Abstract
A crucial period for the development of the immune system occurs in utero. This results in a high fetal vulnerability to immunotoxic exposure, and indeed, immunotoxic effects have been reported, demonstrating negative effects on immune-related health outcomes and immune functionality. Within the NewGeneris cohort BraMat, a subcohort of the Norwegian Mother and Child Cohort Study (MoBa), immunotoxicity was demonstrated for polychlorinated biphenyls and dioxins, showing associations between estimated maternal intake levels and reduced measles vaccination responses in the offspring at the age of 3. The present study aimed to investigate this link at the transcriptomic level within the same BraMat cohort. To this end, whole-genome gene expression in cord blood was investigated and found to be associated with maternal Food Frequency Questionnaires–derived exposure estimates and with vaccination responses in children at 3 years of age. Because the literature reports gender specificity in the innate, humoral, and cell-mediated responses to viral vaccines, separate analysis for males and females was conducted. Separate gene sets for male and female neonates were identified, comprising genes significantly correlating with both 2,3,7,8-tetrachlorodibenzodioxin (TCDD) and polychlorinated biphenyls (PCB) exposure and with measles vaccination response. Noteworthy, genes correlating negatively with exposure in general show positive correlations with antibody levels and vice versa. For both sexes, these included immune-related genes, suggesting immunosuppressive effects of maternal exposure to TCDD and PCB at the transcriptomic level in neonates in relation to measles vaccination response 3 years later.
Key Words: immunotoxicology, toxicogenomics, newborns, maternal exposure, TCDD, PCB.
There is a growing interest in the effects of prenatal exposure to toxicants because the immune system develops extensively in utero, possibly leading to a higher fetal vulnerability to exposure (Fine et al., 1989; Van Loveren and Piersma, 2004; West, 2002). Exposure occurs through the environment and lifestyle habits, and diet presents the main source of exposure to immunotoxicants reaching the fetus by crossing the placental barrier (Covaci et al., 2002; Park et al., 2008). Therefore, the EU-funded ‘‘Newborns and Genotoxic exposure risks’’ (NewGeneris) project investigates molecular events in the unborn child due to maternal exposure to dietary toxicants, leading to an increased risk of cancer, impaired immune functionality, and immune disorders in childhood (Merlo et al., 2009). Investigated toxicants include polycyclic aromatic hydrocarbons, heterocyclic amines, nitrosamines, acrylamide, the mycotoxin deoxynivalenol, dioxin and polychlorinated biphenyls (PCBs), alcohol, and DNA-reactive aldehydes.
Gluckman et al. (2008) proposed that fetal responses to toxicants may result in changes of a persistent nature, possibly leading to predisposition to develop disease such as cancer and immune diseases. This developmental plasticity (i.e., ability of an organism to develop in various ways, depending on the particular environment or setting) in response to toxic exposure may occur through modulation of the fetal transcriptome, warranting investigation of transcriptomic effects as a result of in utero exposure to immunotoxicants (Gluckman et al., 2008).
Based on the concept put forward by Gluckman et al., the present study aimed to investigate the link between maternal immunotoxic exposure and neonatal immune functionality at the transcriptomic level. Within the NewGeneris cohort BraMat, a subcohort of the Norwegian Mother and Child cohort, immunotoxicity was demonstrated for PCB and dioxins (main food groups contributing to exposure in BraMat were seafood, milk and dairy products, egg, and cereals). Associations were found between estimated maternal intake levels based on food frequency questionnaire (FFQ) and increased risk of infections in the offspring after 1 year (Stølevik et al., 2011) and reduced measles vaccination responses at the age of 3 (Stølevik et al., submitted). These findings are in accordance with previous publications, demonstrating negative effects of (in utero) exposure to PCBs and 2,3,7,8-tetra- chlorodibenzodioxin (TCDD) on immune-related health outcomes and immune functionality parameters including antibody responses to vaccines (Heilmann et al., 2006; Weisglas-Kuperus et al., 1995; Weisglas-Kuperus et al., 2000; Weisglas-Kuperus et al., 2004). Based on these findings, the current study focused on PCBs and TCDD exposures and measles vaccination response.
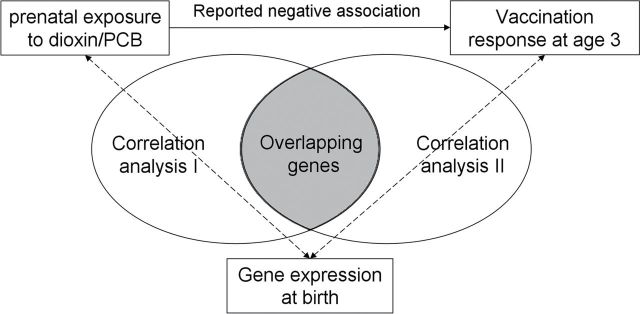
To investigate the link between maternal immunotoxic exposure and neonatal immune functionality at the transcriptomic level in the BraMat cohort, fetal transcriptomic responses were investigated by whole-genome analyses of cord blood samples, associated with maternal dietary exposure to PCBs and TCDD as estimated by FFQ, and measles antibody levels measured at the age of 3 as immune functionality. These transcriptomic profiles were subsequently examined to identify the overlap between genes and processes correlating with maternal exposure and those correlating with neonatal immune functionality as illustrated in Figure 1, thereby focusing on differences between boys and girls. Recently, we reported on transcriptomic responses in relation to fetal exposure to dietary carcinogens demonstrating gender specificity in neonatal gene expression in relation to dioxin and dioxin-like exposure by means of DR CALUX (Hochstenbach et al., submitted). Furthermore, there is a growing awareness that males and females also differ in their innate, humoral, and cell-mediated responses to viral vaccines, warranting separate analysis for males and females (Klein et al., 2010).
Fig. 1.
Study strategy of the data analysis for the identification of the overlap (indicated by the grey area) between genes correlating with exposure data and genes correlating with vaccination response within the BraMat cohort. Correlation analysis I entailed a Pearson correlation analysis of whole-genome gene expression in cord blood with maternal exposure estimates determined by FFQ. Correlation analysis II consisted of a Pearson correlation analysis of whole-genome gene expression in cord blood with measles antibody response measured at 3 years of age.
MATERIALS AND METHODS
Blood sampling and handling for microarray analysis. Umbilical cord blood samples were collected immediately after birth from the cord vein of 45 male and 66 female babies (for population description, see Table 1) whose mothers participated in the Norwegian BraMat cohort. This subcohort is nested within the Norwegian Mother and Child Cohort Study (MoBa), a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health (Magnus et al., 2006). Study protocols were approved by the Regional Committee for Ethics in Medical Research in Oslo, Norway. Informed consent was obtained from all participating mothers. Samples were collected by trained nurses at the maternity wards of the Oslo University Hospital Ullevål and Akershus University Hospital, Norway. For preserving RNA, aliquots of heparin-anticoagulated whole blood of 0.4ml were mixed with 1.2ml of RNAlater (Ambion/Applied Biosystems, Nieuwerkerk aan den Ijssel, The Netherlands) as soon as possible after blood collection and subsequently stored at −80°C until shipment on dry ice to the research laboratory.
