Abstract
Estrogen deficiency changes the regional distribution of tissue mineral density leading to alteration of the mechanical properties of bone at the tissue level. Direct measurement of the regional variation of elastic modulus and viscosity, which is the capacity to resist time-dependent viscoelastic deformation, will aid in our understanding of how estrogen deficiency alters bone quality. It was observed that, compared to bone from other anatomical sites, the jaw bone is less sensitive to estrogen deficiency. Thus, the objective of this study was to examine the effect of estrogen deficiency on 1) the regional variations of tissue modulus and viscosity of bone using nanoindentation, and 2) the modulus-viscosity relationships in jaw and vertebral bones for comparison between different anatomical sites. Mandibular and vertebral bone specimens of sham surgery and ovariectomized (OVX) rat groups were subject to nanoindentation in hydration. Indentation modulus and viscosity were measured at relatively new (less mineralized) tissue regions and at the corresponding pre-existing old (more mineralized) tissue regions of mandibular and vertebral bones. In the mandibular bones, significant regional variations of indentation modulus and viscosity were observed (p<0.039) and OVX increased the indentation viscosity. While significant positive correlations were found between indentation modulus and viscosity (p<0.001), the correlation slopes for the mandibular and vertebral bones were significant different (p<0.001). The current results indicated that changes in viscoelastic property and its regional variation should be examined to obtain a better understanding of estrogen deficiency-dependent alteration of bone quality.
Keywords: Nanoindentation, Estrogen deficiency, Viscosity, Mandible, Vertebra
INTRODUCTION
Estrogen deficiency-dependent active bone remodeling causes unbalanced bone turnover, resulting in bone loss in postmenopausal patients (Lerner, 2006). In addition to this alteration of bone quantity, active bone remodeling can increase resorption of old bone, which is in turn followed by the formation of new bone tissue. This process changes the quality of the bone by amplifying the variability of mineral density at the tissue level, as observed in postmenopausal human and animal bones (Ames et al., 2010; Busse et al., 2009; Deguchi et al., 2008). Recently, it was found that the variability of tissue mineral density determines unrecoverable residual deformation at the macro level of human and animal vertebral bones, resulting from a time-dependent viscoelastic creep deformation (Kim et al., 2011; Kim et al., 2012). It was also found that viscosity, which is a capacity to resist time-dependent viscoelastic deformation, has a strong linear relationship with elastic modulus (Kim et al., 2010), which is linearly correlated with tissue mineral density (Mulder et al., 2008). These findings suggest that the estrogen deficiency-dependent changes of tissue mineral density distribution may alter the distribution of the viscoelastic properties, including viscosity, at the tissue level resulting in the high risk of time-dependent deformation of postmenopausal osteoporotic bone at the organ level. However, the effects of estrogen deficiency on elastic modulus and viscosity of bone at the tissue level have not been fully examined.
The elastic and viscoelastic mechanical properties of bone tissue are mainly determined by the interaction between collagen fibrils and crystalline mineral components, for both human and animal bone (Pathak et al., 2011; Ruppel et al., 2008; Wu et al., 2012). Bone tissue maturity (mineral-to-matrix ratio) was used to reflect bone quality (Pathak et al., 2011; Ruppel et al., 2008). It was indicated that the mineral-to-matrix ratio was lower in newly-formed bone tissue regions than in pre-existing old bone tissue regions when mouse femurs were observed under Raman spectroscopy (Pathak et al., 2011). The mineral-to-matrix ratio of bone tissue also varies depending on anatomical site (Fu et al., 2012; Otomo et al., 2004).Pathak et al. (2011) found a larger viscoelastic response in a region of tissue with a smaller mineral-to-matrix ratio using nanoindentation-based dynamic mechanical analysis. However, a lack of knowledge exists about the effects of estrogen deficiency on the regional variation of the mechanical properties of bone at the tissue level.
Obtaining more information about the mechanical properties of bone at the tissue level can provide baseline knowledge to better understand how the load bearing ability of bone changes with disease, including estrogen deficiency-induced postmenopausal osteoporosis. In this study, we hypothesized that direct measurement of the regional variation of elastic modulus and viscosity at the tissue level will help us to understand how estrogen deficiency-dependent abnormal bone remodeling alters bone quality differently between bone regions and anatomical sites. It was observed that the jaw bone is less sensitive to estrogen deficiency than bone from another anatomical site (Ejiri et al., 2008; Mavropoulos et al., 2007). Thus, the objective of this study was to examine the effect of estrogen deficiency on 1) the regional variations of tissue modulus and viscosity of bone using nanoindentation, and 2) the modulus-viscosity relationships in jaw and vertebral bones for comparison between different anatomical sites.
