Abstract
Rationale
A number of tasks are used to assess working memory in rodents, but the Odor Span task (OST) is unique in studying performance as a function of the number of stimuli to remember.
Objectives
The purpose of the present study was to better characterize the behavioral pharmacology of the OST by exploring the effects of several amnestic agents including an NMDA antagonist (dizocilpine), a positive GABA-A modulator (chlordiazepoxide), an anticholinergic compound (scopolamine) and as a negative control, an opiate receptor agonist (morphine).
Methods
Rats were trained to perform on the OST which is a non-match-to-sample procedure with an incrementing number of sample odors to remember as the session progresses. Trials with a simple odor discrimination task (SD) were interspersed to provide a control for effects unrelated to memory load.
Results
All four drugs disrupted performances on the OST task in a dose-dependent fashion, but only the NMDA antagonist dizocilpine produced impairments that were clearly dependent on the number of stimuli to remember. Dizocilpine impaired OST performance at a dose (0.1 mg/kg) that did not affect SD and that impairment depended on memory-load. Chlordiazepoxide (3.0 mg/kg) also produced amnestic effects that were manifest by shorter memory spans and runs of correct responding. In contrast, morphine and scopolamine impaired OST accuracy only at doses that also disrupted SD (18.0 and 0.3 mg/kg, respectively).
Conclusions
These results provide evidence of NMDA and benzodiazepine modulation of working memory as assessed by the OST.
There is considerable interest in rodent models that permit pharmacological analysis of memory processes with translational significance (c.f. Keeler and Robbins 2011). A number of procedures have been used to assess drug effects on working memory including the radial arm maze, the Morris swim task and delayed matching-to-sample or matching-to-place tasks, to name a few. These tasks are described as working memory assays because they often involve “a short-term memory for an object, stimulus or location that is used within a testing session, but not typically between sessions” (Dudchenko 2004 p. 700). However, Wright (2007) noted that most procedures used to study memory in non-humans focus on the effects of a delay interval on retention of only a single item and, as such, may fail to address important aspects of memory processes. In contrast, the human memory literature includes countless studies of memory for lists of items, complex passages and long episodes. Indeed, the construct of working memory in humans is typically characterized not only as short-lived, i.e., affected by the delay interval, but also as of limited capacity, affected by the number of items to be remembered and the processing required (Gathercole 2009).
Until recently though, few methods of studying memory capacity in animals have been available. Dudchenko et al. (2000) have described a memory span task in rodents that seems to provide such a model. Using an incrementing non-match-to-sample procedure, their technique assessed the number of odors sampled that rats can remember without error within a session (odor span). Thus, the odor span task (OST) permits the researcher to study the effects of drugs and other variables as a function of the memory load, but only a few studies have explored the behavioral pharmacology of the OST. Rushforth et al. (2010; 2011) found that nicotine improved memory span, but that NMDA antagonist ketamine impaired span using the OST in rats. MacQueen et al. (2011) found that another NMDA antagonist, dizocilpine (MK-801), reduced span and also showed that its effects depended on the memory load, i.e., there were no effects with only a few stimuli to remember, but doses of 0.1 and 0.17 mg/kg dizocipline (DZP) produced substantial impairment with higher memory loads. These findings and those of Rushforth et al. are consistent with the hypothesis of Bannerman et al. (2006) that some working memory processes require NMDA receptor activation, but such an interpretation may be premature because so little is known about the OST.
The present study was designed to explore the behavioral pharmacology of the OST by studying several critical drugs: DZP, morphine, chlordiazepoxide (CDZ) and scopolamine. An adaptation of the OST was developed with several controls to enhance interpretation of drug effects (e.g., a five-choice odor discrimination task that was conducted during each OST session as a control for drug effects unrelated to memory load, e.g., sensori-motor impairment, motivational changes, etc.). DZP was studied to determine whether the previous findings with NMDA antagonists (MacQueen et al. 2011; Rushforth et al. 2011) could be replicated with more stringent controls. Scopolamine and CDZ were chosen because of the substantial literature showing amnestic effects with these two drugs using other models of working memory in rodents (Buccafusco 2009; Curran 1991; Hudzik and Wenger 1993; Klinkenberg and Blockland 2010) and morphine was included as a negative control (c.f. Mintzer et al. 2010).
Method
Subjects
Sixteen male Sprague-Dawley rats (Harlan) served as subjects. Six animals were tested with each drug. The animals were between 90 and 120 days old at the onset of the study and were housed individually under a 12/12 hr light/dark environment. Water was continuously available in the home cage, but food was restricted to maintain animals at approximately 85% of their free feeding weight.
Apparatus
The apparatus was a circular open-field arena (94 cm in diameter) described previously (MacQueen et al. 2011). The Formica floor of the arena contained 18 holes arranged in two circular arrays in which plastic stimulus cups (60 ml) were placed. Metal baffling 32 cm high surrounded the arena and white masking noise (70 dB) was presented throughout the session. The apparatus was housed in a small room with a video camcorder positioned in the ceiling so that each session could be digitally recorded.
Stimuli
Odor stimuli were presented by covering the plastic cups with opaque plastic lids which had been scented by storage in plastic containers containing odorants such as aromatic oils and household spices (allspice, almond, anise, banana, bay, beet, bubble gum, caraway, carob, celery, cherry, cinnamon, clove, coriander, cumin, dill, fennel, fenugreek, garlic, ginger, grape, lime, marjoram, nutmeg, onion, orange, oregano, paprika, peach, pineapple, raspberry, rosemary, sage, sassafras, savory, spinach, strawberry, sumac, thyme, tomato, turmeric, vanilla, and Worcestershire) purchased from Great American Spice Co.
