Abstract
The Tsimane of lowland Bolivia are an indigenous forager-farmer population living under conditions resembling pre-industrial European populations, with high infectious morbidity, high infection and inflammation, and shortened life expectancy. Analysis of 917 persons ages 5 to 60+ showed that allele frequencies of 9 SNPs examined in the apolipoprotein E (apoE), C-reactive protein (CRP), and interleukin-6 (IL-6) genes differed from some European, African, and north Asian-derived populations. The apoE2 allele was absent, whereas four SNPs related to CRP and IL-6 were monomorphic: CRP (rs1800947, rs3093061, and rs3093062) and IL-6 (rs1800795). No significant differences in apoE, CRP, and IL-6 variants across age were found CRP levels were higher in carriers of two CRP proinflammatory SNPs, whereas they were lower in carriers of apoE4. Taken together, the evidence for (1) different allele frequencies between the Tsimane and other populations and (2) the correlations of CRP and apoE alleles with blood CRP may suggest that these variants are under selection in response to a high infection environment.
Introduction
This study analyzes the distribution of genetic diversity in a set of single nucleotide polymorphisms (SNPs) in the Tsimane of Bolivia, a population of hunter-forager-farmers with a high infectious burden and shortened lifespan (Gurven, Kaplan, and Supa 2007; Gurven, Kaplan, Winking, Finch, and Crimmins 2008; McDade et al. 2005; Vasunilashorn et al. 2010). Genetic variation within and between populations may be associated with a variety of factors (Drenos and Kirkwood 2010; Finch and Stanford 2004), including natural selection in resistance to pathogens (Pennington et al. 2009). A classic example is malaria, in which individuals who are heterozygous for the β-globin sickle-cell allele are more resistant to malaria and thus have a survival advantage (Flint, Harding, Boyce, and Clegg 1998; López et al. 2010; Wellems, Kayton, and Fairhurst 2009). Other chronic infections may influence the frequencies of genetic variants at different ages within a population through selective advantage from innate immune responses (Drenos, Westendorp, and Kirkwood 2006; Harpending and Cochran 2006; Kuningas et al. 2009). The advent of high throughput DNA technology, along with the growing characterization of genes, has made it possible to determine genetic dispositions to disease (Collins, Guyer, and Charkravarti 1997; Hardy and Singleton 2009) and to determine the associations between genetic variation and human traits (Goldstein 2009; Huang et al. 2007; Khor et al. 2010; Yashin et al. 2000). The present study applies this technology to three gene-encoding blood proteins that were chosen because of their influence on inflammatory responses and lifespan including apolipoprotein E (apoE), C-reactive protein (CRP), and interleukin-6 (IL-6).
Significance
The population genetics of indigenous South American populations has received less attention than the genetics of Eurasian and African populations. The Amazonian Tsimane are of particular interest because of detailed information on their demographics, infectious load, and high level of inflammation. Until recently, the Tsimane have had little access to modern medicine, which has led to low levels of life expectancy. Thus, it may be possible to define signatures of natural selection in gene variants with functional influences across life history. With respect to our results, the different SNP frequencies observed between the Tsimane and other populations as well as the correlations of CRP and apoE alleles with blood CRP levels suggest that these genes (and other inflammatory proteins) may be under genetic selection in response to a highly infectious environment. By addressing questions that are relevant to genetics research in this indigenous population, our study may have broad and important implications for the field of social sciences. Such a study contributes to our understanding of the record of human history and more broadly is relevant to a wider range of disciplines as it deals with the amount of variation in health and aging that is a result of social or selective environmental pressures.
Background
Genetic Markers Associated with Infection and Inflammation
This study analyzes allele frequencies of selected immune response genes in the Tsimane of Bolivia, a population exposed to a very high infectious burden with elevations of serum CRP levels, parasite infection, and white blood cell count (Gurven et al. 2007 2008; McDade et al. 2005; Vasunilashorn et al. 2010). In this study, we examine variants of genes encoding apoE, CRP, and IL-6. Differences in allele frequencies among populations may provide some insight as to the interaction between genes and the environment and the relevance of natural selection in shaping variation in immune-related genes both within and among populations. We also examined genotype frequencies across various age ranges to evaluate possible genetically related survival advantages to living under conditions of infectious environment. Some of the genes that enhance survival from infection by increased inflammatory responses may also promote aging among survivors later in life through their effects on inflammatory mechanisms in chronic conditions of aging (Franceschi et al. 2000, 2005; Finch 2007). Finally, we investigate the relationship between serum markers of infection and inflammation on proinflammatory genotypes among the Tsimane. We hypothesize that there will be a positive association between proinflammatory genotypes and blood markers of inflammation.
C-reactive Protein
CRP, an acute-phase response protein, is a marker of systemic inflammation, and its expression is influenced by genetic factors, infection, trauma, and the presence of chronic conditions (Ridker et al. 2008, 2009). Heritability estimates for serum CRP levels in various populations range from 27 to 40 per cent (Dupuis et al. 2005; Pankow et al. 2001), suggesting the influence of genetic factors. In agreement with this notion, several SNPs and haplotypes at the CRP locus have been associated with blood CRP levels (Crawford et al. 2006; Eirksdottir et al. 2009; Kathiresan et al. 2006; Ridker et al. 2008; Szalai et al. 2005; Teng et al. 2009) including those that are located in introns (Szalai 2002), exons (Zee and Ridker 2002), the upstream promoter (Brull et al. 2003; Kovacs et al. 2005), and the 3′-untranslated regions of the mRNA (Brull et al. 2003). Genetic markers related to CRP production have also been associated with survival from infection as well as with serum levels of other inflammatory markers related to the immune response (Balding et al. 2003; Slattery et al. 2007; Walston et al. 2005).
Interleukin-6
IL-6 is another protein involved in the acute-phase inflammatory response that has broad metabolic roles in normal metabolism and is controlled by genetic variation. For example, a G>C substitution (rs1800795) at position –174 of the IL-6 promoter has been associated with resistance to infection and enhanced survival during bacterial meningitis (Balding et al. 2003). Importantly, this variant has also been associated with serum IL-6 levels (de Craen et al. 2005; Slattery et al. 2007; Walston et al. 2005).
Apolipoprotein E
ApoE has three common alleles with the order of prevalence being apoE3 > apoE4 > apoE2 in most populations. The apoE4 allele is a common genetic risk factor for cardiovascular disease and Alzheimer’s disease (Corder et al. 1996; Eichner et al. 2002; Huang 2006; Lahoz et al. 2001; Rosvall et al. 2009; Wilson et al. 1996) and reduces life expectancy by about 5 years in European-derived populations (Ewbank 2004, 2007; Schachter et al. 1994; Smith 2002). However, apoE4 may also influence infectious conditions, as indicated by other reports. For example, in hepatitis C virus infections, apoE4 carriers developed less severe liver fibrosis (Fabris et al. 2005; Wozniak et al. 2002). In addition, children in a Brazilian favela with at least one apoE4 allele had less Giardia and fewer bouts of diarrhea than those with other apoE alleles (Oriá et al. 2010).
The apoE4 allele influences inflammatory responses and, relative to apoE3, is proinflammatory in some conditions. Among humans after surgery, apoE4 carriers exhibited higher levels of tumor necrosis factor (TNF)-α compared to apoE3/E3 patients (Drabe et al. 2001; Grunenfelder et al. 2004; Olgiati et al. 2010). A mouse model supports these allele effects. For example, brain monocytes (microglia) from transgenic mice expressing apoE4 secreted more TNFα, IL-6, and nitric oxide than mice with the apoE3 allele (Vitek, Brown, and Colton 2009).