MATERIALS AND METHODS
Specimen preparation
Sprague-Dawley female rats (6 months old) were obtained following experimental protocol approval by the Institutional Animal Care and Use Committee (IACUC) of The Ohio State University. A group of rats had a sham operation (sham) and the other group of rats underwent a bilateral ovariectomy (OVX) operation at Harlan Sprague Dawley, Inc (Indianapolis, IN). The rats were fed a normal diet for 2 months post-surgery and were then euthanized. Tooth-bearing 5 mm mandibular bone sections were made in the bucco-lingual direction using a low speed saw with two parallel diamond blades under water irrigation (Fig. 1a). Three lumbar vertebrae (L3, L4 and L5) were also obtained from each rat (Fig. 1b). A total of 20 mandibular specimens (sham: 6 left and 4 right sides, and OVX: 4 left and 6 right sides) and 12 vertebral specimens (sham: 2 L3, 3 L4, 1 L5; and OVX: 2 L3, 3 L4, 1 L5) were prepared from randomly selected rats and subject to nanoindentation. Each specimen was obtained from an individual rat. The fresh specimens were stored −20 °C until nanoindentation.
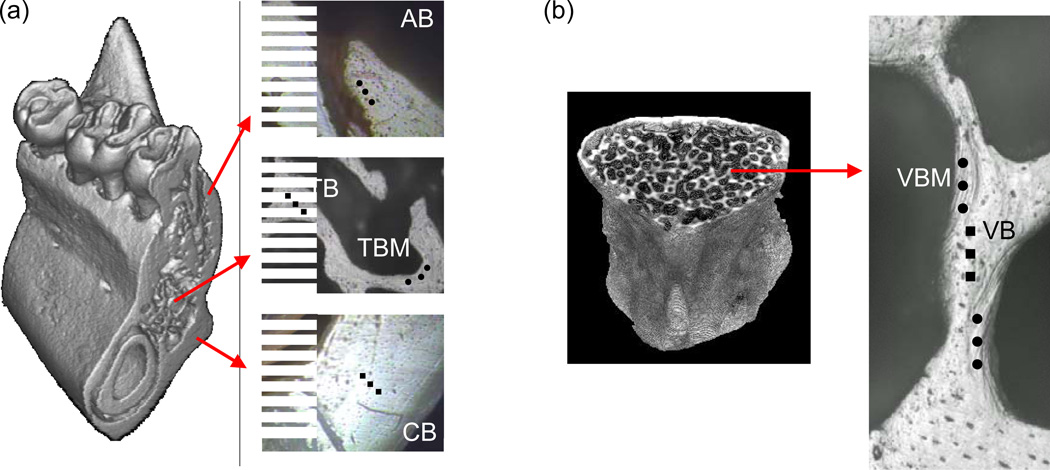
Fig. 1.
Descriptive regions for nanoindentation in 3D micro-CT images and under an indenter microscope; (a) Four mandibular regions (AB, alveolar bone (black dots); CB, cortical basal bone (black squares); TBM, marginal region of trabecular bone (black dots); TB, inner region of trabecular bone (black squares)) and (b) two vertebral regions (VBM, marginal region of vertebral bone (black dots) and VB, inner region of vertebral bone (black squares)).
The sliced surfaces of the bone specimens were polished under wet conditions. The polished specimens were glued onto a polycarbonate holder that has a fluid drainage system as previously introduced (Huja et al., 2006) and mounted on a nanoindenter (Nano-XP, MTS, Oakridge, TN). Indentation regions were determined using the nanoindenter microscope (Fig. 1a, b). Two paired regions in the mandibular bone and one paired region in the vertebral bone were identified. One of the mandibular bone paired regions was the alveolar region (AB), in which rapid bone turnover is stimulated by mastication, and the other was the cortical basal (CB) region, in which routine bone turnover is anticipated (Mavropoulos et al., 2004; Renders et al., 2006). Other paired regions of interest were marginal (TBM) and inner (TB) regions of mandibular trabecular bone, and marginal (VBM) and inner (VB) regions of vertebral trabecular bone. These regions were examined because the alveolar and marginal regions (AB, TBM and VBM) under rapid bone turnover have newer bone tissue relative to the basal and inner regions (Renders et al., 2006). Inter-indent locations were at least 50 µm apart to prevent any interruptions from the adjacent indents. A total of 579 indentations were successfully performed. The 302 indents from the sham group (71 indents from AB, 41 from CB, 42 from TBM, 38 from TB, 62 from VBM and 48 from VB) and the 277 indents from the OVX group (74 indents from AB, 41 from CB, 28 from TBM, 28 from TB, 54 from VBM and 52 from VB) were analyzed.
