Abstract
A novel clinical glucose biosensor fabricated using functionalized metalloid-polymer (silver-silica coated with polyethylene glycol) hybrid nanoparticles on the surface of a graphene oxide nanosheet is reported. The cyclic voltammetric response of glucose oxidase modification on the surface of a functionalized graphene oxide electrode showed a surface-confined reaction and an effective redox potential near zero volts, with a wide linearity of 0.1–20 mM and a sensitivity of 7.66 μA mM−1 cm−2. The functionalized graphene oxide electrode showed a better electrocatalytic response toward oxidation of H2O2 and reduction of oxygen. The practical applicability of the functionalized graphene oxide electrode was demonstrated by measuring the peak current against multiple urine and serum samples from diabetic patients. This new hybrid nanoarchitecture combining a three-dimensional metalloid-polymer hybrid and two-dimensional graphene oxide provided a thin solid laminate on the electrode surface. The easy fabrication process and retention of bioactive immobilized enzymes on the functionalized graphene oxide electrode could potentially be extended to detection of other biomolecules, and have broad applications in electrochemical biosensing.
Keywords: clinical diagnostics, glucose biosensor, metalloid-polymer nanoparticles, glucose oxidase, cyclic voltammetry
Introduction
Development of novel biosensors for early detection of specific analytes and self-monitoring of disease states is widely recognized as an effective method for diagnosis of metabolic disorders such as diabetes.1,2 According to a recent International Diabetes Federation report, 380 million people are expected to suffer from diabetes by 2025.3 Although a large number of glucose sensors, such as urine glucose meters and blood glucose sensors, have been developed and are commercially available, there is growing interest in developing an advanced biosensor system serving as a reusable self-monitoring device with improved analytical performance, ability to resist interference, and possible multiple functionalities in the same device. Of the biosensor methods available, electrochemical analysis has been shown to have inherent benefits of simplicity, high sensitivity, good selectivity, easy integration, and relatively low cost.4–6 Therefore, different chemically modified electrodes have been developed for biomolecular analysis, environmental monitoring, and analysis of food quality.7–11 Functional modification of electrode substrates for the construction of electrochemical biosensors having improved analytical performance with respect to other biosensor designs is highly desirable for developing potential device applications.10,11 In particular, electrodes that are modified or functionalized by nanoscale materials provide a suitable microenvironment for immobilization of biomolecules which can retain their biological activity and enhance electron transfer between biomolecules and the electrode.11–13 This paper reports the results of one such advanced nanomaterial-functionalized electrode for sensitive electrochemical biosensing of clinical glucose samples.
Graphene oxide nanosheets have emerged as a novel material with an expanded active surface area, good dispersibility in water at the molecular level, good biocompatibility, and a potentially low manufacturing cost.14–17 The unique two-dimensional sheet-like nanostructure, high surface-to-volume ratio, and physicochemical properties of graphene oxide make it a promising candidate for constructing electrochemical sensors and biosensors. According to the widely accepted Lerf–Klinowski model, graphene oxide platelets have chemically reactive oxygen functionality, including carboxylic acid groups at their edges and epoxy and hydroxyl groups on the basal planes. Therefore, chemical functionalization of graphene oxide can be mediated by orthogonal reactions of these groups to functionalize one site selectively over another.18 Thus, when such chemically reactive components are modified with other active nanoparticles; a novel three-dimensional hybrid nanoarchitecture with features of increased binding affinity towards bioactive molecules and electrode substrates is achieved.19
Several reports of carbon composite materials, such as graphene-gold nanoparticles-chitosan hybrid film and glucose oxidase-assembled graphene and graphene oxide-Prussian blue hybrid film for glucose biosensing have been published.19–21 Although graphene-based electrodes and other electrodes modified by composite materials have demonstrated their utility as electrochemical glucose biosensors in phosphate-buffered saline, and serum and urine samples,3,19–22 to the best of our knowledge, their use for detection of glucose in urine and serum samples from diabetic patients using a functionalized electrode has not yet been studied. Furthermore, the graphene-based composites utilized so far for electrochemical studies have been fabricated by physical modification19 and are not chemically bonded or functionalized, suggesting lack of long-term binding affinity and stability problems between nanoparticles and graphene oxide during electrochemical analysis. Therefore, it would be worthwhile developing a chemically stable functionalized graphene oxide material with the ability to determine urinary and plasma glucose levels. In addition, studying the direct electrochemical response of such a hybrid material with the potential to act as a biosensing platform would be of interest in a number of interdisciplinary fields.
Here we describe a novel functionalized graphene oxide (FGO)-based electrochemical biosensor for clinical detection of glucose in different types of physiological samples. The impetus for development of this device arose from the established use of metalloid-polymer nanostructures, including thin solid laminates and active materials used in sensor studies.23–25 We used metalloid-polymer hybrid nanoparticles consisting of silver-silica (Ag@SiO2) and polyethylene glycol (PEG) with an average particle diameter of 12 ± 3 nm to functionalize the surface of graphene oxide. The nanoparticles were first silanized by reacting them with 3-aminopropyltriethoxysilane and then covalently reacting them with the oxygen group of graphene oxide. Spectroscopic investigations revealed that the carboxylic acid groups of graphene oxide were directly involved in the covalent reaction with silanized metalloid-polymer hybrid nanoparticles. The electrochemical efficiency of the novel FGO-modified electrode was superior to that of a commercial gold-printed circuit board (Au-PCB) electrode. The synergistic features of the nanohybrid (containing metal, nonmetal, and polymer particles) on the surface of graphene oxide are believed to be the factors involved in its direct electron transfer properties, by providing the smallest difference of oxidation and reduction potential near zero volts. The fabricated FGO material was developed to test the possibility of electrochemical biosensing of glucose molecules in phosphate-buffered saline and in urine and serum samples from diabetic patients.
Materials and methods
Materials
The precursor materials required for the synthesis of the metalloid-polymer hybrid nanoparticles (MPHs), namely silver nitrate (AgNO3), tetraethoxysilane, sodium borohydride, and ammonium hydroxide, as well as the 3-aminopropyltriethoxysilane needed for functionalization of graphene oxide were purchased from Sigma-Aldrich (St Louis, MO). PEG (number average molecular weight 10,000 g/mol), expandable graphite powder, D(+)glucose, and glucose oxidase from Aspergillus niger were also obtained from Sigma-Aldrich. Sulfuric acid, potassium permanganate, hydrogen peroxide, and hydrochloric acid were obtained from Daejung Chemicals and Metals Ltd, Gyeonggi-do, South Korea. Milli-Q water with resistance greater than 18 MΩ was used in all experiments. All chemicals were of analytical grade and used as received without further purification.
The urine and serum samples from diabetic patients utilized in the biosensor study were kindly provided by Bundang Seoul National University Hospital, Seoul, South Korea. All experiments were performed according to a protocol approved by the institutional review committee at Seoul National University Bundang Hospital. Before involvement in any study-related procedures, all patients gave their written informed consent. Table S1 provides the patient details and their respective serum glucose concentrations as measured by a commercially available glucose sensor.