Procedure
Initial training
Subjects were tested five days per week (M-F) throughout the experiment. Rats were first exposed to the arena in which cups were placed containing sucrose pellets (45 mg Bio-Serv). When the rat was readily consuming the pellets, trials were conducted with baited cups partially covered by an unscented plastic lid. As the rat became successful at retrieving pellets, the lid was gradually positioned such that it covered the cup completely and once the rat was reliably removing the lids, OST training began.
OST training
On the first trial of OST sessions, a single baited stimulus cup covered with a scented lid was randomly placed in one location with the remaining 17 holes filled with empty cups. The rat was then placed in the arena until it removed the lid from the stimulus cup and retrieved the sucrose pellet. After an inter-trial interval (ITI) of approximately 1 min (spent in a holding cage in the same room), the rat was returned to the arena in which two stimulus cups were placed in random locations. One cup was covered with a lid of the same odor presented on the previous trial (unbaited, S-) and the other was covered with a differently scented lid (baited, S+). If the rat responded to the new (non-matching) odor, the next trial presented three stimuli in the arena: two cups with lids scented with the previously presented odors and not baited with a food pellet (S-), and the third cup baited and covered with a lid scented with a new odor (S+). During initial training, this arrangement continued with the addition of one new baited stimulus among the previous odors (which were not baited) until an error was made (i.e., when a response, defined as any displacement of a lid by the paws or snout, was made to a previously presented odor or when no response to the new odor was made during the two-min trial). A correction procedure was implemented wherein trials continued until the subject responded to the correct stimulus and received the food pellet or 2 min elapsed, whichever came first. Following an error, the procedure was reset such that the next trial presented a single new odor with no comparison stimuli, and the number of stimuli incremented as before following correct responses. Sessions were terminated after 24 trials.
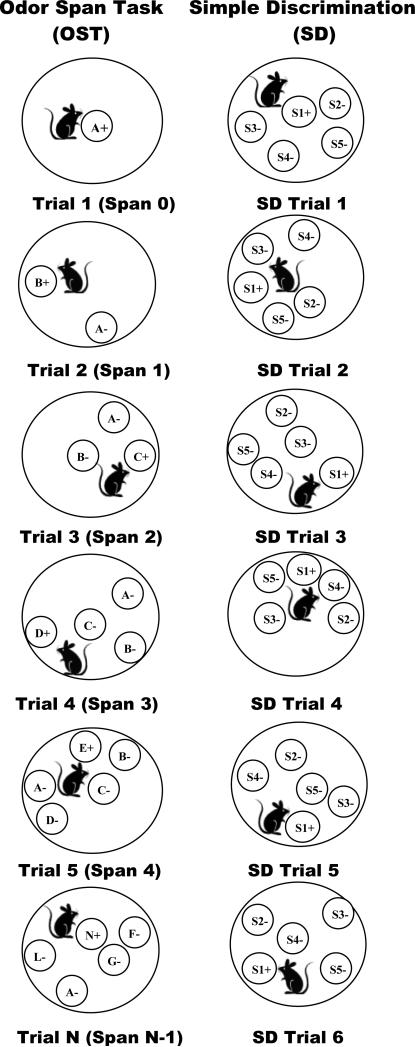
Once animals were achieving runs of five consecutive correct responses or better, the procedure was no longer reset to a single odor following errors, and instead a new stimulus was added following each trial regardless of whether the response was correct or incorrect (a correction procedure remained in effect after errors so that each trial continued until the rat removed the correct lid). In order to avoid a confound between the number of stimuli to remember and the number of comparison stimuli to choose from, the number of stimuli in the arena was limited to five even as the number of new stimuli presented continued to increment. Thus, each trial after the fourth trial included one new stimulus (S+) as well as four previously presented stimuli (S-) randomly chosen from the pool of odors presented in the previous trials of the session (See Figure 1, left column, for a trial by trial illustration of the OST procedure). The stimulus presentation order, the S- comparisons on each trial, and the placement location for stimuli were pseudo-randomly determined for each session. Stimulus lids were replaced after each trial with new lids of the same odor to ensure that scent marks left behind on lids from previous trials could not influence performance. Training under these conditions was conducted for a minimum of ten sessions. When responding became stable, the final pre-drug phase of training was introduced.
Figure 1.
Illustration of OST and simple discrimination (SD) procedures. Left column shows stimulus configurations for the OST over several trials with a single rewarded odor on Trial 1 (A+) and a new odor introduced on Trial 2 (second row, B+). Rows 3-5 show that the number of comparisons in the arena increments until five is reached, and Row 6 illustrates how four previously encountered stimuli are selected along with one new odor on Trials 6-24 (row 6). Right column shows stimulus configurations for the SD task and illustrates that responses to one stimulus (S1) are always reinforced, and that responses to the other stimuli (S2, S3, S4, S5) are never reinforced.