The apoE allele frequencies vary widely between human populations of Europe, Africa, Australia, and North America (Corbo and Seacchi 1999; Finch 2007; Singh, Singh, and Mastana 2006). ApoE2 is the least prevalent (Sakuma et al. 1995) whereas apoE3 is the most prevalent, with >50% frequency in all human populations studied. ApoE4 shows more variation with prevalence varying from 49 per cent among the Huli of New Guinea to absent among the Ache in Paraguay (Demarchi et al. 2005). Geographic distributions indicate that Northern Europe has a greater apoE4 prevalence than Mediterranean regions (Corbo and Scacchi 1999; Demarchi et al. 2005; Gerdes, Klausen, Sihm, and Faergeman 1992; Panza et al. 2003). Less economically developed and traditional pre-industrial societies also vary widely in apoE alleles: apoE2 is absent in the East Greenland Inuit (Gerdes et al. 1996); Mayans of Mexico (Kamboh, Weiss, and Ferrell 1991); Yanomamo of Brazil (Crews, Kamboh, Mancilha-Carvalho, and Kottke 1993); Australian aborigines (Kamboh, Serjeantson, and Ferrell 1991), Hichol Indians from western Mexico (Aceves et al. 2006); and Amerindians from nine different tribes in Central and South America (Asakawa, Takahashi, and Rosenblum 1985). Conversely, apoE4 is relatively frequent in the East Greenland Inuit (Gerdes et al. 1996); Saami of Finland (Lehtinen et al. 1994); Nigerians (Sepehrnia et al. 1988; Kamboh et al. 1989; Sandholzer, Delport, Vermaal, and Utermann 1995); and aborigines from Australia (Kamboh, Serjeantson, and Ferrell 1991a), Papua New Guinea (Kamboh, Bahtia, and Ferrell 1990), and Malaysia (Gajra et al. 1994). These wide distributions in allele frequencies suggest the influence of founder effects and/or potential selective influences on survival via proinflammatory responses in environments with variable pathogenicity.
Mortality and Infection in the Tsimane
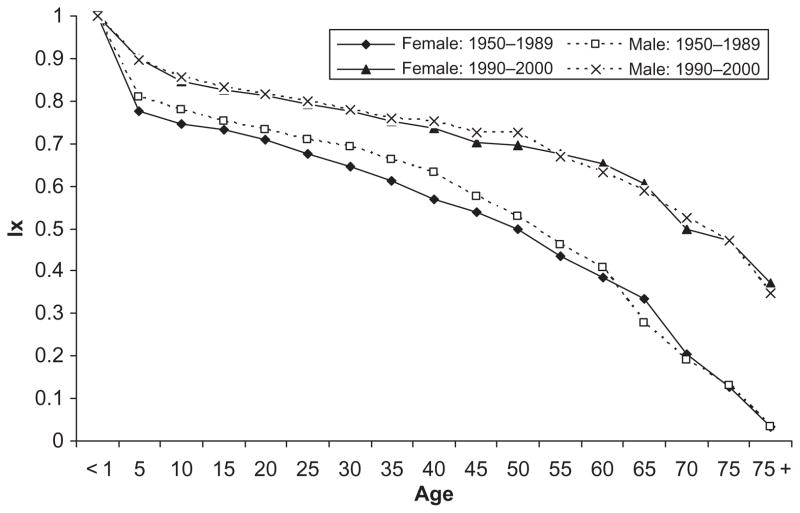
Our analysis focuses on the Tsimane, who live in a highly infectious environment. These indigenous forager-farmers in lowland Amazonian Bolivia live under conditions similar to pre-industrial European populations with high infectious morbidity, limited diets, and short life expectancy (Gurven et al. 2007 2008; McDade et al. 2005). Extensive demographic data document the level of mortality in two periods: 1950–1989 and 1990–2000 (Gurven et al. 2007). Life tables indicate short life expectancy at birth relative to the standards of contemporary developed countries: 44.2 years for males and 42.8 years for females before 1990 and 54.3 for males and 54.0 for females during the 1990–2000 period. Survival curves from life tables for these two periods (Figure 1) show the marked recent improvements in survival. For example, between 1950 and 1989, 20 per cent of the Tsimane (21 per cent of females and 19 per cent of males) died before their fifth birthday; this high early mortality decreased by about 25 per cent during the next decade, 1990–2000. By age 35 in the earlier period (the midpoint of the 10- to 59-year range), the proportion dying was about 37 per cent (39 per cent for women and 34 per cent for men). From 1990 to 2000, this proportion decreased to 25 per cent. During the period 1950 to 1989, 60 per cent of the cohort died before age 60 (61 per cent for females; 59 per cent for males), which was reduced to 40 per cent during the later period. Despite these improvements, the major cause of death at all ages is infectious disease. Among children, respiratory and gastrointestinal conditions are the primary causes of death but, among people beyond age 60, deaths are due to respiratory conditions, including tuberculosis (Gurven et al. 2007). A higher life expectancy for males than females has been observed in other populations with low life expectancy, including Afghanistan (males [M] 46.62, females [F] 45.10 years), Bangladesh (M 60.40, F 59.91), Niger (M 41.43, F 41.11), and Zimbabwe (M 39.18, 36.34) in 2000. In these societies, not only is life expectancy relatively low but fertility is still high, and maternal mortality is an important cause of the low life expectancy of women.
Figure 1.
Probability of survival (lx) by age in 1950–1989 and 1990–2000 among the Tsimane.
The Tsimane are exposed to high levels of inflammation, infection, and parasitic load (Gurven et al. 2008; McDade et al. 2007; Tanner et al. 2009; Vasunilashorn et al. 2010). About 40 per cent have elevated levels of CRP (≥ 3 mg/l), and 60 per cent had high IL-6 (≥2.68 pg/ml). The total white blood cell count averaged 10,344 cells/mm3, which is in the upper normal range of clinical samples from the United States (Table 1). In contrast, mean white blood cell count for individuals in the United States without coronary heart disease is 7,500 cells/mm3 (Friedman et al. 1974). At any given time, the majority (nearly 75 per cent) of subjects had at least one parasite; two or more parasites were carried by 40 per cent of individuals sampled in 2004, and the average number of parasite species per person was 1.3 (Vasunilashorn et al. 2010). The most prevalent parasites include a hookworm (46 per cent), protozoa (Entamoeba coli, 22 per cent, and Iodamoeba butschilii, 17 per cent), and roundworm (Ascaris lumbricoides, 17 per cent). During medical exams, more than 60 per cent had symptoms of respiratory or gastrointestinal illness (Gurven et al. 2008). Malaria and HIV have not been reported among the Tsimane through 2010.
Table 1.
Clinical characteristics of the Tsimane population
| No. | Mean (SD) or % | Range | |
|---|---|---|---|
| Age | 917 | 35.8 (19.6) | 5 – 90 |
| Males (%) | 917 | 49.2 | |
| Anthropometric measures | |||
| Height (cm) | 854 | 149.9 (28.7) | 81.3 – 178.2 |
| Males | 152.6 (17.9) | ||
| Females | 147.3 (35.8) | ||
| Body mass index (BMI, kg/m2) | 852 | 22.2 (4.0) | 0.7 – 38.1 |
| Underweight (BMI <18.5) | 19.8 | ||
| Overweight (BMI ≥ 25) | 21.1 | ||
| Obese (BMI ≥ 30) | 3.1 | ||
| Markers of inflammation and infection | |||
| C-reactive protein (CRP, mg/l) | 635 | 7.4 (16.2) | 0.15 – 150.0 |
| <3 (%) | 58.9 | ||
| 3.0–9.99 (%) | 26.6 | ||
| ≥10.00 (%) | 14.5 | ||
| Interleukin-6 (IL6, pg/ml) | 292 | 5.3 (7.9) | 2.0 – 57.4 |
| <2.68 (%) | 40.4 | ||
| ≥2.68 (%) | 59.6 | ||
| White blood cell count | 829 | 10344 | 4, 400 – 21, 500 |
| Erythrocyte sedimentation rate | 831 | 34.7 (19.9) | 2 – 115 |
Note: The majority of the % underweight are children age ≤ 11.
To investigate the relationship between living in a high infection environment and allele and genotype frequencies associated with inflammation, we used blood samples collected from the Tsimane. As blood was not collected from children younger than age 5, the present study examined the distribution of the genetic markers in three age groups: 5 to 9, 10 to 59, and 60+. We assume that the children currently between the ages of 5 and 9 have lived under the more recent mortality levels and that about 90 per cent of the original birth cohort has survived. At the older ages, the proportion of subjects alive most likely reflects a combination of the mortality schedules from the earlier and the later periods. We estimate that about 30 per cent of the original cohort, who would now be 35-year-olds, have died and that about 55 per cent of those who were in the original cohort of those 60 years of age or older are deceased.