Instrumentation
High-resolution transmission electron microscopic imaging of graphene oxide and the FGO-modified MPHs was performed using a Cs-corrector-equipped Titan 80–300 device (FEI, Hillsboro, OR) operated at 300 kV. The samples used for the measurements were prepared by drop-casting 5–10 μL of graphene oxide (0.1 mg/mL) or FGO suspension onto the surface of a copper grid, and allowing the solvent to evaporate before measurement. Ultraviolet-visible absorbance spectra for aqueous solutions of graphene oxide and FGO were measured using a Varian Cary 50 spectrophotometer. The average particle size distribution of the as-prepared MPHs was studied (dispersing the dried particles in anhydrous ethanol) using the electrophoretic light scattering method with an ELS-8000 (Otsuka Electronics Co, Ltd, Osaka, Japan). Prior to measurement, colloidal solutions were filtered through a Millipore membrane (pore size 0.2 μm) three times and then introduced into the cell. The chemical structure and functional groups of graphene oxide were observed before and after functionalization with MPHs using a Fourier transform-infrared spectrophotometer (Nicolet 6700) and a KBr disk at a resolution of 4 cm−1. Raman spectra for pure graphene oxide and FGO were recorded using a Micro Raman system (Ramboss-500i) with a 50× objective lens at room temperature, a 633 nm He-Ne laser beam, and 1800 lines per mm grating. Aqueous dispersions of graphene oxide and of FGO were drop-casted onto a clean glass substrate (2.0 × 2.0 cm) and the solvent allowed to evaporate at ambient temperature before carrying out the measurements. Cyclic voltammogram (CV) measurement for the commercial Au-PCB electrode modified with graphene oxide and/or FGO-glucose oxidase (GOx) was done using a VersaSTAT 3 in a three-electrode configuration, with the customized Au-PCB used as the working electrode, and a platinum wire and an Ag/AgCl electrode used as the counter and reference electrodes, respectively.
Synthesis of Ag@SiO2-PEG (MPHs)
Metalloid-polymer hybrid nanoparticles composed of Ag@SiO2-PEG (average size 12 ± 3 nm) were synthesized using an ultrasonochemical approach, following our previous report.24 Briefly, 150 μL of a precursor 30 mM AgNO3 aqueous solution was added to a reaction vessel containing 12 mL of the stabilizing agent (PEG, number average molecular weight 10,000 g/mol) and 300 μL of 30 mM sodium borohydride as a reducing agent. Under controlled conditions, including probe temperature (65°C), amplitude (35%), and pulser on-off cycle (5–10 seconds), the reaction mixture was ultrasonicated for 15 minutes to form the initial silver core. Following this, the desired amounts of aqueous tetraethoxysilane (150 μL, 30 mM) and ammonium hydroxide (300 μL) were added. The reaction vessel containing the precursor solutions of metal, nonmetal, PEG, and reducing agent were again subjected to ultrasonication for 30 minutes to ensure a complete reduction reaction and formation of the hybrid structure. The hybrid particles were isolated from the resulting colloidal solution by centrifugation, washed twice with deionized water and ethanol, and then used for the functionalization experimentation.
Functionalization of MPHs onto graphene oxide
The brownish colloidal suspensions of graphene oxide nanosheets utilized in the current experiment were prepared by vigorous oxidation of graphite powder, according to a modified Hummers method.15,26 Functionalization of MPHs onto the surface of graphene oxide was then achieved by a two-step process, following a recent report.25 Briefly, 200 μL of MPHs at a concentration of 5 mg/mL and 40 μL of 3-aminopropyltriethoxysilane (30 μL/mL in C2H5OH) was added to a vial containing 3 mL of anhydrous ethanol and magnetically stirred at 800 rpm and room temperature for 10 hours. Next, an aqueous solution of 200 μL graphene oxide (concentration 2.5 mg/mL) was added and magnetically stirred at 800 rpm for another 10 hours to ensure a covalent reaction between the silanized MPHs and graphene oxide. After completion of the reaction process, the MPHs functionalized with graphene oxide sheets were separated by centrifugation at 12,000 rpm for 30 minutes, washed three times with ethanol, and used for construction of the biosensor platform.
Fabrication of Au-PCB-FGO electrode for biosensor platform
After performing sequential washing with anhydrous ethanol, acetone, and deionized water, the Au-PCB working electrode (approximately 1 mm in diameter) underwent a surface immobilization procedure starting with oxygen plasma treatment for 2 minutes. Several experimental optimizations were carried out to obtain a suitable working potential range (from −0.15 to +0.25 V) and an optimal CV response. Typically, 4 μL of aqueous graphene oxide and/or FGO suspension (2 mg/mL) was drop-casted onto the Au-PCB electrode and allowed to evaporate at ambient temperature for one hour. The resulting substrate was then coated with 4 μL of glucose oxidase (5 mg/mL in phosphate-buffered solution), refrigerated at 4°C for one hour, and the resulting glucose oxidase-modified Au-PCB electrode modified with graphene oxide (Au-PCB-GO-GOx) and/or with functionalized graphene oxide (Au-PCB-FGO-GOx) was used for electrochemical characterization. A reproducible CV was obtained under steady-state conditions after about five cycles. Unless otherwise stated, all CV measurements were recorded at a constant scan rate of 50 mV per second and a potential range from −0.15 to +0.25 V.
Three types of electrochemical CV were recorded for the Au-PCB-GO-GOx and Au-PCB-FGO-GOx electrodes, including for different concentrations and volumes of D(+)glucose and clinical glucosuric samples in phosphate-buffered solution (50 mL, pH 7.4), respectively, and also serum samples from diabetic patients modified with Au-PCB-FGO-GOx in phosphate-buffered solution (50 mL, pH 7.4). Phosphate-buffered solution was used as the common electrolyte for all electrochemical measurements. The solution was prepared by dissolving a phosphate-buffered saline tablet (Sigma-Aldrich) in 200 mL of distilled water, giving a final concentration of 0.0027 M potassium chloride and 0.137 M sodium chloride, with a pH 7.4 at 25°C.
Results and discussion
Optical absorbance, morphology, and Raman spectral analysis of graphene oxide and FGO
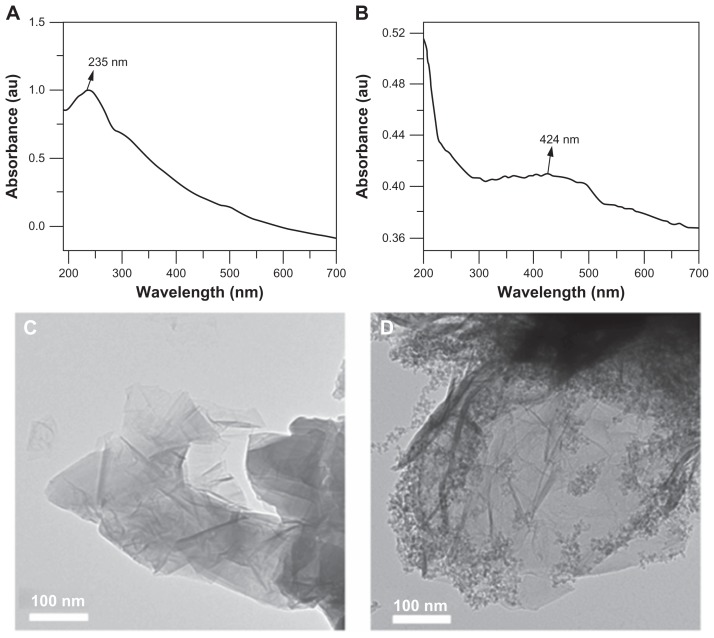
The general strategy for functionalization of MPHs onto the surface of graphene oxide can be explained by two important surface chemical reactions, ie, amidation by a carboxylic group from graphene oxide and silanized MPHs and nucleophilic attack at the α-carbon by the amine groups on silanized MPHs.25Figure 1A shows the ultraviolet-visible absorbance spectrum of graphene oxide synthesized by the modified Hummers method.15,26 The wavelength of maximum absorbance for graphene oxide was found at 235 nm, which corresponds to the characteristic π-π* electron transitions of the aromatic C–C bonds.16,26 As shown in Figure S1A, MPHs in their normal state have a λmax at 418 nm, in agreement with previous reports for MPHs.23–25 Surface functionalization of graphene oxide with MPHs resulted in a shifted peak at 424 nm with a broadened shape (see Figure 1B). In general, transition metal colloidal nanoparticles, such as those containing silver, have a well known surface plasmon resonance depending on particle size and shape. Surface plasmon resonance is seen due to the collective oscillations of conducting electrons from the sample species.27 The optical absorption spectrum of metal nanoparticles is always dominated by surface plasmon resonance, leading to a distinct shift to either red or blue, depending on the dielectric properties of the surrounding matrix or environmental conditions.24,27 In this study, the final hybrid FGO would be expected to have the two characteristics of optical absorbance, such as π-π* electron transitions from the aromatic C–C of graphene oxide, and surface plasmon resonance related to the surface-modified silver nanostructures. However, the peak obtained for FGO showed a broadened signal centered at 424 nm. Possible reasons for this observation could be the surface chemical modification of the silver core by nonmetal silica and the PEG polymer layer. In addition, FGO has MPHs on its surface promoting strong interaction between graphene oxide and MPHs, that may influence the width and overlap between graphene oxide optical absorbance and the MPH peak (Figure 1B).25
Figure 1.