Five-comparison OST with added simple discrimination
In this phase, a simple discrimination task (SD) was introduced to serve as a within-session control for non-mnemonic drug effects. In this task, one odor stimulus was consistently baited across sessions and the other four odor stimuli were never baited (See Figure 1, right column, for an illustration). The odors used to define the simple discrimination task were selected randomly from a set of stimuli that were not used in the odor span task. Six simple discrimination trials were presented in a block following the final odor span trial of each session until subjects were performing accurately and then the six SD trials were interspersed pseudo-randomly within the odor span trial (beginning with Trial 5). After some initial disruption of the OST task for a few training sessions, rats came to readily discriminate which odors cued the SD task and which the OST, and were able to switch between the non-matching task to the simple discrimination with considerable accuracy consistently averaging better than 95% correct on SD trials and above 88% correct on OST trials. This arrangement defined the final baseline for the experiment with each session consisting of 30 trials: 24 OST and 6 SD trials.
No-Bait Control
In order to assess whether subjects were responding to the odor stimuli and not to the odor of the reward sucrose pellet, no-bait control sessions were conducted once weekly. On a given no-bait session six trials were randomly selected to include a correct stimulus cup that did not contain a sucrose pellet. On these trials the pellet was delivered into the cup by hand once a response to the S+ was made. Performances on baited trials were not significantly more accurate than non-baited trials for any subject (p > .05) indicating that performance was not guided by the scent of the sucrose pellet.
Drug Phase
Subjects were trained under baseline conditions until a stability criterion was met on both OST and SD tasks: the mean difference between the percent correct on the most recent five sessions and the immediately preceding five sessions had to be less than 10% of the grand mean of the ten sessions. When these criteria were met, the drug phase of the study began. Generally, i.p. injections preceded the testing session three days each week. Saline injections were administered on Tuesdays, whereas rats received active drug on Wednesdays and Fridays. The experimenter testing the animal was blind as to the dose administered. No injections were given before Monday or Thursday sessions, which provided an ongoing baseline. Dizocilpine (MK801) maleate (Tocris) was administered 30 min prior to the test session at doses of .03, .1, .17, and .3 mg/kg (all doses are expressed in terms of the salt). The remaining three drugs were administered 15 min before the session: morphine sulfate (NIDA—1.0, 3.0, 10.0, and 18.0 mg/kg), chlordiazepoxide hydrochloride (Sigma-1.0, 1.8, 3.0, 10.0, 30.0 mg/kg) and scopolamine hydrobromide (Sigma—0.03, 0.056, 0.1, 0.3 mg/kg). Drugs were dissolved in physiological saline and injected in a volume of 1.0 ml/kg. Doses were determined 2-4 times for each rat and were administered in a semi-random order such that each dose was administered in the first round of determinations before the second round was initiated, and so on. Some animals were tested in more than one drug condition. In such cases, order of drugs studied was randomized across subjects and baseline sessions were conducted with no injections administered for a two week period before a second drug experiment began.
Dependent Measures and Statistical Analysis
The span was determined in each session by the number of correct responses until the first error of the session was made (excluding the first trial with only a single stimulus as there are no stimuli to remember). The longest run was defined as the greatest number of consecutive correct responses in the session, whether or not the run began with the first trial. In each session, the number of correct responses was divided by the number of trials on which a response was made to determine the proportion correct for both OST and SD components. These were multiplied by 100 and are presented as percent correct. The latency from the trial onset until the initial response (correct or incorrect) was recorded for both OST and SD trials and was converted to a mean latency for each session. At high doses of each drug, rats frequently failed to respond to any stimulus during the 2-min trial and these omission trials were not included in the determination of percent correct or latency, but the percent of trials per session in which responding was omitted was plotted separately. One-way, within-subject ANOVAs were computed for the span and longest run measures. Percent correct and latency were analyzed with a Dose X Task factorial ANOVA and LSD post hoc comparisons were conducted following significant omnibus tests. High drug doses that eliminated responding completely for one or more rats were omitted from statistical analysis. Recordings of ten randomly chosen sessions were scored by an observer blind to the condition and stimulus arrangement to determine inter-rater reliability. Agreement was calculated by comparing the scoring of the observer with that of the experimenter present during the actual session. The two raters agreed on 98.7% of the scored trials, indicating the high reliability of the lid removal response definition.
