Summary
Interactions between stem cells and their surrounding microenvironment, or niche, are critical for the establishment and maintenance of stem cell properties1. The adult Drosophila testis contains a discrete morphological stem cell niche, the ‘hub’, that provides an excellent model for the study of niche development2-4. The small cluster of non-dividing, somatic hub cells at the anterior tip of the fly testis is contacted by the germline stem cells (GSCs)5 that retain their stem cell character through the direct association with the hub6. Here we demonstrate that integrin-mediated adhesion is important for maintaining the correct position of embryonic hub cells during gonad morphogenesis. The misplaced hub in integrin mutant embryos directs the orientation of cell divisions in the presumptive GSC, a hallmark of the active germline stem cell niche. A reduction of integrin-mediated adhesion in adult testes, which resulted in a loss of hub cells and the stem cell population, revealed the importance of hub cell anchoring. Finally, we show that an ECM is present around the gonad in late embryogenesis and that this ECM is defective in integrin mutant gonads. Based on our data we propose that integrins are required for the attachment of the hub cells to the ECM, which is critical for maintaining the stem cell niche.
Keywords: Stem cells niche, Integrin, Cell adhesion, Gonad, hub
Results and Discussion
The hub cells in adult gonads are located at the anterior tip of the testes where they directly contact approximately nine germline stem cells5. Adult testes derive from a pair of embryonic gonads that form during the later stages of embryogenesis (stages 12-17). After compaction (coalescence) the embryonic gonads are spherical structures comprised of primordial germline cells that are intermingled with and ensheathed by mesodermally-derived somatic gonadal precursor cells (SGPs)7. Recent studies revealed that hub cells arise during embryogenesis, shortly after gonad compaction, from a subset of anterior SGPs8, 9. We noticed that the Drosophila filamin Cheerio (Cher) was highly expressed in a group of somatic cells located at the anterior of the gonad in half of wild-type embryos from late stage 16 onward (Supplemental Fig. S1). We confirmed that the Cher antibody recognizes hub cells by colocalization of Cher and β-Gal staining in hub cells in the LacZ marker line 25410 (Supplemental Fig. S1). Thus, Cher provides a marker of the hub cells in embryonic gonads, thereby enabling us to use it to study their development and morphogenesis.
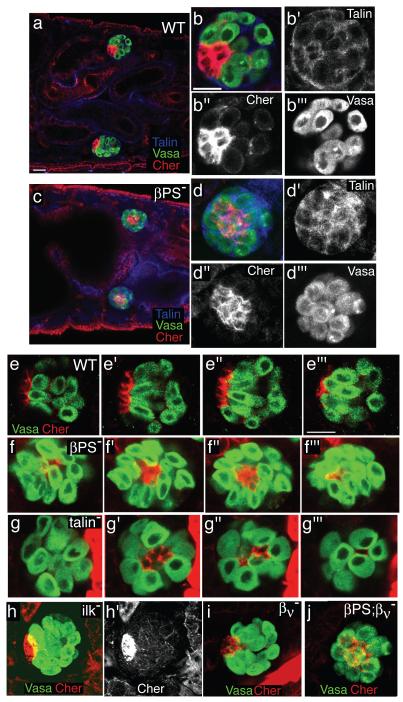
Morphogenesis of most tissues in metazoans requires cell-to-matrix adhesion mediated by the integrin class of adhesion receptors11, 12. Integrins are heterodimeric, transmembrane receptors, comprised of an α and a β subunit that mediate adhesion by simultaneously binding extracellular matrix proteins and the actin cytoskeleton13. In Drosophila, integrins containing the βPS subunit are essential for many morphogenetic processes12. To test the function of integrins in the gonad we examined myospheroid mutant (mysXG43) embryos, which are hemizygous for a null mutation in the gene encoding the βPS integrin subunit and therefore lack all βPS containing heterodimers. In the absence of βPS integrin the embryonic hub cells were mislocalized (Fig. 1). In wild-type embryos, the hub cells are at the anterior edge of the gonad, forming a cap that contacted the anterior germline cells (Fig. 1a, b). In embryos lacking βPS integrin, the hub cells were embedded at the centre of the gonad surrounded by germline cells on all sides (Fig. 1c, d). Although mislocalized, the hub cells formed a tight cluster, suggesting that integrin is not required for adhesion between the hub cells.
