Abstract
The hippocampus has been implicated in many cognitive and emotional behaviors and in the physiology of the stress response. Within the hippocampus, the dentate gyrus has been implicated in the detection of novelty. The dentate is also a major target for stress hormones and modulates the hypothalamic-pituitary-adrenal (HPA) axis response to stress. Whether these functions of the dentate integrate or segregate remains unknown, as most investigations of its role in stress and learning are separate.
Since the exciting discovery of adult neurogenesis in the dentate gyrus, adult-born neurons have been implicated in both novelty detection and the stress response. In this perspective we will discuss the literature that implicates the hippocampus, and potentially, adult-born neurons in these two functions. We will attempt to reconcile the seemingly contradictory behavioral results for the function of adult-born neurons. Finally, we will speculate that a key function of adult-born neurons within hippocampal function may be to modulate the stress response and perhaps assign stress salience to the sensory context.
Keywords: Neurogenesis, Stress, Hippocampus, Learning, Antidepressants, Anxiety, Depression, Stem cells
While the generation of neurons from neural progenitors in the adult hippocampus is widely accepted, the functional importance of adult hippocampal neurogenesis remains hotly contested. Young neurons in the adult hippocampus have been implicated in some forms of complex behaviors like performance on anxiety tasks [1,2] and behavioral responses to antidepressants [3–7], as well as more basic underlying psychological constructs like pattern separation, novelty detection, and memory formation [8–12]. In contrast, other reports have demonstrated that ablation of neurogenesis in the adult brain did not result in behavioral phenotypes using the same tasks [2,4,7,13]. As the field continues to debate the function of adult neurogenesis, one early set of correlative findings persists as the most reproducible: the exquisite sensitivity of the hippocampal stem cell system to chronic stress and exercise with social enrichment. Adult hippocampal neurogenesis is potently inhibited by exposing animals to chronic stress and potently activated by exercise and enrichment [14].
In this perspective we propose that a key function of adult-born neurons within general hippocampal function is to modulate the stress response and perhaps assign stress salience to the sensory context. This speculation is based on (1) the rapidly accumulating often contradictory data on the importance of adult-born neurons for cognitive and emotional learning, (2) the widely accepted effect of stress on adult-born neurons, and (3) the emerging evidence for a role of adult-born neurons in the stress response.
The identification of neurogenesis in the adult brain, and in particular the hippocampus, spurred intense debate about the normal function of adult-born neurons. Early provocative findings that hippocampal neurogenesis is highly responsive to changes in the animal’s environment [15–17] fueled this debate [18]. Numerous studies over the past 15 years have together demonstrated that in the adult hippocampus, cellular proliferation, neuronal maturation, and survival are highly susceptible to changes in the environment [19]. Exposure to toys and exercise or certain learning tasks increases division of neuronal progenitors and the likelihood that they will differentiate into neurons [17,20]. Moreover, while many neuroblasts and maturing neurons die before integrating into hippocampal circuits, survival of immature neurons is increased by these interventions [21]. Finally, neurons that survive in animals exposed to learning tasks have more complex dendritic arbors and increased dendritic spine density [22]. Conversely, chronic stress reduces proliferation of progenitors, decreases survival of immature neurons and reduces their dendritic complexity [23]. Many reports document in detail the impact of stress on neurogenesis (see [24] for a recent review). Together, these results make a compelling argument that exposure to stressful and enriching environments have reciprocal effects on adult neurogenesis.
Although there is consensus that neurogenesis is a dynamic process, the functional significance of adult stem cells and the neurons that they produce for hippocampal circuitry remains hotly contested. Some of the debate has been fueled by seemingly conflicting results from loss of function studies utilizing ablation techniques to deplete neurogenesis in the rodent brain. The role of adult-born neurons in the formation and retrieval of memories, in expression of anxiety, and in the recognition of novelty has been tested and demonstrated using many different approaches. Remarkably, for most of the tasks tested, several groups found that ablating neurogenesis alone did not reveal a behavioral phenotype [7,25–28]. (A comprehensive synthesis of behavioral results from rodent models interfering with adult neurogenesis is compiled on the blog: http://www.functionalneurogenesis.com/blog/.) Two simple interpretations of such discordant results are either that the testing parameters are influencing whether new neurons are being recruited to solve the task, or that it may be difficult to disambiguate the effects of the different ablation techniques from the direct effects of newborn neurons. Another possibility is that the contribution of new neurons is not large enough to have reproducible consequences. In fact, one recent report demonstrated that changing the number of learning trials on the same task could dictate whether the task is sensitive to ablation of neurogenesis [29]. What then governs whether and how much these cells contribute to behaviors, and is there a common theme between the diverse learning and memory tasks that governs dependency on neurogenesis?