Results
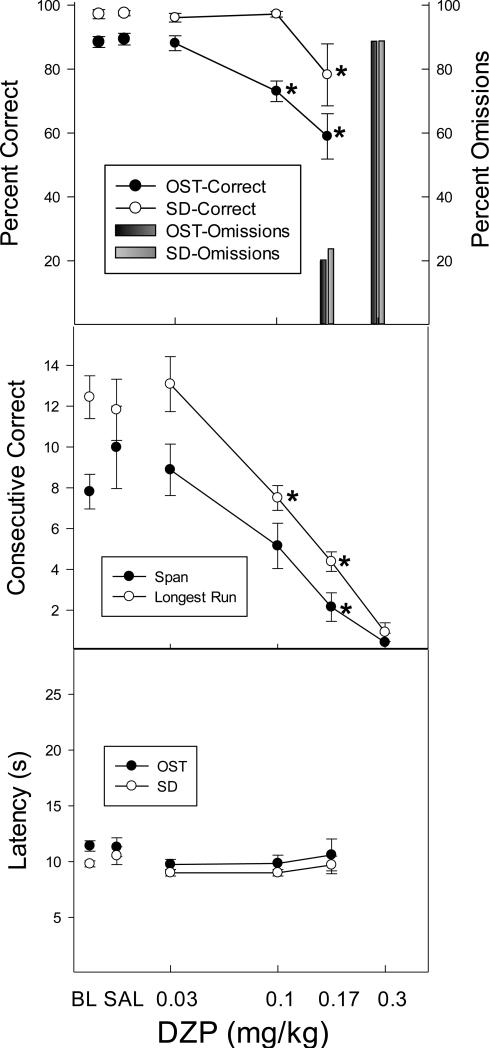
Extensive training was required for rats to progress through the various stages of training to reach final baseline procedures (five comparison OST with SD control) with an average of 39.6 sessions (range 11-66 sessions). Once the baseline procedures began though, the stability requirements to begin the drug administration phase were reached relatively rapidly (Mean = 19.4, range 10-40 sessions). At this point, all rats were maintaining stable, high levels of accuracy on both the OST and SD tasks. Figure 2 shows the effects of DZP on the key dependent variables. The top panel shows percent correct as a function of DZP dose for the OST (black circles) and SD (white circles) tasks. Under control conditions (baseline and saline) accuracies approached 100% on SD trials and were only slightly lower, nearly 90%, on the OST. DZP impaired accuracy on both tasks in a dose-dependent fashion. Percent correct in the OST showed a clear decrease at the 0.1 mg/kg dose and declined still further at higher doses of DZP (0.17 and 0.3 mg/kg). In contrast, note that DZP had no effect on SD accuracy until doses of 0.17 and 0.3 mg/kg were reached; the effects of 0.1 mg/kg DZP were thus selective to the OST. The percentage of trials on which there was a failure to respond to any stimulus within 2 min (omission errors) is indicated by the bars in Figure 2, and it is clear that response omissions began to occur at the 0.17 dose on both OST and SD trials, and that virtually all trials at the highest DZP dose (0.3 mg/kg) were omissions. The selective effects of 0.1 mg/kg DZP were confirmed by a significant Dose X Task interaction: F (4, 20) = 4.47, p < .05, and post hoc tests revealed that this dose reduced accuracy on the OST (p < .05), but not the SD task (p > .05). As noted, higher doses were non-selective: significantly decreasing accuracies on both tasks (p < .05, note that the 0.3 mg/kg dose was excluded from statistical analysis due to high percentage of omissions).
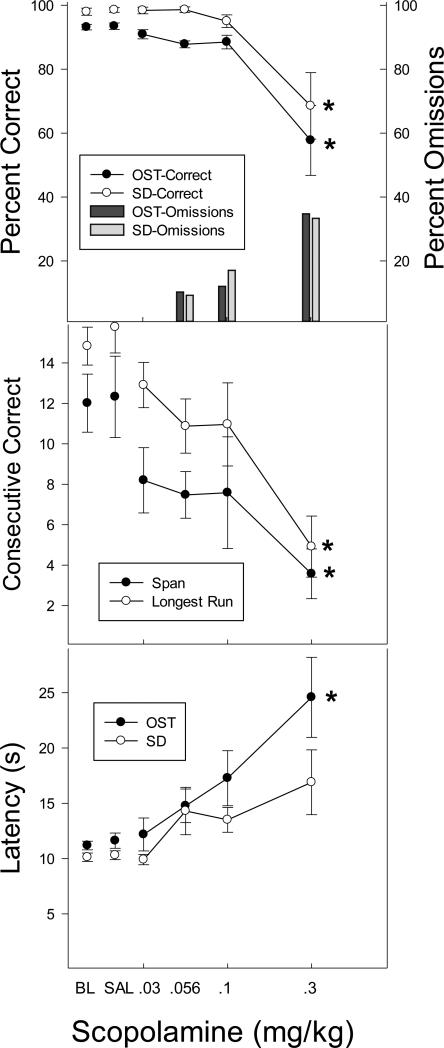
Figure 2.
Top panel shows the effects of DZP on percent correct (circles) and omissions (bars) for the OST (black circles; dark bars) and the SD (white circles; light bars). Middle panel shows span (black circles) and longest run (white circles) and the bottom panel shows latency to the first response for the OST (black) and the SD (white). Vertical lines indicate SEM and stars indicate conditions that differed significantly from saline control.
Under baseline and saline control conditions, mean spans (black circles) ranged from 8-10 odors, whereas longest runs (white circles) were somewhat higher with runs of 12-13 consecutive correct responses (middle panel of Figure 2). DZP produced dose dependent reductions in both span [F (4, 20) = 7.20, p < .05] and longest run [F (4, 20) = 14.82, p < .05]. Span was significantly below saline levels at doses of 0.17 mg/kg, but longest runs differed significantly at doses of 0.1 mg/kg and higher. Latencies to respond (bottom panel of Figure 2) were not elevated at any dose (p > .05). In sum, the effects of DZP were selective to the OST (percent correct and longest run) at the 0.1 mg/kg dose, but higher doses produced more general disruption of performance with impairment of SD accuracies and general increase in response omissions.