TABLE 1 .
Characteristics of the Study Population and Data Distribution of the Various Parameters for Exposure Analysis (A) and Vaccination Response Analysis (B)
| All | Males | Females | |
|---|---|---|---|
| A. Exposure | |||
| N | 111 | 45 | 66 |
| Birth weight | 3629±434 | 3722±416 | 3564±438 |
| Gestational length | 39.8±1.1 | 39.9±1.1 | 39.7±1.1 |
| Age | |||
| Mother | 31.3±4.2 | 31.0±4.3 | 31.5±4.2 |
| Father | 32.8±6.4 | 32.3±4.3 | 33.7±5.7 |
| Mother body mass index BP | 23.4±3.9 | 23.1±3.6 | 23.6±4.1 |
| Parity (≥ 1) | |||
| No | 35% | 34% | 36% |
| Yes | 65% | 66% | 64% |
| Mother smoked DP | |||
| No | 100% | 100% | 100% |
| Occasional | 0% | 0% | 0% |
| Daily | 0% | 0% | 0% |
| Mother smoked BP | |||
| No | 84% | 82% | 85% |
| Occasional | 9% | 11% | 8% |
| Daily | 7% | 7% | 6% |
| TCDD | 2.78±1.44 | 2.79±1.69 | 2.77±1.25 |
| PCB77 | 0.06±0.04 | 0.06±0.06 | 0.05±0.03 |
| PCB126 | 25.17±16.96 | 26.13±19.27 | 24.51±14.27 |
| PCB153 | 78.52±107.20 | 82.27±122.71 | 75.92±95.92 |
| PCB169 | 1.56±1.03 | 1.59±1.13 | 1.53±0.96 |
| B. Vaccination response | |||
| N | 59 | 20 | 39 |
| Birth weight | 3600±443 | 3610±363 | 3594±484 |
| Gestation | 39.8±1.1 | 40.1±1.1 | 39.7±1.2 |
| Age | |||
| Mother | 31.8±4.3 | 30.5±4.8 | 32.5±4.0 |
| Father | 33.6±5.6 | 31.3±4.7 | 34.6±5.7 |
| Mother body mass index BP | 23.4±4.4 | 22.8±3.9 | 23.8±4.7 |
| Parity (≥ 1) | |||
| No | 41% | 55% | 33% |
| Yes | 59% | 45% | 67% |
| Mother smoked DP | |||
| No | 100% | 100% | 100% |
| Occasional | 0% | 0% | 0% |
| Daily | 0% | 0% | 0% |
| Mother smoked BP | |||
| No | 86% | 85% | 87% |
| Occasional | 7% | 5% | 8% |
| Daily | 7% | 10% | 8% |
| Measles antibody levels | 0.81±0.43 | 0.75±0.33 | 0.84±0.47 |
Note. BP, before pregnancy; DP, during pregnancy. Birth weight expressed in g, gestation in weeks, age in years, estimated maternal intake to TCDD, PCB77, PCB126, and PCB169 expressed in pg TEQ/kg bw/day, and estimated maternal intake to PCB153 in ng/kg bw/day.
RNA isolation. Total RNA was isolated from the RNAlater-preserved whole blood samples using the RiboPure-Blood system (Ambion) according to the manufacturer’s instructions. RNA yield and purity were measured by means of NanoDrop equipment (NanoDrop Technologies, Wilmington, DE), and integrity was verified by automated gel electrophoresis (2100 BioAnalyzer; Agilent Technologies, Amstelveen, The Netherlands).
Gene expression analysis. Only RNA samples with a RNA Integrity Number ≥ 6 and a bench time (time from collection until RNA stabilization) of ≤ 6h were used for transcriptomic analysis. Total RNA (1 µg) was used to generate cyanine-labeled cRNA using the Agilent Low RNA Input Linear Amplification kit according to the manufacturer’s instructions. Each individual cord blood sample was labeled by means of cyanine-5 and competitively hybridized against a common reference sample (top 20 samples according to RNA Integrity Number were pooled RNA and labeled with cyanine-3) onto Agilent 4x44k human oligonucleotide microarrays (Agilent Technologies, Palo Alto, CA) according to the manufacturer’s instructions. After hybridization, slides were washed with wash buffers, including Agilent stabilization and drying solution, which contains an ozone-scavenging compound dissolved in acetonitrile in order to protect against ozone-induced degradation of cyanine dyes, in particular Cy5 (Agilent Technologies). Microarrays were scanned immediately using an Axon GenePix 4000B Microarray Scanner (Molecular Devices, Sunnyvale). Laser power was set to 100%. The photo multiplier tube gain was set to a saturation tolerance of 0.02% to minimize background and saturated spots.
Blood sampling and handling for vaccine responses. At 3 years of age, venous blood was collected from the same subjects in whom gene expression was measured at birth (age mean (range); 36 (33–43) months) at their doctor’s office, at home by a technician, or at a commercial laboratory (Fürst medical laboratory), as described by Stølevik et al. (submitted). Blood was collected into BD Vacutainer SSTII serum gel separation tubes with butterfly blood collection sets (BD, Franklin Lakes, NJ. The blood was allowed to clot for at least 30min before centrifugation at 1000–1300 × g for 10min at room temperature. Aliquots of the serum samples were stored at −20°C until further analyses.
Exposure assessment of dietary toxicants. Maternal intake of the dietary toxicants PCBs (PCB77, PCB126, PCB153, and PCB169) and TCDD was estimated from a validated FFQ used in MoBa. The structure and validation of the FFQ are described elsewhere, showing correlation coefficients between FFQ-derived exposure estimates and biomarker-based measurements of 0.3–0.4 (Brantsaeter et al. 2008; Meltzer et al. 2008). The FFQ covers the dietary intake of the participants during the first 5 months of pregnancy. The method for estimation of exposure to PCBs and TCDD has been described by Kvalem et al. (2009). In brief, an extensive database was built comprising all available concentrations of dioxins and PCB congeners in Norwegian foods from 2000 to 2006. Intakes of PCB77, PCB126, PCB153, and PCB169 and TCDD were estimated by multiplying consumption with congener levels in food by use of the online data program FoodCalc (http://www.ibt.ku.dk/jesper/foodcalc).
Determination of measles vaccine responses. The serum samples were analyzed for anti-measles IgG antibodies using Enzygnost Anti-Measles Virus IgG ELISA (Siemens, München, Germany). The assay was performed as recommended by the manufacturers. The cutoff for qualitative evaluation of positivity was an optical density of 0.2 at 450nm, corresponding to approximately 400U/ml, as recommended by the manufacturer.