In the present study, we first compared the allelic frequencies of loci associated with inflammation and survival in the Tsimane to other populations to provide insight as to the relationship between genetic variation and environment. Second, we examined genotype frequencies with age to evaluate possible survival advantages under conditions including significant infectious exposure. Last, we investigated the relationship between genotype and serum levels of CRP.
Methods
Clinical Measurements
Blood was separated and spun in the field and frozen in liquid nitrogen for transport to the University of New Mexico where it was assayed for serum CRP levels. Frozen lymphocytes were sent to the University of Southern California for DNA extraction and genotyping. Serum high sensitivity-CRP and IL-6 were measured using assays with detectable ranges of 0.1 to 150.0 mg/L and 2.0 to 1,000.0 pg/mL, respectively, at the Tricore Reference Laboratories in Albuquerque using Immulite 2000 kits. The mean replicate interassay coefficient of variation was 5.6 per cent for hs-CRP and 5.8 per cent for IL-6 (Diagnostics Products Corporation, Siemens, Deerfield, IL, USA). White blood cell count was analyzed in the field using fresh samples.
Molecular Analysis
Single nucleotide polymorphism (SNP) genotyping was performed using the Applied Biosystems TaqMan system (Livak 2003). Briefly, for each SNP, a polymerase chain reaction containing 2 ng of genomic DNA, amplification primers, and two 20–30-bp oligonucleotides encompassing the polymorphic site were carried out according to manufacturer’s protocols. Genotyping assays were selected through Applied Biosystems “Assays on Demand” database (http://myscience.appliedbiosystems.com/%20navigation/http://myscience.appliedbiosystems.com/navigation/mysciapplications.jsp) or custom-designed using the “Assays by Design” service. Primer and probe sequences can be provided upon request.
Genetic loci examined in this study were chosen based on previously reported associations with blood levels of CRP or IL-6 (Table 2). These included 7 SNPs in the CRP gene (Carlson et al. 2005) and a promoter SNP in IL-6. Determination of the apolipoprotein E2/E3/E4 alleles used genotypes derived from two SNPs, rs429358 and rs7412, as reported previously (Nyholt, Yu, and Visscher 2009).
Table 2.
Proinflammatory genotypes of apolipoprotein E (apoE), C-reactive protein (CRP), and interleukin-6 (IL-6) based on previous studies
| dbSNP Number | Associated with | Proinflammatory or At-risk Genotype (Reference) | Proinflammatory Genotype in Tsimane? |
|---|---|---|---|
| rs429358/rs7412 | apoE | E4/E4 | Yes, E3/E4 alleles present |
| rs405509 | apoE | GG (Bizarro et al. 2009) | Yes, G/T alleles present |
| rs1205 | CRP | GG (Crawford et al. 2006; Eiriksdottir et al. 2009; Lange et al. 2006; Teng et al. 2009) | Yes, G/A alleles present |
| rs1417938 | CRP | TT (Crawford et al. 2006; Lange et al. 2006) | Yes, A/T alleles present |
| rs1800947 | CRP | GG (Crawford et al. 2006; Lange et al. 2006; Rizzello et al. 2007; Teng et al. 2009) | Yes, only G allele present |
| rs3093061 | CRP | AA (Crawford et al. 2006) | Yes, only A allele present |
| rs3093062 | CRP | GG (Szalai et al. 2005) | Yes, only G allele present |
| rs2808630 | CRP | AA (Crawford et al. 2006) | Yes A/G alleles present |
| rs3091244 | CRP | AA (Kathiresan et al 2006; Ridker et al. 2008; Teng et al. 2009) | Yes, A/C/T alleles present |
| rs1800795 | IL-6 | GG (Bamouldi et al. 2006; Walston et al. 2005) | Yes, only G allele present |
To investigate how genotypes vary with age, we examined genotypes for specific SNPs associated with the highest blood CRP and IL-6 levels based on published studies. These genotypes are listed as “proinflammatory genotypes” in Table 3. For genetic variants associated with apoE, the proinflammatory genotype was designated by the genotype associated with the greatest risk for adverse conditions or outcomes in old age. The proinflammatory genotypes for the SNPs examined in the current study are apoE4/E4 and rs405509_GG for apoE (Mahley 1988; Mahley and Rall 2000; Schachter et al. 1994; Song, Stampfer, and Liu 2004); rs1205_GG, rs1417938_TT, rs1800947_GG, rs3093061_AA, rs3093062_GG, rs2808630_AA, rs3091244_AA for CRP (Crawford et al. 2006; Eiriksdottir et al. 2009; Teng et al. 2009; Szalai et al. 2005; Kathiresan et al. 2006; Ridker et al. 2008); and rs1800795_GG for IL-6 (Bamouldi et al. 2006; Walston et al. 2005).
Table 3.
Population-based allele frequencies at C-reactive protein (CRP)-associated SNPs: rs1417938, rs1800947, rs3093061, rs3093062, rs3091244, rs1205, and rs2808630; apolipoprotein E (apoE)-associated SNP: rs405509; interleukin-6 (IL-6)-associated SNP: rs1800795
| Populations
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| African
|
Asian
|
Caucasian
|
|||||||
| Tsimane | African | Yoruba | Asian | Han Chinese | Japanese | Caucasian | European | Hispanic | |
| CRP | |||||||||
| rs1417938 | |||||||||
| A | 62 | na | 4 | na | 3 | 5 | 47 | na | na |
| T | 38 | na | 96 | na | 97 | 95 | 53 | na | na |
| rs1800947 | |||||||||
| C | 0 | na | 0 | na | 7 | 3 | 9 | na | na |
| G | 100 | na | 100 | na | 93 | 97 | 91 | na | na |
| rs3093061 | |||||||||
| A | 100 | 100 | na | 94 | na | na | na | 78 | na |
| G | 0 | 0 | na | 6 | na | na | na | 22 | na |
| rs3093062 | |||||||||
| A | 0 | 6 | na | na | 0 | 0 | 0 | na | 6 |
| G | 100 | 94 | na | na | 100 | 100 | 100 | na | 94 |
| rs3091244 | |||||||||
| A | 62 | na | 0 | na | na | na | 3 | na | na |
| C | 37 | na | 78 | na | na | na | 61 | na | na |
| T | 1 | na | 22 | na | na | na | 36 | na | na |
| rs1205 | |||||||||
| A | 32 | 14 | 15 | 55 | 56 | 73 | 31 | 25 | 33 |
| G | 68 | 86 | 85 | 45 | 44 | 27 | 69 | 75 | 66 |
| rs2808630 | |||||||||
| A | 95 | 94 | 82 | 81 | 76 | 89 | 70 | 64 | 76 |
| G | 5 | 6 | 18 | 19 | 24 | 11 | 30 | 36 | 24 |
| apoE | |||||||||
| rs405509 | |||||||||
| G | 24 | na | 76 | na | 34 | 27 | na | 50 | na |
| T | 76 | na | 24 | na | 66 | 73 | na | 50 | na |
| IL-6 | |||||||||
| rs1800795 | |||||||||
| C | 0 | 4 | 0 | 0 | 0 | 0 | 50 | 52 | 20 |
| G | 100 | 96 | 100 | 100 | 100 | 100 | 50 | 48 | 80 |
CEU = Utah residents of Northern and Western European decent; Yoruba = Yoruba people of Nigeria; na = Not available.
All protocols were approved by the institutional review boards at the University of New Mexico, the University of Southern California, and the University of California, Santa Barbara.
Comparison Populations
We also compared allele frequencies in the Tsimane with various other populations, including Africans, Asians, Caucasians, and Hispanics, using the available data from the HapMap project.
Statistical Analyses
Differences in genotype prevalence as a function of age were determined using chi-squared tests. Ordinary least squares regression was used to determine the associations between proinflammatory genotypes and blood CRP levels. Model I was adjusted for age and gender. Model II additionally adjusted for body mass index, as adiposity can influence CRP levels (Visser et al. 1999; Wee et al. 2008). Given the non-linear distribution of CRP in the population, CRP was log-transformed in multivariate analyses. All analyses were carried out with SAS 9.1 (SAS Institute, Inc., Cary, NC, USA), and p-values less than 0.05 were considered significant.