Figure 1. The hub cells are mispositioned in integrin mutants.
(a, b) In wild-type stage 17 embryos the hub cells (labelled with Cheer, red) are positioned on the outer anterior edge of the male gonad (germline cells labeled with Vasa, green; Talin in blue labels the SGPs). (c, d) In mysXG43 embryos, which lack the βPS integrin subunit, the hub cells are partitioned to the middle of the testis, nestled in between the germline cells. (e-g) Confocal Z-series of 2 μm steps (labelled with Vasa (green) and Cheerio (red)) confirmed that, in contrast to wild-type embryos (e) where the hub cells were situated at the anterior pole of the gonad, the hub cells were found in the center of the gonad in embryos lacking integrins (f, mysXG43) or the integrin-associated protein talin (g, rhea79A). (h, h’) Hub cells were normally positioned in embryos that lack Integrin Linked Kinase (ilk1) and (i) in embryos that lack only the second integrin β subunit βν. (j) Gonad in embryo lacking both integrin β subunits (mysXG43; βν double mutant) had an identical phenotype to that seen in mysXG43 embryos. Scale bars 20 μm.
In addition to the hub cells, embryonic gonads contain SGPs that are interspersed with the germline cells7. To test whether loss of βPS affects the distribution of all somatic cells within the gonad, embryonic gonads were labelled with an antibody against talin, which has elevated expression in SGPs14. Talin expressing SGPs were interspersed with germline cells throughout the gonad in both wild-type and mysXG43 embryos (Fig. 1b’ and 1d’, respectively). To test if integrins were required during earlier stages of gonad development, gonads in mysXG43 mutant embryos were labelled with Vasa, to mark germline cells, and various SGP markers7 including Eya, ZFH1 and Traffic Jam15 (Supplemental Fig. S2; data not shown). No differences were detected between wild-type and mysXG43 mutant gonads until late embryonic stages (stage 17) when the hub cells were observed as a cluster of cells in the middle of mysXG43 mutant gonads (Supplemental Fig. S2). Thus, integrins are specifically required for hub cells to localize at the anterior pole of male embryonic gonads.
To understand the function of integrins in hub development we investigated which integrins were expressed in the gonad and how they were distributed. Of the 5 αPS integrin subunits encoded by the Drosophila genome12 antibodies exist for αPS1, αPS2, and αPS3. The αPS3 subunit was expressed in all SGPs (Fig. 2e), whereas αPS1 or αPS2 were not detected in embryonic gonads (Fig. 2f; data not shown). Because mutations in the gene encoding αPS3 did not affect hub development (data not shown) it is likely that αPS4 and/or αPS5 are expressed, and act redundantly with αPS3, in the gonad. There are two β integrin subunits in Drosophila, βPS integrin is expressed in all SGPs (Fig. 2d) while the second β subunit, αν, is not detected in SGPs16. Moreover, the hub cells form normally in embryos lacking αν, and the mutant hub phenotype is not enhanced in embryos lacking both αν and βPS (Fig. 1i, j). Thus it is likely that βPS integrin is the only β integrin subunit that functions in the gonad.
Figure 2. Integrin and talin expression in the somatic gonadal cells.
(a-c) Talin protein (a’-c’, red in a-c with germline cells (Vasa) in green), encoded by the gene rhea, is expressed in all the SGPs starting at stage 14 (a) and persisting through stage 15 (b) to the end of embryogenesis (c, stage 17). Expression of integrins in the gonad: (d) It was previously reported that the integrin βPS subunit was not expressed in the gonad14. We have now been able to detect its expression in all SGPs using new fixation methods but the staining is weak probably due to poor antibody penetration at late stages of embryogenesis. (e) The αPS3 subunit is also expressed in all SGPs. (f) The αPS2 subunit (f’, red in f with germline cells (Vasa) in green) is expressed in the muscle attachments (f, arrows) but is not detected in the gonad. Scale bars are 20 μm.