Data analysis. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE31836 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31836). Scan images of Cy5- and Cy3-channels were loaded into ImaGene software version 8.0.1 (BioDiscovery, El Segundo) for the extraction of raw pixel intensities and local backgrounds. The data were preprocessed using the Refiner Array module from the Genedata Expressionist system (Genedata AG, Basel, Switzerland). Raw data were assessed for quality and preprocessed as follows. Signals were corrected by subtracting backgrounds without producing negative values. For each feature and array, the contrast, i.e., the quality for each signal according to its signal-to-noise ratio, the distortion, and imbalance were determined. Defective or saturated features detected by the scanner software or by the Refiner Array module were diagnosed and masked. LOWESS correction was used to correct all features so that the signal distortion and imbalance of the two channels are minimized. Quality criteria for the data analysis were set as follows: signal-to-noise ratios > 2, relative errors < 0.5, and saturated and features flagged as masked filtered out.
Dose-response relationships were investigated by Pearson’s correlation analyses of individual gene expressions (Cy5/Cy3 ratios) and individual values for measles antibody levels and exposure estimates based on FFQ for TCDD, PCB77, PCB126, PCB153, and PCB169. Correlation analyses were performed only for transcripts with least 50% valid expression values. Significantly correlating transcripts were selected using an unadjusted p value cutoff of < 0.05. To identify biological processes in which significantly correlating genes are involved, the software suite Metacore was used (Thomson Reuters (Scientific) Inc., Philadelphia). Gene Ontology (GO) terms containing at least two significantly correlating genes and a p value ≤ 0.01 were considered significantly enriched.
RESULTS
Biomarker Analyses
A total of 120 samples were hybridized onto microarrays. Sample/microarray exclusions were based on technical performance of the microarrays and on absence of informed consent forms or reported smoking of the mother at 30 weeks of pregnancy. The number of cases left in the study after exclusions was 111. Estimated dietary intake of TCDD and PCBs of mothers and further characteristics of this study population are presented in Table 1A, for the whole study population and for male and female infants separately. For correlation analysis of gene expression with antibody levels, a total of 59 samples were available. Antibody levels and characteristics of this study population are presented in Table 1B.
Toxicogenomic Profiles in Newborns in Relation to Maternal Dietary Exposure
Both male and female subjects showed significant gene expression changes associated with PCBs and TCDD exposure (Table 2). Only a small overlap of genes was observed between the two sexes, indicating gender-specific toxicogenomic responses to TCDD and PCBs.
TABLE 2 .
Number of Genes Significantly Correlating With Exposure Markers and Measles Antibody Levels
| Total | Positive | Negative | Total | Positive | Negative | Overlap male/female | Inverse male/female | |
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| TCDD | 399 | 235 | 164 | 254 | 146 | 108 | 1 | 7 |
| PCB77 | 313 | 232 | 81 | 214 | 105 | 109 | 1 | 2 |
| PCB126 | 311 | 220 | 91 | 384 | 218 | 166 | 5 | 8 |
| PCB153 | 337 | 239 | 98 | 450 | 174 | 276 | 6 | 7 |
| PCB169 | 358 | 235 | 123 | 484 | 313 | 171 | 13 | 6 |
| Union | 751 | 946 | ||||||
| Intersection | 96 | 30 | ||||||
| Measles | 1750 | 755 | 995 | 2890 | 1848 | 1042 | 3 | 707 |
| Overlap with union exposure | 84 | 206 | ||||||
| Overlap with intersection exposure | 6 | 10 | ||||||
GO term analyses revealed a high number of processes overrepresented for both males and females, including several immune-related processes (Table 3). GO processes found in both males and females include (positive) regulation of I-kappaB kinase/NF-kappaB cascade, regulation of defense response, viral infectious cycle, and negative regulation of multiorganism process (Table 4). Furthermore, a number of closely related immune processes were overrepresented in both sexes, e.g., cytokine signaling and lymphocyte activation/differentiation (Table 4). Male-specific processes include antigen processing and presentation of peptide antigen, several histone acetylation GO terms, and posttranslational regulation of gene expression. For females, apoptosis-related processes were found to be overrepresented. For complete lists, we refer to Supplementary table 1.
TABLE 3 .
Number of Significantly Enriched GO Terms for Exposure Markers and Measles Antibody Response
| Males | Females | Overlap | |
|---|---|---|---|
| TCDD | 111 | 35 | 2 |
| PCB77 | 63 | 17 | 0 |
| PCB126 | 54 | 20 | 0 |
| PCB153 | 66 | 28 | 0 |
| PCB169 | 46 | 26 | 0 |
| Union exposure | 211 | 100 | 2 |
| Intersection exposure | 2 | 0 | 0 |
| Measles | 104 | 244 | 17 |
| Overlap with union exposure | 23 | 4 | 0 |
| Overlap with intersection exposure | 0 | 0 | 0 |
TABLE 4 .
Overrepresented Immune-Related GO Terms Correlating With TCDD and PCB Exposure
| TCDD | PCB77 | PCB126 | PCB153 | PCB169 | |
|---|---|---|---|---|---|
| p value | |||||
| Significant GO processes in males | |||||
| Regulation of I-kappaB kinase/NF-kappaB cascade | 2,500E−03 | ||||
| Positive regulation of I-kappaB kinase/NF-kappaB cascade | 4,574E−03 | ||||
| Regulation of defense response | 9,764E−03 | ||||
| Viral infectious cycle | 1,271E−04 | ||||
| Negative regulation of multiorganism process | 5,379E−03 | 7,308E−03 | |||
| Protein folding | 7,361E−04 | ||||
| Regulation of lymphocyte activation | 6,633E−03 | 8,900E−03 | |||
| Regulation of cytokine production | 8,061E−03 | 4,550E−03 | 7,981E−03 | ||
| Antigen processing and presentation of peptide antigen via major histocompatibility complex class I | 1,925E−04 | 9,157E−03 | 1,682E−04 | ||
| Histone acetylationa | 4,331E−03 | 8,065E−03 | |||
| Posttranscriptional regulation of gene expression | 7,099E−04 | 9,479E−03 | |||
| Significant GO processes in females | |||||
| Regulation of I-kappaB kinase/NF-kappaB cascade | 6,102E−03 | 2,204E−03 | |||
| Positive regulation of I-kappaB kinase/NF-kappaB cascade | 4,029E−03 | 1,251E−03 | |||
| Regulation of defense response | 7,308E−03 | ||||
| Viral infectious cycle | 7,154E−03 | ||||
| Negative regulation of multiorganism process | 4,833E−03 | ||||
| Protein folding | 1,641E−03 | ||||
| Regulation of T-cell proliferation | 5,436E−03 | 3,710E−03 | |||
| Positive regulation of cytokine-mediated signaling pathway | 1,190E−03 | 5,670E−04 | 2,846E−03 | ||
| Activation of caspase activity by cytochrome c | 6,642E−03 | ||||
| Glial cell apoptosis | 3,947E−03 | 6,037E−03 | |||
Note. For the complete list, refer to Supplementary table 1.
aSeveral histone acetylation GO terms were overrepresented; for complete list, refer to Supplementary table 1.
