Abstract
Environmental exposure to arsenic, especially the trivalent inorganic form (As3+), has been linked to human cancers in addition to a number of other diseases including skin lesions, cardiovascular disorders, neuropathy, and internal organ injury. In the present study, we describe a novel signaling axis of the c-Jun NH2 kinase (JNK) and signal transducer and activator of transcription 3 (Stat3) and its involvement in As3+-induced Akt activation in human bronchial epithelial cells. As3+ activates JNK and induces phosphorylation of the Stat3 at serine 727 (S727) in a dose- and time-dependent manner, which occurred concomitantly with Akt activation. Disruption of the JNK signaling pathway by treatment with the JNK inhibitor SP600125, siRNA knockdown of JNK, or genetic deficiency of the JNK1 or JNK2 gene abrogated As3+-induced S727 phosphorylation of Stat3, Akt activation, and the consequent release of vascular endothelial growth factor (VEGF) and migration of the cells. Similarly, pretreatment of the cells with Stat3 inhibitor or Stat3 siRNA prevented Akt activation and VEGF release from the cells in response to As3+ treatment. Taken together, these data revealed a new signaling mechanism that might be pivotal in As3+-induced malignant transformation of the cells by linking the key stress signaling pathway, JNK, to the activation of Stat3 and the carcinogenic kinase, Akt.
Environmental or occupational exposure to arsenic, especially the trivalent inorganic form of arsenic (As3+), continues to be a major public health concern in a number of countries, including the United States (Heck et al., 2009). Accumulating epidemiological data have indicated that As3+ exposure through contaminated drinking water or inhalation can cause human cancers such as cancers in the lung, liver, skin, kidney, and prostate (Celik et al., 2008). Notably, not only high levels of As3+ exposure but also low to moderate levels of As3+ exposure have been linked to an increased risk of lung cancer and other cancers. The International Agency for Research on Cancer (IARC, 2004) has classified As3+ as a group I carcinogen for lung cancer. A population-based case-control study suggested a strong association between As3+ exposure and small cell and non–small cell lung cancers (Heck et al., 2009). In addition, several ecological and cohort studies have confirmed the contribution of As3+ exposure to human lung cancers (Chen et al., 2004; Chiu et al., 2004; Hopenhayn-Rich et al., 1998).
We have previously shown that the treatment of the human bronchial epithelial cell line BEAS-2B with As3+ results in the ubiquitination and degradation of the CDC25C protein, an important cell cycle regulatory protein involved in the cell cycle transition from G2 to M phase (Chen et al., 2002). We have also demonstrated that As3+ is a potent inducer of GADD45α, a cell cycle checkpoint protein that is partially dependent on the activation of JNK (Chen et al., 2001). Furthermore, we and others have demonstrated that As3+ can activate Akt (Wang et al., 2012; Zhang et al., 2006). Our most recent studies have shown that As3+-induced Akt activation is partially achieved through the upregulation of miR-190, an oncogenic microRNA that downregulates the PH domain, and leucine-rich repeat protein phosphatase, a negative regulator of Akt signaling (Beezhold et al., 2011). It has been generally viewed that activation of JNK or induction of CDC25C degradation sensitizes cell death or cell cycle arrest, whereas activation of Akt favors cell growth or malignant transformation. However, it is unclear how the As3+-based activation of several functionally contradictory signaling pathways ultimately results in cell growth and how these pathways might be connected to each other.
The activation of JNK signaling is generally thought to promote cell death or tumor suppression in response to a variety of extracellular and intracellular stress signals. Paradoxically, overwhelming evidence has also implicated JNK signaling in the promotion of growth and the formation of tumors under many physiological and pathological circumstances (Chen, 2012). For example, oncogenic Ras-induced cell proliferation and transformation requires JNK activation (Dérijard et al., 1994). In animal cancer models, JNK activation, especially, JNK1, appears to be essential for tobacco smoke–induced lung cancer, chemical carcinogen–induced gastric tumors, and hepatocellular carcinomas (Luedde et al., 2007; Maeda et al., 2005; Shibata et al., 2008; Takahashi et al., 2010). Two independent studies have also provided compelling evidence demonstrating the importance of JNK1 activation in the pathogenesis of human hepatocellular carcinoma (Chang et al., 2009; Hui et al., 2008). In addition, several recent studies have revealed key contributions of JNK signaling to the self-renewal and proliferative qualities of embryonic stem cells, adult stem cells, and cancer stem cells (Brill et al., 2009; Chen et al., 2009b; Hutchins and Robson, 2009). It has been proposed that the activation of JNK signaling changes the tissue ecosystem habitats that favor the growth of stem cells. This hypothesis is particularly interesting with respect to cancer because such changes may create a selective pressure that selects cells that possess self-renewing capabilities (cancer stem cells) and limits the growth of cells that have the capacity to differentiate (Chen, 2012). In Drosophila, JNK activation triggers two independent signaling pathways: one induces apoptosis and another stimulates compensatory proliferation of the cells (Suissa et al., 2011). Thus, it is very likely that the growth-promoting role of JNK signaling may overwhelm the proapoptotic role of JNK signaling in a cellular context–dependent manner. In the present study, we provide evidence indicating that JNK is an upstream kinase responsible for the phosphorylation of the serine 727 (pS727) of Stat3 and the subsequent activation of Akt in cells treated with As3+. The inactivation of JNK reduced pS727 of Stat3, Akt activation, vascular endothelial growth factor (VEGF) generation, and cell migration. Collectively, these data provide a novel mechanistic explanation for the growth-promoting or tumorigenic properties of the JNK signaling.
MATERIALS AND METHODS
Cell culture and reagents. The BEAS-2B cell line, which is a human bronchial epithelial cell line derived from normal cells immortalized with the SV40 T-antigen, was purchased from the American Type Culture Collection (Manassas, VA). Wild-type (WT), Jnk1 gene knockout (JNK1−/−), and Jnk2 gene knockout (JNK2−/−) mouse embryonic fibroblast (MEF) cells were gifts of Dr Y. Xia (Department of Environmental Health, University of Cincinnati). Cells were cultured in monolayers using Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Grand Island, NY) supplemented with 5% fetal bovine serum and 1% penicillin-streptomycin at 37°C in a humidified incubator in the presence of 5% CO2. Cells were subcultured as they reached confluence by washing with Ca2+- and Mg2+-free PBS and then dislodged with 0.05% trypsin. Arsenic chloride was purchased from Sigma-Aldrich (St Louis, MO). The JNK inhibitor SP600125 and Stat3 inhibitor V (Stattic) were purchased from Millipore (Billerica, MA); antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) or Millipore.