1. Effects of stress on the hippocampus
In order to get a better sense of what may engage new neurons for a specific hippocampal function, one must first consider what brain functions are mediated by the hippocampus and more specifically by the dentate gyrus. Despite more than a half-century of intensive research, the function of the hippocampus remains a matter of active investigation. The hippocampus has alternatively been seen as an important regulator of emotion [30], a regulator of the stress response [31,32], the substrate for encoding of episodic memories [33], and a structure specialized for encoding spatial information [34,35]. Within these diverse frameworks of hippocampal function, there are a number of concepts that are concrete. One of these is that the hippocampus is a highly plastic structure, and that this plasticity is critical for optimal performance [23]. It is also clear that the hippocampus, with its extremely high levels of glucocorticoid receptors, is particularly susceptible to stress-induced damage, which in turn diminishes its plasticity, and impairs its function [36].
The effects of chronic stress on the hippocampus at the cytoarchitectural level are well documented in both rodent and primate models. In the CA1 area of the hippocampus, chronic stress results in loss of complexity and decreased length of apical dendritic branches. Similar changes are seen in the CA3 area of the hippocampus [37,38]. Within the dentate gyrus, chronic stress also results in a decrease in the levels of adult hippocampal neurogenesis [14]. Interestingly, these changes in animal models appear to be reversible upon elimination of the source of the stress. Moreover, chronic treatment with agents efficacious in treating depression in humans can reverse these effects of chronic stress in rodents [14]. Evidence of hippocampal atrophy after chronic stress also exists in humans. In particular, there is evidence for hippocampal atrophy in stress-related pathological conditions such as depression and post-traumatic stress disorder (PTSD). Whether treatment for these disorders reverses the stress-related damage remains to be seen.
In addition to effects on hippocampal cytoarchitecture, chronic stress disrupts physiological plasticity. Specifically, long-term potentiation (LTP) in the hippocampus is impaired and long-term depression (LTD) is enhanced under conditions of high stress or elevated levels of glucocorticoid levels in dorsal and intermediate levels of the hippocampus. Interestingly, in the ventral pole of the hippocampus, a region thought to mediate negative feedback to the HPA axis, this LTP/LTD relationship in response to stress is reversed [39].
The effects of stress on the function of the hippocampus can also be appreciated at the behavioral level. For example, exposure to either explicit stressors, or to artificially elevated glucocorticoid levels disrupts hippocampal dependent spatial memory performance in rodents [40]. Impaired declarative memory performance in humans under conditions of high glucocorticoids has also been demonstrated [41]. Thus, there is consistent evidence across multiple models and multiple species that suggest a deleterious effect of stress on the functioning of the hippocampus.
2. Stress impacts dentate gyrus function
Within the hippocampus, the dentate gyrus and specifically young, adult-born neurons are highly susceptible to the effects of stress and particularly well poised for regulating the stress response. First, the dentate gyrus has the highest concentration of receptors for adrenal stress hormones in the brain. The two major steroid hormone receptors: the low affinity glucocorticoid (GR) and the high affinity mineralocorticoid (MR) receptors are densely expressed by granule cell neurons [23]. Second, a compelling experiment demonstrated that removal of the adrenal glands results in complete selective degeneration of the adult dentate gyrus [42]. Remarkably, adrenalectomy did not have a similar effect on either non-granule cell neurons of the hippocampus, or non-hippocampal granule cell neurons. Further research demonstrated that MR is the obligate receptor for dentate granule cells in vivo [43], and that steroid dependence can be reversed through modulation of neurotrophins or excitatory transmission [44]. While MR occupancy is necessary for granule cell survival in adult rats, GR stimulation has this effect in neonates, indicating that MR and GR can differentially provide survival signals depending on the age of the animal. Hence, within the hippocampus, dentate gyrus granule cells are specifically dependent on adrenal stress hormone signaling for their survival.
