Abstract
Background
Large animals treated with immunosuppressive drugs for preclinical experiments of transplantation have increased risks of infection, which can be compounded by the induction of diabetes in these animals if islet transplantation is planned.
Methods
We report our experience with severe sepsis in two young cynomolgus monkeys and five pigs that were subjected to diabetes induction, immunosuppressive therapy +/− islet allotransplantation.
Results
In two monkeys and five pigs, infection was associated with a syndrome of profound hypoglycemia accompanied by severe acidosis, which was resistant to treatment. We do not believe this syndrome has been reported previously by others.
Conclusions
Despite treatment, this syndrome complicated the interpretation of blood glucose readings as a measure of islet graft function, and resulted in death or the need for euthanasia in all 7 animals. We tentatively suggest that the syndrome may be related to the presence of microorganisms that metabolize glucose and produce lactate.
Keywords: Acidosis, metabolic, Hypoglycemia, Infection, Islet transplantationMonkey
INTRODUCTION
The encouraging results of clinical islet allotransplantation (1) have stimulated renewed experimental efforts in nonhuman primate and other large animal models of allotransplantation (2,3) and xenotransplantation (4,5).
The immunosuppressive drug regimens used in preclinical studies of transplantation involving large animals increase the risk of infection, as can a state of diabetes. Here we report our experience with severe sepsis in two young immunosuppressed, diabetic cynomolgus monkeys in a study of islet allotransplantation. The nature of the experiment, which included the establishment of permanent intravascular access via indwelling catheters and induction of a diabetic state, may have further increased the infectious risks. Although housed in clean facilities, fecal contamination of the animal cages is inevitable and creates suboptimal hygienic conditions.
Sepsis is a well-known complication seen in patients with diabetes (6) and in those receiving immunosuppressive therapy after organ transplantation (7). Similarly, sepsis is not uncommon in immunosuppressed nonhuman primates undergoing transplantation procedures. However, the diagnosis of sepsis in nonhuman primates is more difficult than in humans. Lethargy, withdrawal, and/or anorexia may suggest sepsis, as might a significant rise in white blood cell count, though this may not occur when intensive immunosuppressive therapy is being administered. A positive blood or tissue culture is usually necessary to confirm the diagnosis and identify the causative microorganism.
In both of the monkeys reported here, infection was associated with a syndrome of intractable profound hypoglycemia accompanied by severe metabolic acidosis. In the discussion of these cases we include information on five immunosuppressed pigs that also experienced severe hypoglycemic episodes after diabetes induction and islet allotransplantation (8). Together, we suggest these cases represent a syndrome that has not been reported in the literature.
RESULTS
Case 1
In this monkey, after the transplantation of allo-islets (12,500IEq/kg) into the portal vein (Figure 1), although blood glucose levels during the first 8 post-transplant days were reduced (mean fasting blood glucose 137mg/dL), consistent normoglycemia was not achieved. Insulin requirements were significantly reduced (from 4.4 to 1.5 IU/day). C-peptide was detectable on day 1 (0.8ng/mL), but later was barely detectable (<0.1ng/mL). The monkey remained active, with normal hematological and biochemical parameters. However, it subsequently became profoundly hypoglycemic (blood glucose <20mg/dL) even when no insulin had been administered, and required frequent i.v. boluses or infusions of dextrose. On day 9, hematologic and biochemistry parameters were within normal ranges, but blood analysis revealed metabolic acidosis (pH 7.26, CO2 31mm Hg, HCO3 13mmol/L, base deficit −13). The cause of the acidosis was uncertain.
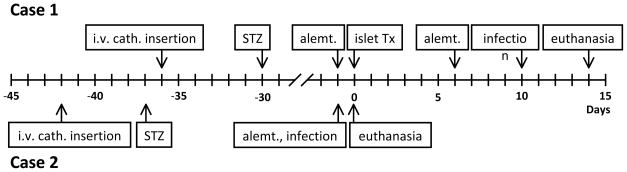
Figure 1. Time line of events.
Timing of i.v. catheter insertion, induction of diabetes with streptozotocin, induction of immunosuppressive therapy, islet transplantation, and onset of clinical features of infection.
alemt = alemtuzumab; cath = catheter; STZ = streptozotocin; Tx = transplantation
On day 10, the monkey became withdrawn, inactive and anorexic, and blood analysis revealed severe metabolic acidosis (Table 1). Although the white blood cell count remained normal, a slight thrombocytopenia and anemia had developed. Physical examination revealed hypothermia (rectal temperature 32 °C), but otherwise stable vital signs. Empiric antibiotic treatment with enrofloxacin i.v. and clindamycin i.v. was initiated after blood was drawn for culture. By day 12, despite the antibiotic regimen, the infusion of warm saline, dextrose and sodium bicarbonate, and maximum supportive therapy, the monkey remained hypoglycemic, acidotic, and hypothermic (Table 1). Diarrhea developed and the clinical status of the monkey deteriorated. Lactobacillus species (Gram-positive rods) had grown from the blood culture, and antibiotic treatment was switched to ampicillin i.v. and metronidazole p.o.