Talin is an integrin-binding cytoskeletal linker that is essential for integrin-mediated adhesion in Drosophila14. In embryos, talin was highly expressed in the gonadal mesoderm and was concentrated at the plasma membrane of all SGPs including in hub cells (Fig 2c). Embryos lacking talin exhibited a mislocalization of the hub identical to that seen in embryos lacking integrin (Fig. 1g). In contrast, mutations that eliminate the integrin-associated proteins ILK and PINCH17, 18 did not perturb hub cell positioning (Fig. 1h; data not shown). Analysis of the temporal and spatial aspects of βPS integrin and talin expression showed that they were both expressed in gonads starting at stage 14 and remained uniformly expressed in the SGPs throughout embryonic development (Fig 2a-c).
In wild-type Drosophila embryos ECM markers are detected around the outer perimeter of the gonad at stage 16. LamininA (LanA) and Nidogen (Ndg), a laminin-associated protein, were abundantly expressed in the gonadal ECM at this time (Fig. 3a,c). The distribution of these proteins suggests that ECM is assembled at a time when integrin-mediated adhesion is important for gonad morphogenesis. This ECM lies in direct contact with the outermost SGPs and the hub cells are positioned such that they contact both the ECM and germline cells (Fig. 1a, b and 3a’,c’).
Figure 3. Integrins assemble an ECM around the embryonic gonad.
(a, c) ECM proteins Ndg (a, a’, red) and LanA (c, c’, red) are localized around the embryonic gonad in wild-type embryos. The embryonic hub cells (labelled with Cher, green in a’ and c’) are in contact with the ECM. In integrin mutant embryos Ndg (b, b’, red) and LanA (d, d’, red) around the gonad was substantially reduced. Scale bars are 20 μm.
To explore the molecular basis for the hub cell mislocalization when integrin function is impaired, we examined the organization of ECM components in integrin mutant gonads. The amount of LanA and Ndg around the gonad was greatly reduced when βPS integrins were absent (Fig. 3b, d). This shows integrins play an active role in ECM accumulation around the gonad. This finding raises the possibility that the main role of integrins is to assemble an ECM around the gonad, and a different ECM receptor anchors the hub cells to the ECM. It is difficult to completely rule this out, but we examined the other known ECM receptor present in Drosophila, Dystroglycan19, and could not detect it in embryonic hub cells (data not shown).
Cells with different adhesive properties in an environment that allows cell mobility can undergo cell sorting, where cells that adhere most strongly to one another separate from, and become enveloped by less adhesive cells20. Two of our observations suggested that differential adhesion-mediated cell sorting occurs during hub development. First, the hub cells form a tight cluster, separate from other SGPs in wild-type and integrin mutant gonads. Second, in integrin mutants the hub cells become enveloped by the other SGPs and germline cells. Cell sorting can be achieved by modulating cadherin-mediated adhesion21, 22. Both DE-and DN-cadherin are expressed at higher levels in the hub compared to other gonadal cells (Supplemental Fig. S3 and ref. 8). To test whether hub cells segregated from other SGPs and the germline cells because of differential cadherin expression we modulated DE- or DN-cadherin levels in integrin mutant embryos. We found that reducing or increasing cadherin levels (Supplemental Fig. S3, and S4, respectively) did not affect the position or segregation of the hub cells. These results suggest that the adhesion molecule that mediates the association of hub cells and their movement to the middle of the gonad in integrins mutants is not a classical cadherin, but do not rule out a contribution by these cadherins. Moreover, the homophilic cell adhesion molecule Fas3 is expressed on the surface of embryonic hub cells8, consistent with the idea that other adhesion molecules contribute to adhesion between hub cells.
