Abstract
OBJECTIVE
Premenstrual dysphoric disorder (PMDD) is characterized by severe, negative mood symptoms during the luteal phase of each menstrual cycle. We recently reported that women with PMDD show a greater increase in relative glucose metabolism in the posterior cerebellum from the follicular to the luteal phase, as compared with healthy women, and that the phase-related increase is proportional to PMDD symptom severity. We extended this work with a study of brain structure in PMDD.
METHODS
High-resolution magnetic resonance imaging (MRI) scans were obtained from 12 women with PMDD and 13 healthy control subjects (whole-brain volume-corrected p<.05). Voxel-based morphometry was used to assess group differences in cerebral grey-matter volume (GMV), using a statistical criterion of p<.05, correcting for multiple comparisons in the whole-brain volume.
RESULTS
PMDD subjects had greater GMV than controls in the posterior cerebellum but not in any other brain area. Age was negatively correlated with GMV within this region in healthy women, but not in women with PMDD. The group difference in GMV was significant for women over age 30 (p=.0002) but not younger participants (p>.1).
CONCLUSIONS
PMDD appears to be associated with reduced age-related loss in posterior cerebellar GMV. Although the mechanism underlying this finding is unclear, cumulative effects of symptom-related cerebellar activity may be involved.
Keywords: premenstrual dysphoric disorder, premenstrual syndrome, cerebellum, neuroimaging, voxel-based morphometry, brain aging
Introduction
It is estimated that 5–8 % of reproductive women suffer from the neuroendocrine affective disorder PMDD, experiencing decreases in the quality of life similar to those associated with major depressive disorder (Halbreich et al. 2003; Wittchen et al. 2002). The symptoms of PMDD include irritability/anger, depression, mood swings, anxiety/tension, feeling “out of control,” difficulty concentrating, food cravings, sleep disturbances, and fatigue (American Psychiatric Association1994). They appear during the luteal phase of the menstrual cycle, reach a zenith within the week prior to menses, and resolve by Day 7 of the subsequent follicular phase (Backstrom et al. 2003). PMDD does not share common biological markers or an identical profile of therapeutic responses to medications or psychotherapy with other affective disorders (Rubinow and Schmidt 1995). PMDD does not respond to psychotherapy or non-serotonergic antidepressants, and differences in concentrations of ovarian sex steroids do not explain the symptoms (Rubinow et al. 1988; Rubinow and Schmidt 2006).
Neuroimaging studies of women with PMDD have begun to provide information about underlying pathophysiology. A protein magnetic resonance spectroscopy study showed that women with PMDD have higher concentrations of cortical γ-aminobutyric acid (GABA) in the luteal phase than in the asymptomatic follicular phase of the menstrual cycle, whereas healthy women exhibited opposite findings (Epperson et al. 2002). The authors concluded that abnormal GABAA receptor functioning could reduce sensitivity to GABA agonists, including neuroactive steroids. Diminished progesterone-mediated GABAergic inhibition was also suggested in a study in which transcranial magnetic stimulation was applied to the motor cortex of women with premenstrual syndrome (Smith et al. 2003).
In a study pairing presentation of emotional words with fMRI, Go/NoGo task, accuracy of performance was lower in the luteal phase than in the follicular phase in women with PMDD (Protopopescu et al. 2008). This finding was interpreted as showing less impulse control via prefrontal “top-down” modulation of the limbic system during the luteal phase than the follicular phase. Consistent with this explanation, negative words elicited more activity during the luteal than the follicular phase in the anterior-medial orbitofrontal cortex of control participants. Women with PMDD did not have more activity in the anterior-medial orbitofrontal cortex during the luteal than the follicular phase, but instead had more activity in the amygdala (Protopopescu et al. 2008).
We recently mapped cerebral function in the absence of explicit provocation, using positron-emission tomography (PET) with [18F]fluorodeoxyglucose, while participants performed an affectively neutral vigilance task. Within parts of the cerebellum that have been previously activated during emotional tasks (Stoodley and Schmahmann 2009), women with PMDD showed a greater increase in relative glucose metabolism from the follicular phase to the late luteal phase than healthy control women, and the degree of metabolic increase was proportional to symptom severity (Rapkin et al. 2011). These findings suggest that activity in cerebellar nuclei that have been implicated in emotion-processing and in other mood disorders also contribute to negative mood or its regulation in PMDD.