Ultraviolet-visible absorbance spectrum (A) graphene oxide and (B) functionalized graphene oxide, and transmission electron microscopic images of (C) graphene oxide and (D) functionalized graphene oxide.
High-resolution transmission electron micrographs were obtained to verify the structural characteristics of graphene oxide before and after functionalization with MPHs. Normal graphene oxide (Figure 1C) had the appearance of a wrinkled sheet-like structure, whereas FGO (Figure 1D) had a surface-functionalized nanoparticle structure. Graphene oxide is a two-dimensional nanosheet material with a large active surface area and good biocompatibility, and is used widely as a building block for surface and interface studies. Functionalization of three-dimensional heterogeneous MPHs (containing a silver metal core, a silica shell, and PEG as polymer layer, ie, a three-in-one system) on the surface of graphene oxide provides an advanced three-dimensional hierarchical structure with multifunctional features.25
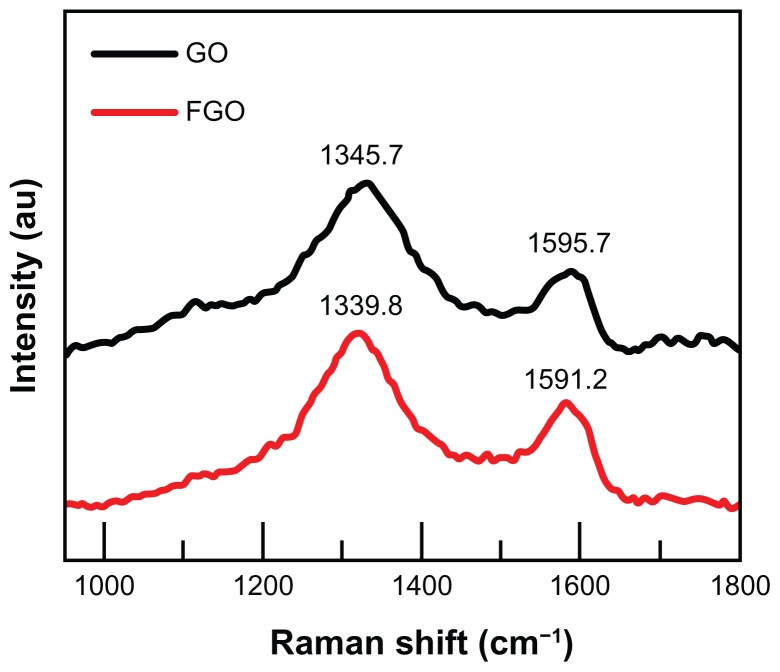
As shown in Figure 2, Raman spectral analysis was used to observe the ordered and disordered crystal structures of graphene oxide and FGO. The Raman spectrum of graphene oxide showed a characteristic G band at 1595.7 cm−1 assigned to the E2 g phonon mode of sp2-hybridized carbon atoms, and a D-band at 1345.7 cm−1 assigned to the vibrations of carbon atoms with dangling bonds in plane terminations of disordered graphite, particularly at the edges.15,16,25 After functionalization of graphene oxide by silanized MPHs, the D bands and G bands were blue-shifted at 1339.8 cm−1 and 1591.2 cm−1, respectively. Furthermore, the D peak intensity (I(D)) was increased in the FGO spectrum compared with that for normal graphene oxide, indicating that our functionalization process had modified the lattice structure of the graphene oxide sheets. The ratio of D band to G band intensity (I(D)/I(G)) for FGO was calculated to be slightly larger than that for graphene oxide, confirming that functionalization of graphene oxide with silanized MPHs significantly influenced its structure. These results are in good agreement with previous reports on functionalization of graphene oxide sheets.25,28 The structural integrity of individual graphene oxide, MPHs, and the combined functional nanosheets were separately characterized for their stretching and bending vibrations by Fourier transform-infrared spectroscopy and the results are included as supporting information (see Figure S2).
Figure 2.
Raman spectrum of graphene oxide and functionalized graphene oxide.
Abbreviations: GO, graphene oxide; FGO, functionalized graphene oxide.
Construction of biosensor platform for glucose detection
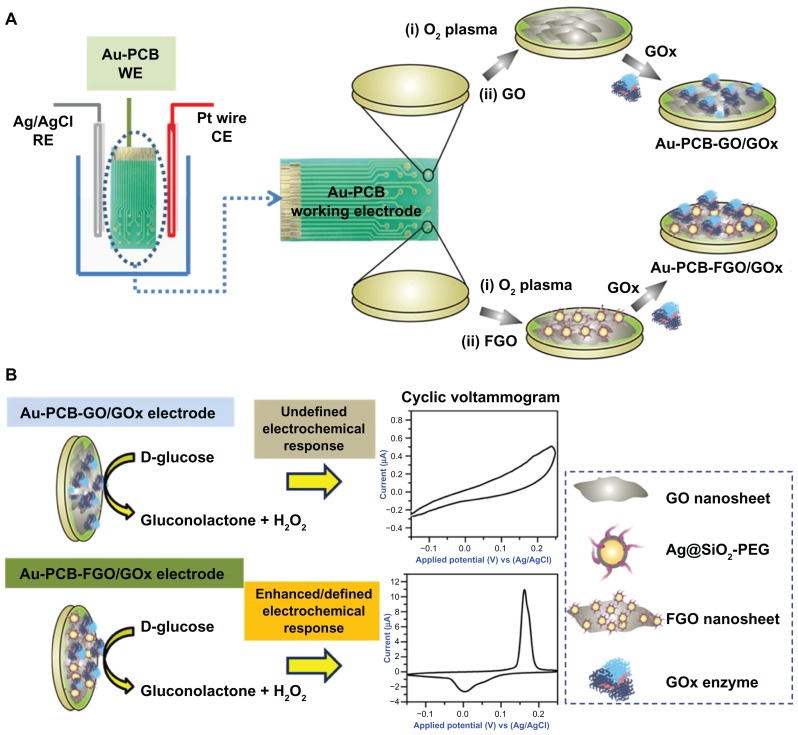
As shown in Figure 3, graphene oxide or FGO was initially immobilized on the surface of the commercial Au-PCB electrode, followed by immobilization of glucose oxidase solution onto the surface of the Au-PCB-GO and/or Au-PCB-FGO substrate by drop-casting. Of note, the electrode substrate utilized in the current study differs from the conventional glassy carbon electrode or indium tin oxide electrode. It has 16 distinct patterns of gold substrate (each with a surface area of about 1 mm in diameter) on a printed circuit board electrode (fabricated by an electroplating method), with the potential for multiple surface modifications using active moieties or enzymes for sensor studies. Depending on the location of the surface modification, the gold substrate is fixed with a wire to connect the electrochemical system. The present Au-PCB electrode (customized as a working electrode) can be reused after performing a sequential wash with an alcoholic solvent or acetone in an ultrasonication bath to remove the immobilized materials. PCB electrodes are more advantageous than thin-film electrodes in terms of cost, design features, fabrication, large-scale production, reliability, and electronic integration.29 Using PCB technology, it is possible to design low-resistance electrodes on insulator substrates which can withstand larger currents than a thin-film electrode.29
Figure 3.