Cell treatment. Cells (5×105) were seeded in 6-well plates (BD Falcon, Sparks, MD) in a volume of 2ml of medium per well. Once the cells reached 80% confluence, they were treated with the indicated concentrations of As3+ for 0–16h. In some experiments, JNK inhibitor SP600125 or the STAT3 inhibitor V were added 4h prior to As3+ treatment. At the end of each experiment, the cell supernatants were collected for cytokine detection and the cells were collected for Western blotting experiments.
Western blotting. BEAS-2B cells were lysed by sonication in 1 × RIPA buffer (Millipore) supplemented with phosphatase and protease inhibitor cocktail and 1mM PMSF. Insoluble debris was removed by centrifugation at 13,000g for 10min at 4°C. Protein concentrations in the clarified lysates were quantified using the Micro BCA Protein Assay Reagent Kit (Pierce Biotechnology, Rockford, IL). Cell lysates were diluted with 4 × NuPage LDS sample buffer (Invitrogen) containing 200mM dithiothreitol and were boiled at 95°C for 7min before they were loaded onto a 10% or 12% SDS-PAGE gel. After electrophoretic separation of the cell lysates, the proteins were transferred onto PVDF membranes (Invitrogen) and were blocked in 5% nonfat milk/Tris-buffered saline with 0.05% Tween-20 (TBS-T), pH 7.4 for 1h at room temperature. Then the membranes were incubated with the appropriate primary antibody at a dilution of 1:1000 overnight at 4°C. After extensive washing with TBS-T, the membranes were incubated with anti-rabbit IgG conjugated with alkaline phosphatase (AP) at a dilution of 1:3000 for 1h at room temperature. CDP-Star Reagent (New England Biolabs, Ipswich, MA) was used for the development of immunoreactive bands and subsequent x-ray film visualization. The primary antibodies used were phospho-Akt (Ser473), Akt, phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, phospho-p38 MAP Kinase (Thr180/Tyr182), p38 MAPK Kinase, phospho-p44/42 MAPK (Erk1/2)(Thr202/Tyr204), p44/42 MAPK (Erk1/2), phospho-Stat3 (Ser727), phospho-Stat3 (Tyr705)(D3A7), Stat3, and β-actin.
Cell transfection and siRNA knockdown. siRNA duplexes targeting JNK or Stat3, as well as the control siRNAs, were purchased from Millipore. BEAS-2B cells were seeded in 6-well tissue culture plates with serum-free and antibiotic-free DMEM and were reverse transfected using the Lipofectamine RNAiMAX reagent (Invitrogen) and 50nM siRNA duplex per well according to the manufacturer’s suggested instructions. Twenty-four hours post-transfection, the cells were treated with As3+ for 4h. Gene silencing was confirmed by Western blotting at the end of the treatment.
ELISA. Serum-starved BEAS-2B cells were treated with various concentrations of As3+ or for various lengths of time with As3+, with or without pretreatment with pharmacological inhibitors or siRNA. Cell-free supernatants were collected for the determination of IL-6 and VEGF. The level of IL-6 was determined by using a human IL-6 EIA Kit (Cayman, Ann Arbor, MI). The level of VEGF was determined by using the DuoSet ELISA development system (R&D systems, Minneapolis, MN), by following the manufacturer’s suggested protocol.
Cell coculture and migration assay. To determine the effect of secreted factors from the As3+-treated cells on the migration of nonstimulated cells, a modified Boyden chamber cell coculture assay was employed by using inserts with a polyethylene terephthalate filter (8-μm pores, BD Biosciences, Sparks, MD) in a 24-well format. Briefly, 1×105 BEAS-2B cells in 500 μl of DMEM were seeded in the lower chamber followed by reverse transfection of the cells with 50nM control siRNA or 50nM siRNA that targets JNK or Stat3 and were incubated at 37°C in the presence of 5% CO2 for 24h. Then the cells were treated with 10μM As3+ for an additional 8h. At the end of the culture, the cells were washed twice with 1× PBS and serum-free DMEM was added to the cells. The inserts containing 5,000 BEAS-2B cells in 500 μl of serum-free DMEM were assembled into each well, and the chambers were incubated at 37°C in the presence of 5% CO2 for 24h. At the end of this incubation, cells that did not migrate through the filters were removed by using cotton swabs. The cells that migrated to the underside of the filter were fixed with 3.7% paraformaldehyde for 20min, washed thrice with PBS, and then stained with 0.1% crystal violet for 30min. The cells were visualized and quantified by microscope.
Statistical analysis. Data were reported as the mean ± SD. Student’s t-test or one-way ANOVA analysis was used, as appropriate, to determine the statistical significance (p < 0.05) of differences between the treatment conditions.
RESULTS
JNK Inhibition Suppresses As3+-Induced Akt Activation
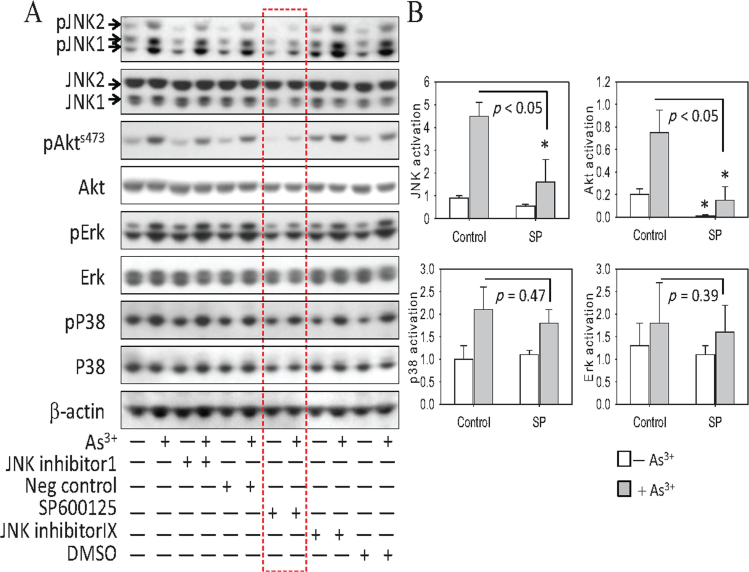
We have previously shown that As3+ is capable of activating JNK and Akt in BEAS-2B cells (Chen et al., 2001; Zhang et al., 2006). Considering the fact that JNK is often associated with stress responses, whereas Akt activation is known to promote cell growth and survival, it is intriguing to know whether the JNK and Akt pathways are interplayed. To do this, we first tested several JNK inhibitors on As3+-induced Akt activation. JNK inhibitor I, a TAT-linked cell-permeable peptide, blocks c-Jun phosphorylation by JNK, whereas both SP600125 and JNK inhibitor IX are ATP-competitive inhibitors that inhibit JNK phosphorylation and activation by upstream kinases (Bogoyevitch and Arthur, 2008). BEAS-2B cells were pretreated with 20μM of each inhibitor or with a negative control peptide for 4h followed by a 4h treatment with 10μM As3+. As expected, the inhibitory effect of JNK inhibitor I on As3+-induced JNK activation, as reflected by the phosphorylation of the JNK proteins, is very marginal (Fig. 1A). A pronounced inhibition of SP600125 on As3+-induced JNK activation was noted (lanes were outlined by a dashed rectangle, Fig. 1A). JNK inhibitor IX, which is selective for JNK3, had no effect on As3+-induced phosphorylation of the JNK1 and JNK2 proteins.