By morphological and molecular criteria the hub cells in embryonic gonads appear similar to adult hub cells (Supplemental Fig. S1; ref. 8). In adult testes, cell divisions of GSCs are oriented perpendicular to the hub and this helps to regulate the balance between GSC self-renewal and differentiation6. In GSCs at early interphase, a single centrosome is always positioned near the cell cortex adjacent to the site of contact with the hub. One daughter centrosome migrates to the opposite side of the nucleus following centrosome duplication so that the spindle is oriented perpendicular to the hub6. To test whether germline cell divisions in the embryonic gonad are also orientated towards the hub, centrosomes were visualized with a centrosomin (CNN) specific antibody, and dividing cells were labelled with Phospho-Histone H3. Serial confocal sections were used to reconstruct the orientation of dividing germline cells. In wild-type gonads, in every germline cell that directly contacted the hub, one centrosome was positioned on the side that contacted the hub (n>100) (Fig. 4a). During mitosis the centrosomes aligned in an orientation perpendicular to the hub (n=23) resulting in a regular pattern of cell divisions along the anterior-posterior axis (Fig. 4a). These results suggest that hub cells may already control spindle orientation of dividing GSCs in the embryo.
Figure 4. Spindle orientation in wild-type and integrin mutant embryonic gonads.
(a) In mitotic GSCs of wild-type gonads (labelled with Phospho-Histone H3 specific antibody, blue) the centrosomes (labelled with CNN, green) are oriented so that the cell division is perpendicular to the anterior hub (labelled with Cheerio, red) and are generally aligned along the anterior-posterior axis of the embryo. (b) In integrin mutant gonads the centrosomes align perpendicular to the mispositioned hub. However, because the hub is the center of the gonad the plane of division is no longer strictly along the anterior-posterior axis. Scale bars are 10 μm.
We examined whether the ectopic hub in integrin mutants retains the ability to orient the mitotic spindle during GSC division. In interphase germline cells that contacted the hub, the centrosome was always positioned towards the side of the cell in contact with the hub, even though the hub was misplaced to the centre of the gonad in integrin mutants (n>100). In addition, mitotic germline cells adjacent to the hub cells divided perpendicular to the hub in all cases (n=21). However, as the hub was in the centre of the gonad the regular anterior-posterior orientation of dividing GSCs was not maintained (Fig. 4b), resulting in an abnormal cell division pattern compared to wild-type gonads. These results indicate that hub cells in embryonic testes direct spindle orientation even when mislocalized. Since GSC divisions orient normally with respect to the hub no matter where the hub is within the testes, integrin dependent hub positioning is critical for the normal anterior-posterior orientation of germline cell divisions.
Next, we examined the importance of integrin-dependent anchoring of the hub for spermatogenesis. In adult testes, hub cells occupy the tip of the gonad and contact somatic and germline stem cells on one side and ECM on the other5. The ECM overlaying the adult hub is thicker than it is around the rest of the testis and has a convoluted appearance suggesting that it makes extensive contacts with the hub cells5. It has been proposed that hub cell-ECM contacts in the adult serve to maintain the hub at the anterior tip of the gonad5, consistent with the function integrins have in embryonic testes. We could not study integrin function in adults using integrin null homozygous mutants as they die during embryogenesis. Moreover, the hub cells in the adult never divide5, 23 preventing the use of the FLP/FRT system to generate adult hub cells lacking integrin. We therefore used dsRNA interference (RNAi) to disrupt integrin-mediated adhesion.