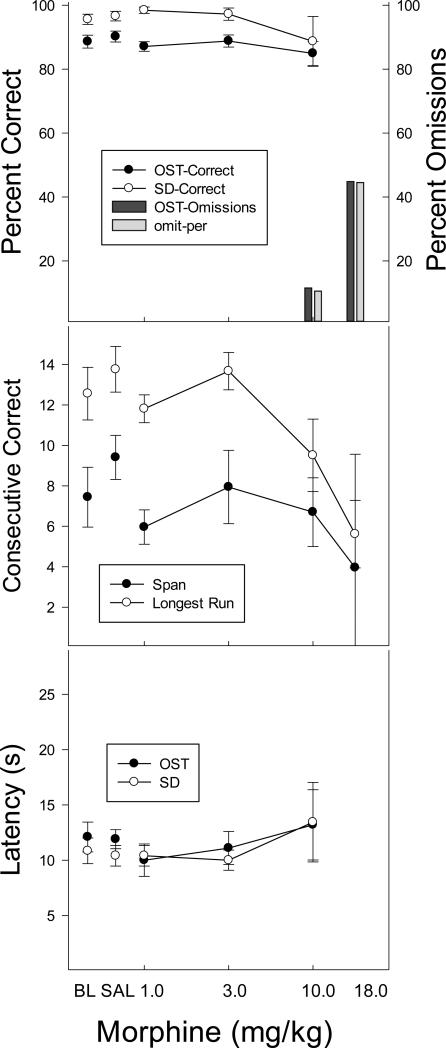
Morphine was included in the study as a negative control and, as expected, its effects were non-selective (Figure 3). The top panel of Figure 3 shows that morphine did not affect percent correct at any dose (p > .05). Omissions began to occur at the 10.0 mg/kg dose level and increased sharply at the highest (18.0 mg/kg) dose, at which several animals failed to respond entirely. Span and longest run were rather variable across the dose range, with no significant effects obtained with either measure (middle panel). Response latency (bottom panel) was also not significantly affected at any dose (p > .05). In sum, the only effect of morphine was to eliminate responding at high doses on both OST and SD trials.
Figure 3.
Top panel shows the effects of morphine on percent correct (circles) and omissions (bars) for the OST (black circles; dark bars) and the SD (white circles; light bars). Middle panel shows span (black circles) and longest run (white circles) and the bottom panel shows latency to the first response for the OST (black) and the SD (white). Vertical lines indicate SEM and stars indicate conditions that differed significantly from saline control.
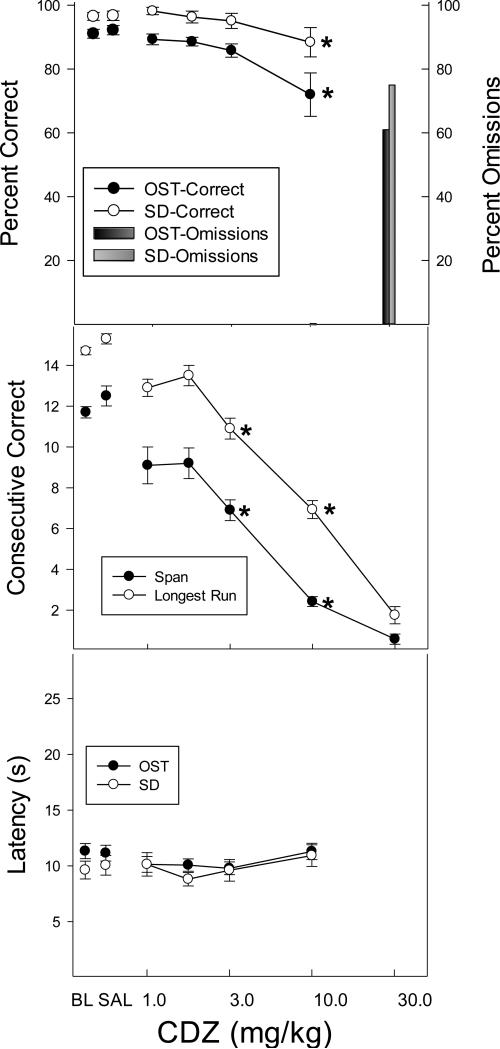
The effects of CDZ on accuracy are summarized in the top panel of Figure 4 and are characterized by a dose-dependent decrease in accuracy on both tasks [F (5, 25) = 7.17, p < .05]. Accuracies were significantly below control levels only at the 10.0 mg/kg dose (p < .05) and response omissions became frequent at the 30.0 mg/kg dose. Span and longest run also declined with CDZ dose [span: F (5, 25) = 6.33, p < .05; longest run: [F (5, 25) = 9.31, p < .05], post hoc tests revealed significant decreases in both measures relative to saline at the 3.0 and 10.0 mg/kg doses. Latency to respond was not affected by CDZ (bottom panel). In sum, CDZ effects on overall accuracy did not appear to be selective as OST accuracy decreased only at a dose that had comparable effects on SD (10.0 mg/kg). However, some evidence of CDZ effects that were selective to the OST comes from the observation that measures of consecutive correct responding (span and longest run) were disrupted after 3.0 mg/kg CDZ: a dose that did not affect overall accuracy or response latency.
Figure 4.
Top panel shows the effects of CDZ on percent correct (circles) and omissions (bars) for the OST (black circles; dark bars) and the SD (white circles; light bars). Middle panel shows span (black circles) and longest run (white circles) and the bottom panel shows latency to the first response for the OST (black) and the SD (white). Vertical lines indicate SEM and stars indicate conditions that differed significantly from saline control.