Increasing regional brain activity produced by effortful processing over weeks, learning a motor task (Draganski et al. 2004), and hours learning a cognitive task (Kwok et al. 2011) have been shown to produce congruent increases in local grey-matter volume (GMV). To our knowledge, however, there have been no studies of brain structure in women with PMDD. Women with PMDD experience about 3000 days of severe symptoms throughout their lives (Rapkin and Winer 2009). We hypothesized that cumulative increases in the activity of a distributed cerebellar network associated with emotional processing, operationalised as lobule VI, and the CrusI and vermis portions of lobule VII (Stoodley and Schmahmann 2009), would result in greater local cerebellar GMV in women with PMDD as compared to those without PMDD. On the other hand, increased neural activity might increase glutamate-release and associated excitotoxicity, and have the opposite effect on GMV. There also could be structural abnormalities in parts of the brain other than the cerebellum, such as the amygdala and frontal cortex, which have also been implicated in mood disorders. Therefore, as a conservative test of our hypothesis, we employed a whole-brain analysis and required evidence of higher GMV in either group to retain significance after correction for whole-brain search volume.
Methods and Materials
General Experimental Design
As described earlier (Rapkin et al. 2011), research participants were screened prospectively for two menstrual cycles using the Daily Rating of Severity of Problems (DRSP) (Endicott et al. 2006) and assigned to either a PMDD group or a healthy control comparison group. All patients in the PMDD group were diagnosed with severe premenstrual syndrome, and also met the DSM IV criteria for PMDD. Participants continued to complete the DRSP for the next 1–2 months while undergoing the scanning sessions. During both the follicular and late luteal phases of the menstrual cycle, PET scans were performed to measure relative regional cerebral glucose metabolism as an index of regional brain function. A composite mood summary score was created by summing the ratings from the DRSP on the day of each PET scan for the core mood symptoms of depression, anxiety, mood swings, and irritability. A structural MRI scan of the brain was acquired on a separate day within one month of the initial PET scan (Rapkin et al. 2011), and the MRI data are the subject of this manuscript. Although MRI was recorded only once per subject, the proportion of MRI scans acquired during the luteal phase was balanced in the two groups (69% in controls versus 67% in woman with PMDD).
Participants
Participants were healthy, English-speaking, right-handed women with regular menstrual cycles of 24 – 32 days. They were recruited through local newspaper advertisements. Participants were screened over the telephone and scheduled for an on-site visit to determine study eligibility, using medical history and physical examination, including a brief neurological examination, and a handedness inventory (Oldfield 1971). After a thorough explanation of the protocol, written informed consent was obtained as approved by the UCLA Office for Protection of Research Subjects. Psychiatric evaluation was performed using the Mini International Neuropsychiatric Interview for DSM IV, English version 5.0 (Sheehan et al. 1998).
Participants were excluded if they reported any of the following conditions: 1) current or past history of any major Axis 1 psychiatric disorder, 2) use of medication for premenstrual syndrome, including herbal treatments and oral or injectable contraceptives, 3) use of psychoactive medications or recreational drugs, including ethanol more than two times per week, or marijuana more than once per week, 4) contraindications to MRI scanning, including implanted ferromagnetic devices, pacemakers, or claustrophobia. Self-report measures over the first two months of the study were used to confirm the diagnosis of PMDD and to ensure that the control participants were asymptomatic throughout the follicular and late luteal phases, as previously described (Rapkin et al. 2011).
Data from one PMDD participant, studied with PET, was excluded from structural analysis due to an abnormal MRI image (as detailed in the next section). Data from another participant with PMDD, and one without PMDD, who were excluded from the PET study due to unusable PET scans, were included in this study. The twenty-five usable participants were 20–41 years of age (mean = 30.1, standard deviation [SD] = 6.48). The age of the twelve participants with PMDD (30.9 +/− 6.63) did not differ significantly from the thirteen control participants (29.2 +/− 6.50) as assessed by a 2-tailed t-test (p > 0.5).
Data Acquisition and Analysis
Brain grey matter structure was assessed by a high-resolution, sagittal T1-weighted, 3D volumetric MRI scan (1.5 T, Siemens Sonata) using a whole-brain MPRAGE sequence (repetition time/echo time = 25/11 ms, number of excitations = 1, slice thickness = 1.2 mm contiguous, in-plane resolution = 1 × 1 mm2, runtime = 10 min). Analyses were performed using publicly available software for voxel-based morphometry (VBM dbm.neuro.uni-jena.de/vbm8/VBM8-Manual.pdf) within a commonly used brain image analysis package for Statistical Parametric Mapping (SPM8 http://www.fil.ion.ucl.ac.uk/spm/software/spm8/).