While some stimulation of adrenal steroid receptors is necessary for granule cell survival, exposure to chronic stress results in changes in neuronal structure and likely deficient function of the DG. Decreased dentate volume without major cell loss after chronic stress suggests atrophy of the dentate neuropil. In fact, synaptic field potentials in the dentate are decreased following exposure to chronic stress [45]. Moreover, the well-characterized morphological sequelae of chronic stress in CA3 are likely governed by the mossy fiber outputs of the dentate granule neurons [46]. Since MRs are occupied by baseline physiological levels of circulating glucocorticoids, atrophy of the neuropil is likely to be mediated by GR [23,47] indicating that exposure to chronic stress decreases DG volume; alters DG plasticity; and produces atrophy in neurons receiving inputs from the DG.
3. Dentate gyrus participates in hippocampal regulation of the stress response
In addition to being highly susceptible to changes in adrenal function, the role of the hippocampus in regulating the response to stress has been appreciated for close to 40 years [48]. Direct stimulation of the hippocampus can depress corticosterone levels in rodents [49]. Moreover, humans with bilateral hippocampal lesions exhibit a blunted cortisol response to psychosocial stress [50]. Hippocampal, and ventral subiculum lesions in rodents result in increased corticosterone levels after stress without changing baseline levels or diurnal variation [51–54]. Remarkably, these lesions affected HPA responses to some, but not other stressors. Amongst the stressors tested, exposure to novelty had a particularly pronounced hippocampal dependence of adrenal responses, as lesioned animals exhibited a tripling in plasma corticosterone levels [52]. This increase was sustained twice as long as increases produced by other stressors. Moreover, the lesioned animals exhibited a dramatic decrease in exploration. Novelty detection through pattern separation and pattern completion has been intimately tied to normal DG function in rodents and humans [55–58] and emerging evidence suggests a specific role for immature adult-born neurons in rodents [8,12]. Strikingly, selective lesions of the dentate gyrus resulted in decreased corticosterone responses to novelty and obliterated habituation that is commonly seen with repeated exposure [59]. Based on differences in the directionality of steroid responses to stress between human hippocampus, rodent subiculum, and rodent DG data, the physiology of how the hippocampus governs the HPA response remains to be worked out. However, the data are consistent in implicating the hippocampus in regulating HPA axis function and establishing a role for the DG in contributing to the HPA axis response to stress.
4. Adult-born neurons are affected by stress and control the stress response
The ability of stress to suppress neurogenesis was already discussed. It is still unclear whether stress acts directly on stem cells and/or adult-born neurons or indirectly through secreted factors or changes in network activity to this end. One way to explore the mechanism by which stress could impact adult-born neurons is to examine GR and MR expression patterns on stem cells and the neurons that they produce. While MR expression is restricted to the hippocampus, it is absent from radial astocytes (neural stem cells), intermediate progenitors, and immature neurons [60,61]. GR is expressed by neural stem cells, intermediate progenitors, and mature neurons, but is absent from immature neurons. Therefore, neural stem cells and progenitors can potentially serve as targets for stress hormones through activation of cell autonomous GRs, while mature neurons are putative targets via both GR and MR. Importantly, deletion of GR from the brain did not decrease cellular proliferation in the adult hippocampus [62] suggesting that the effects of glucocorticoids on stem cell proliferation and on the morphology of immature neurons is indirect.
In addition to being affected by stress hormones, some intriguing new data suggest that newly generated adult-born hippocampal neurons regulate the HPA axis. Initial experiments suggested that X-irradiation of the hippocampus to ablate neurogenesis did not interfere with the normal stress-induced corticosterone response [7]. However, more specific suppression of adult neurogenesis using inducible tissue-specific mice results in increased corticosteroid secretion in response to open field stress, suggesting dysregulation of negative feedback in the absence of newly generated neurons [63]. Moreover, partial depletion of immature neurons inhibits the susceptibility of animals to the behavioral sequelae of social stressors [64], while complete ablation impairs recovery from a social stressor when placed in an enriched environment [65]. These seemingly paradoxical results may highlight the difference between the physiology of stress response and stress recovery or may reflect a technical difference in approaches used to suppress neurogenesis. Nevertheless, the two findings together highlight the importance of the stress state of the animal when probing the function of adult-born neurons, since adult-born neurons appear to be unnecessary for behavioral effects of environmental enrichment in animals that are stress-naive [66].