Fig. 1.
JNK inhibition blocks As3+-induced Akt activation. (A) BEAS-2B cells were pretreated with the indicated JNK inhibitors (20μM), negative control, or dimethyl sulfoxide (DMSO) (used as vehicle solution for these inhibitors) for 4h. Cell lysates were prepared for Western blotting after incubating the cells in the presence or absence of 10μM As3+ for an additional 4h. Data are representative of three independent experiments. (B) Quantification of the effect of SP600125 (SP) on As3+-induced JNK, Akt, p38, and Erk by densitometry of the Western blotting images. *p < 0.05. n = 3.
The JNK inhibitor SP600125 also exhibited a pronounced inhibition of Akt S473 phosphorylation in response to As3+ but showed a negligible degree of inhibition of either Erk or p38 (Figs. 1A and B). These data suggest that the inhibition of Akt by SP600125 is a result of JNK inhibition, rather than by a nonspecific effect of SP600125 on multiple kinases.
Stat3 Mediates the JNK-Dependent Akt Activation Induced by As3+
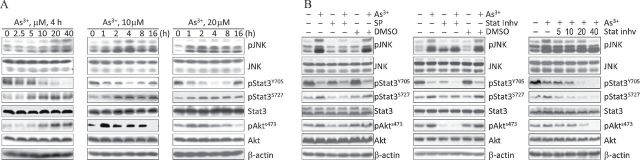
The activation of Akt occurs near the cell membrane. In contrast, activated JNK is most likely located in the nucleus of the cells. Therefore, it is unlikely that Akt is a direct substrate of activated JNK. Stat3 has been linked to Akt activation in mouse models of ischemia/reperfusion injury and cancer (Blando et al., 2011; Ke et al., 2012; Iliopoulos et al., 2010), and previous studies have indicated that the S727 phosphorylation (pS727) of Stat3 is dependent on JNK activation (Lim and Cao, 1999; Zhang et al., 2001). Thus, we speculated that Stat3 might mediate the JNK-dependent activation of Akt in the presence of As3+. To test this hypothesis, we measured Stat3 phosphorylation in parallel with JNK and Akt activation in cells treated with As3+ for different lengths of time and at various concentrations. We noted that As3+ activated JNK and Akt in a concentration- and time-dependent manner (Fig. 2A). Similar to the patterns of JNK and Akt activation, As3+ also promoted pS727 of Stat3, whereas basal levels of pS727 were nearly undetectable. pS727 was clearly detected in cells treated with 5μM As3+ for 4h and peaked in cells treated with 20μM As3+. Time course experiments revealed that induction of pS727 was sustained in the cells treated with As3+ for 4–16h. In contrast, we observed a concentration- and time-dependent inhibition of tyrosine 705 (Y705) phosphorylation (pY705) of Stat3 by As3+ (Fig. 2A). Basal levels of pY705 were significantly higher than pY705 levels in As3+-treated cells. The peak inhibition of pY705 of Stat3 upon As3+ treatment occurred at 4h. After 8h of As3+ treatment, pY705 levels increased and reached to the basal level by 16h.
Fig. 2.
As3+-induced pS727 of Stat3 correlates with JNK and Akt activation. (A) Dose-dependent (left panel) and time-dependent (middle and right panels) studies of As3+ induces activation of JNK, Stat3, and Akt in BEAS-2B cells. (B) Inhibition of JNK by SP600125 (left panel) or Stat3 by Stat3 inhibitor V (middle panel) results in a reduction in As3+-induced Akt activation. BEAS-2B cells were pretreated with 20μM of the indicated inhibitors for 4h and then were treated with 10μM As3+ for an additional 4h. The right panel shows the dose-dependent inhibition of the Stat3 inhibitor V on As3+-induced pS727 of Stat3 and Akt activation.
To explore the potential interactions between As3+-activated JNK, Stat3, and Akt, we pretreated cells with the JNK inhibitor SP600125 (20μM) or the Stat3 inhibitor V (20μM) and determined the effect of these inhibitors on As3+-induced Akt activation. As shown in Figure 2B, inhibition of JNK by SP600125 reduced both pS727 of Stat3 and Akt activation (Fig. 2B, left panel). An inhibition of the Akt activation by Stat3 inhibitor V was also observed (Fig. 2B, middle panel). A moderate degree of inhibition of As3+-induced JNK activation by the Stat3 inhibitor V was also observed, which is in agreement with other studies that indicated mutual activation between JNK and Stat3 (Kim et al., 2010; Zhang et al., 2001). However, in a Stat3 inhibitor V dose-dependence experiment, we noted that As3+-induced pS727 of Stat3 and Akt activation could be inhibited by as little as 5μM of Stat3 inhibitor V, at which dose it showed a negligible effect on As3+-induced JNK activation (Fig. 2B, right panel). Taken together, these data suggest that pS727 of Stat3 is downstream of JNK but upstream of Akt.