Using a high-dimensional DARTEL normalization procedure included in the VBM8 software (Ashburner, 2007), the original T1 images were transformed into the template space developed at the Montreal Neurological Institute (MNI space), and segmented into separate component images representing grey matter, white matter, and cerebrospinal fluid comprised of 1.5 mm3 voxels. Segmented grey matter images were modulated using nonlinear transforms in order to analyze differences in regional GMV, corrected for individual brain size. Recommended automated SPM quality checks were used to assess the output images visually and through box plots and covariance matrices. One outlier was identified (>2.0 SD in structural covariance from the mean whole-brain image, whereas all other images were <0.7 SD). This PMDD participant was also a clinical outlier (>2SD), the only participant in either group whose composite mood summary score was worse during the follicular as compared to luteal phase assessment. For these reasons, the participant was dropped from analysis, resulting in sample sizes of 12 PMDD participants and 13 healthy comparison participants.
The modulated images were smoothed with a Gaussian kernel of 8×8×8 mm3, and entered into a whole-brain SPM analysis that assessed differences in GMV between the PMDD and control group. Because the cerebellum is one of the brain areas with the highest rate of age-related shrinkage, decreasing approximately 2% per decade during adulthood (Eckert 2011; Raz et al. 2001; Raz et al. 2010), a second whole-brain SPM analysis was performed to assess covariance between GMV and age in the two groups. In this analysis, the covariates were the ages of the PMDD and control women. Separate contrasts were used to quantify positive and negative covariation with age across both groups (2 contrasts), positive and negative covariation with age in each group (4 contrasts), and differences between the groups in the covariation of GMV with age (2 contrasts). For further characterization of group differences in GMV with respect to age, GMV values at the location of the largest group difference in GMV was compared between the groups separately for the 13 women under and the 12 women over the age of 30 (median split).
To assess statistical significance, a cluster-forming threshold of p<0.005, uncorrected, was applied voxel-wise. The sizes of resulting clusters were adjusted to correct for the varying degrees of smoothness in different parts of the brain (Hayasaka et al., 2004), and family-wise error (FWE) was applied to correct for multiple comparisons in testing significance of cluster extent (p<0.05).
Because both analyses generated significant whole-brain clusters that were predominantly cerebellar, and the cerebellum was the region of greatest a priori structural interest (see introduction) the location of effects within the cerebellum were further characterized using post hoc region-of-interest (ROI) analyses of the 18 subregions that comprise the cerebellum in an anatomy toolbox that is distributed with SPM8. The toolbox uses a probabilistic atlas of the cerebellar lobules as defined in an fMRI atlas based on an individual cerebellum (Schmahmann et al. 2000), after transformation into MNI-space (Diedrichsen et al. 2009).
Results
Group Differences in GMV
In a whole-brain volumetric analysis, women with PMDD had higher GMV than control women in a cluster of 2,228 voxels in the cerebellum (whole-brain corrected p = 0.035; peak t = 5.07 at location −22, −80, −20). There were no other significant group differences in GMV.
Relationship of GMV to Age (Table 1)
Table 1.
Covariation of Age With Brain GMV in Women With PMDD and Healthy Control Participants.
| Voxel of Peak Effect | Cluster | |||||
|---|---|---|---|---|---|---|
| x | y | z | t | p | k | |
| Main effect: Negative covariationa | 21 | −48 | −6 | 5.19 | .033 | 2303 |
| Interaction with Groupb | −22 | −80 | −20 | 5.16 | .041 | 2135 |
| Negative covariation: control onlyc | 21 | −48 | −6 | 5.24 | .021 | 2692 |
| −10 | −46 | 72 | 5.49 | .041 | 2132 | |
Statistical parametric t-maps were thresholded at p < .005 (t > 2.82) with clusters containing > 100 contiguous voxels. All clusters that were significant for spatial extent (size) after whole-brain volume correction are listed in the table. The x, y, and z coordinates represent approximate millimeters to the right, anterior, and superior, respectively, of the midpoint of the anterior commissure,
p = whole-brain volume-corrected, spatial-extent probability; k = number of voxels; GMV, grey-matter volume; PMDD, premenstrual dysphoric disorder
This contrast represents negative covariation across both groups of participants. The other possible main effect (positive covariation) generated no significant clusters.
This contrast yields greater negative covariation in control than PMDD participants (or greater positive covariation in PMDD than control participants). The other possible interaction (greater positive covariation in control than PMDD participants or greater negative covariation in PMDD than control participants) generated no significant clusters.
This simple effect contrast is the only one that generated significant clusters – i.e., there were no significant clusters of negative covariation in PMDD participants, or positive covariation in either group.