This later observation that neurogenesis is important for the behavioral effects of stress and for stress recovery is especially intriguing given the possibility that MR and GR may have separate roles in the stress response [47] and differential expression on young neurons [60,61]. The initiation of the stress response is thought to have rapid onset of action that engages the sympathetic nervous system. While both GR and MR are nuclear receptors, in the dentate MRs were recently implicated in miniature excitatory post-synaptic currents, which are thought to be rapid and transcription/translation-independent [67]. In contrast, activation of GRs is thought to increase calcium influx over time, thereby contributing to parasympathetic activation and stress adaptation [68]. Given that GR was hypothesized to mediate stress recovery and the slow onset effects of stress exposure, the ability of immature adult-born neurons to impact stress recovery and the slowly developing sequelae of stress may be mediated by GR. Moreover, emergence of GR expression prior to MR during maturation of adult-born neurons may hint that immature neurons serve as autonomous sensors for the hippocampal feedback on the HPA axis. Therefore, adult-born neurons are perfectly positioned to both sense the stress state of the animal and adjust the stress response accordingly. In humans, both GR and MR polymorphisms have been associated with behavioral changes in stress responses and mental illness [69,70]. Given the previously established role for adult-generated neurons in mediating some aspects of response to antidepressant drugs, the possible links to mediating feedback to the HPA axis remains an intriguing new avenue of investigation [71].
5. Adult-born neurons as stress integrators
Above we speculate that DG may serve to integrate novelty detection with regulation of the HPA. This was primarily based on data measuring glucocorticoid stress responses in which the stressors that elicit the largest response involve exposure to novelty [59]. The dentate is also anatomically well-positioned to integrate stress responses with novelty detection due to its reciprocal connections with emotional brain centers and its extensive collateral connectivity [72]. Within the DG, new neurons may play an important role in this type of integration since HPA responses to novelty stress are increased when new neurons are absent [63]. Moreover, immature neurons were recently implicated in the detection of novelty as measured by pattern separation. In a compelling study Clelland et al. [8] demonstrated that mice made more errors while distinguishing similar contexts in a navigation task when neurogenesis was ablated. There was no difference between the groups when the contexts were markedly different. Importantly, two different forms of ablation (genetic and focused X-rays) were used. More-over, the result was repeated in an unrelated task that did not rely on navigation adding construct validity to the finding. Conversely, a recent study demonstrated that genetically increasing the numbers of adult-born neurons was sufficient to enhance the animals’ ability to distinguish similar contexts [73]. Thus in addition to being affected by stress, adult-born neurons appear to be involved in pattern separation (i.e. novelty detection), and in providing feedback about the stress of novelty to the HPA axis. In summary, the dentate gyrus is used by the brain to assist in distinguishing familiar aspects within the environment; is highly susceptible to stress; and exerts control over the stress response. Given the ability of young adult-born neurons to carry out all of these functions, the young cells are strong candidates to assign stress salience to novelty during learning.
The ability of young neurons to assign stress salience to novel cues may help to reconcile some of the discrepant findings from studies examining their behavioral significance. Assigning a stress value to a contextual cue can alter the strength of the representation of that cue. An analogous type of valuation system is well-characterized in the amygdala where assigning an emotional value to a sensory cue dramatically increases the strength of a memory trace [74]. The mechanisms underlying this phenomenon in the amygdala have been characterized in both rodents and humans [74]. If the strength of the hippocampal representation for novelty depends on the stress state of the animal, then a similar test performed under more or less stressful conditions would be expected to have discrepant behavioral consequences (Fig.1). If the role of young neurons was to assign stress valuation then these cells would become specifically engaged under only certain stress conditions. Moreover, their absence would only lead to a measurable difference in behavioral tasks in accordance with the appropriate stress state of the animal. Having neurogenesis highly regulated by stressful and enriching experiences over prolonged periods of time would then enable the brain to incorporate the stress state of the animal into stable representations made by the dentate gyrus.