Results
Allele Frequencies of CRP SNPs in the Tsimane and Other Populations
The allele frequencies of the selected SNPs in the CRP gene for the Tsimane and other populations are summarized in Table 3. Though the frequencies of SNPs rs1800947, rs3093061, rs3093062, and rs1205 were comparable between the Tsimane and other populations, there were also some notable differences. For example, the A allele of rs1417938 was present in 62 per cent of the Tsimane and 47 per cent of Caucasians but in less than 5 per cent of Asians and Africans. In addition, the A allele of the tri-allelic SNP rs3091244 was twice as frequent in the Tsimane compared to Caucasians and was completely absent in Africans, whereas rs2808630 was least frequent in the Tsimane (see Table 3).
Proinflammatory Genotypes
Of three SNPs in the CRP gene and one in the IL-6 gene, only the genotypes considered to be proinflammatory were present in the Tsimane (rs1800947_GG, rs3093062_GG, and rs3093061_AA, and rs1800795_GG). In addition, the AA genotype of the tri-allelic SNP rs3091244 was present at 38 per cent.
The apoE4/E4 proinflammatory genotype was present in 2.9 per cent of the Tsimane sample whereas the at-risk genotype (GG) of the SNP in the promoter region (rs405509), which has also been associated with Alzheimer’s disease (Bizzarro et al. 2009), was found in 6.4 per cent of the Tsimane (see Table 3).
Distributions of Genotypes by Age
Based on the allele frequency variations observed in the Tsimane, we examined the age distribution of proinflammatory genotypes at several loci. These included the apoE4 allele, apoE rs405509, and CRP SNPs rs1417938_TT, rs3091244_AA, rs1205_GG, and rs2808630_AA, which have been associated with serum CRP levels. We did not examine the age distribution for CRP SNPs rs1800947 and rs3093062 and IL-6 SNP rs1800795, as the Tsimane were monomorphic at these loci. ApoE allele frequencies by age group are shown in Table 4. ApoE2 was absent from the sample, and E3/E3 was the most prevalent for all age groups (range: 68 to 76 per cent), followed by E3/E4 (range: 21 to 27 per cent). E4/E4 was the least prevalent (range: 0 to 5 per cent), and there was a slightly higher proportion of E4/E4 at ages 60+ compared to earlier ages. There was a non-significant positive trend between age and the frequency of E4/E4 (r = 0.05, p = 0.13). CRP genotypes showed divergent trends with age (see Table 4), with rs3091244_AA exhibiting the strongest association with age: 42.0 per cent among ages 5 to 9, 42.8 per cent among ages 10 to 59, and 30.3 per cent among adults age 60+; p < 0.03). For the other genotypes at rs3091244, less age group variation was observed, with the range of frequencies by age being 0 to 1.7 per cent for rs3091244_CT; 16.0 to 19.3 per cent for CC; 0 to 2.5 per cent for AT; and 39.6 to 48.7 per cent for AC. These preliminary findings do not support the hypothesis that proinflammatory CRP genotypes favor survival to later ages.
Table 4.
Frequency (%) of apolipoprotein E (apoE) and C-reactive protein (CRP) polymorphisms by age group
| Age
|
X2 | p-value | |||
|---|---|---|---|---|---|
| 5–9 | 10–59 | 60+ | |||
| Apolipoprotein E | |||||
| apoE | |||||
| E3/E3 | 74.0 | 76.0 | 68.1 | 6.13 | 0.19 |
| E3/E4 | 26.0 | 21.3 | 26.9 | ||
| E4/E4* | 0.0 | 2.7 | 5.0 | ||
| rs405509 | |||||
| GG* | 6.1 | 6.4 | 5.9 | 3.6 | 0.46 |
| GT | 22.4 | 35.0 | 36.4 | ||
| TT | 71.4 | 58.6 | 57.6 | ||
| C-reactive protein | |||||
| rs1417938 | |||||
| AA | 42.0 | 42.6 | 30.3 | 6.62 | 0.16 |
| AT | 42.0 | 40.4 | 48.7 | ||
| TT* | 16.0 | 17.0 | 21.0 | ||
| rs3091244 | |||||
| AA* | 42.0 | 42.8 | 30.3 | 16.81 | 0.03 |
| AC | 42.0 | 39.6 | 46.2 | ||
| AT | 0.0 | 0.8 | 2.5 | ||
| CC | 16.0 | 16.7 | 19.3 | ||
| CT | 0.0 | 0.1 | 1.7 | ||
| rs1205 | |||||
| GG* | 42.9 | 50.0 | 43.3 | 4.39 | 0.36 |
| AG | 49.0 | 37.5 | 42.5 | ||
| AA | 8.1 | 12.5 | 14.2 | ||
| rs2808630 | |||||
| GG | 0.0 | 0.3 | 0.0 | 5.14 | 0.27 |
| AG | 6.0 | 9.4 | 15.1 | ||
| AA* | 94.0 | 90.3 | 84.9 | ||
Proinflammatory genotype in some populations (U.S. National Health and Nutrition Examination Survey, U.S. Framingham Heart Study, and a Taiwanese population).
Blood CRP and Genotypes
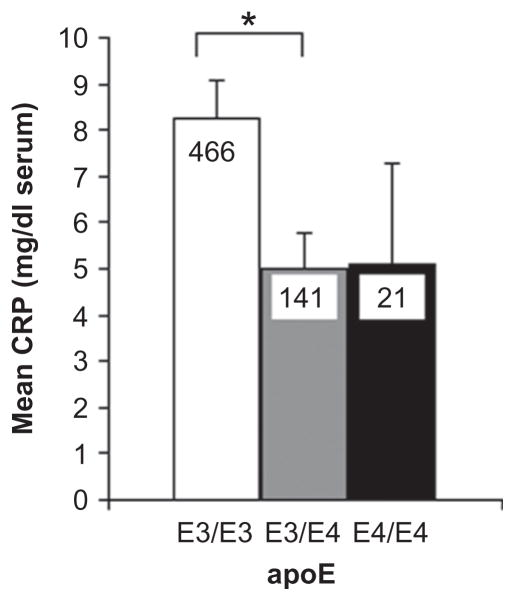
We next determined whether serum CRP levels differed as a function of genotype. Subjects homozygous for the apoE3 allele had significantly higher CRP levels compared to apoE3/E4 and apoE4/E4 subjects (Figure 2; Table 5). By comparison, there was no relationship between proinflammatory genotypes of apoE SNP rs405509 and CRP SNPs rs1417938, rs3091244, and rs2808630 and blood CRP levels (p = 0.81, p = 0.48, p = 0.21, and p = 0.44, respectively; see Table 5). Adjusting for body mass index did not attenuate the relationship between the apoE4 allele and blood CRP levels or alter the results with the other four SNPs. The proinflammatory genotype of CRP rs1205 was associated with higher levels of blood CRP (p = 0.04, Model I) but was no longer significant after adjusting for body mass index.
Figure 2.
Serum C-reactive protein (CRP) levels are associated with apolipoprotein E (apoE) alleles. *p < 0.05. Legend: Blood CRP (mg/dl serum) from all ages in association with apoE alleles. No apoE2 was detected. Further statistical analysis is provided in Table 5.
Table 5.