Toxicogenomic Profiles in Newborns in Relation to Measles Vaccine Response
A much higher number of genes were found to correlate with vaccination response in females compared with that in males, with only 3 genes overlapping between the sexes and 707 genes inversely correlating (Table 2). Accordingly, a higher number of processes were found for females, which to a large extent are immune related. In fact, the most significant GO term is the immune system, whereas in males mostly general cellular processes were overrepresented like translation and RNA splicing (Supplementary table 2). GO term analyses on the 707 inversely correlating genes reveal these genes to be involved in hormone secretion, (lymphocyte) apoptosis, and immunity (Table 5; Supplementary table 3).
TABLE 5 .
Significantly Overrepresented GO Processes Among the Inversely Correlating Genes Between Males and Females for the Measles Antibody Levels
| GO processes | p value |
|---|---|
| Hormone secretion | 1,004E−04 |
| Regulation of lymphocyte apoptosis | 9,373E−04 |
| Negative regulation of apoptosis | 1,350E−03 |
| Regulation of leukocyte-mediated immunity | 5,294E−03 |
| Positive regulation of adaptive immune response | 7,654E−03 |
| Immune system process | 7,905E−03 |
| Type I interferon-mediated signaling pathway | 8,792E−03 |
| Cellular response to type I interferon | 8,792E−03 |
| Cell proliferation | 8,856E−03 |
| Antigen processing and presentation of peptide antigen via major histocompatibility complex class Ib | 9,049E−03 |
| Response to type I interferon | 9,620E−03 |
Note. For the complete list, refer to Supplementary table 3.
Linking Transcriptomic Effects of Maternal TCDD and PCB Exposure and Antibody Response to Measles Vaccination at Age 3
Next we investigated which genes were related both to exposure and to vaccination response as illustrated in Figure 1. For both males and females, almost without exception, all genes in the overlap show opposite correlation between exposure and vaccination response. A number of 84 genes for males and 206 genes for females were found to correlate significantly with both exposure and vaccination response (Table 2). When comparing this list to the online Comprehensive List of Immune-Related Genes of the Immunology Database and analysis Portal (www.immport.org), 21 out of 84 male genes appear immune related, presented in Table 6 and Supplementary Table 4. Evaluation of these immune-related genes for specific pathways (as categorized by Immport) shows human leukocyte antigen (HLA)-E and MICA to be part of the antigen processing and presentation pathway, LCK of the T-cell receptor signaling pathway and natural killer cell cytotoxicity pathway, IL27, PLXNB2, and IL16 of the cytokine (receptor) pathway, and IL27 of the antimicrobials pathway.
Forty out of 206 female genes are immune related (Table 7; Supplementary table 4), with PSMD4 part of the antigen processing and presentation pathway, PDCD1 and NCK1 of the T-cell receptor signaling pathway, SH3BP2 of the natural killer cell cytotoxicity pathway, 6 genes part of the cytokine (receptor) pathway, and 7 genes of the antimicrobials pathway (including signal transducer and activator of transcription 1 [STAT1] and toll-like receptor 4 [TLR4]).
Next to the immune-related genes, GO analysis revealed histone modification by acetylation to be predominantly overrepresented in the male gene list (Supplementary table 5). Concordantly, histone acetylation in newborns was found in association with maternal exposure and with vaccination response at 3 years of age. In females, overlapping genes between exposure and antibody response to vaccination are involved in response to drug and leukocyte activation (Supplementary table 5). Leukocyte activation was found in association with exposure and vaccination response.
DISCUSSION
The present study aimed to investigate a possible link between the transcriptomic effects in neonates due to maternal exposure to immunotoxicants, in particular TCDD and PCB, and antibody response to measles vaccination in the offspring at the age of 3. Separate gene sets for male and female neonates were identified comprising genes significantly correlating with both TCDD and PCB exposure and with measles vaccination response at 3 years of age. Almost without exception, genes correlating negatively with estimated maternal dietary intake of TCDD and PCBs showed positive correlations with antibody levels and vice versa. This suggests that the transcriptomic effects of maternal exposure to dioxins on the fetus might result in suppression of immune functionality because these particular genes are associated with immune functionality later in life. Although for both sexes immune-related gene expressions were found to be modified, histone acetylation was overrepresented in the male set.
Toxicogenomic Profiles in Newborns in Relation to Maternal Dietary Exposure
Previous publications demonstrate negative effects of (in utero) exposure to PCBs and TCDD on immune-related health outcomes and immune functionality parameters including antibody responses to vaccines (Heilmann et al., 2006; Weisglas-Kuperus et al., 1995; Weisglas-Kuperus et al., 2000; Weisglas-Kuperus et al., 2004). Importantly, within BraMat, the cohort investigated in the current study, PCBs and dioxins were associated with a reduced response to measles vaccination (Stølevik et al., submitted). The associations found in the current study at the transcriptomic level, between estimated maternal intake levels of TCDD and PCBs, and effects on the immune system for both male and female newborns may be related or even (partially) explain reported immunosuppressive effects of these immunotoxicants.
The most distinct commonly overrepresented GO process between male and female newborns in relation to maternal TCDD and PCB exposure estimates was the IKB/NFKB cascade. This is in accordance with the literature because this signal transduction cascade has been shown to interact with the Ah receptor, which mediates most of dioxin-induced immune suppression (Tian et al., 2002). The NFKB signal transduction pathway is involved in the development of the immune system and adaptive and innate immunity. It has a central role in coordinating the expression of a wide variety of genes that control immune responses (Tian et al., 2002).
Although maternal exposure estimates did not differ significantly between newborn males and females, gender-specific responses were observed. The lack of overlap between the genes correlating with maternal TCDD and PCB exposure for males and females is in agreement with a recent study performed by our group, investigating gene expression in relation to dioxin-like exposure in utero by means of DR CALUX (Hochstenbach et al., submitted).
The generally proposed mechanism of action for dioxin and dioxin-like PCBs involves the activation of the aromatic hydrocarbon receptor (Ahr), leading to (regulatory) T-cell expansion (Funatake et al., 2005; Kerkvliet et al., 2009). Accordingly, we found the GO term lymphocyte activation correlating with maternal exposure to PCB126 and PCB153 in males although PCB153 is a nondioxin-like PCB. T-cell proliferation was found to correlate with exposure to PCB77 and TCDD in females. In agreement with a recent publication on transcriptomic effects in the thymus of mice (Frawley et al., 2011), antigen presentation was affected upon exposure to TCDD and PCBs. However, this was only found in males. The associations found at the transcriptomic level between estimated maternal intake levels of TCDD and PCBs and effects on the neonatal immune system, thus, possibly suggest gender-specific immune responses although the current data do not allow any definite conclusions.