Scopolamine disrupted performance on both tasks across the dose range (Figure 5). Percent correct declined with scopolamine dose on both OST and SD tasks [F (5, 25) = 9.15, p < .05]. Only the 0.3 mg/kg dose produced a significant disruption in accuracy relative to saline, but the percentage of omissions increased in a dose-dependent fashion with many omissions occurring at doses as low as 0.056 mg/kg. There was no significant dose X task interaction. Scopolamine also impaired span and longest run, but significant effects were obtained only at the highest scopolamine dose in both cases (0.3 mg/kg). Finally, latency to respond was elevated in dose-dependent fashion in both tasks with a main effect of dose [F (5, 25) = 19.11, p < .05] and a significant dose X task interaction [F (5, 25) = 3.84, p < .05], which was largely due to a pronounced increase in latency in OST responding at the 0.3 mg/kg dose. In general, scopolamine disrupted response accuracy and increased response omissions on both OST and SD tasks.
Figure 5.
Top panel shows the effects of scopolamine on percent correct (circles) and omissions (bars) for the OST (black circles; dark bars) and the SD (white circles; light bars). Middle panel shows span (black circles) and longest run (white circles) and the bottom panel shows latency to the first response for the OST (black) and the SD (white). Vertical lines indicate SEM and stars indicate conditions that differed significantly from saline control.
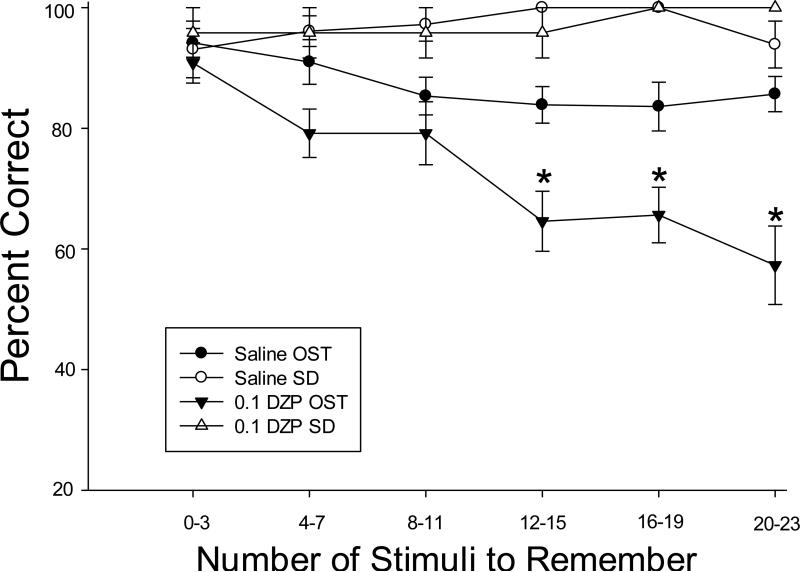
DZP was the only drug in the present study that selectively affected overall accuracy on the OST. In order to assess the way in which DZP interacted with memory load, Figure 6 shows percent correct across the session after saline (circles) and 0.1 mg/kg DZP (triangles), the dose which produced effects that were selective to the OST. For the OST (black symbols), this presents accuracy as a function of the number of stimuli to remember and the SD (white symbols) provides an index of performance at the same point in the session without an increasing memory load. OST accuracy declined as the number of stimuli to remember increased under saline conditions (black circles), but the function was relatively shallow. In contrast, note the increased OST decline with increasing memory load after 0.1 mg/kg DZP (black triangles). A drug X trial block ANOVA confirmed the steeper forgetting function for 0.1 DZP with a significant interaction [F (5, 25) = 4.28, p < .05]. Post hoc comparisons revealed no significant differences between saline and 0.1 DZP in blocks 1-3 (0-11 stimuli to remember), but accuracy on blocks 4, 5 and 6 (12-23 stimuli to remember) was significantly reduced by 0.1 DZP (p < .05). No within-session effects were apparent for SD performance in either condition (white symbols).
Figure 6.
Black symbols show mean percent correct on the OST as a function of the number of stimuli to remember after saline (black circles) and 0.1 mg/kg DZP (black triangles). Data are presented in blocks of four consecutive trials (the first block includes only trials 2-4 because there is nothing to remember on Trial 1). White symbols show percent correct for the SD task after saline (white circles) and 0.1 mg/kg DZP (white triangles). Vertical lines represent SEM and stars indicate memory loads at which .1 DZP differed from saline.
Although CDZ did not produce selective effects in the analysis of overall accuracy, it did reduce both span and longest run at a dose (3.0 mg/kg) that did not affect overall percent correct, omissions or response latency. In order to determine whether CDZ effects depended on memory load, an analysis of the effects of 3.0 mg/kg CDZ as a function of the number of stimuli to remember was conducted. Percent correct in the OST decreased as the memory load increased after both saline and 3.0 mg/kg CDZ. Accuracy was consistently lower under CDZ conditions, but the slopes were comparable and both relatively shallow. This observation was confirmed by a significant main effect of dose [F (1, 5) = 6.59, p < .05] and no significant interaction. Performance on SD trials was not affected at any point in the session.