Gene Silencing of JNK or Stat3 Prevents As3+-Induced Akt Activation
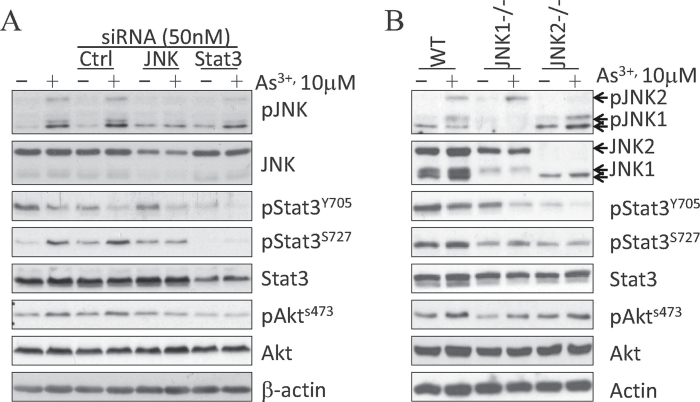
Our data suggest a link between JNK and Stat3 to As3+-induced Akt activation. Both JNK inhibitor SP600125 and Stat3 inhibitor V are relatively specific for their respective target. However, these inhibitors might have off-target effects that could directly or indirectly interfere with other signaling pathways. To address this concern, we used a siRNA-based gene silencing strategy to silence JNK or Stat3 expression in BEAS-2B cells. As showed in Figure 3A, the control siRNA with a scramble sequence that does not target any mRNA showed no effect on As3+-induced JNK activation, pS727 of Stat3, or Akt activation. However, JNK-specific siRNA reduced the basal level of JNK protein and As3+-induced pJNK. Transfection of the cells with JNK-specific siRNA also reduced As3+-induced pS727 of Stat3 and Akt activation (Fig. 3A). As expected, Stat3-specific siRNA reduced the protein levels of total Stat3 and phosphorylated Stat3. The As3+-induced Akt activation was effectively prevented by Stat3 siRNA, which showed very marginal effect on JNK activation.
Fig. 3.
Silencing JNK or Stat3 prevents As3+-induced Akt activation. (A) BEAS-2B cells were transfected with 50nM control siRNA, JNK siRNA, or Stat3 siRNA by reverse transfection. After 24h, cells were treated with 10μM As3+ for an additional 4h. At the end of the culture, cell lysates were prepared for Western blotting with the indicated antibodies. (B) JNK activation, pS727 of Stat3, and Akt activation in WT, JNK1−/−, and JNK2−/− MEFs treated with 10μM As3+ for 4h.
To verify the physiological importance of JNK activation on As3+-induced pS727 of Stat3 and Akt activation, we determined the levels of pS727 of Stat3 and Akt activation in MEFs derived from WT, JNK1 gene knockout (JNK1−/−), and JNK2 gene knockout (JNK2−/−) mice in response to As3+. WT MEFs expressed nearly equal levels of JNK1 (46-kDa) and JNK2 (54-kDa) (lanes 1 and 2, Fig. 3B), whereas JNK1−/− MEFs lacked the expression of the 46-kDa proteins (JNK1α1/β1) and JNK2−/− MEFs lacked the expression of the 54-kDa proteins (JNK2α2/β2 proteins) (lanes 3–6, Fig. 3B) (Chen et al., 2009a). As expected, pJNK1 levels in the JNK1−/− MEFs and pJNK2 levels in the JNK2−/− MEFs were greatly reduced in response to the treatment of the cells with 10μM As3+ for 4h. Both pS727 of Stat3 and Akt activation induced by As3+ were decreased in the JNK1−/− and JNK2−/− MEFs when compared with WT MEFs. These data support our conclusion that JNK is the upstream kinase for As3+-induced Akt activation, which is mediated by Stat3.
Silencing JNK Does Not Prevent As3+-Induced IL-6 Production
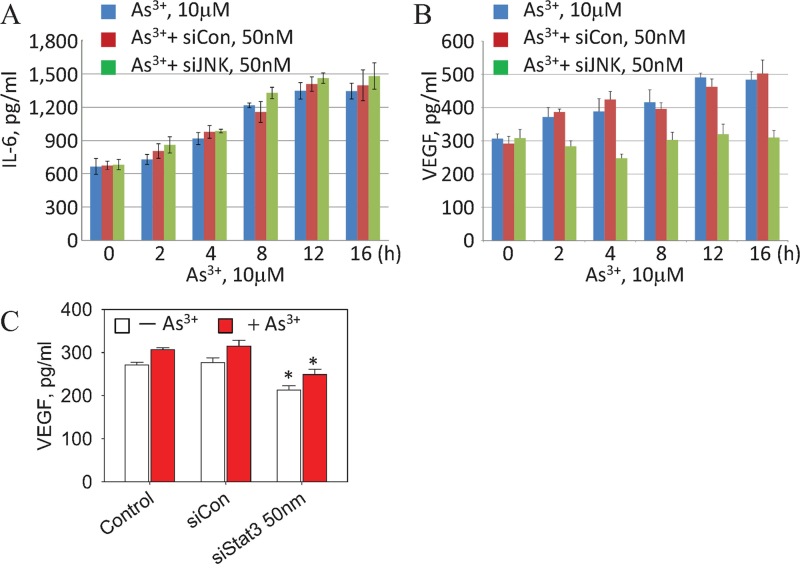
During inflammatory responses, JNK activation has been implicated as an essential step for the generation of IL-6, one of the key activators for Janus kinase (JAK) that phosphorylates Y705, and possibly S727 of Stat3 (Chen, 2012; Park et al., 2010). To determine whether IL-6 is involved in the JNK-Stat3 signaling axis for As3+-induced Akt activation, we measured the levels of IL-6 secreted from cells that were transfected with control (siCon) or JNK-specific siRNA (siJNK) and cultured in the presence or absence of 10μM As3+ for 0–16h. Figure 4A shows that As3+ induces IL-6 generation in a time-dependent manner with a peak in IL-6 secretion by 12–16h. However, silencing JNK by siJNK had no effect on As3+-induced IL-6 generation from the BEAS-2B cells. This result indicates that instead of JNK, other upstream signals, such as NF-κB, might be involved in the As3+-induced IL-6 generation.
Fig. 4.