(A) Illustration of the electrochemical setup under electrolyte solution. Dotted circle depicts the digital image of Au-PCB working electrode and the process of glucose oxidase modification at two different stages, ie, Au-PCB-GO and Au-PCB-FGO biosensor platform, respectively. (B) Enzymatic reaction at the electrode interface and its respective cyclic voltammogram.
Abbreviations: Ag/AgCl, silver/silver chloride; RE, reference electrode; Pt, platinum; CE, counter electrode; Au-PCB, gold printed circuit board electrode; WE, working electrode; Au-PCB-GO, gold printed circuit board electrode modified with graphene oxide; Au-PCB-FGO, gold printed circuit board electrode modified with functionalized graphene oxide; GOx, glucose oxidase; Ag@SiO2-PEG, silver-silica coated with polyethylene glycol; FGO, functionalized graphene oxide; GO, graphene oxide.
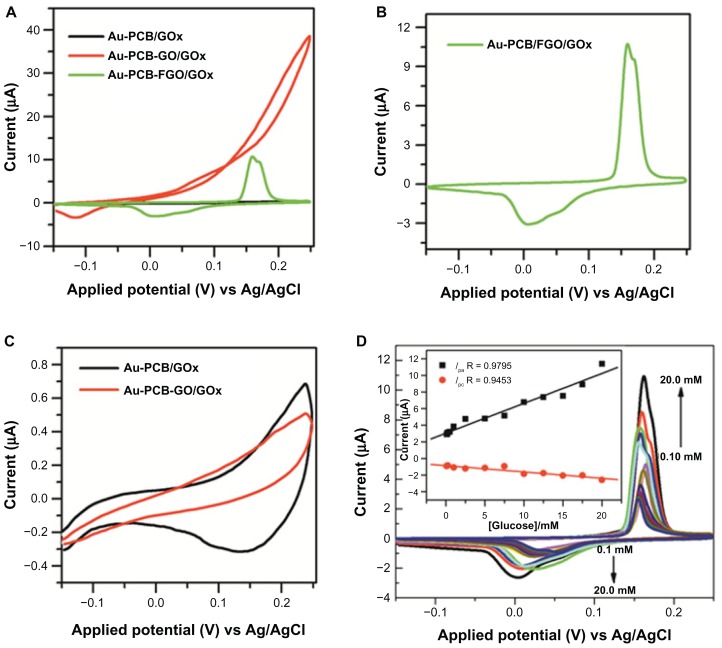
After performing the necessary washing step as described in the experimental section, oxygen plasma treatment was performed to modify the gold substrate into a hydrophilic one for enhancing immobilization of graphene oxide. In general, graphene oxide possesses chemically reactive oxygen functional groups at the edges and basal planes of a sheet-like nanostructure, making it suitable for several types of chemical functionalization. We used CVs to analyze the electrochemical response of the fabricated electrodes. Figure 4A shows the fundamental electrochemical responses of glucose oxidase-modified Au-PCB, Au-PCB-GO, and Au-PCB-FGO in phosphate-buffered solution. As observed in Figure 4B, the CV curve for Au-PCB-FGO-GOx (Figure 4A, green trace) exhibits the well-defined redox peak (corresponding to characteristic cathodic peak potential (Epc) and anodic peak potential (Epa) centered at 0.158 V and at 0.006 V, respectively. In contrast, glucose oxidase-modified bare Au-PCB and Au-PCB-GO do not exhibit any characteristic redox signal either without (Figure 4A) or with D(+)glucose (Figure 4C). The mechanism of redox wave generation from the Au-PCB-FGO electrode may involve oxidation and reduction reactions of active MPH on the surface of graphene oxide.25 In general, achieving direct electrochemistry of enzymes on conventional electrodes is very difficult due to problems concerning accessibility of active centers, which are located deep within the hydrophobic cavity of the molecule, affecting the local concentration and rate of electron transport.30–32 Therefore, researchers have modified the electrode surface with different composites of carbon nanomaterials, such as nanotubes and graphene-based hybrid and metal nanoparticles, to enable significant enhancement of the electron transfer reaction at electrochemical interfaces, such as between enzymes and electrodes.31,32 It has been demonstrated that the electrical properties of graphene-based materials are stronger than those of other nanomaterials.33 Chemical species adsorbed onto the surface of a graphene system act as electron donors or acceptors.34 Due to an extended π-system, graphene materials have charge transfer interactions with electron donor and electron acceptor molecules.35 Chemical modification of graphene systems depends strongly on the types of dopants and concentrations used, and their locations within the graphene systems. Chemically modified graphene materials are reported to have enhanced electronic and quantum transport properties.36 Similarly, in the current study, chemical functionalization of MPHs on graphene oxide nanosheets allowed a synergistically enhanced electrochemical reaction at the interface between the glucose oxidase enzyme and the electrode than that seen when using pure Au-PCB and Au-PCB-GO. Structurally, the MPH is composed of silver metal as the core and SiO2 as the shell, and is coated by PEG as an inert stabilizing layer to avoid agglomeration of the individual composite particles.24,25 The PEG layer on the silver-silica composite surface not only prevents particle aggregation but also promotes active immobilization of glucose oxidase on the functionalized electrode surface. For instance, preliminary research has demonstrated the active role of PEG as a spacer molecule, not only enhancing active immobilization of glucose oxidase in an organic matrix at the electrode surface, but also preserving the bioactivity of glucose oxidase, depending on chain length.37 Redox wave generation from conventional silver metal nanoparticles or silver-doped composites with silane-modified electrodes has been reported previously in the literature.38,39 In short, the mechanism of redox reaction associated with silver-doped aminosilica in phosphate-buffered solution is demonstrated to involve oxidation and reduction potentials at 0.05 V and −0.09 V, respectively (Ag + Cl− → AgCl and AgCl → Ag).38 On the other hand, when the silver-silica composite electrode is immersed in phosphate solution (without NaCl), redox reactions are associated with Ag → Ag2O and Ag2O → Ag at 0.37 V and −0.03 V.38 Furthermore, studies have revealed that incorporation of silver nanoparticles with aminosilica film improves the electron transfer rate because of the smallest difference in the oxidation and reduction potential.39–41
Figure 4.
(A) CVs of GOx-modified Au-PCB (black trace), Au-PCB-GO (red trace), and Au-PCB-FGO electrode in PBS buffer (pH 7.4). (B) Focused CV curve of Figure 4A (green trace) GOx-modified Au-PCB-FGO electrode. (C) CVs of bare Au-PCB/GOx (black trace) and Au-PCB-GO/GOx (red trace) versus 20 mM D(+) glucose in PBS (pH 7.4). (D) CVs of Au-PCB-FGO-GOx versus D(+) glucose in PBS buffer at different concentration ranges from 20 mM to 0.1 mM, and the inset shows the corresponding plot of peak currents against molar concentrations of glucose.