Talin dsRNA was expressed in somatic cells of the testes under the control of a tj-GAL4 driver (P{GawB}NP1624-5-124) to disrupt integrin-mediated adhesion. The activity pattern of the tj-Gal4 driver reflects that of tj (D.G., unpublished data), which means tj-GAL4 is expressed throughout development in somatic gonadal cells that contact the germline cells, including the hub15. In tj-GAL4/+; UAS-talin RNAi/+ (talin-RNAi) embryos we observed no reduction in talin, and the hub cells localized normally (Fig. 5a). However, when talin-RNAi males were allowed to develop to adulthood they developed progressive defects in spermatogenesis. In adult control (tj-GAL4/tj-GAL4) flies, aged 1, 3 and 14 days, the testes contained a hub, GSCs, and differentiating germline cells at all stages of spermatogenesis (Fig 5b,b’, d”, f). While in tj-GAL4/tj-GAL4 adult testes talin staining was detected in all somatic cells including the hub (Fig. 5d’), talin staining was not detected in somatic cells in talin-RNAi testes (Fig. 5e’). Most one-day-old talin-RNAi adult testes exhibited normal spermatogenesis but a small number (9%, n=100) appeared to lack a hub. In 3 day-old talin-RNAi flies, the number of testes that did not have a hub had substantially increased (33%, n=100). In testes lacking a hub no GSCs or germline cells at early stages of differentiation were observed, and instead the distal region contained germline cells at mid to late stages of spermatogenesis (Fig. 5g). Moreover, even when the hub was present it was sometimes mislocalized (Fig. 5h). Finally, after 2 weeks more than half the testes from talin-RNAi adults (56%, n=100) lacked a hub, GSCs, and differentiating germline cells and instead contained only differentiated sperm, which filled the entire gonad (Fig. 5c,c’ and e”). Inspection of the distal region showed that expressing talin-RNAi caused a complete loss of GSCs (Fig 5e vs d). Although tj-GAL4 is expressed in cyst cells in addition to hub cells our data suggested that cyst cells are not affected by talin-RNAi expression as the germline cells continue to differentiate into sperm. It is unclear why the hub disappearance is gradual, the hub cells are non-dividing and it may take a long time to obtain a significant reduction in talin protein if its turnover was low. Alternatively, there may be another mechanism to retain the hub in place that is only partially effective and fails to retain the hub over time. Thus, based on our data we propose that depletion of talin leads to progressive loss of the hub in adult testes which results in the gradual differentiation of all GSCs into sperm.
Fig. 5. Phenotypes of talin RNAi in adult testes.
UAS-Talin RNAi was expressed in embryonic and adult testis using Traffic Jam-Gal4. (a) In tj-GAL4/+; UAS-talin RNAi/+ (talin RNAi) embryos the hub cells localize to the anterior part of the gonad (Vasa, Green; Cher, red). (b) In a 2 week old adult tj-GAL4/tj-GAL4 testis the distal part of the testis holds the hub cells (b’, Cher, red), the GSCs, dividing gonial cells, and primary spermatocytes (b’, Vasa, green) and the rest of the testis fills with differentiated sperm bundles (arrow shows the distal tip of the testis). (c) A 2 week old adult talin RNAi testis contains only differentiated sperm, the hub cells (c’, Cher in red) are not at the distal testis tip (arrow), and no GSCs, gonial cells or primary spermatocytes are seen (c’, Vasa, green). (d, e) A magnified, close-up view of the distal tip of a 2 week old tj-GAL4 testis (d) and a talin RNAi testis (e). (d) In a tj-GAL4/tj-GAL4 testis Vasa (green) stains the GSCs, gonial cells and primary spermatocytes, no differentiated sperm bundles are observed. (e) In a talin RNAi testis the distal testis tip contains only differentiated sperm bundles (arrow in e) that extend to the apex of the testis (apex marked with an asterisk in d and e). (d’, e’) All somatic cells express talin in a tj-Gal4 testis (d’) but talin staining is absent in a talin RNAi testis (e’), though the gonadal sheath is stained. (d”, e”) In a tj-GAL4/tj-GAL4 testis (d”) the hub is located at the apex but in a talin RNAi testis (e”) the hub is missing (Vasa, green; Cher, red; and Talin, blue). (f-g) In a 3-day-old tj-GAL4tj-GAL4 testis (f) the tip of the testis contains the GSCs and germline cells at early stages of differentiation (arrow) but these are missing in a 3 day old talin RNAi. (h) An example of a 3-day-old talin RNAi testis where the hub is not localized to the distal tip (asterisk). (f-h; Vasa, green; Cher, red). Scale bars in b and c: 100μm, in a, d-h 20 μm.