Table 1.
Results of laboratory tests in two diabetic monkeys with hypoglycemia and acidosis associated with sepsis
| Case 1 | Case 2 | ||
|---|---|---|---|
| Day | 10* | 12* | 0** |
| Parameter | |||
|
| |||
| pH | 7.14 | 7.22 | 7.16 |
| Blood glucose (mg/dL) | <20 | <20 | <20 |
| Base excess (mmol/L)# | −20 | −13 | −16 |
| HCO3−(mmol/L) | 8.9 | 14.9 | 13 |
| Na+ (mmol/L) | 150 | 153 | 127 |
| K+ (mmol/L) | 2.8 | 2.0 | 4.0 |
| iCa2+ (mmol/L) | 1.38 | 1.20 | 0.83 |
| Hemoglobin (g/dL) | 8.5 | 6 | 6.8 |
| WBC (×109/L) | 4.9 | 3.5 | 3.5 |
| Platelets (×109/L) | 183 | 143 | 13 |
Post islet transplantation
Islet transplantation was not carried out. Blood test results relate to the day after receiving alemtuzumab.
Lactate levels and anion gap were not measured.
Because of a lack of clinical improvement, the monkey was euthanized for humane reasons on day 14 after transplantation. Necropsy showed sporadic bilateral hemorrhagic infarction in the lungs. On histology, portions of the lungs appeared atelectatic and alveoli contained a proteinaceous fluid. In the renal tubules, features of a regenerative epithelium were seen, which suggested recovery from renal injury probably caused by streptozotocin treatment. No growth was observed from lung culture swabs. A blood culture drawn at the time of necropsy grew Klebsiella oxytoca (Gram-negative rods). Normal bacterial flora were cultured from the feces. Culture of the indwelling intravenous catheter remained negative. Histological examination of the liver showed multiple small viable islets with no cellular infiltration.
Case 2
In this monkey, the induction of diabetes was not followed by any untoward effect and the monkey appeared quiet but healthy. Thirty-five days after the successful induction of diabetes, immunosuppressive therapy was initiated (Figure 1). Two days earlier, there had been no abnormalities in hematologic parameters, except for a white blood count of 14.4×109/L, but biochemical parameters were not tested. Two hours after the infusion of alemtuzumab on the day before the planned islet transplantation the monkey became acutely dyspneic, anorexic, hypothermic (rectal temperature 33 °C), and demonstrated decreased alertness and lethargy. Laboratory tests showed profound hypoglycemia, severe acidosis, raised serum creatinine, leucopenia (primarily a lymphopenia), and profound thrombocytopenia (Table 1).
The biochemical changes were so severe that we suspect they had been increasing for several days, but had gone unnoticed, although this is conjecture. We suggest that the rapid deterioration might have been associated with cytokine release stimulated by the administration of the alemtuzumab, though we have no data to support this conclusion. There were no differences in selection of the monkey or administration of the monoclonal antibody from our previous study using alemtuzumab in non-diabetic monkeys (9) that might have accounted for this change in clinical condition.
Insulin treatment (up to then at a cumulative dose of 3.83 IU/kg/day) was discontinued and dextrose and bicarbonate infusions established. Blood for culture was drawn and empiric antibiotic treatment with enrofloxacin i.v. was started. In view of the features of sepsis, islet transplantation was postponed (and indeed was never carried out). Despite antibiotic therapy, the monkey’s condition deteriorated rapidly and, after consultation with the veterinary staff, for humane reasons it was euthanized on the following day. Blood cultures grew Gram-negative rods, later classified as Klebsiella oxytoca. At necropsy, severe pulmonary edema with pulmonary alveolar hemorrhages were the main findings. Histologic exmination of lung tissue confirmed interstitial edema. In the kidneys, acute thromboemboli in most arteries and necrotic glomeruli and tubules were seen, indicating acute diffuse renal cortical infarction, possibly as a consequence of sepsis and/or alemtuzumab administration.
DISCUSSION
In this report we describe the occurrence of severe hypoglycemia and metabolic acidosis associated with sepsis in two young cynomolgus monkeys in which diabetes had been induced 4–6 weeks previously, immunosuppressive therapy initiated, and islet transplantation carried out in one case.