Regression models predicting levels of log-transformed c-reactive protein (CRP) from genotypes of apoE and CRP
| Gene | Log CRP
|
|||
|---|---|---|---|---|
| Model I (N = 624)
|
Model II (N = 578)
|
|||
| b | p-value | b | p-value | |
| apoE | ||||
| Age | 0.004 | <.01 | 0.003 | <.01 |
| Male | −0.042 | 0.32 | −0.010 | 0.77 |
| Body mass index | 0.010 | 0.11 | ||
| apoE4 presence | −0.153 | <.01 | −0.154 | <.01 |
| apoE | ||||
| Age | 0.004 | <.01 | 0.003 | <.01 |
| Male | −0.049 | 0.26 | −0.024 | 0.60 |
| Body mass index | 0.011 | 0.11 | ||
| rs405509_GG | 0.0218 | 0.82 | −0.005 | 0.96 |
| CRP | ||||
| Age | 0.004 | <.01 | 0.003 | 0.01 |
| Male | −0.045 | 0.30 | −0.019 | 0.68 |
| Body mass index | 0.011 | 0.09 | ||
| rs1417938_TT | −0.040 | 0.48 | −0.023 | 0.70 |
| CRP | ||||
| Age | 0.004 | <.01 | 0.003 | <.01 |
| Male | −0.044 | 0.31 | −0.019 | 0.68 |
| Body mass index | 0.011 | 0.10 | ||
| rs3091244_AA | 0.056 | 0.21 | 0.039 | 0.40 |
| CRP | ||||
| Age | 0.004 | <.01 | 0.003 | 0.01 |
| Male | −0.050 | 0.25 | −0.023 | 0.61 |
| Body mass index | 0.012 | 0.07 | ||
| rs2808630_AA | −0.055 | 0.44 | −0.073 | 0.33 |
| CRP | ||||
| Age | 0.004 | <.01 | 0.003 | 0.01 |
| Male | −0.044 | 0.31 | −0.022 | 0.64 |
| Body mass index | 0.012 | 0.08 | ||
| rs1205_GG | 0.091 | 0.04 | 0.074 | 0.11 |
Discussion
This preliminary study of genetic markers in the Tsimane suggests some notable differences in allele frequencies relative to other populations. For example, the apoE2 allele is absent in this relatively large sample of Tsimane subjects, which is consistent with studies of five Brazilian Amazonian tribes (Yanomami, Wayan-Apalai, Wayampi, Arara, Kayapo; Asakawa et al. 1985; Crews et al. 1993; Marin et al. 1997); Mayans of Mexico (Kamboh, Weiss, and Ferrell 1991b); and Australian aborigines (Kamboh et al. 1991a). The absence of the E2 allele in the Tsimane and these other populations may be due to selection or genetic drift. However, based on our results, we cannot distinguish between these possibilities. It is also possible that apoE2 is very rare in the Tsimane, which precluded its detection in the number of samples we analyzed in this study. It is interesting to note that in European-derived populations, apoE2 is associated with higher triglyceride levels (Genest 2003; Eichner et al. 2002), which is a metabolic phenotypes not exhibited by the Tsimane.
In addition, we did not find support for our hypothesis that proinflammatory alleles of apoE and CRP would favor survival to later ages, although the apoE4 allele was observed at slightly, but not significantly, higher frequency in older Tsimane. With a larger sample, this difference could be significant, which could indicate that, under the environmental conditions in which the Tsimane live, subjects carrying apoE4 have greater survival to older age (Finch 2007; Finch and Morgan 2007). Such an observation would be opposite to trends for lower survival of E4 carriers at older ages in United States and European populations where infection levels are lower (Ewbank 2004; Gerdes et al. 2000; Rontu et al. 2006).
The different genotype distribution with age for rs3091244 (CRP) suggests an age difference in the distribution of the AA proinflammatory genotype, but additional studies will be required to confirm this relationship. We also observed an association of the apoE4 allele with CRP in the Tsimane, where apoE3/E4 and apoE4/E4 carriers had lower levels compared to apoE3/E3 subjects (see Figure 2); the correlation remained significant after adjustment for body mass index. Similar associations with apoE4 have been reported in Finnish nonagenarians (Rontu et al. 2006), U.S. Latinos, and Japanese-Americans (Aiello, Nguyen, and Haan 2008; Austin et al. 2004). These associations could arise from various mechanisms, including differential binding of apoE4 to very-low-density lipoprotein cholesterol (Austin et al. 2004) and hepatic clearance of CRP with involvement of the mevalonate pathway (Rontu et al. 2006).
We note some limitations of our study. For example, infants and children younger than than age 5 were not included in our analysis but will be included in future studies. In addition, CRP levels based on a single sampling may not accurately represent relationships between genetic variants and inflammatory phenotypes, as CRP levels can vary substantially during infection and are influenced by other genes, such as interleukin (IL)-1, and IL-6. We also acknowledge that the observed allelic associations (or lack thereof) need to be validated with additional samples to draw any firm conclusions. Moreover, examining individual SNPs and their associations with a given phenotype must be interpreted cautiously due to linkage disequilibrium with other variants, which can vary across genomic regions and have not been determined in an isolated and unique population such as the Tsimane. Thus, carrying out dense SNP genotyping across loci of interest (e.g., IL-6 or IL-1) in conjunction with quantitating additional biomarkers/cytokines may help to provide a better understanding of the complex relationship between genetic variation and inflammation within the context of Tsimane life history.
A unique aspect of our study pertained to examining the genetic association of single nucleotide polymorphisms with blood CRP levels in an indigenous population living in a highly infectious environment and with elevated levels of inflammation. It is possible that in populations with a high inflammatory burden such as the Tsimane, the association of CRP genotypes that predispose individuals to high basal levels of serum CRP may not be detectable (Hage and Szalai 2007, 2009). By comparison, studies of the Bimoba of Ghana have reported an association between cytokine levels and single nucleotide polymorphisms in the IL-10 gene (Kuningas et al. 2009; May et al. 2009, 2010), although allele frequency distributions were not evaluated as a function of age. Compared to other populations, the indigenous peoples of the Amazonian basin may also harbor unique SNPs and/or lack additional variants as a result of selective pressures from pathogens present in their environment over time.
Future studies in this distinct population that utilize novel genetic/genomics methods, such as genome-wide association studies (Collins et al. 1997; Goldstein 2009; Hardy and Singleton 2009), will contribute to expanding our understanding of the links between environmental factors, genes, selection, and traits relevant to human disease. These data are being further examined in conjunction with a life-history analysis of physical, cognitive, and social factors. In examining the influence of the environment on selective pressures and human genetic diversity, we can begin to better understand and resolve the influence of genetics and the environment on health and age-associated health outcomes.
Acknowledgments
We gratefully acknowledge the Tsimane people for their participation in this study. We also thank the members of the Tsimane Health and Life History Project, especially Dr. Daniel Eid Rodriguez, Dr. Edhitt Cortez Linares, Dr. Karen Arce Ardaya, Jhon Aguilar and Ivan Maldonado. This work was supported by grants from the National Institute on Aging (R01AG024119-01, P30AG17265, R21AG031988, T32AG0037) (HK, MG, EMC); the National Heart, Lung, and Blood Institute (R01HL079353) (HA);, the National Center for Research Resources (M01-RR-00043, RR10600-01, CA62528-01, RR14514-01) (HA); the National Science Foundation (BCS-0136274 and BCS-0422690) (HK, MG); the Keck Foundation (CEF, EMC); the USC Oakley Fellowship Fund (SV); the Ellison Medical Foundation (CEF); and the Ziegler Fund (CEF, EMC).