Nanoindentation
All nanoindentations were performed under wet conditions by dripping 0.5 mg/ml solution of gentamicin sulfate (Sigma, St. Louis, MO) solution to maintain wet specimens (Huja et al., 2007). All indentations were conducted under load control with indentation depth equivalent to 500 nm, with corresponding displacement rates of 50 nm/sec for the mandibular specimens and 100 nm/sec for the vertebral specimens. After a 30-second hold period under a constant peak load, the indenter was unloaded with the corresponding displacement rates of 50 nm/sec for the mandibular specimens and 10 nm/sec for the vertebral specimens. Nanoindentation modulus was measured using Eq. 1.
| (Eq.1) |
where Er (reduced modulus) is measured as the unloading slope from the displacement-force curve. The indices s and i refer to the specimen and the indenter material, respectively, and ν is Poisson’s ratio. For diamond, values of Ei = 1141 GPa and νi = 0.07 are typically used. Poisson’s ratio for bone was assumed to be 0.3 following a previous study (Huja et al., 2007).
Creep was measured as the displacement during the 30-second hold period at peak load (Fig. 2). The creep displacement-time curve of each indentation was fitted using the following Voigt model (Eq.2).
| (Eq.2) |
where h(t) is creep displacement (nm) as a function of time, Pmax is the peak load during the hold period, α is an equivalent cone semi-angle (70.3°) to the face angle of the Berkovich indenter (65.27 °) (Fischer-Cripps, 2004), E2 is an elastic element of the Voigt model (GPa) and η is the indentation viscosity (GPaS) term.
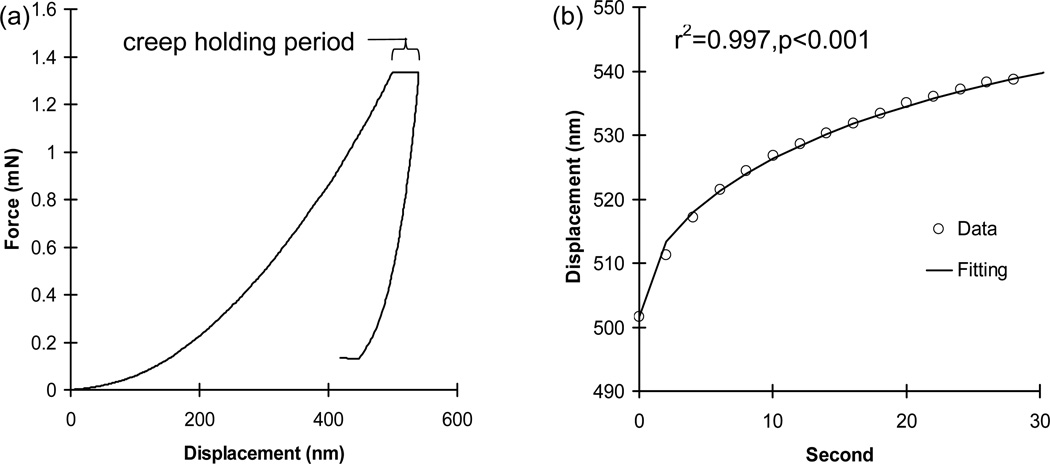
Fig. 2.
(a) A typical nanoindentation creep curve (b) with an excellent curve fitting.
Statistical analysis
A Student’s t-test was performed to compare the BV/TV and BMD between the sham and OVX groups. Analysis of variance (ANOVA), followed by Fisher’s PLSD post hoc test, was utilized to compare the regional variations of nanoindentation modulus (E) and viscosity (η). Correlations of η with E were examined using a linear regression test for the mandibular and vertebral groups. If the correlations turned out to be significant for both groups, analysis of covariance (ANCOVA) was used to test whether the correlations were different between the groups. If the correlation was not significantly different, the data of both groups were pooled. Additional ANCOVA was performed to test whether the correlations were changed by OVX. Significance was set at p<0.05.