As3+-induced VEGF, but not IL-6 secretion, is JNK dependent. (A) BEAS-2B cells were either untransfected or transfected with 50nM control siRNA (siCon) or JNK siRNA (siJNK) followed by As3+ treatment for the indicated times. IL-6 in the cell culture medium was determined by ELISA. (B) Cells were treated as in (A). The levels of VEGF were determined by ELISA. (C) VEGF generation from the control cells, cells transfected with Stat3 siRNA (siStat3), or control siRNA (siCon) in the presence or absence of 10μM As3+ for 8h. Data are expressed as the mean ± SD, n = 3. *p < 0.05.
Silencing JNK or Stat3 Abrogates VEGF Generation Induced by As3+
Our results show that the inhibition of JNK by the chemical inhibitor, SP600125, siRNA, or genetic deficiency reduces As3+-induced Akt activation. To determine whether the suppression of JNK-mediated Akt activation has downstream effects, such as the release of VEGF, a cytokine regulated mainly by the activated Akt, the effect of JNK inhibition on VEGF induction by As3+ was analyzed. As shown in Figure 4B, VEGF induction occurred within 2h of treatment with 10μM As3+, which gradually increased until a peak was reached at 12–16h. Transfection of the cells with control siRNA (siCon) prior to As3+ treatment had no effect on As3+-induced VEGF generation. However, a significant inhibition of the As3+-induced VEGF generation was observed in cells transfected with the siJNK (Fig. 4B). The inhibitory effect of siJNK on As3+-mediated VEGF generation suggests that JNK is an upstream kinase that mediates As3+-induced VEGF generation through Akt.
We also determined VEGF induction by As3+ in the cells where the expression of Stat3 was silenced by Stat3-specific siRNA (siStat3). As depicted in Figure 4C, siStat3 suppressed both basal and As3+-induced VEGF generation in the cells. Cells transfected with siCon showed no inhibition of As3+-induced VEGF production.
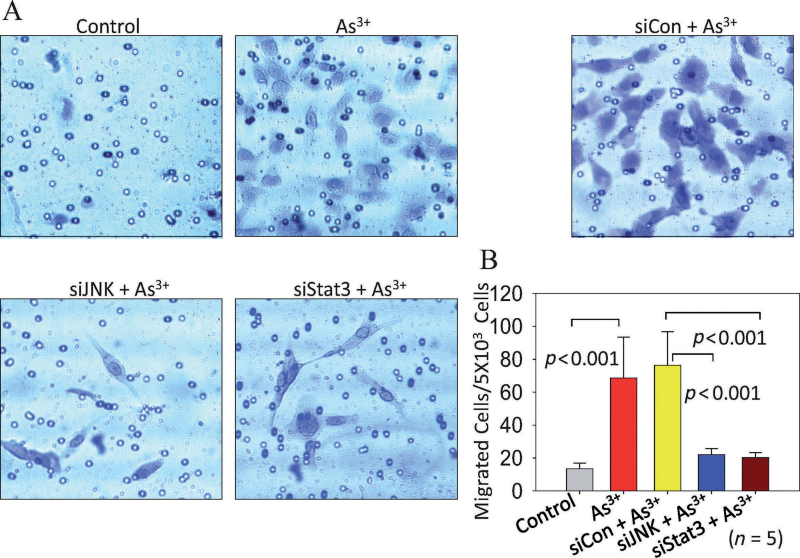
As3+-Activated JNK and Stat3 Contribute to Migration of the Neighboring Cells
Emerging evidence suggest that aberrant activation of Akt is a key step for tumorigenesis by enhancing angiogenesis through the release of VEGF and the migration of endothelial cells (Olsson et al., 2006). We speculated that VEGF released from cells where the JNK, Stat3, and Akt were activated by As3+ might also be capable of inducing migration of neighboring nonendothelial cells in a nonautonomous or paracrine fashion. To test this possibility, we used a cell coculture strategy to assess the induction of cell migration in nontreated cells by cells separated by a filter membrane and treated with As3+ for 8h. To rule out any effect of As3+ in the lower chambers on the nontreated cells seeded in the upper chamber inserts, the medium in the lower chambers was replaced with fresh serum-free and As3+-free medium before the assembly of the upper chambers into the lower chambers (Supplementary figure 1A). Relative to the control cells, the As3+-treated cells induced a 5- to 14-fold increase in migration of the nontreated cells (Figs. 5A and B; Supplementary figures 1B and C). Next, we sought to determine the role of JNK and Stat3 activation on this As3+-induced cell migration by knocking down JNK and Stat3 in the cells with siRNA prior to assessing migration. We observed a significant decrease in the migration of the nontreated cells (upper chamber cells) in response to the lower chamber cells, in which the JNK or Stat3 was silenced by siRNA (Figs. 5A and B). This reduction in migration was not due to the nonspecific effects of the siRNAs because the scramble siRNA (siCon) exhibited no effect on the migration induced by As3+-treated cells.
DISCUSSION
In this study, we provided evidence showing that JNK and pS727 of Stat3 are involved in As3+-induced Akt activation in the human bronchial epithelial cell line BEAS-2B. Pharmacological inhibition or genetic disruption of JNK signaling by JNK inhibitor, siRNA, or gene deficiency in JNK1 or JNK2 reduced pS727 of Stat3 and the subsequent Akt activation induced by As3+. In addition, abrogating Stat3 activity by Stat3 inhibitor or siRNA-based silencing blocked As3+-induced Akt activation. Furthermore, the downstream effects of As3+-induced Akt activation, including VEGF release and cell migration, were diminished in cells in which the expression of JNK or Stat3 was silenced by siRNA. Thus, these data revealed a new signaling mechanism in As3+-induced Akt activation, which addressed the important role of the JNK activation in pS727 of Stat3 that linked to Akt activation in response to As3+.
As3+ is an environmental hazard that has been shown to cause human cancers. Exposure to As3+ is relatively common due to the widespread presence of As3+ in drinking water and the emission of As3+ into the air by certain industrial processes (Heck et al., 2009). JNK is one of the earliest kinases activated by As3+ (Bernstam and Nriagu, 2000). Although some studies have demonstrated that the activation of JNK is largely associated with cellular stress responses and cell death, the growth-promoting role of JNK signaling has been supported by several recent studies (Chen, 2012; Chen et al., 2009a). Aberrant activation of JNK has been frequently observed in a number of human and experimentally induced cancers (Chen, 2012; Chen et al., 2009a). In addition, JNK signaling is essential for the compensatory growth of normal tissues that are adjacent to sites of injury (Chen, 2012). Furthermore, several recent studies have implicated JNK in the growth regulation of embryonic stem cells (ESCs), adult stem cells, and cancer stem cells. For example, enhanced activation of JNK has been observed in some human embryonic stem cell lines (hESCs). Inhibition of JNK signaling resulted in a substantial suppression in the expression of NANOG and OCT4, two transcription factors critical for the self-renewal capacity of hESCs (Brill et al., 2009; Hutchins and Robson, 2009). However, the mechanism by which JNK activation may be advantageous for stem cell or cancer cell growth remains to be fully understood. The findings in the present study indicate that JNK signaling contributes to Akt activation through the activation of Stat3, which may provide a new mechanistic explanation on the pro-proliferative and procarcinogenic role of JNK signaling in cellular response to As3+ and potentially other carcinogens.