References
- Aceves D, Ruiz B, Nuño P, Roman S, Zepeda E, Panduro A. Heterogenity of apolipoprotein E polymorphism in different Mexican populations. Hum Biol. 2006;78:65–75. doi: 10.1353/hub.2006.0021. [DOI] [PubMed] [Google Scholar]
- Aiello AE, Nguyen HO, Haan MN. C-reactive protein mediates the effect of apolipoprotein E on cytomegalorvirus infection. J Infect Dis. 2008;197:34–41. doi: 10.1086/524144. [DOI] [PubMed] [Google Scholar]
- Asakawa J, Takahashi N, Rosenblum BB, Neel JV. Two-dimensional gel studies of genetic variation in the plasma proteins of Amerindians and Japanese. Hum Genet. 1985;70:222–230. doi: 10.1007/BF00273446. [DOI] [PubMed] [Google Scholar]
- Austin MA, Zhang C, Humphries SE, Chadler WL, Talmud PJ, Edwards KL, Leonetti DL, McNeely MJ, Fujimoto WY. Heritability of C-reactive protein and association with apolipoprotein E genotypes in Japanese Americans. Ann Hum Genet. 2004;68:179–188. doi: 10.1046/j.1529-8817.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- Balding JC, Healy M, Livingstone WJ, White B, Mynett-Johnson L, Cafferkey M, Simth OP. Genomic polymorphic profiles in an Irish population with meningococaemia: Is it possible to predict severity and outcome of disease? Genes Immun. 2003;4:533–540. doi: 10.1038/sj.gene.6364020. [DOI] [PubMed] [Google Scholar]
- Bamouldi J, Courivaud C, Deschamps M, Mercier P, Ferrand C, Penfornis A, Tiberghien P, Chaolpin JM, Saas P, Ducloux D. IL-6 promoter polymorphism -174 is associated with new-onset of diabetes after transplantation. J Am Soc Nephrol. 2006;17:2333–2340. doi: 10.1681/ASN.2006010066. [DOI] [PubMed] [Google Scholar]
- Bizzarro A, Seripa D, Acciarri A, Matera MG, Pilotto A, Tiziano FD, Brahe C, Masullo C. The complex interaction between APOE promoter and AD: an Italian case-control study. Eur J Hum Genet. 2009;17:938–945. doi: 10.1038/ejhg.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brull D, Serrano N, Zito F, Jones L, Montgomery HE, Rumley A, Sharma P, Lowe GD, World MJ, Humphries SE, et al. Human CRP gene polymorphism influences CRP levels: implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:2063–2069. doi: 10.1161/01.ATV.0000084640.21712.9C. [DOI] [PubMed] [Google Scholar]
- Carlson CS, Aldred SF, Lee PK, Tracy RP, Schwartz SM, Rieder M, Liu K, Williams D, Iribarren C, Lewis EC, et al. Polymorphisms with the C-reactive protein promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005;77:64–77. doi: 10.1086/431366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. Evolution in age-structured populations. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Charlesworth B. Evolution of senescence: Alzheimer’s disease and evolution. Curr Biol. 1996;6:20–22. doi: 10.1016/s0960-9822(02)00411-6. [DOI] [PubMed] [Google Scholar]
- Chiappelli M, Tampieri C, Tumini E, Porcellini E, Caldarera CM, Nanni S, Branzi A, Lio D, Caruso M, Hoffmann ME, et al. Interleukin-6 gene polymorphism is an age-dependent risk factor for myocardial infarction in men. J Immunogenet. 2005;32:349–353. doi: 10.1111/j.1744-313X.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- Collins FS, Guyer MS, Charkravarti A. Variations on a theme: Cataloging human DNA sequence variation. Science. 1997;278:1580–1581. doi: 10.1126/science.278.5343.1580. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63:301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- Corder EH, Lannfelt L, Viitanen M, Corder LS, Manton KG, Winblad B, Basun H. Apolipoprotein E genotype determines survival in the oldest old (85 years or older) who have good cognition. Arch Neurol. 1996;53:418–422. doi: 10.1001/archneur.1996.00550050048022. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Sanders CL, Qin X, Smith JD, Shephard C, Wong M, Witrak L, Rieder MJ, Nickerson DA. Genetic variation is associated with C-reactive protein levels in the Third National Health and Nutrition Examination Survey. Circulation. 2006;114:2458–2465. doi: 10.1161/CIRCULATIONAHA.106.615740. [DOI] [PubMed] [Google Scholar]
- Crews DE, Kamboh MI, Mancilha-Carvalho JJ, Kottke B. Population genetics of apolipoprotein A-4, E, and H polymorphisms in Yanomami Indians of Northwestern Brazil: Associations with lipids, lipoproteins, and carbohydrate metabolism. Hum Biol. 1993;65:211–224. [PubMed] [Google Scholar]
- Das M, Pal S, Ghosh A. Apolipoprotein E gene polymorphism and dyslipidaemia in adult Asian Indians: A population based study from Calcutta, India. Indian J Hum Genet. 2008;14:87–91. doi: 10.4103/0971-6866.45000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Craen AJM, Posthuma D, Remarque EJ, van den Biggelaar AHJ, Westendorp RGJ, Boomsma DI. Heritability estimates of innate immunity: An extended twin study. Genes Immun. 2005;6:167–170. doi: 10.1038/sj.gene.6364162. [DOI] [PubMed] [Google Scholar]
- Demarchi DA, Salzano FM, Altuna ME, Fiegenbaum M, Hill K, Hurtado AM, Tsunetto LT, Petzl-Erler ML, Hutz MH. APOE polymorphism distribution among Native Americans and related populations. Ann Hum Biol. 2005;32:351–365. doi: 10.1080/03014460500097237. [DOI] [PubMed] [Google Scholar]
- Drabe N, Zund G, Grunenfelder J, Sprenger M, Hoerstrup SP, Bestmann I, Maly FE, Turina M. Genetic predisposition in patients undergoing cardiopulmonary bypass surgery is associated with an increase of inflammatory cytokines. Eur J Cardio-Thorac Surg. 2001;20:609–613. doi: 10.1016/s1010-7940(01)00842-9. [DOI] [PubMed] [Google Scholar]
- Drenos F, Kirkwood TBL. Selection on alleles affecting human longevity and late-life disease: The example of Apolipoprotein E. PLoS One. 2010;5:10022. doi: 10.1371/journal.pone.0010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenos F, Westendorp R, Kirkwood TBL. Trade-off mediated effects on the genetics of human survival caused by increasingly benign living conditions. Biogerontology. 2006;7:287–295. doi: 10.1007/s10522-006-9027-9. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Larson MG, Vasan RS, Massaro JM, Wilson PW, Lipinska I, Corey D, Vita JA, FKeaney J, Jr, Benjamin EJ. Genome scan of systemic biomarkers of vascular inflammation in the Framingham Heart Study: evidence for susceptibility loci on 1q. Atherosclerosis. 2005;182:307–314. doi: 10.1016/j.atherosclerosis.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: A HuGE Review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- Eiriksdottir G, Smith AV, Aspelund T, Hafsteinsdottir SH, Olafsdottir E, Launer LJ, Harris TB, Gudnason V. The interaction of adiposity with the CRP gene affects CRP levels: Age, Gene/Environment Susceptibility-Reykjavik Study. Int J Obesity. 2009;33:267–272. doi: 10.1038/ijo.2008.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- Ewbank DC. The APOE gene and differences in life expectancy in Europe. J Gerontol Biol Sci Med Sci. 2004;59:16–20. doi: 10.1093/gerona/59.1.b16. [DOI] [PubMed] [Google Scholar]
- Ewbank DC. Differences in the association between apolipoprotein E genotype and mortality across populations. J Gerontol Biol Sci Med Sci. 2007;62:899–907. doi: 10.1093/gerona/62.8.899. [DOI] [PubMed] [Google Scholar]
- Fabris C, Toniutto P, Bitetto D, Minisini R, Smirne C, Caldato M, Pirisi M. Low fibrosis progression of recurrent hepatitis C in apolipoprotein E epsilon4 carriers: Relationship with the blood lipid profile. Liver Int. 2005;25:1128–1135. doi: 10.1111/j.1478-3231.2005.01156.x. [DOI] [PubMed] [Google Scholar]
- Finch CE. The biology of human longevity. Burlington, MA: Elsevier; 2007. [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Finch CE, Morgan TE. Systemic inflammation, infection, apoE alleles, and Alzheimer disease: A position paper. Curr Alzheimer Res. 2007;4:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biol. 2004;79:3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- Flint J, Harding RM, Boyce AJ, Clegg BB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol. 1998;11:1–51. doi: 10.1016/s0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottavini E, Benedictis G. Inflammaging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Olivieri F, Marchegiani F, Cardelli M, Cavallone L, Capri M, Salvioli S, Valensin S, De Bendicitis G, Di Iorio A, et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Aging Develop. 2005;126:351–361. doi: 10.1016/j.mad.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Friedman GE, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974;290:1275–1278. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- Gajra B, Candlish JK, Sha N, Mak JW, Tay JSH. Effect of apolipoprotein E variants on plasma lipids and apolipoproteins in the Orang Asli (‘Aborigines’) of Malaysia. Hum Hered. 1994;44:209–213. doi: 10.1159/000154219. [DOI] [PubMed] [Google Scholar]
- Genest J. Lipoprotein disorders and cardiovascular risk. J Inherit Metab Dis. 2003;26:267–287. doi: 10.1023/a:1024449603891. [DOI] [PubMed] [Google Scholar]
- Gerdes LU, I, Klausen C, Sihm I, Faergeman O. Apolipoprotein E polymorphism in a Danish population compared to findings in 45 other study populations around the world. Genet Epidemiol. 1992;9:155–167. doi: 10.1002/gepi.1370090302. [DOI] [PubMed] [Google Scholar]
- Gerdes LU, Gerdes C, Hansen PS, Klausen IC, Faergaeman O, Dyerberg J. The apolipoprotein E polymorphism in Greenland Inuit in its global perspective. Hum Genet. 1996;98:546–550. doi: 10.1007/s004390050257. [DOI] [PubMed] [Google Scholar]
- Gerdes LU, Jeune B, Ranberg KA, Nybo H, Vaupel JW. Estimation of apolipoprotein E genotype-specific relative mortality risks from the distribution of genotypes in centenarians and middle-aged men: Apolipoprotein E genes is a “frailty gene,” not a “longevity gene”. Genet Epidemiol. 2000;19:202–210. doi: 10.1002/1098-2272(200010)19:3<202::AID-GEPI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360:1696–1698. doi: 10.1056/NEJMp0806284. [DOI] [PubMed] [Google Scholar]
- Grunenfelder J, Umbehr M, Plass A, Bestmann L, Maly FE, Zèund G, Turina M. Genetic polymorphisms of apolipoprotein E4 and tumor necrosis factor beta as predisposing factors for increased inflammatory cytokines after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2004;128:92–97. doi: 10.1016/j.jtcvs.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Supa AZ. Mortality experience of Tsimane Amerindians of Bolivia: Regional variation and temporal trends. Am J Hum Biol. 2007;19:376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Finch C, Crimmins EM. Aging and inflammation in two epidemiological worlds. J Gerontol Biol Sci Med Sci. 2008;63:196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage FG, Szalai AJ. C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol. 2007;50:1115–1122. doi: 10.1016/j.jacc.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Hage FG, Szalai AJ. The role of C-reactive protein polymorphisms in inflammation and cardiovascular risk. Curr Atheroscler Rep. 2009;11:124–130. doi: 10.1007/s11883-009-0020-z. [DOI] [PubMed] [Google Scholar]
- [accessed April 15, 2010];HapMap Website. http://hapmap.ncbi.nlm.nih.gov/cgi-perl/gbrowse/hapmap27_B36/#search.