RESULTS
Excellent fittings were obtained for all nanoindentation creep curves using the Voigt model (r2>0.96, p<0.001, Fig. 2b). Means of indentation moduli of the relatively new bone tissue regions (AB, TBM, and VBM) were significantly lower than those of the corresponding pre-existing old bone tissue regions (CB, TB, and VB) for the sham group (p<0.039) (Table 1). The alveolar (AB) regions and the marginal regions of the mandibular trabecular bones (TBM) of the OVX group had significantly and marginally lower means of indentation moduli than the mandibular cortical basal (CB) regions (p<0.001) and the inner regions of the mandibular trabecular bones (TB) (p=0.068), respectively. However, no significant differences were found for the means of indentation moduli between the vertebral trabecular bone regions (VBM and VB) of the OVX group (p=0.205). Means of indentation viscosities of the relatively new mandibular regions (AB and TBM) were significantly lower than those of the corresponding pre-existing old mandibular regions (CB and TB) for both the sham and OVX groups (p<0.039), while those between vertebral regions (VBM and VB) were not significantly different for both the sham and OVX groups (p≥0.284) (Table 1).
Table 1.
Comparison of indentation modulus (E) (GPa) and viscosity (η) (GPa S) between mandibular alveolar bone and cortical basal bone (AB vs. CB), marginal region and the inner region of mandibular trabecular bone (TBM vs. TB), and marginal region and inner region of vertebral bone (VBM vs. VB) for sham and OVX groups.
| Organ | Region | Treatment | ||||
|---|---|---|---|---|---|---|
| sham | OVX | |||||
| E (GPa) | Mandible | AB | 13.21±4.85 | p<0.003 | 14.50±7.58 | p<0.001 |
| CB | 16.91±7.83 | 18.68±5.83 | ||||
| TBM | 14.28±3.95 | p<0.039 | 15.40±5.32 | p=0.068 | ||
| TB | 17.15±4.97 | 18.42±3.58 | ||||
| Vertebra | VBM | 18.02±7.72 | p<0.018 | 16.95±6.26 | p=0.205 | |
| VB | 20.84±6.46 | 18.47±5.59 | ||||
| η (GPa S) | Mandible | AB | 11672.82±6420.32 | p<0.001 | 13629.23±9185.23 | p<0.001 |
| CB | 17577.52±11494.92 | 20906.67±9367.91 | ||||
| TBM | 12334.02±5305.39 | p<0.002 | 16759.50±8639.70 | p<0.039 | ||
| TB | 17420.50±8653.06 | 20719.08±7038.08 | ||||
| Vertebra | VBM | 8222.69±4633.85 | p=0.284 | 7996.94±3966.98 | p=0.473 | |
| VB | 9699.37±4550.68 | 8997.02±4054.34 | ||||
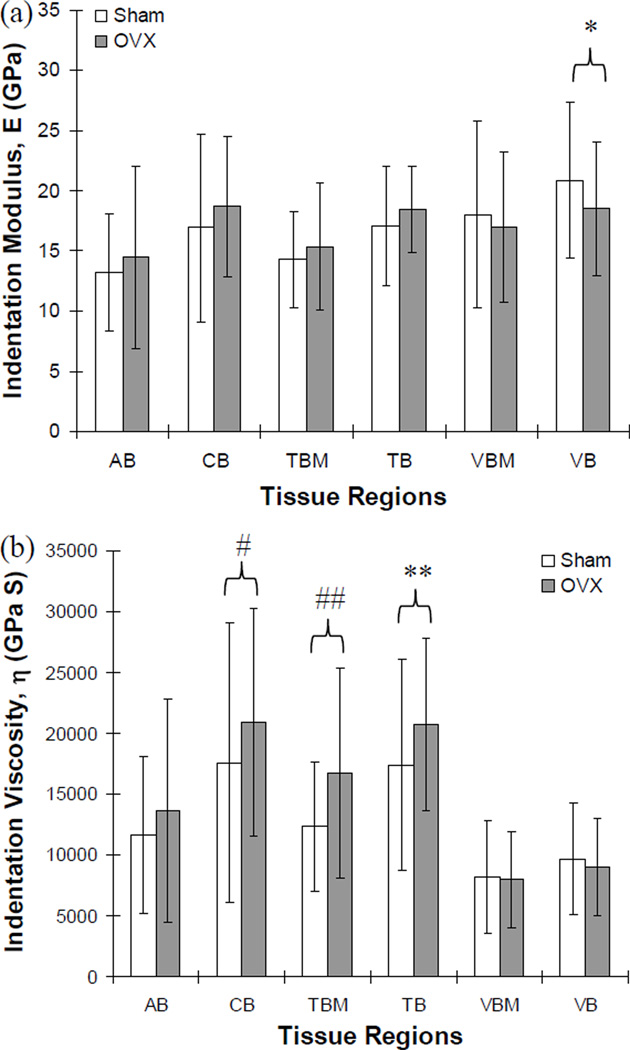
Means of indentation moduli were not significantly different between the sham and OVX group for most of the examined regions (p>0.193), while the sham group had a marginally higher mean of indentation moduli than the OVX group (p=0.055) at the inner regions of the vertebral bones (VB) (Fig. 3a). However, the sham group had significantly lower means of indentation viscosities at the mandibular cortical basal regions (CB, p<0.036) and at the marginal regions of the mandibular trabecular bones (TBM, p<0.012), and marginally lower means at the inner regions of the mandibular trabecular bones (TB, p=0.065) than those for the OVX group (Fig. 3b). All other regions did not have significantly different means of indentation viscosities between the sham and OVX groups (p>0.1).