Next to immune-related processes, we found an epigenetic response in neonates to maternal TCDD exposure, showing histone acetylation processes significantly enriched in males. This was previously demonstrated in a study from our group after in utero exposure to dioxin and dioxin-like compounds by means of DR CALUX measurements in the BraMat cohort (Hochstenbach et al., submitted). Effects on the epigenetic level are in concordance with the known mechanism of action of dioxins; activation of transcription by dioxin is accompanied by changes in chromatin structure, which depend upon a functional Ahr (Morgan and Whitlock, 1992). An epigenetic response in male neonates was also found for PCB153. Gender specificity in this epigenetic response is emphasized by the inverse correlation between males and females of a number of genes involved in histone and chromatin modification, including CPA4, SF3B1, H3F3A and B, and HIST1H3A, G, H, and K (data not shown). Epigenetic modulation upon exposure to TCDD and PCBs may represent a persistent effect on the genome.
Toxicogenomic Profiles in Newborns in Relation to Measles Vaccine Response
Gender-specific gene expression profiles were associated with antibody responses to measles vaccination. A significantly higher number of genes were found regulated in females, which intuitively may be in accordance with the fact that elevated immune responses in female humans and animals compared with males have been reported, including enhanced innate recognition and cytokine response to viral infections (McClelland and Smith, 2011). Also, gender may play a significant role in how the immune system responds to viral vaccines and their side effects, as recently reviewed by Klein et al. (2010), describing more robust immune responses in females to antigenic challenges through a higher antibody production and increased cell-mediated immunity. A stronger humoral immune response to single measles vaccine and MMR vaccine has been reported in females (Dominguez et al., 2006; Green et al., 1994). Accordingly, the mean antibody level was higher in females compared with males although this did not reach significance, possibly due to the relatively low number of subjects.
Differential transcriptomic responses between males and females to viral vaccination have recently been reported (Klein et al., 2010). A more robust transcriptomic profile was found in females upon yellow fever virus 17D vaccination, including genes associated with toll-like receptors, interferon, cytokines, macrophages, dendritic cells, natural killer cells, B cells, and inflammation. In contrast, in males only few genes were found to be differentially expressed. In the present study, the identified profiles in neonates in relation to vaccination response show similar results: in females GO terms corresponding to all the above mentioned functional categories were found to be overrepresented in addition to cell death and apoptosis. Apoptosis is a highly important phenomenon in the immune function: it is an important mechanism in activation-induced cell death, the negative selection of T cells, and B-cell maturation. Apoptosis is also important in killing infected target cells by cytotoxic T cells and natural killer cells (Feig and Peter, 2007). In accordance with the findings of Klein et al., a significantly lower number of processes were found in males, all not directly related to the immune system.
Interestingly, oppositely correlating genes between males and females are involved in immunity and apoptosis, suggesting high gender specificity regarding these processes relevant to the immune response to vaccination.
Linking Transcriptomic Effects in Neonates due to Maternal TCDD and PCB Exposure and Antibody Response to Measles Vaccination at the Age of 3
Strikingly, for both males and females, almost without exception all genes in the overlap show opposite correlation between exposure and vaccination response, i.e., genes correlating negatively with exposure show positive correlation with antibody levels and vice versa as shown in Tables 6 and 7. This suggests that transcriptomic effects in the fetus, i.e., the modulation of immune-related genes due to maternal exposure, may result in suppressed immune functionality at 3 years of age.
TABLE 6 .
Immune-Related Transcripts Significantly Correlating With Antibody Response and One or More Exposure Markers for Males
| Entrez gene ID | Gene name | Gene symbol | Measles males | TCD Dmales | PCB77 males | PCB126 males | PCB153 males | PCB169 males | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3603 | Interleukin 16 lymphocyte chemoattractant factor | IL16 | 0.68 | −0.34 | −0.34 | −0.34 | −0.40 | |||||||||
| 246778 | Interleukin 27 | IL27 | 0.52 | −0.47 | −0.43 | −0.40 | −0.40 | |||||||||
| 976 | CD97 molecule | CD97 | 0.76 | −0.24 | −0.24 | −0.30 | ||||||||||
| 3932 | Lymphocyte-specific protein tyrosine kinase | LCK | 0.46 | −0.31 | −0.24 | −0.29 | ||||||||||
| 6404 | Selectin P ligand | SELPLG | 0.57 | −0.29 | −0.28 | |||||||||||
| 8106 | Poly A binding protein, nuclear 1 | PABPN1 | 0.50 | −0.28 | −0.29 | |||||||||||
| 984 | Cell division cycle 2–like 1 PITSLRE proteins | CDC2L1 | 0.47 | −0.19 | −0.22 | |||||||||||
| 27128 | Pleckstrin homology, Sec7 and coiled-coil domains 4 | PSCD4 | 0.58 | −0.19 | ||||||||||||
| 708 | Complement component 1, q subcomponent binding protein | C1QBP | 0.56 | −0.30 | ||||||||||||
| 10628 | Thioredoxin-interacting protein | TXNIP | 0.50 | −0.30 | ||||||||||||
| 3133 | Major histocompatibility complex class I, E | HLA-E | 0.47 | −0.27 | ||||||||||||
| 51604 | Phosphatidylinositol glycan anchor biosynthesis, class T | PIGT | 0.46 | 0.26 | ||||||||||||
| 5692 | Proteasome prosome, macropain subunit, beta type, 4 | PSMB4 | 0.45 | 0.21 | ||||||||||||
| 11146 | Glomulin, FKBP-associated protein | GLMN | −0.62 | 0.43 | 0.44 | 0.41 | 0.34 | 0.36 | ||||||||
| 4300 | Myeloid/lymphoid or mixed-lineage leukemia trithorax homolog, Drosophila; translocated to, 3 | MLLT3 | −0.63 | 0.45 | 0.45 | 0.46 | 0.41 | 0.42 | ||||||||
| 25909 | AT hook containing transcription factor 1 | AHCTF1 | −0.47 | 0.18 | 0.29 | 0.26 | 0.32 | |||||||||
| 5055 | Serpin peptidase inhibitor, clade B ovalbumin, member 2 | SERPINB2 | −0.55 | 0.35 | 0.30 | 0.28 | 0.32 | |||||||||
| 57824 | Histocompatibility minor HB-1 | HMHB1 | −0.59 | 0.23 | 0.32 | 0.23 | 0.30 | |||||||||
| 56940 | Dual specificity phosphatase 22 | DUSP22 | −0.65 | 0.32 | 0.39 | |||||||||||
| 7185 | TNF receptor–associated factor 1 | TRAF1 | −0.54 | 0.30 | ||||||||||||
| 60412 | Exocyst complex component 4 | EXOC4 | −0.67 | 0.36 |
Note. For the complete list, refer to Supplementary table 4.