Stat3 is an oncogenic transcription factor involved in the inflammatory response, malignant transformation, and the pluripotency of stem cells (He and Karin, 2011). One of the oncogenic roles of Stat3 is mediated by Akt, which promotes cell growth, cell migration, and angiogenesis (Iliopoulos et al., 2010). In response to cytokines or growth factors, Stat3 becomes phosphorylated on tyrosine 705 (Y705) and/or S727, which results in the enhanced transcriptional activity of Stat3 (Decker and Kovarik, 2000). The significance of pS727 of Stat3 in cell growth or tumorigenesis has not been appreciated until recently. By introducing the prostate cancer cell line LNCaP with several different Stat3 mutant constructs, a study by Qin et al. (2008) has suggested that relative to pY705, pS727 is a much more potent inducer of proliferation, anchorage-independent growth, invasion, and tumorigenesis of LNCap cells in mice. It was believed that pS727 might be indispensable for the nuclear localization of Stat3 and transcriptional upregulation for the expression of genes associated with tumorigenesis, such as Mcl-1, survivin, and c-myc (Qin et al., 2008). On the other hand, pY705 appears to be dispensable for the survival of macrophages or neuronal stem cells (Androutsellis-Theotokis et al., 2006; Liu et al., 2003).
Several lines of evidence have attributed JNK activation to pS727 of Stat3 protein. The treatment of cells with extracellular stress inducers such as UV irradiation, TNF-α, or LPS resulted in a pronounced activation of JNK and pS727 of Stat3 (Lim and Cao, 1999). In addition, the activation of the upstream kinases of the JNK signaling pathway, such as MEKK1, MKK7, or MKK4, caused pS727 of Stat3 (Turkson et al., 1999). Furthermore, the level of UVA-induced pS727 of Stat3 was substantially reduced in cells derived from JNK1−/− or JNK2−/− mice, as well as cells expressing a dominant negative JNK construct (Zhang et al., 2001). Finally, the S727 residue resides within a Pro-Met-Ser-Pro motif, which is a conserved consensus phosphorylation motif of JNK and other MAP kinases (Bogoyevitch and Kobe, 2006). In agreement with these observations, our data from BEAS-2B cells where JNK expression was silenced by siRNA knockdown or from the JNK1 or JNK2 knockout cells (Fig. 3) clearly indicated that JNK is the upstream kinase responsible for the As3+-induced pS727 of Stat3.
In mammalian cells, JNK is a key regulator of the expression of IL-6 or the members of the unpaired family of cytokines Upd, Upd2, and Upd3, which activate Stat3 through the JAK-dependent pY705 of Stat3 (Chen, 2012; Park et al., 2010). In the case of the As3+-induced activation of JNK and Stat3 that connected to Akt activation, however, IL-6 might not be the intermediate signal in mediating JNK-induced Stat3 activation. This conclusion was supported by two observations. First, despite the fact that As3+ is able to induce both JNK activation and IL-6 generation, silencing JNK by siRNA did not affect As3+-induced IL-6 production (Fig. 4A). Secondly, the level of pY705 of Stat3, which reflects IL-6-dependent JAK activation, was not induced by As3+ treatment. In contrast, similar to previous reports of UVA irradiation of JB6 fibroblast cells (Zhang et al., 2001), the treatment of BEAS-2B cells with As3+ decreased the level of pY705 of Stat3 in a time- and dose-dependent manner (Fig. 2). In fact, studies in JB6 cells have already suggested that JNK can directly phosphorylate S727, leading to Stat3 activation independent of cytokines (Zhang et al., 2001).
The finding that As3+ induces Akt activation through JNK-dependent pS727 of Stat3 has not been reported before. Although there are reports showing concomitant activation of JNK, Stat3, and Akt in some cell types in response to leptin (Miyazaki et al., 2008), Friend spleen focus-forming virus (Jelacic et al., 2008), ischemia-reperfusion injury (Iñiguez et al., 2006), rheumatoid inflammation (Galligan et al., 2009), fibroblast growth factor-2 (Vlotides et al., 2009), and G-CSF (Hara et al., 2011), a signaling axis of Akt activation through JNK and Stat3 has not been established. Loss-of-function studies in the present report suggest the requirement of JNK and Stat3 for As3+-induced Akt activation. Inhibition or silencing of JNK or Stat3 not only abrogated Akt activation but also impaired the downstream effects of Akt including VEGF generation and the induction of neighboring cell migration in response to the As3+-treated cells (Figs. 2–5).
The carcinogenic role of As3+ has been well established. How environmental or occupational exposure to As3+ causes human cancers, such as lung cancer or bladder cancer, however, remains to be fully elucidated. The importance of a single signaling molecule or protein kinase for As3+ or other carcinogen-induced carcinogenesis has been addressed in many cellular or animal experiments, which usually overlooked the interplays among different intracellular signaling pathways. The present report revealed that As3+ activates a new signaling axis from JNK to Stat3 that contributes to Akt activation, followed by release of VEGF and cell migration. These findings, thus, will yield new insights into the mechanism, prevention, and treatment of malignancies resulted from exposures to As3+ or other environmental cues.
Fig. 5.
Silencing JNK or Stat3 reduces the migration of neighboring cells induced by cells treated with As3+. (A) siRNA transfection and cell cocultures were performed as described in the Materials and Methods section. Data shown are representative microscopic images of the migrated cells induced by the control cells, cells treated with As3+ for 8 h, and cells transfected with control siRNA (siCon), JNK siRNA (siJNK), or Stat3 siRNA (siStat3) prior to As3+ treatment. (B) Quantification of the migrated neighboring cells. Data are expressed as mean ± SD, n = 5.