Across all participants (13 control, 12 PMDD) GMV was negatively correlated with age in a cluster that extended from the right posterior cerebellar hemisphere anterosuperiorly into the lingual gyrus and parahippocampal gyrus of the cerebral cortex (see Table 1, first line). There was a group difference in the slope of the relationship of age to GMV, with the relationship being more negative in the healthy control than the PMDD group for a cluster restricted to the left posterior cerebellar hemisphere (see Table 1, second line). The peak t-score for this group difference was at the same location as the peak t-score for the group difference in GMV reported in the preceding section; −22,−80,−20. The clusters representing the spatial extent of the two effects were almost identical (see Figure 1). Finally, in the healthy control group assessed alone, there were two significant clusters where GMV decreased with age. One of these clusters was congruent with the cluster representing the main effect of negative covariation across groups with a peak t-score at the same voxel (compare first line to third line in Table 1); the second was in the left superior parietal lobe.
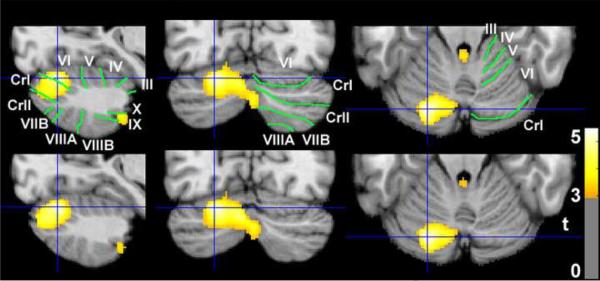
Figure 1.
Differences between women with premenstrual dysphoric disorder (PMDD) and healthy control women in grey-matter volume (GMV) and the covariation of age with GMV. The grey-scale images (neurological orientation) depict cerebellar slices through a T1 structural magnetic resonance image in standard space (Montreal Neurological Institute) where positive values of the x, y, and z coordinates approximately represent millimeters to the right, anterior and superior to the sagittal midpoint of the anterior commissure. The color bar indicates t-values exceeding 2.82 (p<.005). The slices were selected at MNI −22, −80, −20, the location of the highest t-score for group differences in both GMV (PMDD > Control; top row) and covariation of GMV with age (Age × Group interaction, inverse covariation in control, but not PMDD group; bottom row). Colored clusters other than the cluster in the crosshairs were not significant for spatial extent after whole-brain volume correction. Green lines demarcate and white text labels the fissures and lobules of the cerebellum (Schmahmann et al. 2000).
In women under 30, the mean GMV at the location of maximum group differences (voxel −22, −80, −20) was not significantly higher in the PMDD group than in the control group (mean [sd] = .72 [.06] vs .66 [.05] 2-tailed t-test p = .12). In contrast, for women over 30, the mean GMV was significantly higher in the PMDD group than the control group (mean [sd] = .76 [.03] vs .62 [.04] p = .0002).
There were no areas of positive covariation (i.e., where GMV increased with age) across groups or in either individual group. There were no areas of negative covariation in the PMDD group, and no areas where the slope of the relationship of GMV to age was more positive in the control group than the PMDD group.
Localization of Effects within the Cerebellum
Both whole-brain analyses found significant group differences only in the cerebellum. Post hoc ROI analyses were employed to characterize where in the cerebellum these effects were localized. Group differences attaining p < .005 in GMV and the relationship of GMV to age within the cerebellar lobules were very similar in spatial localization (Figure 1). Both effects covered a greater proportion of the cerebellar vermis than the cerebellar hemispheres (22% vs 4% for GMV differences, 20% vs 5% for GMV by age differences), and covered a greater proportion of six ROIs previously associated with emotional processing (Stoodley and Schmahmann 2009) as compared to the other 12 ROIs (8% compared to 5% for GMV, 9% vs 5% for the relationship of GMV to age) (see Table 2).
Table 2.
Group Differences in GMV and the Relationship of GMV in the Cerebellum to Age.