In summary, our results show integrins have a critical function in maintaining the position of the germline stem cell niche within fly testes. Multiple lines of evidence presented here suggest that the germline stem cell niche in Drosophila testis is anchored to the anterior of the gonad by adhering to the ECM. 1) An ECM assembles around the gonad late in embryonic development at a time when the hub cells become morphologically distinct. 2) The hub cells line up along the anterior periphery of the gonad and directly contact this ECM. 3) Integrins and their associated proteins, such as talin, are expressed in SGPs, including hub cells. 4) Integrins are necessary for the accumulation of ECM components around the gonad. 5) Integrin and talin are required for maintaining the hub cells at an anterior position. 6) Loss of integrin-mediated adhesion by depleting talin in adult testes results in loss of the hub cells. During gonad development cell sorting can explain the differential adherence of hub cells to one another. Our model is that the hub cells, derived from cells that form at8, and are restricted to9, the anterior of the gonad, subsequently sort from other somatic and germline cells, form a cohesive unit, and attach to the ECM adjacent to the anterior of the gonad. The function of the ECM attachment is in anchoring hub cells and preventing their sorting to the middle of the gonad. The mispositioning of the hub in integrin mutant embryos and the loss of the hub and GSCs in talin RNAi treated adult testes illustrates the severe consequences of a failure in this anchoring to the ECM.
Materials and methods
Fly stocks and Genetics
The following mutants and transgenic lines were used: rhea79a (ref. 14), mysXG43, cadN-cadN2Δ14 (this deficiency removes both cadN genes25), shg317, twist-GAL4, nanos-GAL4-VP16, UAS-DE-Cadherin (gift of Ulrich Tepass), UAS-DN-cadherin (gift of Tadashi Uemura), and 254-LacZ10. UAS-Talin RNAi lines were obtained from the VDRC26 (Transformant ID 40399 and 40400, Construct ID 12050).
Female germline clones lacking talin were generated using the FRT/FLP OvoD method27. Female flies of the genotype hs-FLP/+; FRT2A OvoD/FRT2A rhea79a were heat shocked and their female progeny crossed to rhea79a/TM3-GFP. To drive expression in the gonadal mesoderm twist-GAL4 (on 2nd chromosome) was used and nanos-GAL4-VP16 (on 3rd chromosome) was used for germ cell overexpression. Talin RNAi testes obtained by crossing tj-GAL4 (P{GawB}NP1624-5-124) the UAS-RNAi lines. Adult testes were dissected 14 days after eclosion.
Immunohistochemistry and Microscopy
The following antibodies were used: anti-βPS (CF6G1128, mouse mAb; 1:10), anti-αPS2 (7A1029, rat mAb; 1:10), anti-αPS3 (gift of R. Davis, rabbit pAB; 1:300), anti-Talin (E16B14, mouse mAb; 1:10), anti-Ndg (gift of S. Baumgartner, rabbit pAB; 1:200), anti-LanA (gift of S. Baumgartner; rabbit pAB; 1:500), anti-Cher (gift of Lynn Cooley; rat pAB N-fil & C-fil; 1:1000), anti-DN-cadherin (Developmental Studies Hybridoma Bank, DN-ex#8, rat mAb; 1:50), anti-DE-cadherin (Developmental Studies Hybridoma Bank, DCAD2, rat mAb; 1:50), anti-Vasa (gift of R. Lehmann; rabbit pAb; 1:5000), anti-βgal (Cappel; mouse Mab; 1:1000), anti-CNN (gift of Jordan Raff; rabbit mAb; 1:4000), anti-Phospho Histone H3 (Upstate Biotechnology; mouse mAb; 1:2000). Anti-Tj (G515; Guinea Pig pAb; 1:2500). Fluorescently conjugated Alexa Fluorophore 488, Cy3 and Cy5 secondary antibodies were used at 1:400 (Molecular Probes).
Antibody staining of adult testes and embryos was carried out according to standard procedures. For stage 17 embryos, when standard fixation methods cannot penetrate the cuticle for antibody stainings, we employed a heat fixation protocol. Briefly, embryos were dechorionated in 50% bleach for one minute, rinsed in water, immersed in boiling 1X E-wash buffer (100 mM NaCl, 0.1% Tween-20) for a few seconds, then immediately cooled by adding 3X volume of ice cold E-wash, and placed on ice. Embryos were then devitellenized in methanol/heptane. For anti-βPS staining embryos were fixed in standard 4% formaldyhyde/PBS solution and dechorinated in ethanol. For DE-cadherin staining Phosphate Buffer was used instead of PBS. For every gonad imaged a confocal Z-series extending through the entire gonad was collected. Confocal images were collected using a BioRad MRC Radiance 1024 system and were processed using Adobe Photoshop 8.0.