Discussion
All four drugs impaired performance on the OST task in a dose-dependent fashion, but only the NMDA antagonist DZP produced impairments that were clearly dependent on the number of stimuli to remember. Significant reductions in overall OST percent correct and longest run were obtained at the 0.1 mg/kg DZP dose, but neither SD accuracy nor overall response latency was affected until higher doses were reached. This selective effect of DZP on OST performance was further clarified by the within-session analysis which showed that 0.1 mg/kg DZP only decreased OST accuracy late in the session as the number of odors to remember increased to 12-23 stimuli. This effect cannot be attributed to changes in DZP activity within the session, as SD performance was unaffected at any point. Interestingly, span was less sensitive to the effects of DZP, as significant decreases were seen only at the 0.17 mg/kg level--a dose which also increased errors on the SD task. The present results replicate the findings of MacQueen et al. (2011) who also found selective OST effects with a 0.1 and 0.17 mg/kg dose of DZP. In the MacQueen et al. study, a two-choice simple discrimination served as the control and because the OST task used has five comparison stimuli on most trials, it could be argued that the selective effects obtained in that study were due to the number of choices, rather than the number of stimuli to remember. The more stringent five-choice simple discrimination control used in the present study provides stronger support for conclusions about the selectively of the DZP effects on working memory at the 0.1 mg/kg dose (at 0.17 mg/kg in the present study both the OST and the SD tasks were affected). The value of the five-choice SD control is that it requires the same perceptual-motor, motivational, and reference memory requirements as the OST. Thus, when OST is affected at a dose that spares SD, it provides strong evidence of the selectivity of the drug effect on processes involved with working memory, or at least some form of load-dependent recognition memory. The present results are also consistent with those of Rushforth et al. (2011) with NMDA antagonist ketamine using the OST, but in that study the only dependent measure was span and, in the absence of stringent controls for non-cognitive effects, the selectivity of these effects remains open to question. It is well established that NMDA antagonists produce many interfering behavioral actions that make it notoriously difficult to isolate those effects specific to memory (Keith and Rudy 1990; Dix et al. 2010) and different NMDA antagonists have been shown to produce very different behavioral effects across various procedures (Gilmour et al. 2009). Thus, further study of the effects of ketamine and perhaps other NMDA antagonists using the procedures developed here would be of value.
Of more than passing interest was the finding that morphine and scopolamine failed to produce clear evidence of selective effects on memory capacity. Morphine was included in the study as a negative control because opiates are not generally associated with specific effects on working memory (e.g. Mintzer et al. 2010), and indeed even fairly high doses of morphine had no effect on OST performance in the present study. Indeed, no significant effects of morphine were observed except at doses that were sufficiently high to produce response omissions on most trials (18.0 mg/kg morphine); errors of commission were relatively rare at any dose of morphine.
Surprisingly, the effects of scopolamine were similar to those of morphine in that OST performance was affected only at doses that also impaired SD, and as with morphine, the effects of scopolamine were primarily to eliminate responding. The failure of scopolamine to produce selective effects on working memory in OST was unexpected because numerous studies with other rodent models of working memory have shown scopolamine-induced impairment (see Buccafusco 2009; Klinkenberg and Blokland 2010 for reviews), and because Turchi and Sarter (2000) found that lesions of cholinergic pathways in basal forebrain impaired olfactory span in rats. However, most of these studies did not use such stringent controls for non-mnemonic drug effects as the SD control in the present study, so it is possible that the effects reported are not truly specific to working memory processes. As Klinkenberg and Blokland (2010) note, scopolamine produces a variety of non-specific effects including locomotor and perceptual impairments and anxiogenic effects which might have interfered with accuracy on both simple discrimination as well as the OST in the present study. It would be of interest to explore the effects of cholinergic compounds with more selective sub-receptor actions than scopolamine such as m1 antagonist, biperiden, (Klinkenberg and Blokland 2011) to further characterize the role of cholinergic neurotransmission in memory load-dependent tasks like the OST.
It also was expected that CDZ would show effects selective to the OST much like those of DZP, as benzodiazepines often produce amnestic effects in other models of working memory (Hudzik and Wenger 1991; Keith et al 2003). In the present study, overall OST accuracy was affected by CDZ only at doses (10.0 mg/kg and higher) that also impaired simple discrimination accuracy, but the effects were complex. The 3.0 mg/kg dose of CDZ had no overall effect on OST or SD accuracy or latency, but produced significant reductions in both span and longest run. In other words, although overall OST accuracy was not significantly reduced, error patterns were altered by this low dose of CDZ. Within-session analysis revealed that 3.0 mg/kg CDZ produced small, but consistent reductions in accuracy that emerged relatively early in the session and paralleled the saline memory load function throughout the session. This was in contrast to the effects of 0.1 DZP which only emerged as the number of stimuli to remember became relatively high. Thus, although both DZP and CDZ affected remembering in the OST, their actions were different: CDZ produced small disruptions in performance throughout the OST session, whereas DZP effects depended on the number of stimuli to remember. This outcome also emphasizes the importance of using multiple dependent measures in the OST, as odor span, longest run and overall accuracy were not always affected in the same way. Many OST studies focus on span as the key dependent variable, but the present findings suggest that span may not be the best index of memory load effects.
That CDZ and scopolamine effects did not depend on memory load may be viewed by some as diminishing the translational value for the OST. Indeed, studies with human subjects often do show effects of benzodiazepines and anticholinergic drugs on working memory. However, recent studies suggest that the effects of neither drug increase with memory load, suggesting an absence of effect on human working memory capacity (Green et al. 2005; Mintzer and Griffiths 2007) that is consistent with the present results. In contrast, studies of ketamine in human subjects have shown memory-load dependent effects using the n-back procedure that were similar to the DZP effects in the present study (Lofwall et al. 2006; Morgan et al. 2004). The consistency between the present findings and the human literature suggest that the OST may in fact have considerable translational value.