Fig. 3.
Comparisons of indentation (a) modulus and (b) viscosity between sham and OVX groups. The indentation modulus was marginally different at the inner regions of vertebral trabecular bone (VB, *; p=0.055) between sham and OVX groups while the indentation viscosity was significantly different at the mandibular cortical basal bone (CB, #; p<0.036) and at the marginal region of mandibular trabecular bone (TBM, ##; p<0.012), and marginally different at the inner regions of mandibular trabecular bone (TB, **; p=0.065). No significant difference was found at all other regions (p>0.1).
Overall, the mandibular bones of the sham group had a significantly lower mean of indentation modulus (p<0.001) and a significantly higher mean of indentation viscosities (p<0.001) than the vertebral bones. The mandibular bones of the OVX group did not have significantly different means of indentation moduli (p=0.073), but had significantly higher means of indentation viscosities (p<0.001) than the vertebral bones.
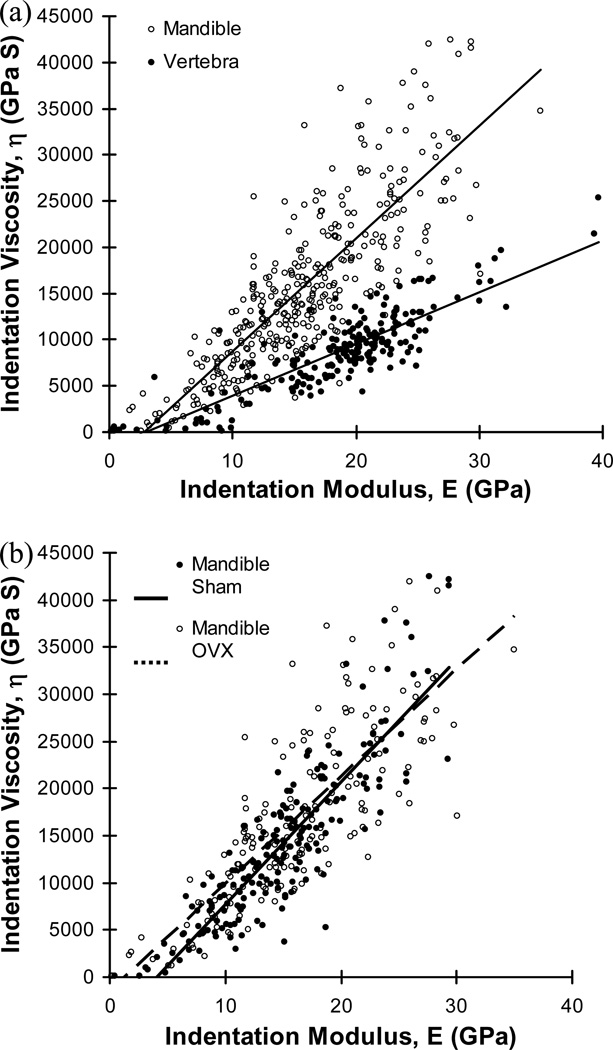
Significant positive correlations between indentation modulus and viscosity were found for both mandibular bones (r2=0.698, p<0.001, n=363) and vertebral bones (r2=0.743, p<0.001, n=216). Mandibular bones had a significantly higher slope of correlation between indentation modulus and viscosity than the vertebral bones (ANCOVA, p<0.001) (Fig. 4a). The slopes of correlation were not significantly different between mandibular regions (ANCOVA, p>0.135), while the sham group had a slightly higher slope of correlation for the mandibular bones than the OVX group (ANCOVA, p=0.05), but the difference was trivial (Fig. 4b). No significantly different correlations were found between regions or between sham and OVX groups of the vertebral bones (ANCOVA, p>0.112).
Fig. 4.
(a) Strong positive correlations between indentation modulus and viscosity were found for mandibles (η=1221.307E-3520.293, r2=0.698, p<0.001, n=363) and vertebrae (η=557.625E-1629.957, r2=0.743, p<0.001, n=216) while the slopes of correlation were significantly different between the two bones (ANCOVA, p<0.001) and (b) significantly different slopes of correlation between sham and OVX groups were found for mandibular bones (ANCOVA, p=0.05) while no significantly different correlation was found for the vertebral bones (ANCOVA, p=0.667).