Notes: Scan rate: 50 mV/s. All the individual CV responses were measured by different electrodes prepared appropriately.
Abbreviations: CVs, cyclic voltammograms; GOx, glucose oxidase; Au-PCB, gold printed circuit board electrode; Au-PCB-GO, gold printed circuit board electrode modified with graphene oxide; Au-PCB-FGO, gold printed circuit board electrode modified with functionalized graphene oxide; Au-PCB-FGO-GOx, gold printed circuit board electrode modified with functionalized graphene oxide and glucose oxidase; PBS, phosphate-buffered saline; Ag/AgCI, silver/silver chloride.
The electrochemical response of the Au-PCB-FGO-GOx electrode against different concentrations of glucose in phosphate-buffered solution (pH 7.4) is shown in Figure 4D. The CV of the Au-PCB-FGO-GOx electrode is observed to have a significant shift in peak redox potentials and broadness of the peak, especially at an anodic potential (Figure 4B). The reason for the shift in peak currents at the expected redox potential [such as 0.158 V (Epc) and 0.006 V (Epa)] and the broadness of the anodic peak could reflect concentration-dependent electron transport of molecules and the associated redox reaction at the functionalized electrode surface and interface. Because there were no chemical redox species directly involved in the current study, all the redox reactions observed on CV come from the MPHs on the surface of the Au-PCB-GO electrode.25 Thus, depending on the increase in glucose concentration in the bulk electrolyte, the enzymatic reaction increases the electrochemical redox reaction at the working electrode in a linear manner, such as for oxidation of glucose and reduction of oxygen from the solution. This combined reaction at the working electrode reflects the increased redox wave signal of MPHs with specific broadness in the anodic peak. Earlier reports, such as for metalloid (silver-doped) aminosilica nanocomposites on a fluorine-doped tin oxide electrode39 and for a glassy carbon electrode modified by silver nanoparticles, have observed a similar redox wave with specific broadness of the anodic peak in phosphate-buffered solution.41 Although showing a shift in peak currents at different potentials, it is clear that the Au-PCB-FGO-GOx electrode in our study ensured the linear relationship of Ip versus molar concentrations of glucose, with a wide linear range from 0.1 mM to 20 mM (R 0.9795 and R 0.9453 for Ipc and Ipa, respectively) and a sensitivity of 7.66 μA mM−1 cm−2, suggesting that the synergistic effect from MPHs functionalized with graphene oxide provided the necessary conduction pathways and electron transfer kinetics at the active substrate. Moreover, the sensitivity obtained for this biosensor was much better than that for other reported glucose biosensors, such as poly(3,4-ethylenedioxythiophene)-Prussian blue multiwalled carbon nanotubes (2.67 μA mM−1 cm−2)42 and glucose oxidase-modified Prussian blue screen-printed electrodes (3.21 ± 0.16 μA mM−1 cm−2).43 In addition, the linear range (up to 20 mM) is much wider than for similar biosensors, such as glucose oxidase immobilized onto a nanocomposite graphene-gold nanoparticle-chitosan film (10 mM),19 graphene (10 mM),20 graphene oxide-Prussian blue hybrid film (1.2 mM),21 silver-DNA hybrid nanoparticles (1.2 mM),44 a copper self-assembled monolayer nanocomposite on a gold substrate (0.0018 mM),45 poly(3,4-etheylenedioxythiophene)-Prussian blue multiwalled carbon nanotubes (10.0 mM),42 highly ordered polyaniline nanotubes (5.5 mM),46 a chitosan-gold nanoparticle hybrid film on a Prussian blue-modified electrode (1.6 mM),47 a highly ordered porous anodic alumina membrane-Prussian blue nanoarray (8.0 mM),48 and a Prussian blue-modified screen-printed electrode (3.0 mM).43
The diagnosis of diabetes is made on identification of fasting plasma glucose concentrations ≥ 126 mg/dL (7.0 mM) on at least two occasions.49 Our fabricated biosensor is therefore suitable for practical application in the detection of human blood sugar concentrations. The linear response with respect to various molar concentrations of glucose and the change in the peak current at the Au-PCB-FGO-GOx electrode surface indicate that enzymes immobilized on the surface of FGO provide a suitable environment for electron transfer between the glucose analyte and the sensor surface, which in turn detects the H2O2 obtained during the enzymatic reaction (glucose oxidase: glucose + O2 → gluconolactone + H2O2).3 In the present study, with increasing glucose concentrations (from 0.1 mM to 20mM), the peak oxidation currents also increased due to oxidation of the H2O2 generated, while the peak reduction current decreased due to consumption of O2. FGO-modified electrode has exhibited an enhanced electrocatalytic current and earlier onset of redox potential. Thus, similar to other electrochemical devices it can be a potential candidate for application in glucose detection.19–21
Clinical glucose detection
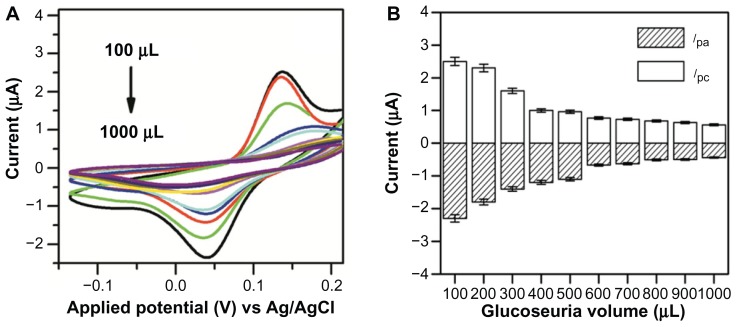
The fabricated Au-PCB-FGO-GOx biosensor platforms were then utilized for detecting glucose from two types of clinical diabetic samples, namely urine and serum. Interferents such as ascorbic acid or acetaminophen, or adherents such as urea compounds, are critical factors causing difficulty in urinary glucose detection.3 After performing preliminary volumetric assessments using 10, 60, and 160 μL urine samples from five diabetic patients (see Figure S3), the CV for a sample from a glucosuric patient was selected as the model clinical sample to confirm glucose detection by our novel biosensor platform. Although the density of pure glucose in urine samples is usually detected using a differential amperometric method, in the current study we used the CV method to ensure the electrochemical response (oxidation-reduction) of the Au-PCB-FGO-GOx electrode for feasibility in different physiological fluids.
Figure 5A shows the CV of Au-PCB-FGO-GOx versus different glucosuric sample volumes ranging from 100 to 1000 μL. A linear relationship was observed between the glucosuric sample and the peak current of the FGO-modified electrode. It is worth noting that, in contrast with commercial D(+) glucose in phosphate-buffered solution (Figure 4D), the peak current for Au-PCB-FGO-GOx versus that of the glucosuric sample decreased in a linear manner (see Figure 5B). Similarly, an earlier report based on glucose oxidase immobilized on hexamine ruthenium (III) chloride [Ru(NH3)6]Cl3 containing a nitrocellulose strip for urine glucose monitoring has also demonstrated a linear decrease in peak current with increasing glucose density.50 Possible reasons accounting for the decrease in peak redox current with a respective volumetric increase in urine glucose samples might include mixing of analyte urine glucose sample in bulk electrolyte phosphate-buffered solution and its dependent electrochemical reaction at the Au-PCB-FGO-GOx electrode that influences the concentration variation in oxidation of produced H2O2 and reduction of O2. In addition, air saturation in phosphate-buffered solution and the existence of interferents in the urine samples may have decreased the peak electrocatalytic current for detection of H2O2 and reduction of dissolved O2.