TABLE 7 .
Immune-Related Transcripts Significantly Correlating With Antibody Response and One or More Exposure Markers for Females
| Entrez gene ID | Gene name | Gene symbol | Measles females | TCDD females | PCB77 females | PCB126 females | PCB153 females | PCB169 females | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 203190 | Leucine-rich repeat LGI family, member 3 | LGI3 | 0.48 | −0.48 | −0.42 | −0.51 | −0.49 | −0.42 | ||||||||
| 3801 | Kinesin family member C3 | KIFC3 | 0.39 | −0.28 | −0.34 | −0.34 | −0.30 | −0.25 | ||||||||
| 54472 | Toll interacting protein | TOLLIP | 0.33 | −0.19 | −0.22 | −0.30 | −0.28 | −0.33 | ||||||||
| 2200 | Fibrillin 1 | FBN1 | 0.47 | −0.20 | −0.24 | −0.29 | −0.24 | |||||||||
| 56892 | Chromosome 8 open reading frame 4 | C8orf4 | 0.45 | −0.24 | −0.34 | −0.45 | −0.38 | |||||||||
| 5133 | Programmed cell death 1 | PDCD1 | 0.42 | −0.36 | −0.45 | −0.45 | −0.43 | |||||||||
| 55846 | Integrin alpha FG-GAP repeat containing 2 | ITFG2 | 0.47 | −0.23 | −0.30 | −0.24 | ||||||||||
| 3351 | 5-hydroxytryptamine serotonin receptor 1B | HTR1B | 0.42 | −0.31 | −0.34 | −0.36 | ||||||||||
| 4842 | Nitric oxide synthase 1 neuronal | NOS1 | 0.54 | −0.47 | −0.44 | |||||||||||
| 112464 | Protein kinase C, delta binding protein | PRKCDBP | 0.58 | −0.23 | ||||||||||||
| 8200 | Growth differentiation factor 5 | GDF5 | 0.52 | −0.20 | ||||||||||||
| 581 | BCL2-associated X protein | BAX | 0.51 | −0.34 | ||||||||||||
| 27202 | G protein–coupled receptor 77 | GPR77 | 0.50 | −0.24 | ||||||||||||
| 25794 | Fascin homolog 2, actin-bundling protein, retinal Strongylocentrotus purpuratus | FSCN2 | 0.50 | −0.32 | ||||||||||||
| 10887 | Prokineticin receptor 1 | PROKR1 | 0.47 | −0.12 | ||||||||||||
| 3909 | Laminin, alpha 3 | LAMA3 | 0.46 | −0.20 | ||||||||||||
| 2065 | v-erb-b2 erythroblastic leukemia viral oncogene homolog 3 avian | ERBB3 | 0.45 | −0.24 | ||||||||||||
| 5600 | Mitogen-activated protein kinase 11 | MAPK11 | 0.45 | −0.19 | ||||||||||||
| 6753 | Somatostatin receptor 3 | SSTR3 | 0.45 | −0.22 | ||||||||||||
| 23786 | BCL2-like 13 apoptosis facilitator | BCL2L13 | 0.45 | −0.14 | ||||||||||||
| 2730 | Glutamate-cysteine ligase, modifier subunit | GCLM | 0.43 | 0.21 | ||||||||||||
| 5710 | Proteasome prosome, macropain 26S subunit, non-ATPase, 4 | PSMD4 | 0.42 | −0.11 | ||||||||||||
| 145741 | Nuclear localized factor 1 | NLF1 | 0.37 | −0.25 | ||||||||||||
| 51668 | Chromosome 1 open reading frame 41 | C1orf41 | 0.35 | 0.27 | ||||||||||||
| 9135 | Rabaptin, RAB GTPase binding effector protein 1 | RABEP1 | 0.35 | −0.30 | ||||||||||||
| 6929 | Transcription factor 3 E2A immunoglobulin enhancer binding factors E12/E47 | TCF3 | 0.34 | −0.14 | ||||||||||||
| 55023 | Pleckstrin homology domain interacting protein | PHIP | −0.34 | 0.20 | 0.24 | 0.16 | 0.24 | |||||||||
| 5515 | Protein phosphatase 2 formerly 2A, catalytic subunit, alpha isoform | PPP2CA | −0.37 | 0.28 | 0.18 | 0.23 | ||||||||||
| 3516 | Recombination signal binding protein for immunoglobulin kappa J region | RBPJ | −0.38 | 0.26 | 0.23 | 0.27 | ||||||||||
| 8106 | Poly A binding protein, nuclear 1 | PABPN1 | −0.34 | 0.25 | ||||||||||||
| 80762 | Nedd4 family–interacting protein 1 | NDFIP1 | −0.34 | 0.26 | ||||||||||||
| 7534 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide | YWHAZ | −0.36 | 0.30 | ||||||||||||
| 7155 | Topoisomerase DNA II beta 180kDa | TOP2B | −0.37 | 0.15 | ||||||||||||
| 6672 | SP100 nuclear antigen | SP100 | −0.37 | 0.14 | ||||||||||||
| 7099 | Toll-like receptor 4 | TLR4 | −0.39 | 0.11 | ||||||||||||
| 4690 | NCK adaptor protein 1 | NCK1 | −0.40 | 0.22 | ||||||||||||
| 81542 | Thioredoxin domain containing 1 | TXNDC1 | −0.40 | 0.14 | ||||||||||||
| 493 | ATPase, Ca++ transporting, plasma membrane 4 | ATP2B4 | −0.40 | 0.15 | ||||||||||||
| 9516 | Lipopolysaccharide-induced TNF factor | LITAF | −0.45 | −0.25 | ||||||||||||
| 6772 | Signal transducer and activator of transcription 1, 91kDa | STAT1 | −0.46 | −0.22 |
Note. For the complete list, refer to Supplementary table 4.
Human leukocyte antigen (HLA) genes have been associated with the ability to respond to measles antigens by antibody production. A large number of studies have been reported on associations between HLA alleles and antibody levels after vaccination (Alper et al., 1989; Ovsyannikova et al., 2004; Poland, 1998). Both in males and females, genes part of the HLA system and involved in antigen presentation were found to be negatively correlated with exposure, including HLA-E and MICA (males), RXRB and PSMD4 (females). These genes were all positively correlated with vaccination response.
Antigens stimulate a number of cells in the immune system, including macrophages, T cells, and B cells. Perinatal exposure to TCDD suppresses cell-mediated immunity, which is essential in immunization response and is more severe and persistent than that caused by adult exposure (Fine et al., 1989). Accordingly, a number of genes were identified in the overlap involved in cell-mediated immunity, mostly negatively correlated with exposure and positively with immune response. In males, LCK, GLMN, and IL27 were found, involved in T-cell activation and proliferation, whereas in females, PDCD1, BAX, TLR4, and NCK1 were found. In females, TCF3 was also found, involved in B-lymphocyte proliferation (Massari et al., 1998).