- Hardy J, Singleton A. Genomewide association studies and human disease. N Engl J Med. 2009;360:1759–1768. doi: 10.1056/NEJMra0808700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpending H, Cochran G. Genetic diversity and genetic burden in humans. Infect Genet Evol. 2006;6:154–162. doi: 10.1016/j.meegid.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Huang HY, Thuita L, Strickland P, Hoffman SC, Comstock GW, Helzlsouer K. Frequencies of single nucleotide polymorphisms in genes regulating inflammatory responses in a community-based population. BMC Genet. 2007;8:7. doi: 10.1186/1471-2156-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YD. Apolipoprotein E and Alzheimer disease. [accessed January 21, 2008];Neurology. 2006 66:79–85. International Still’s Disease Foundation Web site: http://www.stillsdisease.org/lab_tests/cbc. [Google Scholar]
- Kamboh MI, Sepehrnia B, Ferrell RE. Genetic studies of human apolipoproteins: VI. Common polymorphism of apolipoprotein E in blacks. Dis Markers. 1989;7:49–55. [PubMed] [Google Scholar]
- Kamboh MI, Bhatia KK, Ferrell RE. Genetic of human apolipoproteins: XII. Population genetics of apolipoproteins in Papua New Guinea. Am J Hum Biol. 1990;2:17–23. doi: 10.1002/ajhb.1310020103. [DOI] [PubMed] [Google Scholar]
- Kamboh MI, Serjeantson SW, Ferrell RE. Genetic studies of human apolipoproteins: XVIII. Apolipoprotein polymorphisms in Australian Aborigines. Hum Biol. 1991a;63:179–186. [PubMed] [Google Scholar]
- Kamboh MI, Weiss KM, Ferrell RE. Genetic studies of human apolipoproteins: XVI. APOE polymorphism and cholesterol levels in the Mayans of the Yucatan peninsula, Mexico. Clin Genet. 1991b;39:26–32. doi: 10.1111/j.1399-0004.1991.tb02981.x. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Larson MG, Vasan RS, Guo C-Y, Gona P, Keaney JF, Jr, Wilson PWF, Newton-Cheh C, Musone Camargo SLAL, et al. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation. 2006;116:1415–1423. doi: 10.1161/CIRCULATIONAHA.105.591271. [DOI] [PubMed] [Google Scholar]
- Khor CC, Vannberg FO, Chapman SJ, Guo H, Wong SH, Walley AJ, et al. CISH and susceptibility to infectious diseases. N Engl J Med. 2010;362:2092–2101. doi: 10.1056/NEJMoa0905606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs A, Green F, Hansson LO, Lundman P, Samnegard A, Boquist S, Ericsson CG, Watkins H, Hamsten A, Tornvall P. A novel common single nucleotide polymorphism in the promoter region of the C-reactive protein gene associated with the plasma concentration of C-reactive protein. Atherosclerosis. 2005;178:193–198. doi: 10.1016/j.atherosclerosis.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kuningas M, May Tamm L, Dvan Bodegom R, van den Biggelaar AHJ, Meij JJ, Frölich M, Ziem JB, Suchiman HED, Metspalu A, et al. Selection for genetic variation inducing pro-inflammatory response under adverse environmental conditions in a Ghanaian population. PLoS One. 2009;4:7795. doi: 10.1371/journal.pone.0007795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoz C, Schaefer EJ, Cupples LA, Wilson PWF, Levy D, Osgood D, Parpos S, Pedro-Botet J, Daly JA, Ordovas JM. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis. 2001;154:529–537. doi: 10.1016/s0021-9150(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Lange LA, Carlson CS, Hindorff LA, Lange EM, Walston J, Durda JP, Cushman M, Bis JC, Zeng D, Lin D, et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA. 2006;296:2703–2711. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- Lehtinen S, Luoma P, Lehtimäki T, Näyhä S, Hassi J, Nikkari T. Differences in genetic variation of apolipoprotein E in Lapps and Finns [abstract] Atherosclerosis. 1994;109:263. [Google Scholar]
- Livak KJ. SNP genotyping by the 5–nuclease reaction. Methods Mol Biol. 2003;212:129–147. doi: 10.1385/1-59259-327-5:129. [DOI] [PubMed] [Google Scholar]
- López C, Saravia C, Gomez A, Hoebeke J, Patarroyo MA. Mechanisms of genetically-based resistance to malaria. Gene. 2010;467:1–12. doi: 10.1016/j.gene.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: Far more than a lipid transport protein. Annu Rev Ergon Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Marin GB, Tavella MH, Guerreiro JF, Santos SEB, Zago MA. Absence of the E2 allele of apolipoprotein in Amerindians. Brazilian J Genet. 1997;20:741–743. [Google Scholar]
- May L, van den Biggelaar AHJ, van Bodegom D, Meij HJ, de Craen AJM, Amankwa J, Frölich M, Kuningas M, Westendorp RGJ. Adverse environmental conditions influence age-related innate immune responsiveness. Immun Aging. 2009;6:7. doi: 10.1186/1742-4933-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L, van Bodegom D, Frölich M, van Lieshout L, Slagboom PE, Westendorp RG, Kuningas M. Polymorphisms in TLR4 and TLR2 genes, cytokine production and survival in rural Ghana. Eur J Hum Genet. 2010;18:480–495. doi: 10.1038/ejhg.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Leonard WR, Burhop J, Reyes-Garciá VV, Huanca T, Godoy RA. Predictors of C-reactive protein in Tsimane’ 2 to 15 year-olds in lowland Bolivia. Am J Phys Anthropol. 2005;128:906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- Nyholt DR, Yu CE, Visscher PM. On Jim Watson’s APOE status: Genetic information is hard to hide. Eur J Hum Genetics. 2009;17:147–150. doi: 10.1038/ejhg.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oligati P, Politis A, Malitas P, Albani D, Dusi S, Polito L, De Mauro S, Zisaki A, Piperi C, Samouli E, et al. APOE epsilon-4 allele and cytokine production in Alzheimer’s disease. Int J Geriatr Psychiatry. 2010;25:338–344. doi: 10.1002/gps.2344. [DOI] [PubMed] [Google Scholar]
- Oriá RB, Patrick PD, Oriá MO, Lorntz B, Thompson MR, Azevedo OG, Lobo RN, Pinkerton F, Guerrant RL, Lima AA. ApoE polymorphisms and diarrheal outcomes in Brazilian shanty town children. Braz J Med Biol Res. 2010;43:249–256. doi: 10.1590/s0100-879x2010007500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN, Eckfeldt JH, Tracy RP. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–689. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Colacicco A, Basile A, D’Introno A, Capurso C, Sabba M, Capurso S, Capurso A. Apolipoprotein E (APOE) polymorphism influences serum APOE levels in Alzheimer’s disease patients and centenarians. Neuroreport. 2003;14:605–608. doi: 10.1097/00001756-200303240-00016. [DOI] [PubMed] [Google Scholar]