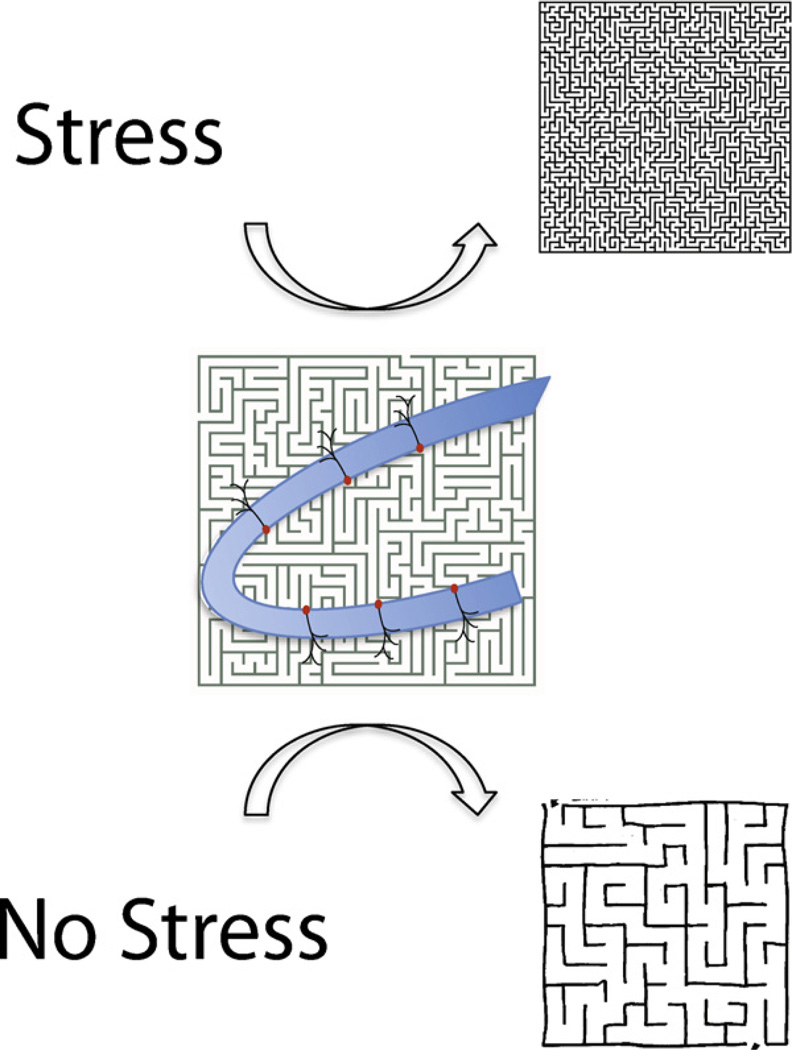
Fig. 1.
Cartoon depicting the contribution of young hippocampal neurons to two distinct representations of a single object, when acquired in the presence or absence of stress. We propose that, in addition to participating in the formation of hippocampal representations, recruitment of young neurons contributes information about the internal stress state of the animal. Thus the same object would be represented differently if encountered by an animal under distinct states of stress. In this illustration, newborn neurons contribute to the perception of the maze as either a simple (in the absence of stress) or a complex (in the presence of stress) object.
Acknowledgements
The authors thank Ben Samuels and Rebecca Shansky for helpful suggestions. This work was supported by K08MH079088 and R01MH091844 (AD) and K08MH076083 and R01MH091427 (EDL).