Stimulated immune cells secrete a variety of signal molecules called cytokines. In male and female newborns, genes associated with cytokines were all negatively associated with maternal exposure and positively with vaccination response. In males, genes encoding for interleukins IL16 and IL27 were found, as well as GLMN and PLXNB2. In females, RXRB, GDF5, GPR77, ROBO3, GNRH2, RABEP1, and STAT1 were found. The latter was negatively associated with exposure and vaccination response.
Importantly, besides at gene level, overlap between exposure and immune response was found at pathway level. In females, the GO term regulation of defense response was significantly overrepresented in association with exposure and vaccination response. This term is defined as the regulation of reactions, triggered in response to the presence of a foreign body or the occurrence of an injury, which result in restriction of damage to the organism attacked or prevention/recovery from the infection caused by the attack.
In males, histone H4-K12 acetylation was found in the overlap. This might suggest that maternal exposure to TCDD and PCBs leads to gender-specific epigenetic changes in the fetus, which influences immune functionality at 3 years of age.
General Considerations
FFQ-based estimates for PCBs and dioxins are a proxy for blood values. The FFQ used in the present study covers the mother’s self-reported dietary intakes during the first 5 months of pregnancy thus reflecting intakes over a relatively short time period. In addition, pregnant women may change their dietary habits. However, unpublished data from MoBa suggest that 70–80% of the women do not alter their fish intake, which is the main contributor to PCB and dioxin exposure from food.
The database used for estimating the dietary intake levels is based on all available concentrations of dioxin and PCB congeners measurements from a large array of food product found on the Norwegian market from 2000 to 2006. Although most food items were based on sufficient underlying concentration data, some were based on a small number of samples and other items had to be estimated. We acknowledge that calculated exposure values using FFQs have limitations compared with measured blood values. However, our results are in accordance with published data based on measured levels of PCBs and dioxins in biological samples, which indicate that the estimated intake levels may reflect the exposure to these toxicants.
The median dietary intake of dioxin-like compounds was 0.58 pg TEQ/kg bw/day (Stølevik et al., 2011), which is lower than the intakes reported in other studies of the general population in Western European countries and United States (Baars et al., 2004; Darnerud et al, 2006; Kiviranta et al., 2004; Lloblet et al., 2008; Schecter et al., 2001). However, the intake estimates in the cited studies are produced using the 1998 WHO Toxic Equivalency Factor (TEF) values, which were higher than the 2005 WHO (TEF) values used in the present study. The estimated value of nondioxin-like PCBs (2.59ng/kg bw/day) (Stølevik et al., 2011) is lower compared with other reported values (Baars et al., 2004; European Food Safety Authority, 2005).
This study only investigated the estimated maternal intake during pregnancy in relation to vaccination responses, whereas exposures between birth and the age of 3 (the time of antibody measurements) were not taken into account. The children may have been exposed to TCDD, PCB, and other immunotoxicants in variable degrees through breast milk in the first year of life and later through the diet (Ayotte et al., 2003).
Long-chain n-3 fatty acids from fish may influence the associations with PCBs and dioxins found due to their suggested anti-inflammatory effects (Chapkin et al., 2009). As described by Stølevik et al. (submitted), a significant association was only found between the prenatal exposure to n-3 fatty acids from food items and wheeze during the first three years of life. No associations were found for n-3 fatty acids from dietary supplements (purified to remove PCBs and dioxins), which may suggest that exposure to PCBs and dioxins, rather than n-3 fatty acids, is most important for the immunosuppression found in BraMat.
CONCLUSION
The current study links transcriptomic effects of maternal exposure to TCDD and PCBs and neonatal vaccination response at the age of 3. For both males and females, genes correlating negatively with exposure show positive correlation with antibody levels and vice versa. This suggests that transcriptomic effects in neonates due to maternal exposure, i.e., the modulation of immune-related genes upon exposure to TCDD and PCB, may suppress measles vaccination response at 3 years of age.
Apart from these immune-related genes, in males an epigenetic response was found through histone acetylation, which was also linked to immune functionality. This might suggest that maternal exposure to TCDD and PCBs leads to gender-specific epigenetic changes in the fetus, which subsequently influences immune functionality up until 3 years of age, possibly mechanistically underlying why males show lower responses to measles vaccination.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
Funding
EU Integrated Project NewGeneris, 6th Framework Programme, Priority 5: Food Quality and Safety. NewGeneris is the acronym of the project “Newborns and Genotoxic exposure risks” (FOOD-CT-2005-016320). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no NO-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10).
Supplementary Material
Acknowledgments
We are grateful to all the participating families in Norway, in particular the mothers, who take part in this ongoing cohort study.