Figure 5.
(A) CVs of Au-PCB-FGO-GOx against glucosuria sample (injected into supporting electrolyte) and (B) histogram of peak currents for Au-PCB-FGO/GOx electrode against glucosuria sample of different volumes from one diabetic patient, ensuring the linear relationship between the volume and peak current.
Notes: Where error bars are not visible, they are smaller than the symbols. Scan rate: 50 mV/s.
Abbreviations: CVs, cyclic voltammograms; Au-PCB-FGO-GOx, gold printed circuit board electrode modified with functionalized graphene oxide and glucose oxidase; Ag/AgCI, silver/silver chloride.
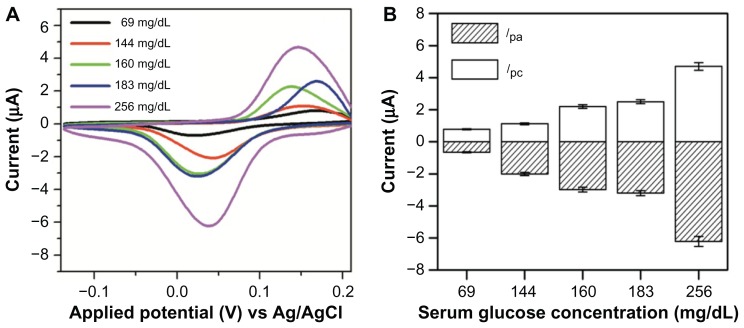
In order to evaluate the electrochemical response of the Au-PCB-FGO-GOx electrode against blood glucose, serum samples from five diabetic patients with different glucose levels at 69, 144, 160, 183, and 256 mg/dL (measured using a commercial glucose sensor, Accu-Chek Performa, Roche, Basel, Switzerland) were tested. To mimic the analysis function of a commercial blood glucose sensor system, all serum glucose samples in the current study were modified separately on the surface of the Au-PCB-FGO-GOx electrode and measured for their electrochemical response. As can be seen from the CV measurement in Figure 6A, the lowest glucose concentration (69 mg/dL) among the five serum samples showed the lowest peak cathodic current at 0.83 μA, whereas the other glucose samples (144, 160, 183, and 256 mg/dL) had higher peak cathodic currents (1.11, 2.28, 2.61 and 4.67 μA, respectively). This ensured a linear response against the serum glucose concentration and peak current obtained for the Au-PCB-FGO-GOx electrode. As discussed in the previous section, the peak redox current of Au-PCB-FGO-GOx versus the serum glucose samples is observed to have a significant shift at different redox potentials (Figure 4D). However, the peak currents become relatively increased with increasing glucose concentration. These CVs (Figure 6A) are in relation to the commercial glucose CVs and are contrary to the urine samples. Although the same type of functional electrode comprised of Au-PCB-FGO-GOx has been utilized for the three different types of physiological glucose tested in this study, the individual response is distinctive. We suggest that the mechanism for the linear response of the peak current according to glucose concentration is highly dependent on the mode of electrochemical interaction, such as analyte molecules on the functional electrode or mixed into the electrolyte solution. The good concordance between detection at the commercial and clinical levels (glucosuria and serum glucose) confirms the reliability of our advanced hybrid material (FGO)-based electrochemical sensor. This method provides a novel sensitive platform for the development of an FGO-based electrochemical biosensor for clinical detection of glucose levels. Furthermore, the durability of our Au-PCB-FGO-GOx electrode for glucose detection in human serum was analyzed at the time of preparation (Figure S4A) and after 50 days of storage at 4°C (Figure S4B). As can be seen, the peak redox current intensity after the electrode had been incubated for 50 days is slightly decreased in comparison with that on the day of preparation; however, no fundamental change was observed in the position of the peak redox potential. This ensures the stability and binding efficiency of the active material modified on the electrode. As emphasized previously, the redox wave generation is attributed to the presence of MPHs on the surface of the graphene oxide. Unlike preparation of a conventional graphene oxide nanocomposite, ie, by physical adsorption, the present work involved a covalent reaction between silanized MPHs and carboxylic acid groups in graphene oxide,25 and the resulting chemical bonds enhanced the long-term binding affinity of MPHs at the graphene oxide-electrode interface. The high molecular weight PEG layer on the surface of the metalloid composite preserves the binding and bioactivity of glucose oxidase.37 Further, oxygen plasma pretreatment provides an excellent hydrophilic nature on the bare Au-PCB substrate which is also mediated by the strong affinity between the oxygenated graphene nanosheets and the Au-PCB substrate. Taken together, all these factors resulted in long-term stability of the glucose oxidase-modified Au-PCB-FGO electrode and provided better durability for studying the clinical glucose sample.
Figure 6.
(A) CVs of Au-PCB-FGO-GOx against serum glucose samples (modified on electrode) from diabetic patients and (B) histogram of peak currents for Au-PCB-FGO/GOx electrode against serum samples from low to high concentration from different diabetic patients.
Notes: Where error bars are not visible, they are smaller than the symbols. Scan rate: 50 mV/s.
Abbreviations: CVs, cyclic voltammograms; Au-PCB-FGO-GOx, gold printed circuit board electrode modified with functionalized graphene oxide and glucose oxidase; Ag/AgCI, silver/silver chloride.
Conclusion
In summary, an electrochemical biosensor based on Ag@SiO2-PEG (MPH) functionalized with graphene oxide was fabricated for glucose detection. Our electrochemical investigations demonstrate that functionalized graphene oxide provided a suitable electrocatalytic platform for glucose detection, which greatly facilitated electron exchange between the immobilized glucose oxidase enzyme and the electrode, and increased the detection ability for commercial and clinical glucose concentrations at multiple concentration ranges. Furthermore, properties such as inherent redox wave generation in phosphate-buffered solution and a hybrid nanoenvironment such as metal (Ag), non-metal (SiO2), and polymer (PEG) on the surface of novel graphene oxide make FGO a promising candidate for construction of other biosensor platforms.
Acknowledgment
This research was supported by grants from the Ministry of Knowledge and Economy (10039863) to KSYun and the Ministry of Knowledge and Economy (10032112) to M-HLee.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Table S1.
Shows the patient details in terms of their age, gender and glucose concentration measured by commercial sensor Accu-Chek® from Roche (Basel, Switzerland)
| Diabetic patient serum sample no | Patient age | Gender | Accu-Chek performa (Roche) measured glucose concentration |
|---|---|---|---|
| 1 | 68 | F | 69 mg/dL |
| 2 | 49 | F | 144 mg/dL |
| 3 | 69 | M | 160 mg/dL |
| 4 | 66 | M | 183 mg/dL |
| 5 | 43 | M | 256 mg/dL |
(A) Ultraviolet-visible absorbance spectrum, and (B) particle size distribution histogram of metalloid polymer hybrid nanoparticles (Ag@SiO2-PEG) from electrophoretic light scattering measurements. (C) Field emission scanning electron microscopic (scale bar 30 nm) and (D) high resolution transmission electron microscopic images (scale bar 10 nm) of metalloid polymer hybrid nanoparticles.
Abbreviations: Ag@SiO2-PEG, silver-silica coated with polyethylene glycol; LS int., light scattering intensity.
Fourier transform infrared absorbance spectra of (A) GO, (B) MPHs, and (C) FGO.
Abbreviations: GO, graphene oxide; MPHs, metalloid polymer hybrid nanoparticles; FGO, functionalized graphene oxide.