Supplementary Material
Acknowledgements
We would like to thank S. Baumgartner for LamininA and Nidogen antibodies, Lynn Cooley for Cher antibodies, Ruth Lehmann for the Vasa antibodies, and Jordan Raff for the CNN antibody. The Developmental Studies Hybridoma Bank for monoclonal antibodies. Barry Dickson for RNAi fly stocks, DGRC (Kyoto, Japan) for the Tj-Gal4 line, and Thomas Clandinin and Ulrich Tepass for cadherin fly stocks. Semil Choksi, Leanne Jones, and Ulrich Tepass for critical reading of the manuscript. This work was supported by a Natural Sciences and Engineering Research Council of Canada grant to DG, a Wellcome Trust grants to NHB (69943) and DD (72817), an HFSP Long Term Fellowship and a Development Travelling Fellowship to GT.
References
- 1.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 2.Xie T, Kawase E, Kirilly D, Wong MD. Intimate relationships with their neighbors: tales of stem cells in Drosophila reproductive systems. Dev Dyn. 2005;232:775–90. doi: 10.1002/dvdy.20317. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 4.Gilboa L, Lehmann R. How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development. 2004;131:4895–905. doi: 10.1242/dev.01373. [DOI] [PubMed] [Google Scholar]
- 5.Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–90. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–50. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 7.Starz-Gaiano M, Lehmann R. Moving towards the next generation. Mech Dev. 2001;105:5–18. doi: 10.1016/s0925-4773(01)00392-6. [DOI] [PubMed] [Google Scholar]
- 8.Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev Biol. 2006;294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 9.Kittadate Y, Shigenobu S, Arita K, Kobayashi S. Boss/Sev Signaling from Germline to Soma Restricts Germline-Stem-Cell-Niche Fromation in the Anterior Region of Drosophila Male Germline. Developmental cell. 2007;13:151–159. doi: 10.1016/j.devcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Gonczy P, Viswanathan S, DiNardo S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development. 1992;114:89–98. doi: 10.1242/dev.114.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–41. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 12.Brown NH, Gregory SL, Martin-Bermudo MD. Integrins as mediators of morphogenesis in Drosophila. Dev Biol. 2000;223:1–16. doi: 10.1006/dbio.2000.9711. [DOI] [PubMed] [Google Scholar]
- 13.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 14.Brown NH, et al. Talin is essential for integrin function in Drosophila. Dev Cell. 2002;3:569–79. doi: 10.1016/s1534-5807(02)00290-3. [DOI] [PubMed] [Google Scholar]
- 15.Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- 16.Devenport D, Brown NH. Morphogenesis in the absence of integrins: mutation of both Drosophila beta subunits prevents midgut migration. Development. 2004;131:5405–15. doi: 10.1242/dev.01427. [DOI] [PubMed] [Google Scholar]
- 17.Clark KA, McGrail M, Beckerle MC. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development. 2003;130:2611–21. doi: 10.1242/dev.00492. [DOI] [PubMed] [Google Scholar]
- 18.Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol. 2001;152:1007–18. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng WM, et al. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–84. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–8. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 21.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 22.Godt D, Tepass U. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature. 1998;395:387–91. doi: 10.1038/26493. [DOI] [PubMed] [Google Scholar]
- 23.Gonczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–47. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi S, et al. GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis. 2002;34:58–61. doi: 10.1002/gene.10137. [DOI] [PubMed] [Google Scholar]
- 25.Prakash S, Caldwell JC, Eberl DF, Clandinin TR. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. 2005;8:443–50. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–6. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 27.Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–9. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brower DL, Wilcox M, Piovant M, Smith RJ, Reger LA. Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc Natl Acad Sci U S A. 1984;81:7485–9. doi: 10.1073/pnas.81.23.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogaert T, Brown N, Wilcox M. The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell. 1987;51:929–40. doi: 10.1016/0092-8674(87)90580-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.