References
- 1.Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci USA. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 3.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 4.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 8.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, et al. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci USA. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2010 doi: 10.1002/hipo.20895. Epub 2010 Nov 3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 14.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 16.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 19.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 21.Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2006;103:780–785. doi: 10.1073/pnas.0510291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci USA. 2010;107:7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 26.Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–743. [Google Scholar]
- 31.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 32.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Eichenbaum H. Conscious awareness, memory and the hippocampus. Nat Neurosci. 1999;2:775–776. doi: 10.1038/12137. [DOI] [PubMed] [Google Scholar]
- 34.O’Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- 35.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space. Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 36.McEwen BS. Corticosteroids and hippocampal plasticity. Ann N Y Acad Sci. 1994;746:134–142. doi: 10.1111/j.1749-6632.1994.tb39223.x. [discussion 42–4, 78–9]. [DOI] [PubMed] [Google Scholar]
- 37.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 39.Maggio N, Segal M. Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus. J Neurosci. 2007;27:5757–5765. doi: 10.1523/JNEUROSCI.0155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Sollas AL, Paul LA, et al. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- 43.Woolley CS, Gould E, Sakai RR, Spencer RL, McEwen BS. Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res. 1991;554:312–315. doi: 10.1016/0006-8993(91)90207-c. [DOI] [PubMed] [Google Scholar]
- 44.Gould E, Tanapat P, Cameron HA. Adrenal steroids suppress granule cell death in the developing dentate gyrus through an NMDA receptor-dependent mechanism. Brain Res Dev Brain Res. 1997;103:91–93. doi: 10.1016/s0165-3806(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 45.Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 46.McEwen BS, Gould E. Adrenal steroid influences on the survival of hippocampal neurons. Biochem Pharmacol. 1990;40:2393–2402. doi: 10.1016/0006-2952(90)90079-z. [DOI] [PubMed] [Google Scholar]
- 47.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 48.Casady RL, Taylor AN. Effect of electrical stimulation of the hippocampus upon corticosteroid levels in the freely-behaving, non-stressed rat. Neuroendocrinology. 1976;20:68–78. doi: 10.1159/000122470. [DOI] [PubMed] [Google Scholar]
- 49.Dunn JD, Orr SE. Differential plasma corticosterone responses to hippocampal stimulation. Exp Brain Res. 1984;54:1–6. doi: 10.1007/BF00235813. [DOI] [PubMed] [Google Scholar]
- 50.Buchanan TW, Tranel D, Kirschbaum C. Hippocampal damage abolishes the cortisol response to psychosocial stress in humans. Horm Behav. 2009;56:44–50. doi: 10.1016/j.yhbeh.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kant GJ, Meyerhoff JL, Jarrard LE. Biochemical indices of reactivity and habituation in rats with hippocampal lesions. Pharmacol Biochem Behav. 1984;20:793–797. doi: 10.1016/0091-3057(84)90201-6. [DOI] [PubMed] [Google Scholar]
- 52.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary- adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 53.Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci USA. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 55.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- 57.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 58.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 59.Johnson LL, Moberg GP. Adrenocortical response to novelty stress in rats with dentate gyrus lesions. Neuroendocrinology. 1980;30:187–192. doi: 10.1159/000122998. [DOI] [PubMed] [Google Scholar]
- 60.Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- 61.Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004;3:363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 62.Gass P, Kretz O, Wolfer DP, Berger S, Tronche F, Reichardt HM, et al. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep. 2000;1:447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 67.Pasricha N, Joels M, Karst H. Rapid effects of corticosterone in the mouse dentate gyrus via a nongenomic pathway. J Neuroendocrinol. 2011;23:143–147. doi: 10.1111/j.1365-2826.2010.02091.x. [DOI] [PubMed] [Google Scholar]
- 68.Hesen W, Karst H, Meijer O, Cole TJ, Schmid W, de Kloet ER, et al. Hippocampal cell responses in mice with a targeted glucocorticoid receptor gene disruption. J Neurosci. 1996;16:6766–6774. doi: 10.1523/JNEUROSCI.16-21-06766.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeRijk RH, Wust S, Meijer OC, Zennaro MC, Federenko IS, Hellhammer DH, et al. A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. J Clin Endocrinol Metab. 2006;91:5083–5089. doi: 10.1210/jc.2006-0915. [DOI] [PubMed] [Google Scholar]
- 70.van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004;59:333–357. doi: 10.1210/rp.59.1.333. [DOI] [PubMed] [Google Scholar]
- 71.Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neuron to improve stress response regulation. Mol Psychiatry. doi: 10.1038/mp.2011.48. Epub 2011 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011 doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McGaugh JL. Make mild moments memorable: add a little arousal. Trends Cogn Sci. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]