As both monkeys had bacterial growth from blood cultures, we conclude that septicemia played an important role in the development of hypoglycemia and acidosis. However, possible side effects of alemtuzumab infusion in Case 2 may have contributed to the clinical condition, although we have not seen this previously in monkeys (n=6) that remained well and non-infected for periods of >1 year after receiving alemtuzumab with or without mycophenolate mofetil (9). Acute alveolar hemorrhage, renal failure and coagulopathy have all been reported after alemtuzumab treatment in human patients (10–12). Nevertheless, we suspect that the monkey in Case 2 had developed an infection before administration of alemtuzumab that had gone unnoticed, but clinical deterioration only became obvious at this time. Early signs of disease or infection may not always be obvious in nonhuman primates (13). In our previous studies of alemtuzumab, we did not see any infectious complications, but these monkeys were not diabetic (9).
Although the release of stress hormones during sepsis most commonly leads to a hyperglycemic state, hypoglycemic sepsis has been described (14,15), and has been shown to be independently associated with death in critically ill patients (14). Hypoglycemia should be considered a biomarker of disease severity (16), especially in diabetic patients (17). The young age and small size (body weight 1.9kg and 1.6kg, respectively – lower body weights than any of our previous studies) of the two monkeys in this report may have contributed to the severe derangements in glucose metabolism, acid-base balance and body temperature, as we have not observed these signs so severely in our broad experience with nonhuman primate transplant recipients of >2.5kg. Furthermore, their young age and low body weight might have rendered them more vulnerable to the effects of infection.
In previously reported experiments of islet allotransplantation into the gastric submucosal space of young (<2 months, mean 13kg) diabetic pigs immunosuppressed with tacrolimus and mycophenolate mofetil, we observed episodes of profound hypoglycemia in five pigs (8). The clinical picture seen in these pigs was almost identical to that seen in the two monkeys reported here. Diarrhea was observed in four of the five pigs. In the pigs, with functioning or partially-functioning islet grafts, blood glucose levels fell to 25–50mg/dL, resulting in sudden death in two pigs, and the need for euthanasia in the remaining three. Blood cultures grew Klebsiella oxytoca in one, and Streptococcus viridans and coagulase-negative Staphylococci in another. Blood cultures remained negative in the other three; however, at necropsy, signs of infection, such as colitis, cellulitis, and lobar pneumonia, were present. Although at the time we reasoned that infection would have led to hyperglycemia, our more recent experience in the monkeys reported here suggests that sepsis from Klebsiella, Lactobacillus, or possibly other pathogens likely played a role in the development of hypoglycemia.
Importantly, we have not seen this syndrome in numerous other nonhuman primates and pigs that have received identical or similar immunosuppressive regimens, but that were not rendered diabetic. We have no suggestions as to how this syndrome can be prevented as the monkeys and pigs were treated similarly to others at our facility that did not develop hypoglycemia and acidosis. We suggest that the nature of the infecting organism may be key in the development of the syndrome.
Our group has considerable experience with induction of diabetes by streptozotocin (in the form of Zanosar) in monkeys (18–20). Although renal injury has been documented on two occasions, the agent has otherwise not been associated with significant complication. In Case 1 of the present report, however, histopathological examination of the kidneys suggested some renal injury may have occurred which may have enhanced the risk of, or reduced the response to, sepsis.
The combination of diabetes and immunosuppressive therapy therefore appears to be important in the development of the syndrome, most likely contributing to an infection with a specific microorganism (or microorganisms). Nonhuman primates would appear to be much less susceptible to this (potential) infecting agent in the absence of either of these two contributory factors. We have not seen this syndrome in nonhuman primates that have been immunosuppressed but not diabetic. Nevertheless, it seems possible that hypoglycemia could develop in the presence of a positive infection and immunosuppressive therapy if the causative microorganisms were lactobacilli (or similar) which metabolize glucose.
Our vigorous efforts to maintain a state of normoglycemia (by i.v. dextrose infusion) and correct the acidosis (by i.v. sodium bicarbonate infusion) unexpectedly proved inadequate, attesting to the aggressive and progressive nature of the biochemical changes taking place. Whether alternative antibiotic therapy, lymphocyte-depleted whole blood transfusion (to correct anemia), and other measures would have proved successful remains speculative, but in our opinion appears unlikely.
Lactobacillus species are Gram-positive rods that colonize the gastrointestinal and genitourinary tracts and are considered normal human flora (21). Although rarely seen, Lactobacillus infections are opportunistic infections associated particularly with the use of immunosuppressive drugs after transplantation (22), in HIV infection (23), the use of probiotics (24), or the long-term use of indwelling intravascular catheters (25). To our knowledge, Lactobacillus infection in nonhuman primates after organ or cell transplantation has not been reported previously. Lactobacilli are (facultative) anaerobic bacteria and have a high saccharoclastic activity, i.e., they ferment glucose with at least one-half of the end-product carbon being lactate, which is not further fermented (26). The fermentation of glucose into lactate may have contributed to the treatment-resistant hypoglycemia and metabolic acidosis observed in our Case 1. Furthermore, although not positively cultured, we cannot exclude the possibility that Lactobacilli were playing a role in Case 2.