| GMV | Age × Group Interaction | |||||||
|---|---|---|---|---|---|---|---|---|
| Cerebellum ROI | Size | %<0.005 | t | P(t)FWE | %<0.005 | T | p(t)FWE | |
| I–IV | Hemispheres | 6796 | 1% | 3.26 | NS | 0% | 3.01 | NS |
| V | Hemispheres | 11790 | 0% | 3.17 | NS | 1% | 3.54 | NS |
| VI | Vermis | 2550 | 9% | 4.06 | 0.045 | 10% | 4.40 | 0.023 |
| VI | Hemispheres | 20040 | 5% | 5.07 | 0.037 | 6% | 5.16 | 0.031 |
| VIIA_CrusI | Vermis | 198 | 18% | 4.08 | 0.013 | 22% | 4.31 | 0.008 |
| VIIA_CrusI | Hemispheres | 21248 | 8% | 5.07 | 0.050 | 9% | 5.16 | 0.042 |
| VIIA_CrusII | Vermis | 677 | 31% | 4.00 | 0.025 | 38% | 4.40 | 0.011 |
| VIIB | Vermis | 1223 | 39% | 4.08 | 0.025 | 43% | 4.40 | 0.014 |
| VIIA_CrusII | Hemispheres | 15338 | 7% | 4.20 | NS | 9% | 4.43 | NS |
| VIIB | Hemispheres | 12891 | 3% | 4.08 | NS | 4% | 4.40 | NS |
| VIIIA | Verm | 2533 | 31% | 4.05 | 0.040 | 30% | 4.30 | 0.024 |
| VIIIA | Hemispheres | 13892 | 2% | 4.05 | NS | 3% | 4.30 | NS |
| VIIIB | Vermis | 2022 | 28% | 3.91 | 0.040 | 22% | 4.16 | 0.025 |
| VIIIB | Hemispheres | 11678 | 3% | 4.37 | NS | 2% | 4.18 | NS |
| IX | Vermis | 1970 | 13% | 3.41 | NS | 6% | 3.47 | NS |
| IX | Hemispheres | 8642 | 4% | 4.57 | 0.045 | 2% | 4.43 | NS |
| X | Vermis | 839 | 5% | 3.26 | NS | 3% | 2.99 | NS |
| X | Hemispheres | 2499 | 3% | 3.73 | NS | 3% | 3.94 | NS |
Statistical parametric t-maps of differences between the PMDD and control groups in GMV (left columns) and the relationship of Age to GMV (right columns) were thresholded at p < .005 (t > 2.82 with no extent threshold) within the 18 regions-of-interest (ROIs) that comprise a probabilistic atlas of the cerebellum in MNI-space (Diedrichsen et al. 2009). The size of each ROI is given in 1.5 mm3 voxels, followed by the suprathreshold proportion of the ROI, the peak voxel t-score and it's associated probability, after familywise error correction for the ROI volume. The six bolded rows represent ROIs associated with emotional processing tasks according to a recent meta-analysis (Stoodley and Schmahmann 2009).
PMDD, premenstrual dysphoric disorder; GMV, grey-matter volume; NS, p > .05.
Table two shows that of the 18 cerebellar partitions assessed, peak effects retained statistical significance (p<.05 after ROI volume-correction) in the six partitions associated with emotional processing and the two comprising vermis lobule VIII. We did not define this region as “emotional” a priori because the meta-analysis of Stoodley and Schmahmann (2009) reported that emotional processing was associated with activity in lobule VI and the Crus I and vermis portions of lobule VII. However, examination of their Table 3 revealed that one of the clusters associated with emotion had a peak (MNI −4,−80, −34) that was labeled as being in vermis lobule VII in their paper, whereas it is localized to vermis lobule VIII according to the SPM anatomy toolbox (Diedrichsen et al. 2009). This observation suggests that emotional processing in the vermis may extend posterior and inferiorly into lobule VIII. The vermis of lobule VIII comprises only 3.3 % of the total cerebellum. However, including it with the other six regions we defined a priori as belonging to the “emotional” cerebellum would increase the disparity between the proportion of the emotional and non-emotional cerebellum showing group structural differences attaining p < .005 (10% compared to 3% for GMV, 11% vs 3% for the relationship of GMV to age). This idea is consistent with a previous report of abnormally low gray matter density in lobule VIII in first-episode patients with major depressive disorder (Peng et al. 2010).
Discussion
We previously showed that women with PMDD have a greater increase in cerebellar activity from the follicular phase to the symptomatic late luteal phase than healthy control women, the degree of this increase being correlated with symptom severity (Rapkin et al. 2011). The group difference in menstrual phase-related increase in activity was localized primarily to midline vermis and fastigial cerebellar regions, which have been described as being part of the “limbic” or “emotional” cerebellum (Schmahmann and Sherman 1998; Schmahmann et al. 2007). Our finding of a cerebellar effect in a mood disorder, along with reports of elevated glucose metabolism in the midline cerebellum and vermis in unipolar and bipolar depressed patients (Bench et al. 1992; Ketter et al. 2001; Kimbrell et al. 2002), adds to the literature implicating the cerebellum in a wide range of behaviors involving emotion, pain, and cognition (Schmahmann et al. 2007; Strick et al. 2009).
We now show that the cerebellar regions that have been associated with emotional processing, particularly in the vermis, also show greater GMV in women with PMDD as compared to healthy control women. There was no evidence for group differences elsewhere in the brain. A recent report found women with PMDD had greater GM density than women without PMDD in a small cluster in the left hippocampal gyrus, and less GM density in a smaller cluster in the left parahippocampal gyrus (Jeong et al. 2012). However, that study used an early version of SPM, without the high dimensional DARTEL algorithm which improves VBM registration, and the results were not corrected for multiple-comparisons in whole brain.