DISCUSSION
Substantial regional variation of indentation moduli and viscosities was observed in the jaw bone. The OVX affected the mandibular bone by significantly increasing the indentation viscosities at its cortical basal (CB), marginal (TBM) and inner (TB) regions. The significantly higher slope of correlation between indentation modulus and viscosity for the mandibular bone tissues indicates that the mandibular bones have higher sensitivity of viscoelastic response than the vertebral bones with the same elastic property.
Nanoindentation creep behavior of the bone specimens in hydration was described very well by the traditional viscoelastic Voigt model which revealed the significant positive correlation of indentation viscosity with modulus, consistent with observations using different types of bone (Kim et al., 2010).
It was observed that a newly formed marginal region of trabecular bone has less mineralized bone tissue and lower indentation modulus than the old pre-existing tissue of a pig mandibular condyle (Mulder et al., 2007). It was also indicated that the indentation modulus had a positive linear correlation with the degree of bone mineralization. These observations suggest that the regional variation of indentation modulus found in the current study reflects the heterogeneous distribution of tissue mineralization. This regional analysis revealed that tissue modulus decreased only at the inner vertebral bone region, which may result from estrogen deficiency induced osteopenia in OVX rat vertebral bone (Lelovas et al., 2008).
The values and regional variations of indentation viscosity for the mandibular bone were higher than those for the vertebral bone. It was suggested that the masticatory functional demand of the jaw bone can produce regions of differing composition in the bone tissue, as compared to a vertebral bone, which has a load bearing function (Mavropoulos et al., 2007). The amount of strain in a jaw bone under mastication was estimated to be up to 6000 microns, while strain in a vertebra under moderate exercise was measured to be between 2000 and 3000 microns (Daegling and Hylander, 1997; Ehrlich and Lanyon, 2002). These results indicated that more energy is transmitted to a jaw bone than a vertebral bone under functional loading. It was also indicated that bone tissue with a higher indentation viscosity can absorb more energy under loading (Isaksson et al., 2010). Taken together, the current findings suggested that mastication may stimulate mandibular bone remodeling to develop its tissue composition with a higher magnitude of viscosity, which can help to effectively absorb the high impact masticatory energy. However, further biological studies are needed to clarify the underlying mechanism for this mechanobiologic observation.
The higher indentation viscosity of the mandibular bones in the OVX group suggests that the viscoelastic property should be considered an important parameter in characterizing estrogen deficiency-dependent changes of bone. It was observed that estrogen deficiency decreased the bone’s ability to absorb energy-to–failure while increasing the viscoelastic damage in human and rat vertebral bones (Busse et al., 2009; Kim et al., 2011; Kim et al., 2012). In the current study, the OVX mandibular bone tissue has a greater viscosity than the sham groups and the OVX vertebral bone, which could lead to an enhanced capacity for absorbing loading energy and resisting fracture and viscoelastic damage. This finding provides an insight into why estrogen deficiency has less effect on jaw bone than on other load-bearing bones, as observed in previous studies (Binte Anwar et al., 2007; Ejiri et al., 2008; Mavropoulos et al., 2007).
The positive correlation between indentation modulus and viscosity, independent of tissue regions and anatomical sites, was consistent with previous results utilizing a canine femoral bone (Kim et al., 2010). The higher slope of correlation between indentation modulus and viscosity of mandibular bone can account for why the mandibular tissue had higher indentation viscosity while its indentation modulus was the same or even lower than the vertebral tissue. It was found that higher viscoelastic energy dissipation occurs in tissue regions with a lower mineral-to-matrix ratio (Pathak et al., 2011). The mineral-to-matrix ratios of rat mandibles and lumbar vertebrae were measured to be 20 and 7.9, respectively (Fu et al., 2012; Otomo et al., 2004). In the current study, the slopes of viscosity-to-modulus curves for rat mandibles and lumbar vertebrae were found to be 1221.307 and 557.625, respectively (Fig. 4). As viscosity has an inverse relationship with viscoelastic creep displacement (Kim et al., 2010), it is likely that a modulus-to-creep ratio follows trends similar to the viscosity-to-modulus ratio. Combined together, the mandible-to-vertebra ratio of the rat mineral-to-matrix ratio (approximately 2.51 (=20/7.9)) is similar to that of the rat modulus-to-creep (viscosity-to-modulus) ratio (approximately 2.19 (=1221.307/557.625)). This analysis indicates that the current nanoindentation-based direct measurement of elastic modulus, which is controlled by tissue mineralization, and viscoelastic creep, which is likely determined by interaction between collagen fibrils and crystalline mineral components of bone matrix, could reflect the variations of bone quality (material composition) at the tissue level.
