testosterone treatment. Biol Reprod. 2007;77:83–92. doi: 10.1095/biolreprod.106.058594. doi:10.1095/biolreprod.106.058594. [DOI] [PubMed] [Google Scholar]
- Kaitu'u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–1179. doi: 10.1530/REP-06-0385. doi:10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367:1412–1420. doi: 10.1016/S0140-6736(06)68614-5. doi:10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- Lue Y, Hikim AP, Wang C, Im M, Leung A, Swerdloff RS. Testicular heat exposure enhances the suppression of spermatogenesis by testosterone in rats: the "two-hit" approach to male contraceptive development. Endocrinology. 2000;141:1414–1424. doi: 10.1210/endo.141.4.7416. doi:10.1210/en.141.4.1414. [DOI] [PubMed] [Google Scholar]
- Lue Y, Wang C, Liu YX, Hikim AP, Zhang XS, Ng CM, Hu ZY, Li YC, Leung A, Swerdloff RS. Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macaca fascicularis) J Clin Endocrinol Metab. 2006;91:539–545. doi: 10.1210/jc.2005-1808. doi:10.1210/jc.2005-1808. [DOI] [PubMed] [Google Scholar]
- McCabe MJ, Tarulli GA, Meachem SJ, Robertson DM, Smooker PM, Stanton PG. Gonadotropins regulate rat testicular tight junctions in vivo. Endocrinology. 2010;151:2911–2922. doi: 10.1210/en.2009-1278. doi:10.1210/en.2009-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Greenlee AR, Taub CJ, Braun RE. Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice. Biol Reprod. 2011;85:254–260. doi: 10.1095/biolreprod.110.090621. doi:10.1095/biolreprod.110.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, Tsukita S. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am J Physiol. 1998;274:C1708–C1717. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- Nah WH, Lee JE, Park HJ, Park NC, Gye MC. Claudin-11 expression increased in spermatogenic defect in human testes. Fertil Steril. 2011;95:385–388. doi: 10.1016/j.fertnstert.2010.08.023. doi:10.1016/j.fertnstert.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Page ST, Kalhorn TF, Bremner WJ, Anawalt BD, Matsumoto AM, Amory JK. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J Androl. 2007;28:734–741. doi: 10.2164/jandrol.107.002790. doi:10.2164/jandrol.107.002790. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. doi:10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell BP, Voglmayr JK, Waites GM. A blood-testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol. 1969;200:73–85. doi: 10.1113/jphysiol.1969.sp008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- Takehashi M, Kanatsu-Shinohara M, Miki H, Lee J, Kazuki Y, Inoue K, Ogonuki N, Toyokuni S, Oshimura M, Ogura A, et al. Production of knockout mice by gene targeting in multipotent germline stem cells. Dev Biol. 2007;312:344–352. doi: 10.1016/j.ydbio.2007.09.029. doi:10.1016/j.ydbio.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. doi:10.1016/S0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 2000;149:13–16. doi: 10.1083/jcb.149.1.13. doi:10.1083/jcb.149.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Cui YG, Wang XH, Jia Y, Sinha HA, Lue YH, Tong JS, Qian LX, Sha JH, Zhou ZM, et al. Transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab. 2007;92:3292–3304. doi: 10.1210/jc.2007-0367. doi:10.1210/jc.2007-0367. [DOI] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant'Agnese PA, Mesy-Bentley KL, Tzeng CR, et al. Androgen receptor in sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. doi:10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. 5th edn. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- Xia W, Wong CH, Lee NP, Lee WM, Cheng CY. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model. J Cell Physiol. 2005;205:141–157. doi: 10.1002/jcp.20377. doi:10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. doi:10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]