Funding
National Institutes of Health (RO1 ES-017217 to F.C.); Wayne State University (F.C.); Chinese Scholarship Council (J.L.).
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENT
We are indebted to Dr Ying Xia at the Department of Environmental Health, University of Cincinnati, for providing the wild-type, JNK1−/−, and JNK2−/− mouse embryonic fibroblast cells.
References
- Androutsellis-Theotokis A.,, Leker R. R.,, Soldner F.,, Hoeppner D. J.,, Ravin R.,, Poser S. W.,, Rueger M. A.,, Bae S. K.,, Kittappa R.,, McKay R. D.(2006)Notch signalling regulates stem cell numbers in vitro and in vivo.Nature 442, 823–826 [DOI] [PubMed] [Google Scholar]
- Beezhold K.,, Liu J.,, Kan H.,, Meighan T.,, Castranova V.,, Shi X.,, Chen F.(2011)miR-190-mediated downregulation of PHLPP contributes to arsenic-induced Akt activation and carcinogenesis.Toxicol. Sci. 123, 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstam L.,, Nriagu J.(2000)Molecular aspects of arsenic stress.J. Toxicol. Environ. Health. B. Crit. Rev. 3, 293–322 [DOI] [PubMed] [Google Scholar]
- Blando J. M.,, Carbajal S.,, Abel E.,, Beltran L.,, Conti C.,, Fischer S.,, DiGiovanni J.(2011)Cooperation between Stat3 and Akt signaling leads to prostate tumor development in transgenic mice.Neoplasia 13, 254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch M. A.,, Arthur P. G.(2008)Inhibitors of c-Jun N-terminal kinases: JuNK no more? Biochim. Biophys. Acta 1784, 76–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch M. A.,, Kobe B.(2006)Uses for JNK: The many and varied substrates of the c-Jun N-terminal kinases.Microbiol. Mol. Biol. Rev. 70, 1061–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L. M.,, Xiong W.,, Lee K. B.,, Ficarro S. B.,, Crain A.,, Xu Y.,, Terskikh A.,, Snyder E. Y.,, Ding S.(2009)Phosphoproteomic analysis of human embryonic stem cells.Cell Stem Cell 5, 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik I.,, Gallicchio L.,, Boyd K.,, Lam T. K.,, Matanoski G.,, Tao X.,, Shiels M.,, Hammond E.,, Chen L.,, Robinson K. A.,, et al. (2008)Arsenic in drinking water and lung cancer: A systematic review.Environ. Res. 108, 48–55 [DOI] [PubMed] [Google Scholar]
- Chang Q.,, Zhang Y.,, Beezhold K. J.,, Bhatia D.,, Zhao H.,, Chen J.,, Castranova V.,, Shi X.,, Chen F.(2009)Sustained JNK1 activation is associated with altered histone H3 methylations in human liver cancer.J. Hepatol. 50, 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L.,, Hsu L. I.,, Chiou H. Y.,, Hsueh Y. M.,, Chen S. Y.,, Wu M. M.,, Chen C. J. Blackfoot Disease Study Group(2004)Ingested arsenic, cigarette smoking, and lung cancer risk: A follow-up study in arseniasis-endemic areas in Taiwan.JAMA 292, 2984–2990 [DOI] [PubMed] [Google Scholar]
- Chen F.(2012)JNK-induced apoptosis, compensatory growth, and cancer stem cells.Cancer Res. 72, 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.,, Beezhold K.,, Castranova V. (2009a). JNK1, a potential therapeutic target for hepatocellular carcinoma.Biochim. Biophys. Acta 1796, 242–251 [DOI] [PubMed] [Google Scholar]
- Chen L.,, Ovesen J. L.,, Puga A.,, Xia Y. (2009b). Distinct contributions of JNK and p38 to chromium cytotoxicity and inhibition of murine embryonic stem cell differentiation.Environ. Health Perspect. 117, 1124–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.,, Lu Y.,, Zhang Z.,, Vallyathan V.,, Ding M.,, Castranova V.,, Shi X.(2001)Opposite effect of NF-kappa B and c-Jun N-terminal kinase on p53-independent GADD45 induction by arsenite.J. Biol. Chem. 276, 11414–11419 [DOI] [PubMed] [Google Scholar]
- Chen F.,, Zhang Z.,, Bower J.,, Lu Y.,, Leonard S. S.,, Ding M.,, Castranova V.,, Piwnica-Worms H.,, Shi X.(2002)Arsenite-induced Cdc25C degradation is through the KEN-box and ubiquitin-proteasome pathway.Proc. Natl. Acad. Sci. U.S.A. 99, 1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H. F.,, Ho S. C.,, Yang C. Y.(2004)Lung cancer mortality reduction after installation of tap-water supply system in an arseniasis-endemic area in Southwestern Taiwan.Lung Cancer 46, 265–270 [DOI] [PubMed] [Google Scholar]
- Decker T.,, Kovarik P.(2000)Serine phosphorylation of STATs.Oncogene 19, 2628–2637 [DOI] [PubMed] [Google Scholar]
- Dérijard B.,, Hibi M.,, Wu I. H.,, Barrett T.,, Su B.,, Deng T.,, Karin M.,, Davis R. J.(1994)JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain.Cell 76, 1025–1037 [DOI] [PubMed] [Google Scholar]
- Galligan C. L.,, Siebert J. C.,, Siminovitch K. A.,, Keystone E. C.,, Bykerk V.,, Perez O. D.,, Fish E. N.(2009)Multiparameter phospho-flow analysis of lymphocytes in early rheumatoid arthritis: Implications for diagnosis and monitoring drug therapy.PLoS ONE 4, e6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M.,, Yuasa S.,, Shimoji K.,, Onizuka T.,, Hayashiji N.,, Ohno Y.,, Arai T.,, Hattori F.,, Kaneda R.,, Kimura K.,, et al. (2011)G-CSF influences mouse skeletal muscle development and regeneration by stimulating myoblast proliferation.J. Exp. Med. 208, 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G.,, Karin M.(2011)NF-kappaB and STAT3—Key players in liver inflammation and cancer.Cell Res. 21, 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck J. E.,, Andrew A. S.,, Onega T.,, Rigas J. R.,, Jackson B. P.,, Karagas M. R.,, Duell E. J.(2009)Lung cancer in a U.S. population with low to moderate arsenic exposure.Environ. Health Perspect. 117, 1718–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopenhayn-Rich C.,, Biggs M. L.,, Smith A. H.(1998)Lung and kidney cancer mortality associated with arsenic in drinking water in Córdoba, Argentina.Int. J. Epidemiol. 27, 561–569 [DOI] [PubMed] [Google Scholar]