One limitation of the current study was that the loading and unloading indentation rates were different between the mandibular and vertebral bones. No significant sensitivity of indentation loading rates to viscosity of bone tissue was reported (Isaksson et al., 2010) and the indentation modulus of bone tissue was not significantly changed by unloading rates higher than 10 nm/sec (Hoffler et al., 2005). As the loading and unloading rates used in the current study were higher than 10 nm/sec, it is likely that the different loading and unloading rates did not influence the current results.
Another limitation was that the detailed composition of bone tissue materials was not investigated. Recently, through nanoindentation dynamic mechanical analysis, Pathak et al (Pathak et al., 2011) found that collagen content and the mineral-to-matrix ratio could control the viscoelastic response of mouse femur bone tissue. Further works are needed to examine whether the viscoelastic property changes based upon the composition of bone tissues that may be altered from bone disease and that may differ among anatomical sites.
In conclusion, the current results indicated that changes in the viscoelastic tissue property and its regional variation should be examined to obtain a better understanding of estrogen deficiency-dependent alteration of bone quality. The slopes of correlation between elastic and viscoelastic tissue properties of bone were different between anatomical sites, reflecting the different functional demands at the organ level. The significant correlation between tissue modulus and viscosity in combination with the viscoelastic Voigt model (Eq.2) can provide a time-dependent function to assist in developing more accurate computational models, including non-linear finite element simulations of the viscoelastic response of bones under time dependent loading conditions.
ACKNOWLEDGMENTS
The project described was, in part, supported by Grant Number AG033714 from National Institute on Aging (Kim, D-G). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest Statement
None declared
REFERENCES
- Ames MS, Hong S, Lee HR, Fields HW, Johnston WM, Kim DG. Estrogen deficiency increases variability of tissue mineral density of alveolar bone surrounding teeth. Archives of Oral Biology. 2010;55:599–605. doi: 10.1016/j.archoralbio.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binte Anwar R, Tanaka M, Kohno S, Ikegame M, Watanabe N, Nowazesh Ali M, Ejiri S. Relationship between porotic changes in alveolar bone and spinal osteoporosis. Journal of Dental Research. 2007;86:52–57. doi: 10.1177/154405910708600108. [DOI] [PubMed] [Google Scholar]
- Busse B, Hahn M, Soltau M, Zustin J, Puschel K, Duda GN, Amling M. Increased calcium content and inhomogeneity of mineralization render bone toughness in osteoporosis: mineralization, morphology and biomechanics of human single trabeculae. Bone. 2009;45:1034–1043. doi: 10.1016/j.bone.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Daegling DJ, Hylander WL. Occlusal forces and mandibular bone strain: is the primate jaw "overdesigned"? Journal of Human Evolution. 1997;33:705–717. doi: 10.1006/jhev.1997.0164. [DOI] [PubMed] [Google Scholar]
- Deguchi T, Takano-Yamamoto T, Yabuuchi T, Ando R, Roberts WE, Garetto LP. Histomorphometric evaluation of alveolar bone turnover between the maxilla and the mandible during experimental tooth movement in dogs. American Journal of Orthodontics and Dentofacial Orthopedics. 2008;133:889–897. doi: 10.1016/j.ajodo.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporosis International. 2002;13:688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- Ejiri S, Tanaka M, Watanabe N, Anwar RB, Yamashita E, Yamada K, Ikegame M. Estrogen deficiency and its effect on the jaw bones. Journal of Bone and Mineral Metabolism. 2008;26:409–415. doi: 10.1007/s00774-008-0870-4. [DOI] [PubMed] [Google Scholar]
- Fischer-Cripps AC. A simple phenomenological approach to nanoindentation creep. Materials Science and Engineering A-Structural Materials Properties Microstructure and Processing. 2004;385:74–82. [Google Scholar]
- Fu X, Chen J, Wu D, Du Z, Lei Q, Cai Z, Schultze-Mosgau S. Effects of ovariectomy on rat mandibular cortical bone: a study using Raman spectroscopy and multivariate analysis. Analytical Chemistry. 2012;84:3318–3323. doi: 10.1021/ac300046x. [DOI] [PubMed] [Google Scholar]