CVs of Au-PCB-FGO-GOx versus different clinical glucosuria samples (urine samples from five patients named A–E) at volumes of 10, 60, and 160 μL in phosphate-buffered solution. (F) Cathodic peak current histogram for five glucosuria samples.
Notes: This result shows the preliminary clinical diagnostics of urine samples from hyperglycemic patients. Scan rate: 50 mV/s.
Abbreviations: CVs, cyclic voltammograms; Au-PCB-FGO-GOx, gold printed circuit board electrode modified with functionalized graphene oxide and glucose oxidase; Ag/AgCI, silver/silver chloride.
Multiple cycle CV segments of Au-PCB-FGO-GOx versus glucose serum sample (glucose concentration in serum 131 mg/dL, measured by Accu-Chek®) electrode measured on day 0 (A, black trace) and after 50 days (B, red trace) stored in refrigeration at 4°C.
Note: Scan rate: 50 mV per second.
Abbreviations: CV, cyclic voltammogram; Au-PCB-FGO-GOx, gold printed circuit board electrode modified with functionalized graphene oxide and glucose oxidase; Ag/AgCI, silver/silver chloride.
References
- 1.Uenoyama H, Nankai S. In: Chemical Sensor Technology. Aizawa M, editor. Vol. 5. Tokyo, Japan: Kodansha Ltd; 1994. pp. 177–185. [Google Scholar]
- 2.Cui G, Kim SJ, Choi S, et al. A disposable amperometric sensor screen printed on a nitrocellulose strip: a glucose biosensor employing lead oxide as an interference-removing agent. Anal Chem. 2000;72:1925–1929. doi: 10.1021/ac991213d. [DOI] [PubMed] [Google Scholar]
- 3.Miyashita M, Ito N, Ikeda S, et al. Development of urine glucose meter based on micro-planer amperometric biosensor and its clinical application for self-monitoring of urine glucose. Biosens Bioelectron. 2009;24:1336–1340. doi: 10.1016/j.bios.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 4.Nekrassova O, Lawrence NS, Compton RG. Analytical determination of homocysteine: a review. Talanta. 2003;60:1085–1095. doi: 10.1016/S0039-9140(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 5.Nekrassova O, Lawrence NS, Compton RG. Selective electroanalytical assay for cysteine at a boron doped diamond electrode. Electroanalysis. 2004;16:1285–1291. [Google Scholar]
- 6.Sun TP, Shieh HL, Ching CTS, et al. Carbon nanotube composites for glucose biosensor incorporated with reverse iontophoresis function for noninvasive glucose monitoring. Int J Nanomedicine. 2010;5:343–349. [PMC free article] [PubMed] [Google Scholar]
- 7.Schreier TM, Rach JJ, Howe GE. Efficacy of formalin, hydrogen peroxide, and sodium chloride on fungal-infected rainbow trout eggs. Aquaculture. 1996;140:323–331. [Google Scholar]
- 8.Ndamanisha JC, Bai J, Qi B, et al. Application of electrochemical properties of ordered mesoporous carbon to the determination of glutathione and cysteine. Anal Biochem. 2009;386:79–84. doi: 10.1016/j.ab.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Maleki N, Safavi A, Sedaghati F, et al. Efficient electrocatalysis of L-cysteine oxidation at carbon ionic liquid electrode. Anal Biochem. 2007;369:149–153. doi: 10.1016/j.ab.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Zhang K, Yao YL, et al. Multilayer assembly of Prussian blue nanoclusters and enzyme-immobilized poly(toluidine blue) films and its application in glucose biosensor construction. Langmuir. 2004;20:7303–7307. doi: 10.1021/la049667f. [DOI] [PubMed] [Google Scholar]
- 11.Veerapandian M, Subbiah R, Lim GS, et al. Copper-glucosamine microcubes: synthesis, characterization, and C-reactive protein detection. Langmuir. 2011;27:8934–8942. doi: 10.1021/la2009495. [DOI] [PubMed] [Google Scholar]
- 12.Park HS, Park TJ, Huh YS, et al. Immobilization of genetically engineered fusion proteins on gold-decorated carbon nanotube hybrid films for the fabrication of biosensor platforms. J Colloid Interf Sci. 2010;350:453–458. doi: 10.1016/j.jcis.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 13.Feng D, Wang F, Chen Z. Electrochemical glucose sensor based on one-step construction of gold nanoparticle-chitosan composite film. Sens Actuators B Chem. 2009;138:539–544. [Google Scholar]
- 14.Joung D, Chunder A, Zhai L, et al. Space charge limited conduction with exponential trap distribution in reduced graphene oxide sheets. Appl Phys Lett. 2010;97:093105. [Google Scholar]
- 15.Krishnamoorthy K, Mohan R, Kim SJ. Graphene oxide as a photocatalytic material. Appl Phys Lett. 2011;98:244101. [Google Scholar]
- 16.Krishnamoorthy K, Veerapandian M, Mohan R, et al. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl Phys A. 2012;106:501–506. [Google Scholar]
- 17.Pimenta MA, Dresselhaus G, Dresselhaus MS, et al. Studying disorder in graphite-based systems by Raman spectroscopy. Phys Chem Chem Phys. 2007;9:1276–1291. doi: 10.1039/b613962k. [DOI] [PubMed] [Google Scholar]
- 18.Dreyer DR, Park SJ, Bielawski CW, et al. The chemistry of graphene oxide. Chem Soc Rev. 2010;39:228–240. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 19.Shan CS, Yang HF, Han DX, et al. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens Bioelectron. 2010;25:1070–1074. doi: 10.1016/j.bios.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Wu P, Shao Q, Hu Y, et al. Direct electrochemistry of glucose oxidase assembled on graphene and application to glucose detection. Electrochim Acta. 2010;55:8606–8614. [Google Scholar]
- 21.Zhang Y, Sun X, Zhu L, et al. Electrochemical sensing based on graphene oxide/Prussian blue hybrid film modified electrode. Electrochim Acta. 2011;56:1239–1245. [Google Scholar]
- 22.Ashok Kumar S, Cheng HW, Chen SM, et al. Preparation and characterization of copper nanoparticles/zinc oxide composite modified electrode and its application to glucose sensing. Mater Sci Eng C Mater Biol Appl. 2010;30:86–91. [Google Scholar]
- 23.Veerapandian M, Yun KS. Ultrasonochemical-assisted fabrication and evaporation-induced self-assembly (EISA) of POSS-SiO2@Ag core/ABA triblock copolymer nanocomposite film. Polymer Composites. 2010;31:1620–1627. [Google Scholar]
- 24.Veerapandian M, Yun KS. Ultrasonochemically conjugated metalloid/triblock copolymer nanocomposite and subsequent thin solid laminate growth for surface and interface studies. Langmuir. 2010;26:14216–14222. doi: 10.1021/la1026587. [DOI] [PubMed] [Google Scholar]
- 25.Veerapandian M, Lee MH, Krishnamoorthy K, et al. Synthesis, characterization and electrochemical properties of functionalized graphene oxide. Carbon. 2012;50:4228–4238. [Google Scholar]
- 26.Hirata M, Gotou T, Horiuchi S, et al. Thin-film particles of graphite oxide 1: high-yield synthesis and flexibility of the particles. Carbon. 2004;42:2929–2937. [Google Scholar]
- 27.Veerapandian M, Lim SK, Nam HM, et al. Glucosamine-functionalized silver glyconanoparticles: characterization and antibacterial activity. Anal Bioanal Chem. 2010;398:867–876. doi: 10.1007/s00216-010-3964-5. [DOI] [PubMed] [Google Scholar]