- Pennington R, Gatenbee C, Kennedy B, Harpending H, Cochran G. Group differences in proneness to inflammation. Infect Genet Evol. 2009;9:1371–1380. doi: 10.1016/j.meegid.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson Fonseca E, Genest FAHJ, Gotto JAM, Jr, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. on behalf of the JUPITER Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- Rizzello V, Liuzzo G, Giannuario GD, Trabetti E, Brugaletta S, Santamaria M, Piro M, Pignatti PF, Maseri A, Biasucci LM, Crea F. 1059G/C polymorphism within the exon 2 of the C-reactive protein gene: relationship to C-reactive protein levels and prognosis in unstable angina. Coronary Artery Dis. 2007;18:533–538. doi: 10.1097/MCA.0b013e3282f08eb9. [DOI] [PubMed] [Google Scholar]
- Rontu R, Ojala P, Hervonen A, Goebeler S, Karhunen PJ, Nikkilèa M, Kunnas T, Jylhä M, Eklund C, Hurme M, et al. Apoplipoprotein E genotype is related to plasma levels of C-reactive protein and lipids and to longevity in nonagenarians. Clin Endocrinol. 2006;64:265–270. doi: 10.1111/j.1365-2265.2006.02455.x. [DOI] [PubMed] [Google Scholar]
- Rosvall L, Rizzuto D, Wang HX, Winblad B, Graff C, Fratiglioni L. APOE-related mortality: Effect of dementia, cardiovascular disease and gender. Neurobiol Aging. 2009;20:1545–1551. doi: 10.1016/j.neurobiolaging.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Russell AI, Graham DSC, Shepherd C, Robertson CA, Whittaker J, Meeks J, Powell RJ, Isenberg DA, Walport MJ, Vyse TJ. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–147. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma N, Iwata S, Ikeuchi R, Ichikawa T, Hibino T, Kamiya Y, Ohte N, Kawaguchi M, Kunimatsu M, Kawahara H, et al. Coexisting type III hyperlipoproteinemia and familial hypercholesterolemia: a case report. Metabolism. 1995;44:460–465. doi: 10.1016/0026-0495(95)90052-7. [DOI] [PubMed] [Google Scholar]
- Sandholzer C, Delport R, Vermaa H, Utermann G. High frequency of the apo epsilon 4 allele in Khoi San from South Africa. Hum Genet. 1995;95:46–48. doi: 10.1007/BF00225073. [DOI] [PubMed] [Google Scholar]
- Schachter F, Faure-DiDelanef L, Guenot F, Rouger H, Froguel P, Lesueur-Ginot L, Cohen D. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- Scholz W. Interleukin 6 in diseases: Cause or cure? Immunopharmacology. 1996;31:131–150. doi: 10.1016/0162-3109(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Sepehrnia B, Kamboh MI, Adams Campbell LL, Nwankwo M, Ferrell RE. Genetic studies of human apolipoproteins. VII. Population distribution of polymorphisms of apolipoproteins A-I, A-II, A-IV, C-II, E, and H in Nigeria. Am J Hum Genet. 1988;43:847–853. [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol. 2006;33:279–308. doi: 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Wolff RK, Herrick JS, Caan BJ, Potter JD. IL6 genotypes and colon and rectal cancer. Cancer Causes Control. 2007;18:1095–1105. doi: 10.1007/s10552-007-9049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD. Apolipoproteins and aging: Emerging mechanisms. Aging Res. 2002;1:345– 365. doi: 10.1016/s1568-1637(02)00005-3. [DOI] [PubMed] [Google Scholar]
- Song YQ, Stampfer MJ, Liu SM. Meta-analysis: Apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141:137–147. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- Szalai AJ. The biological functions of C-reactive protein. Vascul Pharmacol. 2002;39:105–107. doi: 10.1016/s1537-1891(02)00294-x. [DOI] [PubMed] [Google Scholar]
- Szalai AJ, Wu J, Lange EM, McCrory MA, Langefeld CD, Williams A, Zakharkin SO, George V, Allison DB, Cooper GS, et al. Single-nucleotide polymorphisms in the C-reactive protein (CRP) gene promoter that affect transcription factor binding, alter transcriptional activity, and associate with difference in baseline serum CRP level. J Mol Med. 2005;83:440–447. doi: 10.1007/s00109-005-0658-0. [DOI] [PubMed] [Google Scholar]
- Tanner S, Leonard WR, McDade TW, Reyes-Garcia V, Godoy R, Huanca T. Influence of helminth infections on childhood nutritional status in lowland Bolivia. Am J Hum Biol. 2009;21:651–656. doi: 10.1002/ajhb.20944. [DOI] [PubMed] [Google Scholar]
- Teng M-S, Hsu L-A, Wu S, Chang H-H, Chou HH, Ko Y-L. Association between C-reactive protein gene haplotypes and C-reactive protein gene haplotypes and C-reactive protein levels in Taiwanese: Interaction with obesity. Atherosclerosis. 2009;204:64–69. doi: 10.1016/j.atherosclerosis.2008.10.034. [DOI] [PubMed] [Google Scholar]
- van Bodegom D, May L, Meij HJ, Westendorp RGJ. Regulation of human life histories. The role of the inflammatory host response. Ann N Y Acad Sci. 2007;1100:84–97. doi: 10.1196/annals.1395.007. [DOI] [PubMed] [Google Scholar]
- Vasunilashorn S, Finch CE, Kim JK, Winking J, Gurven M, Kaplan H, Crimmins EM. Biomarkers of aging in two societies. The US and the Tsimane of Bolivia (forthcoming) 2010 doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S, Crimmins E, Kim JK, Winking J, Gurven M, Kaplan H, Finch C. Blood lipids, infection and inflammatory markers in the Tsimane of Bolivia. Am J Hum Biol. 2010;22:731–740. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Werner MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2009;30:1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Arking DE, Fallin D, Li T, Beamer B, Xue Q, Ferrucci L, Fried LP, Chakravarti A. IL-6 gene variation is not associated with increased serum levels of IL-6 muscle, weakness, or frailty in older women. Exp Gerontol. 2005;40:344–352. doi: 10.1016/j.exger.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Wee CC, Mukamal KJ, Huang A, Davis RB, McCarthy EP, Mittleman MA. Obesity and C-reactive protein levels among white, black, and Hispanic US adults. Obesity. 2008;16:875–880. doi: 10.1038/oby.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems TE, Hayton K, Fairhurst RM. The impact of malaria parasitism: from corpuscles to communities. J Clin Invest. 2009;119:2496–2505. doi: 10.1172/JCI38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PWF, Schaefer EJ, Larson MG, Ordovas JM. Apolipoprotein E alleles and risk of coronary disease—a meta-analysis. Arterioscler Thromb Vascul Biol. 1996;16:1250–1255. doi: 10.1161/01.atv.16.10.1250. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Itzhaki RF, Faragher EB, Jame MW, Ryder SD, Irving WL. Apolipoprotein E-epsilon 4 protects against severe liver disease caused by hepatitis C virus. Hepatology. 2002;36:456–463. doi: 10.1053/jhep.2002.34745. [DOI] [PubMed] [Google Scholar]
- Yashin AI, De Benedictis G, Vaupel JW, Tan Q, Andreev KF, Iachine IA, Bonafe M, Valensin S, De Luc M, Carotenuto L, et al. Genes and longevity: Lessons from studies of centenarians. J Gerontol Biol Sci. 2000;55:319–328. doi: 10.1093/gerona/55.7.b319. [DOI] [PubMed] [Google Scholar]
- Zee RY, Ridker PM. Polymorphism in the human C-reactive protein (CRP) gene, plasma concentrations of CRP, and the risk of future arterial thrombosis. Atherosclerosis. 2002;162:217–219. doi: 10.1016/s0021-9150(01)00703-1. [DOI] [PubMed] [Google Scholar]