In sum, the present findings provide support for the hypothesis that NMDA receptors play a critical role in some forms of working memory (Bannerman et al. 2006) and in particular, in sustaining accurate stimulus control under conditions of increasing memory load (MacQueen et al. 2011). The selective and memory-load dependent effects of DZP observed in the present study are consistent with such an hypothesis, and the finding that drugs acting through benzodiazepine, muscarinic, and opiate receptors failed to produce comparable effects add a measure of pharmacological selectivity to the conclusion. Further research is needed to determine the generality of the DZP effects observed here and the ketamine effects noted by Rushforth et al. (2011) in order to further evaluate the link between NMDA receptor activity and memory load. Although the effects of CDZ were less clearly dependent on the memory load, they were selective to the OST task and the reductions in span and longest run seem to represent amnestic effects as well. Further research with benzodiazepines is needed to clarify the nature of these effects. It would also be of considerable interest to study additional putative amnestic compounds from other drug classes in order to more fully characterize the behavior pharmacology of the OST. Indeed, the OST procedure appears to hold considerable promise as a technique to evaluate the interaction between drug effects and memory load.
Acknowledgements
This research was supported by DA029252 to Mark Galizio. The authors thank Magda Bonk, Lauren Goldstein, Kevin Jacobs, Tim Lefever, Elizabeth Toop and Luke Watterson for assistance in data collection and analysis.
References
- Bannerman DM, Rawlins JNP, Good MA. The drugs don't work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A-containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology. 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ. The revival of scopolamine reversal for the assessment of cognitive-enhancing drugs. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. 2nd Ed. CRC press; Boca Raton (FL): 2009. [Google Scholar]
- Curran HV. Benzodiazepines, memory and mood: A review. Psychopharmacology. 1991;105:1–8. doi: 10.1007/BF02316856. [DOI] [PubMed] [Google Scholar]
- Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M. A within-subject cognitive battery in the rat: differential effects of NMDA receptor antagonists. Psychopharmacology. 2010;212:227–242. doi: 10.1007/s00213-010-1945-1. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci. 2000;20:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE. Working memory. In: Byrne JH, editor. Concise learning and memory: Editor's selection. Elsevier Press Amsterdam; 2009. [Google Scholar]
- Gilmour G, Pioli EY, Dix SL, Smith JW, Conway MW, Jones WT, Loomis S, Mason R, Shahabi S, Tricklebank MD. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: implications for “NMDA antagonist modeling” of schizophrenia. Psychopharmacology. 2009;205:203–216. doi: 10.1007/s00213-009-1530-7. [DOI] [PubMed] [Google Scholar]
- Green A, Ellis KA, Bartholomeusz CF, Ilic S, Croft RJ, PHan KL, Nathan PJ. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Bio Behav. 2005;81:575–584. doi: 10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Wenger GR. Effects of drugs of abuse and cholinergic agents on delayed matching-to-sample responding in the squirrel monkey. J Pharm Exp Ther. 1993;265:120–127. [PubMed] [Google Scholar]
- Keeler JF, Robbins TW. Translating cognition from animals to humans. Biochem Pharmacol. 2011;81:1356–1366. doi: 10.1016/j.bcp.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Keith JR, Pitts RC, Pezzuti T, Galizio M. Effects of positive GABA(A) modulators on a multiple-component, repeated-acquisition test of spatial learning. Behav Pharmacol. 2003;14:67–75. doi: 10.1097/00008877-200302000-00007. [DOI] [PubMed] [Google Scholar]
- Keith JR, Rudy JW. Why NMDA-receptor-dependent long-term potentiation may not be a mechanism of learning and memory: Reappraisal of the NMDA-receptor blockade strategy. Psychobiol. 1990;18:251–257. [Google Scholar]
- Klinkenberg I, Blockland A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Blockland A. A comparison of scopolamine and biperiden as a rodent model for cholinergic cognitive impairment. Psychopharmacology. 2011;215:549–566. doi: 10.1007/s00213-011-2171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Griffiths RR, Mintzer MZ. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Exp Clin Psychopharm. 2006;14:439–449. doi: 10.1037/1064-1297.14.4.439. [DOI] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiol Learn Mem. 2011;95:57–63. doi: 10.1016/j.nlm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Differential effects of scopolamine and lorazepam on working memory maintenance versus manipulation processes. Cog Aff Behav Neurosci. 2007;7:120–129. doi: 10.3758/cabn.7.2.120. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Lanier RK, Lofwall MR, Bigelow GE, Strain EC. Effects of repeated tramadol and morphine administration on psychomotor and cognitive performance in opioid-dependent volunteers. Drug Alcohol Depend. 2010;111:265–268. doi: 10.1016/j.drugalcdep.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharm. 2004;29:208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacut S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett. 2011;471:114–118. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmcol. 2011;36:2774–2781. doi: 10.1038/npp.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Malik N, Tricklebank M. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology. 2011;217:255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- Steckler T, Sahgal A, Aggleton J, Drinkenburg W. Recognition memory in rats—III. Neurochemical Substrates. Prog Neurobiol. 1998;54:333–348. doi: 10.1016/s0301-0082(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Wright AA. An experimental analysis of memory processing. J Exp Anal Behav. 2007;88:405, 433. doi: 10.1901/jeab.2007.88-405. [DOI] [PMC free article] [PubMed] [Google Scholar]