References
- Alper C. A., Kruskall M. S., Marcus-Bagley D., Craven D. E., Katz A. J., Brink S. J., Dienstag J. L., Awdeh Z., Yunis E. J. (1989). Genetic prediction of nonresponse to hepatitis B vaccine N. Engl. J. Med. 321 708–712 [DOI] [PubMed] [Google Scholar]
- Ayotte P., Muckle G., Jacobson J. L., Jacobson S. W., Dewailly E. (2003). Assessment of pre- and postnatal exposure to polychlorinated biphenyls: Lessons from the Inuit Cohort Study Environ. Health Perspect. 111 1253–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars A. J., Bakker M. I., Baumann R. A., Boon P. E., Freijer J. I., Hoogenboom L. A., Hoogerbrugge R., van Klaveren J. D., Liem A. K., Traag W. A., et al. (2004). Dioxins, dioxin-like PCBs and non-dioxin-like PCBs in foodstuffs: Occurrence and dietary intake in The Netherlands Toxicol. Lett. 151 51–61 [DOI] [PubMed] [Google Scholar]
- Brantsaeter A. L., Haugen M., Alexander J., Meltzer H. M. (2008). Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa) Matern. Child Nutr. 4 28–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin R. S., Kim W., Lupton J. R., McMurray D. N. (2009). Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation Prostaglandins Leukot. Essent. Fatty Acids 81 187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaci A., Jorens P., Jacquemyn Y., Schepens P. (2002). Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum Sci. Total Environ. 298 45–53 [DOI] [PubMed] [Google Scholar]
- Dominguez A., Plans P., Costa J., Torner N., Cardenosa N., Batalla J., Plasencia A., Salleras L. (2006). Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: Results of a cross-sectional study Eur. J. Clin. Microbiol. Infect. Dis. 25 310–317 [DOI] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., Lash A. E. (2002). Gene Expression Omnibus: NCBI gene expression and hybridization array data repository Nucleic Acids Res. 30 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (2005). Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the presence of non-dioxin-like polychlorinated biphenyls (PCB) in feed and food EFSA J. 284 1–137 [Google Scholar]
- Feig C., Peter M. E. (2007). How apoptosis got the immune system in shape Eur. J. Immunol. 37(Suppl. 1)S61–S70 [DOI] [PubMed] [Google Scholar]
- Fine J. S., Gasiewicz T. A., Silverstone A. E. (1989). Lymphocyte stem cell alterations following perinatal exposure to 2,3,7,8-tetra- chlorodibenzo-p-dioxin Mol. Pharmacol. 35 18–25 [PubMed] [Google Scholar]
- Frawley R., White K., Jr, Brown R., Musgrove D., Walker N., Germolec D. (2011). Gene expression alterations in immune system pathways in the thymus after exposure to immunosuppressive chemicals Environ. Health Perspect. 119 371–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatake C. J., Marshall N. B., Steppan L. B., Mourich D. V., Kerkvliet N. I. (2005). Cutting edge: Activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells J. Immunol. 175 4184–4188 [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A., Cooper C., Thornburg K. L. (2008). Effect of in utero and early-life conditions on adult health and disease N. Engl. J. Med. 359 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. S., Shohat T., Lerman Y., Cohen D., Slepon R., Duvdevani P., Varsano N., Dagan R., Mendelson E. (1994). Sex differences in the humoral antibody response to live measles vaccine in young adults Int. J. Epidemiol. 23 1078–1081 [DOI] [PubMed] [Google Scholar]
- Heilmann C., Grandjean P., Weihe P., Nielsen F., Budtz-Jorgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. (2006);3:e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N. I., Steppan L. B., Vorachek W., Oda S., Farrer D., Wong C. P., Pham D., Mourich D. V. (2009). Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes Immunotherapy 1 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirante H., Ovaskainen M. A. L., Vartiainen T. (2004). Market basket study on dietary intake of PCDD/Fs, PCBs, and PBDEs in Finland Environ. Int. 30 923–932 [DOI] [PubMed] [Google Scholar]
- Klein S. L., Jedlicka A., Pekosz A. (2010). The Xs and Y of immune responses to viral vaccines Lancet Infect. Dis. 10 338–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvalem H. E., Knutsen H. K., Thomsen C., Haugen M., Stigum H., Brantsaeter A. L., Froshaug M., Lohmann N., Papke O., Becher G., et al. (2009). Role of dietary patterns for dioxin and PCB exposure Mol. Nutr. Food Res. 53 1438–1451 [DOI] [PubMed] [Google Scholar]
- Llobet J. M., Marti.Cid R., Castell V., Domingo J. L. (2008). Significant decreasing trend in human dietary exposure to PCDD/PCDFs and PCBs in Catalonia, Spain Toxicol. Lett. 178 117–126 [DOI] [PubMed] [Google Scholar]
- Magnus P., Irgens L. M., Haug K., Nystad W., Skjaerven R., Stoltenberg C. (2006). Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) Int. J. Epidemiol. 35 1146–1150 [DOI] [PubMed] [Google Scholar]
- Massari M. E., Rivera R. R., Voland J. R., Quong M. W., Breit T. M., van Dongen J. J., de Smit O., Murre C. (1998). Characterization of ABF-1, a novel basic helix-loop-helix transcription factor expressed in activated B lymphocytes Mol. Cell Biol. 18 3130–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland E. E., Smith J. M. (2011). Gender specific differences in the immune response to infection Arch. Immunol. Ther. Exp. (Warsz) 59 203–213 [DOI] [PubMed] [Google Scholar]
- Meltzer H. M., Brantsaeter A. L., Ydersbond T. A., Alexander J., Haugen M. (2008). Methodological challenges when monitoring the diet of pregnant women in a large study: Experiences from the Norwegian Mother and Child Cohort Study (MoBa) Matern. Child Nutr. 4 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo D. F., Wild C. P., Kogevinas M., Kyrtopoulos S., Kleinjans J. (2009). NewGeneris: A European study on maternal diet during pregnancy and child health Cancer Epidemiol. Biomarkers Prev. 18 5–10 [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Whitlock J. P., Jr (1992). Transcription-dependent and transcription-independent nucleosome disruption induced by dioxin Proc. Natl. Acad. Sci. U.S.A. 89 11622–11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova I. G., Jacobson R. M., Vierkant R. A., Shane Pankratz V., Jacobsen S. J., Poland G. A. (2004). Associations between human leukocyte antigen (HLA) alleles and very high levels of measles antibody following vaccination Vaccine 22 1914–1920 [DOI] [PubMed] [Google Scholar]
- Park J. S., Bergman A., Linderholm L., Athanasiadou M., Kocan A., Petrik J., Drobna B., Trnovec T., Charles M. J., Hertz-Picciotto I. (2008). Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from eastern Slovakia Chemosphere 70 1676–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland G. A. (1998). Variability in immune response to pathogens: Using measles vaccine to probe immunogenetic determinants of response Am. J. Hum. Genet. 62 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A., Cramer P., Boggess K., Stanley J., Päpke O., Olson J., Silver A., Schmitz M. (2001). Intake of dioxins and related compounds from food in the U.S. population J. Toxicol. Environ. Health A 63 1–18 [DOI] [PubMed] [Google Scholar]
- Stølevik S. B., Nygaard U. C., Namork E., Haugen M., Kvalem H. E., Meltzer H. M., Alexander J., van Delft J. H., Loveren H., Lovik M., et al. (2011). Prenatal exposure to polychlorinated biphenyls and dioxins is associated with increased risk of wheeze and infections in infants Food Chem. Toxicol. 49 1843–1848 [DOI] [PubMed] [Google Scholar]
- Tian Y., Rabson A. B., Gallo M. A. (2002). Ah receptor and NF-kappaB interactions: Mechanisms and physiological implications Chem. Biol. Interact. 141 97–115 [DOI] [PubMed] [Google Scholar]
- Van Loveren H., Piersma A. (2004). Immunotoxicological consequences of perinatal chemical exposures Toxicol. Lett. 149 141–145 [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N., Patandin S., Berbers G. A., Sas T. C., Mulder P. G., Sauer P. J., Hooijkaas H. (2000). Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children Environ. Health Perspect. 108 1203–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisglas-Kuperus N., Sas T. C., Koopman-Esseboom C., van der Zwan C. W., De Ridder M. A., Beishuizen A., Hooijkaas H., Sauer P. J. (1995). Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants Pediatr. Res. 38 404–410 [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N., Vreugdenhil H. J., Mulder P. G. (2004). Immunological effects of environmental exposure to polychlorinated biphenyls and dioxins in Dutch school children Toxicol. Lett. 149 281–285 [DOI] [PubMed] [Google Scholar]
- West L. J. (2002). Defining critical windows in the development of the human immune system Hum. Exp. Toxicol. 21 499–505 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.