- Hoffler CE, Guo XE, Zysset PK, Goldstein SA. An application of nanoindentation technique to measure bone tissue Lamellae properties. Journal of Biomechanical Engineering. 2005;127:1046–1053. doi: 10.1115/1.2073671. [DOI] [PubMed] [Google Scholar]
- Huja SS, Beck FM, Thurman DT. Indentation properties of young and old osteons. Calcified Tissue International. 2006;78:392–397. doi: 10.1007/s00223-006-0025-3. [DOI] [PubMed] [Google Scholar]
- Huja SS, Fernandez SA, Hill KJ, Gulati P. Indentation modulus of the alveolar process in dogs. Journal of Dental Research. 2007;86:237–241. doi: 10.1177/154405910708600308. [DOI] [PubMed] [Google Scholar]
- Isaksson H, Nagao S, Malkiewicz M, Julkunen P, Nowak R, Jurvelin JS. Precision of nanoindentation protocols for measurement of viscoelasticity in cortical and trabecular bone. Journal of Biomechanics. 2010;43:2410–2417. doi: 10.1016/j.jbiomech.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Kim DG, Huja SS, Lee HR, Tee BC, Hueni S. Relationships of viscosity with contact hardness and modulus of bone matrix measured by nanoindentation. Journal of Biomechanical Engineering. 2010;132:024502. doi: 10.1115/1.4000936. [DOI] [PubMed] [Google Scholar]
- Kim DG, Navalgund AR, Tee BC, Noble GJ, Hart RT, Lee HR. Increased variability of bone tissue mineral density resulting from estrogen deficiency influences creep behavior in a rat vertebral body. Bone. 2012;51:868–875. doi: 10.1016/j.bone.2012.08.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DG, Shertok D, Ching Tee B, Yeni YN. Variability of tissue mineral density can determine physiological creep of human vertebral cancellous bone. Journal of Biomechanics. 2011;44:1660–1665. doi: 10.1016/j.jbiomech.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comparative Medicine. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- Lerner UH. Bone remodeling in post-menopausal osteoporosis. Journal of Dental Research. 2006;85:584–595. doi: 10.1177/154405910608500703. [DOI] [PubMed] [Google Scholar]
- Mavropoulos A, Kiliaridis S, Bresin A, Ammann P. Effect of different masticatory functional and mechanical demands on the structural adaptation of the mandibular alveolar bone in young growing rats. Bone. 2004;35:191–197. doi: 10.1016/j.bone.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Mavropoulos A, Rizzoli R, Ammann P. Different responsiveness of alveolar and tibial bone to bone loss stimuli. Journal of Bone and Mineral Metabolism. 2007;22:403–410. doi: 10.1359/jbmr.061208. [DOI] [PubMed] [Google Scholar]
- Mulder L, Koolstra JH, den Toonder JM, van Eijden TM. Intratrabecular distribution of tissue stiffness and mineralization in developing trabecular bone. Bone. 2007;41:256–265. doi: 10.1016/j.bone.2007.04.188. [DOI] [PubMed] [Google Scholar]
- Mulder L, Koolstra JH, den Toonder JM, van Eijden TM. Relationship between tissue stiffness and degree of mineralization of developing trabecular bone. Journal of Biomedical Materials Research Part A. 2008;84:508–515. doi: 10.1002/jbm.a.31474. [DOI] [PubMed] [Google Scholar]
- Otomo H, Sakai A, Ikeda S, Tanaka S, Ito M, Phipps RJ, Nakamura T. Regulation of mineral-to-matrix ratio of lumbar trabecular bone in ovariectomized rats treated with risedronate in combination with or without vitamin K2. Journal of Bone and Mineral Metabolism. 2004;22:404–414. doi: 10.1007/s00774-004-0502-6. [DOI] [PubMed] [Google Scholar]
- Pathak S, Swadener JG, Kalidindi SR, Courtland HW, Jepsen KJ, Goldman HM. Measuring the dynamic mechanical response of hydrated mouse bone by nanoindentation. Journal of the Mechanical Behavior of Biomedical Materials. 2011;4:34–43. doi: 10.1016/j.jmbbm.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renders GA, Mulder L, van Ruijven LJ, van Eijden TM. Degree and distribution of mineralization in the human mandibular condyle. Calcified Tissue International. 2006;79:190–196. doi: 10.1007/s00223-006-0015-5. [DOI] [PubMed] [Google Scholar]
- Ruppel ME, Miller LM, Burr DB. The effect of the microscopic and nanoscale structure on bone fragility. Osteoporosis International. 2008;19:1251–1265. doi: 10.1007/s00198-008-0579-1. [DOI] [PubMed] [Google Scholar]
- Wu Z, Ovaert TC, Niebur GL. Viscoelastic properties of human cortical bone tissue depend on gender and elastic modulus. Journal of Orthopedic Research. 2012;30:693–699. doi: 10.1002/jor.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]