In the current study, control women but not women with PMDD exhibit a negative correlation of age with GMV in the cerebellum, producing a significant GMV by Age group difference in essentially the same location as the GMV group difference. In addition, the peak GMV difference between the groups remains significant for women over but not under age 30. Therefore, we conclude that PMDD is associated with reduced age-related loss of GMV in the “emotional” cerebellum.
Our previous demonstrations of greater increase in cerebellar activity from the follicular to the late luteal phase in women with PMDD as compared with healthy control women, and the proportionality of relative glucose metabolism to symptom severity (Rapkin et al. 2011) both attained maximal t-values in a more anterior part of the “emotional” cerebellum than the maximal values of the structural differences reported here. If the current results are viewed at a lower threshold of p < 0.05, however, it can be seen that relative protection against age-related GMV loss in the women with PMDD (see Figure 2) extends into much of the area that showed significant effects in our prior study. In combination with demonstrations that effort-related increases in local activity over periods as brief as a few hours can increase local GMV (Draganski et al. 2004; Kwok et al. 2011), this observation suggests that cumulative monthly periods of greater symptom-related cerebellar activity may be responsible for the preserved GMV in the cerebellum of older women with PMDD, as compared to control women.
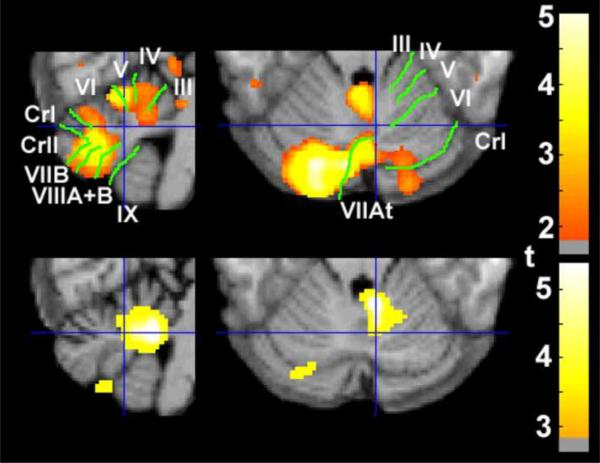
Figure 2.
Relative protection against age-related loss in grey-matter volume (GMV) overlaps with the area of greater activity during the symptomatic phase of the menstrual cycle in women with premenstrual dysphoric disorder (PMDD), relative to healthy control women. The grey-scale images (neurological orientation) depict cerebellar slices through a T1 structural magnetic resonance image in standard space (Montreal Neurological Institute) 8 mm to the right (left panels), and 24 mm inferior (right panels) to the sagittal midpoint of the anterior commissure. The Group × Age interaction for GMV depicted using a liberal threshold (top row; p < .05 uncorrected) covers most of the area where we previously reported greater symptom-related functional activity (bottom row; p < .005, reprinted with permission (Rapkin et al. 2011)). Green lines demarcate and white text labels the fissures and lobules of the cerebellum (Schmahmann et al. 2000).
What could preserve cerebellar GMV during aging of woman with PMDD? Phase-related difficulty in concentrating is one of the standard diagnostic criteria for PMDD, and complaints of impaired memory and motor coordination are common in the disorder (American Psychiatric Association 1994; Diener et al. 1992). Assessments of the extent to which cognitive performance is impaired during the symptomatic luteal phase of the menstrual cycle have been inconsistent, however, showing either no impairment (Morgan and Rapkin 2002; Rapkin et al. 1989) or mild deficits on isolated tasks (Diener et al. 1992; Evans et al. 1998; Man et al. 1999; Posthuma et al. 1987; Resnick et al. 1998). Comparisons of cognitive performance between women with PMDD and healthy women have similarly yielded mixed findings. Women with PMDD did show worse cognitive performance in the late luteal phase than control women in two recent studies that attempted to improve on earlier studies. One study increased power by testing a sample of 120 women (Yen et al. 2011), and the other tried to better simulate real-life working conditions through prolonged cognitive assessments over 4 h on each of four different days during each menstrual phase (Reed et al. 2008). Relatively modest deficits in both studies, combined with the earlier inconsistent findings, suggest that during symptomatic days most women with PMDD can employ more effort, or use other compensatory mechanisms which preserve performance when cognitive demands are not extreme or prolonged. Since a woman with PMDD can experience 3000 days of severe symptoms over her lifetime (Rapkin and Winer 2009), it seems possible that compensatory mechanisms employed to regulate negative emotions and counteract symptom-related cognitive difficulties may act as effortful mental “exercises” that preserve structure in the cognitive/emotional cerebellum during aging just as running with leg weights for part of each month would preserve muscle structure.