- Hui L.,, Zatloukal K.,, Scheuch H.,, Stepniak E.,, Wagner E. F.(2008)Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation.J. Clin. Invest. 118, 3943–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins A. P.,, Robson P.(2009)Unraveling the human embryonic stem cell phosphoproteome.Cell Stem Cell 5, 126–128 [DOI] [PubMed] [Google Scholar]
- Iliopoulos D.,, Jaeger S. A.,, Hirsch H. A.,, Bulyk M. L.,, Struhl K.(2010)STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer.Mol. Cell 39, 493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez M.,, Berasain C.,, Martinez-Ansó E.,, Bustos M.,, Fortes P.,, Pennica D.,, Avila M. A.,, Prieto J.(2006)Cardiotrophin-1 defends the liver against ischemia-reperfusion injury and mediates the protective effect of ischemic preconditioning.J. Exp. Med. 203, 2809–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelacic T. M.,, Thompson D.,, Hanson C.,, Cmarik J. L.,, Nishigaki K.,, Ruscetti S.(2008)The tyrosine kinase sf-Stk and its downstream signals are required for maintenance of friend spleen focus-forming virus-induced fibroblast transformation.J. Virol. 82, 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B.,, Shen X. D.,, Ji H.,, Kamo N.,, Gao F.,, Freitas M. C.,, Busuttil R. W.,, Kupiec-Weglinski J. W.(2012)HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: Regulation of TLR4 innate responses through PI3K/PTEN signaling.J. Hepatol. 56, 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H.,, Lee S. C.,, Ro J.,, Kang H. S.,, Kim H. S.,, Yoon S.(2010)Jnk signaling pathway-mediated regulation of Stat3 activation is linked to the development of doxorubicin resistance in cancer cell lines.Biochem. Pharmacol. 79, 373–380 [DOI] [PubMed] [Google Scholar]
- Lim C. P.,, Cao X.(1999)Serine phosphorylation and negative regulation of Stat3 by JNK.J. Biol. Chem. 274, 31055–31061 [DOI] [PubMed] [Google Scholar]
- Liu H.,, Ma Y.,, Cole S. M.,, Zander C.,, Chen K. H.,, Karras J.,, Pope R. M.(2003)Serine phosphorylation of STAT3 is essential for Mcl-1 expression and macrophage survival.Blood 102, 344–352 [DOI] [PubMed] [Google Scholar]
- Luedde T.,, Beraza N.,, Kotsikoris V.,, van Loo G.,, Nenci A.,, De Vos R.,, Roskams T.,, Trautwein C.,, Pasparakis M.(2007)Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma.Cancer Cell 11, 119–132 [DOI] [PubMed] [Google Scholar]
- Maeda S.,, Kamata H.,, Luo J. L.,, Leffert H.,, Karin M.(2005)IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis.Cell 121, 977–990 [DOI] [PubMed] [Google Scholar]
- Miyazaki T.,, Bub J. D.,, Iwamoto Y.(2008)c-Jun NH(2)-terminal kinase mediates leptin-stimulated androgen-independent prostate cancer cell proliferation via signal transducer and activator of transcription 3 and Akt.Biochim. Biophys. Acta 1782, 593–604 [DOI] [PubMed] [Google Scholar]
- Olsson A. K.,, Dimberg A.,, Kreuger J.,, Claesson-Welsh L.(2006)VEGF receptor signalling - in control of vascular function.Nat. Rev. Mol. Cell Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- Park E. J.,, Lee J. H.,, Yu G. Y.,, He G.,, Ali S. R.,, Holzer R. G.,, Osterreicher C. H.,, Takahashi H.,, Karin M.(2010)Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression.Cell 140, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H. R.,, Kim H. J.,, Kim J. Y.,, Hurt E. M.,, Klarmann G. J.,, Kawasaki B. T.,, Duhagon Serrat M. A.,, Farrar W. L.(2008)Activation of signal transducer and activator of transcription 3 through a phosphomimetic serine 727 promotes prostate tumorigenesis independent of tyrosine 705 phosphorylation.Cancer Res. 68, 7736–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata W.,, Maeda S.,, Hikiba Y.,, Yanai A.,, Sakamoto K.,, Nakagawa H.,, Ogura K.,, Karin M.,, Omata M.(2008)c-Jun NH2-terminal kinase 1 is a critical regulator for the development of gastric cancer in mice.Cancer Res. 68, 5031–5039 [DOI] [PubMed] [Google Scholar]
- Suissa Y.,, Ziv O.,, Dinur T.,, Arama E.,, Gerlitz O.(2011)The NAB-Brk signal bifurcates at JNK to independently induce apoptosis and compensatory proliferation.J. Biol. Chem. 286, 15556–15564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H.,, Ogata H.,, Nishigaki R.,, Broide D. H.,, Karin M.(2010)Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation.Cancer Cell 17, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkson J.,, Bowman T.,, Adnane J.,, Zhang Y.,, Djeu J. Y.,, Sekharam M.,, Frank D. A.,, Holzman L. B.,, Wu J.,, Sebti S.,, et al. (1999)Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein.Mol. Cell. Biol. 19, 7519–7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlotides G.,, Chen Y. H.,, Eigler T.,, Ren S. G.,, Melmed S.(2009)Fibroblast growth factor-2 autofeedback regulation in pituitary folliculostellate TtT/GF cells.Endocrinology 150, 3252–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.,, Yang J.,, Fisher T.,, Xiao H.,, Jiang Y.,, Yang C.(2012)Akt activation is responsible for enhanced migratory and invasive behavior of arsenic-transformed human bronchial epithelial cells.Environ. Health Perspect. 120, 92–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.,, Bhatia D.,, Xia H.,, Castranova V.,, Shi X.,, Chen F.(2006)Nucleolin links to arsenic-induced stabilization of GADD45alpha mRNA.Nucleic Acids Res. 34, 485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.,, Liu G.,, Dong Z.(2001)MSK1 and JNKs mediate phosphorylation of STAT3 in UVA-irradiated mouse epidermal JB6 cells.J. Biol. Chem. 276, 42534–42542 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.