- 28.Jiang G, Lin Z, Chen C, et al. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon. 2011;49:2693–2701. [Google Scholar]
- 29.Pittet P, Lu GN, Galvan JM, et al. PCB technology-based electrochemiluminescence microfluidic device for low-cost portable analytical systems. IEEE Sens J. 2008;8:565–571. [Google Scholar]
- 30.Ghindilis AL, Atanasov P, Wilkins E. Enzyme-catalyzed direct electron transfer: fundamentals and analytical applications. Electroanalysis. 1997;9:661–674. [Google Scholar]
- 31.Shan CS, Yang HF, Song JF, et al. Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal Chem. 2009;81:2378–2382. doi: 10.1021/ac802193c. [DOI] [PubMed] [Google Scholar]
- 32.Shao Y, Wang J, Wu H, et al. Graphene based electrochemical sensors and biosensors: a review. Electroanalysis. 2010;22:1027–1036. [Google Scholar]
- 33.Rao CN, Subrahmanyam KS, Ramakrishna Matte HS, et al. A study of the synthetic methods and properties of graphenes. Sci Technol Adv Mater. 2010;11:054502. doi: 10.1088/1468-6996/11/5/054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Liu Z, Jauregui LA, et al. Wafer-scale synthesis of graphene by chemical vapor deposition and its application in hydrogen sensing. Sens Actuators B Chem. 2010;150:296–300. [Google Scholar]
- 35.Rao CN, Ramakrishna Matte HS, Subrahmanyam KS. Synthesis and selected properties of graphene and graphene mimics. Acc Chem Res. 2012 Jun 27; doi: 10.1021/ar300033m. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 36.Lu R, Terrones M. Towards new graphene materials: doped graphene sheets and nanoribbons. Mat Lett. 2012;78:209–218. [Google Scholar]
- 37.Sung WJ, Bae YH. A glucose oxidase electrode based on polypyrrole with polyanion/PEG/enzyme conjugate dopant. Biosens Bioelectron. 2003;18:1231–1239. doi: 10.1016/s0956-5663(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 38.Wang GF, Wang W, Wu JF, et al. Self assembly of a silver nanoparticles modified electrode and its electrocatalysis on neutral red. Microchim Acta. 2009;164:149–155. [Google Scholar]
- 39.Choi YJ, Luo T-JM. Electrochemical properties of silver nanoparticle doped aminosilica nanocomposite. Int J Electrochem. 2011;404937:1–6. [Google Scholar]
- 40.Guo L, Nie J, Du B, et al. Thermoresponsive polymer-stabilized silver nanoparticles. J Colloid Interface Sci. 2008;319:175–181. doi: 10.1016/j.jcis.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Wang GF, Li MG, Gao YC, et al. Amperometric sensor used for determination of thiocyanate with a silver nanoparticles modified electrode. Sensors. 2004;4:147–155. [Google Scholar]
- 42.Chiu JY, Yu CM, Yen MJ, et al. Glucose sensing electrodes based on a poly(3,4-ethylenedioxythiophene)/Prussian blue bilayer and multi-walled carbon nanotubes. Biosens Bioelectron. 2009;24:2015–2020. doi: 10.1016/j.bios.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 43.O’Halloran MP, Pravda M, Guilbault GG. Prussian blue bulk modified screen-printed electrodes for H2O2 detection and for biosensors. Talanta. 2001;55:605–611. doi: 10.1016/s0039-9140(01)00469-6. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Zhao H, Ju H, et al. Electrodeposition of silver-DNA hybrid nanoparticles for electrochemical sensing of hydrogen peroxide and glucose. Electrochem Commun. 2006;8:1197–1203. [Google Scholar]
- 45.Vijayalakshmi A, Karthikeyan R, Berchmans S. Nonenzymatic reduction of hydrogen peroxide produced during the bioelectrocatalysis of glucose oxidase on urchin-like nanofibrillar structures of cu on au substrates. J Phys Chem C Nanomater Interfaces. 2010;114:22159–22164. [Google Scholar]
- 46.Wang Z, Liu S, Wu P, et al. Detection of glucose based on direct electron transfer reaction of glucose oxidase immobilized on highly ordered polyaniline nanotubes. Anal Chem. 2009;81:1638–1645. doi: 10.1021/ac802421h. [DOI] [PubMed] [Google Scholar]
- 47.Xue MH, Xu Q, Zhou M, et al. In situ immobilization of glucose oxidase in chitosan-gold nanoparticle hybrid film on Prussian blue modified electrode for high-sensitivity glucose detection. Electrochem Commun. 2006;8:1468–1474. [Google Scholar]
- 48.Xian YZ, Hu Y, Liu F, et al. Template synthesis of highly ordered Prussian blue array and its application to the glucose biosensing. Biosens Bioelectron. 2007;22:2827–2833. doi: 10.1016/j.bios.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Niewoehner CB. Endocrine Pathophysiology. 2nd ed. Raleigh, NC: Hayes Barton Press; 2004. [Google Scholar]
- 50.Park HD, Lee KJ, Yoon HR, et al. Design of a portable urine glucose monitoring system for health care. Comput Biol Med. 2005;35:275–286. doi: 10.1016/j.compbiomed.2004.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Shows the patient details in terms of their age, gender and glucose concentration measured by commercial sensor Accu-Chek® from Roche (Basel, Switzerland)
| Diabetic patient serum sample no | Patient age | Gender | Accu-Chek performa (Roche) measured glucose concentration |
|---|---|---|---|
| 1 | 68 | F | 69 mg/dL |
| 2 | 49 | F | 144 mg/dL |
| 3 | 69 | M | 160 mg/dL |
| 4 | 66 | M | 183 mg/dL |
| 5 | 43 | M | 256 mg/dL |
(A) Ultraviolet-visible absorbance spectrum, and (B) particle size distribution histogram of metalloid polymer hybrid nanoparticles (Ag@SiO2-PEG) from electrophoretic light scattering measurements. (C) Field emission scanning electron microscopic (scale bar 30 nm) and (D) high resolution transmission electron microscopic images (scale bar 10 nm) of metalloid polymer hybrid nanoparticles.
Abbreviations: Ag@SiO2-PEG, silver-silica coated with polyethylene glycol; LS int., light scattering intensity.
Fourier transform infrared absorbance spectra of (A) GO, (B) MPHs, and (C) FGO.
Abbreviations: GO, graphene oxide; MPHs, metalloid polymer hybrid nanoparticles; FGO, functionalized graphene oxide.
CVs of Au-PCB-FGO-GOx versus different clinical glucosuria samples (urine samples from five patients named A–E) at volumes of 10, 60, and 160 μL in phosphate-buffered solution. (F) Cathodic peak current histogram for five glucosuria samples.
Notes: This result shows the preliminary clinical diagnostics of urine samples from hyperglycemic patients. Scan rate: 50 mV/s.
Abbreviations: CVs, cyclic voltammograms; Au-PCB-FGO-GOx, gold printed circuit board electrode modified with functionalized graphene oxide and glucose oxidase; Ag/AgCI, silver/silver chloride.
Multiple cycle CV segments of Au-PCB-FGO-GOx versus glucose serum sample (glucose concentration in serum 131 mg/dL, measured by Accu-Chek®) electrode measured on day 0 (A, black trace) and after 50 days (B, red trace) stored in refrigeration at 4°C.
Note: Scan rate: 50 mV per second.
Abbreviations: CV, cyclic voltammogram; Au-PCB-FGO-GOx, gold printed circuit board electrode modified with functionalized graphene oxide and glucose oxidase; Ag/AgCI, silver/silver chloride.