Another possible explanation for the preserved cerebellar structure in women with PMDD involves the hormone leptin. High metabolic demands make cerebellar Purkinje cells extremely vulnerable to damage through oxidative stress during aging (Andersen et al. 2003; Cui et al. 2009; Lee et al. 2000; Sim et al. 2007). Brain imaging studies suggest leptin, which is neuroprotective and expresses the highest receptor density in the cerebellum (Oldreive et al. 2008), may reduce oxidative stress. Cerebellar GMV has been correlated with plasma leptin concentration in older adults (Narita et al. 2009), and cerebellar GMV was increased when genetically leptin-deficient adults began receiving daily supplements (Matochik et al. 2005), but reversibly reduced when leptin supplements were withheld for a month (London et al. 2011). Both of the studies that have reported plasma leptin concentration in women with PMDD found higher median values than control women, with the highest values during the luteal phase (Anim-Nyame et al. 2000; Tommaselli et al. 2003). These findings suggest that elevated leptin in women with PMDD may contribute to protection against age-related gray matter loss in the cerebellum.
The study presented here is limited by relatively small sample size and failure to explicitly assess the effect of menstrual cycle phase on brain structure. However, the proportion of subjects who were scanned during the luteal phase was equivalent in the two groups. Further studies addressing cerebellar circuitry and age-related cognitive functioning in women with PMDD are indicated, and may shed light on mechanisms of GM preservation and cognitive preservation during aging.
Acknowledgements
This study was supported in part by the National Institute of Health M01-RR00865 (General Clinical Research Centers Program), a grant from the Oppenheimer Foundation (AR), and endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies and the Marjorie M. Greene Family Trust (EDL). None of the sponsors had any involvement with the design, collection, analysis, or interpretation of data, writing the manuscript, or the decision to submit the manuscript for publication.
The Ahmanson-Lovelace Brain Mapping Center, where imaging data were collected, is supported by the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, the Pierson-Lovelace Foundation, the Ahmanson Foundation, the Tamkin Foundation, the Jennifer Jones-Simon Foundation, the Capital Group Companies Charitable Foundation, the Robson Family, and the Northstar Fund.
We thank Linda Goldman RNP for help with the screening of participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: There are no conflicts of interest for any of the authors for the paper “Elevated Gray Matter Volume of the Emotional Cerebellum in Women with Premenstrual Dysphoric Disorder Disorder”
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, DC: 1994. p. 888. [Google Scholar]
- Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466(3):356–65. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- Anim-Nyame N, Domoney C, Panay N, Jones J, Alaghband-Zadeh J, Studd JW. Plasma leptin concentrations are increased in women with premenstrual syndrome. Hum Reprod. 2000;15(11):2329–32. doi: 10.1093/humrep/15.11.2329. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A Fast Diffeomorphic Image Registration Algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Andersson A, Andree L, Birzniece V, Bixo M, Bjorn I, et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia--focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22(3):607–15. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Cui L, Hofer T, Rani A, Leeuwenburgh C, Foster TC. Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiol Aging. 2009;30(6):903–9. doi: 10.1016/j.neurobiolaging.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Diener D, Greenstein FL, Turnbough PD. Cyclical variation in digit-span and visual-search performance in women differing in the severity of their premenstrual symptoms. Percept Mot Skills. 1992;74(1):67–76. doi: 10.2466/pms.1992.74.1.67. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5:25. doi: 10.3389/fnins.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9(1):41–9. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851–8. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Levin FR, Foltin RW, Fischman MW. Mood and performance changes in women with premenstrual dysphoric disorder: acute effects of alprazolam. Neuropsychopharmacology. 1998;19(6):499–516. doi: 10.1016/S0893-133X(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28(Suppl 3):1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Ham BJ, Yeo HB, Jung IK, Joe SH. Gray matter abnormalities in patients with premenstrual dysphoric disorder: An optimized voxel-based morphometry. J Affect Disord. 2012 Feb 28; doi: 10.1016/j.jad.2012.02.010. Epub. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biological Psychiatry. 2001;49(2):97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51(3):237–52. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Kwok V, Niu Z, Kay P, Zhou K, Mo L, Jin Z, et al. Learning new color names produces rapid increase in gray matter in the intact adult human cortex. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1103217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25(3):294–7. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Chakrapani S, Delibasi T, Monterosso J, Erol HK, et al. Short-term plasticity of grey matter associated with leptin deficiency and replacement. The Journal of Clinical Endocrinology & Metabolism. 2011;96(8):E1212–20. doi: 10.1210/jc.2011-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man MS, MacMillan I, Scott J, Young AH. Mood, neuropsychological function and cognitions in premenstrual dysphoric disorder. Psychol Med. 1999;29(3):727–33. doi: 10.1017/s0033291798007715. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. The Journal of Clinical Endocrinology & Metabolism. 2005;90(5):2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- Morgan M, Rapkin A. Cognitive flexibility, reaction time, and attention in women with premenstrual dysphoric disorder. J Gend Specif Med. 2002;5(3):28–36. [PubMed] [Google Scholar]
- Narita K, Kosaka H, Okazawa H, Murata T, Wada Y. Relationship between plasma leptin level and brain structure in elderly: a voxel-based morphometric study. Biol Psychiatry. 2009;65(11):992–4. doi: 10.1016/j.biopsych.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oldreive CE, Harvey J, Doherty GH. Neurotrophic effects of leptin on cerebellar Purkinje but not granule neurons in vitro. Neurosci Lett. 2008;438(1):17–21. doi: 10.1016/j.neulet.2008.04.045. [DOI] [PubMed] [Google Scholar]
- Peng J, Liu J, Nie B, Li Y, Shan B, Wang G, et al. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: A voxel-based morphometry study. Eur J Radiol. 2010;80(2):395–9. doi: 10.1016/j.ejrad.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Posthuma BW, Bass MJ, Bull SB, Nisker JA. Detecting changes in functional ability in women with premenstrual syndrome. Am J Obstet Gynecol. 1987;156(2):275–8. doi: 10.1016/0002-9378(87)90267-5. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1–2):87–94. doi: 10.1016/j.jad.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Berman SM, Mandelkern MA, Silverman DH, Morgan M, London ED. Neuroimaging evidence of cerebellar involvement in premenstrual dysphoric disorder. Biol Psychiatry. 2011;69(4):374–80. doi: 10.1016/j.biopsych.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ, Chang LC, Reading AE. Mood and cognitive style in premenstrual syndrome. Obstet Gynecol. 1989;74(4):644–9. [PubMed] [Google Scholar]
- Rapkin AJ, Winer SA. Premenstrual syndrome and premenstrual dysphoric disorder: quality of life and burden of illness. Expert Rev Pharmacoecon Outcomes Res. 2009;9(2):157–70. doi: 10.1586/erp.09.14. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol. 2001;22(6):1161–7. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. White matter deterioration in 15 months: latent growth curve models in healthy adults. Neurobiol Aging. 2010;33(2):429, e1–5. doi: 10.1016/j.neurobiolaging.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Levin FR, Evans SM. Changes in mood, cognitive performance and appetite in the late luteal and follicular phases of the menstrual cycle in women with and without PMDD (premenstrual dysphoric disorder) Horm Behav. 2008;54(1):185–93. doi: 10.1016/j.yhbeh.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick A, Perry W, Parry B, Mostofi N, Udell C. Neuropsychological performance across the menstrual cycle in women with and without Premenstrual Dysphoric Disorder. Psychiatry Res. 1998;77(3):147–58. doi: 10.1016/s0165-1781(97)00142-x. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, et al. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. American Journal of Obstetrics and Gynecology. 1988;158:5–11. doi: 10.1016/0002-9378(88)90765-x. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. The neuroendocrinology of menstrual cycle mood disorders. Ann N Y Acad Sci. 1995;771:648–59. doi: 10.1111/j.1749-6632.1995.tb44717.x. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Front Neuroendocrinol. 2006;27(2):210–6. doi: 10.1016/j.yfrne.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans A. MRI Atlas of the human cerebellum. Academic Press; San Diego: 2000. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum-insights from the clinic. Cerebellum. 2007;6(3):254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan H, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry. 2003;54(7):757–62. doi: 10.1016/s0006-3223(02)01924-8. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tommaselli GA, Di Carlo C, Bifulco G, Di Spiezio Sardo A, Pellicano M, Nappi C. Serum leptin levels in patients with premenstrual syndrome treated with GnRH analogues alone and in association with tibolone. Clin Endocrinol (Oxf) 2003;59(6):716–22. doi: 10.1046/j.1365-2265.2003.01911.x. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. 2002;32(1):119–32. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]
- Yen JY, Chang SJ, Long CY, Tang TC, Chen CC, Yen CF. Working memory deficit in premenstrual dysphoric disorder and its associations with difficulty in concentrating and irritability. Compr Psychiatry. 2011 Aug 5; doi: 10.1016/j.comppsych.2011.05.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]