Klebsiella oxytoca, that was cultured from the blood in both of the monkeys reported here and in one of the pigs reported previously (8), is among the Gram-negative rods that can colonize the gastro-intestinal tract (27). The extent of colonization increases markedly during hospitalization, and it is a cause of infection mainly in patients who are immunocompromised or suffering from a debilitating disease, such as diabetes (27). Unlike Lactobacilli, Klebsiella does not ferment glucose into lactate, but may possibly have contributed to hypoglycemia and metabolic acidosis through a different mechanism, such as endotoxemia.
Because of the ability of Lactobacilli to ferment glucose and produce lactate, we suspect that this microorganism may be playing a role in this syndrome. However, the variety of flora cultured in the blood in the monkeys and pigs that developed hypoglycemia and acidosis suggests that perhaps the syndrome can be associated with other infectious agents. Our microbiological data are admittedly limited, and any conclusions drawn must be provisional and cautious.
Other potential factors in the development of the syndrome that might be considered are the absence of the inhibitory effect of insulin on proinflammatory cytokines (28–30). Partial restraint of the monkey in the jacket and tether system, which may possibly be associated with some stress, seems an unlikely factor as we have employed this system for many years without seeing this complication (20, 31–33); furthermore, the pigs that developed the same clinical features were not restrained in any way.
In summary, we have observed a syndrome in which young, diabetic, immunosuppressed monkeys and pigs develop profound hypoglycemia and severe metabolic acidosis, which in several cases was associated with documented sepsis. We suggest that the syndrome might be related to the presence of microorganisms that metabolize glucose and produce lactate. Although the immunosuppressive state almost certainly increases the susceptibility of the animal to an infectious complication, we have not seen this syndrome in non-diabetic immunosuppressed nonhuman primates and pigs. Despite treatment, this intractable syndrome complicated the interpretation of blood glucose readings as a measure of islet graft function, and resulted in death or the need for euthanasia in all animals in which it occurred.
MATERIALS AND METHODS
All animal care procedures were in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1985), and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Case 1
A 22 month-old (1.9kg) Indonesian cynomolgus monkey (Macaca fascicularis) underwent placement of an indwelling central venous catheter through the internal jugular vein and diabetes induction with streptozotocin (Zanosar, Sicor Pharmaceuticals, Irvine, CA, 150mg/kg) (Figure 1). (This dose has been utilized by our group with safety in the majority of cases [20] and also by others [34].) Diabetes was confirmed by the absence of C-peptide responses on intravenous glucose tolerance and arginine stimulation tests. Insulin therapy was begun to maintain blood glucose levels at <200mg/dL.
Profound lymphopenia (CD3+T cells <15cells/mm3) was induced by alemtuzumab (Campath-1H, Genzyme, Cambridge, MA) at a dose of 20mg/kg on the day before transplantation (i.e., day −1) (1). Pretreatment was with diphenhydramine 5mg/kg i.v. and metoclopramide 0.5mg/kg i.v.. Alemtuzumab administration (10mg/kg) was repeated on day 6 after transplantation to maintain low T cell counts (CD3+T cells <15cells/mm3 after the second infusion) (1).
On day 0, allogeneic islets were infused into the portal vein through a mini-laparotomy under heparin anticoagulation, and anti-TNF-α anti-inflammatory treatment with etanercept (Enbrel, Amgen, Thousand Oaks, CA) and 48h cefazolin antibiotic prophylaxis was initiated. Maintenance immunosuppression consisted of rapamycin 0.5mg/kg/i.m. (aiming for, and achieving, plasma trough levels of 5–15ng/mL). There was definite evidence of islet graft function, with reduction in mean blood glucose level and insulin requirements; C-peptide was detectable on day 1, but later was barely detectable.
Case 2
This 36 month-old (1.6kg) Indonesian cynomolgus monkey was included in the same research protocol, including placement of an indwelling intravascular catheter and induction of diabetes with streptozotocin. Thirty-five days after induction and confirmation of diabetes, alemtuzumab 20mg/kg was infused after pretreatment as in Case 1.
Acknowledgments
Work on transplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959-01, #U01 AI068642, and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
Footnotes
HZ, DJvdW, EMD, RB, and MW performed the monkey experiments and collected the data. HZ, DJvdW, EMD, and DKCC wrote the manuscript. GJE provided data on pigs. LHR performed pathological analyses. RW supervised veterinary support. The authors declare that they have no conflict of interest.
References
- 1.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35:1436. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman DM, Willman MA, Han D, et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59:2558. doi: 10.2337/db10-0136. Epub 2010 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowe M, Badell IR, Thompson P, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079. doi: 10.1111/j.1600-6143.2012.04054.x. Epub 2012 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson P, Badell IR, Lowe M, et al. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. Am J Transplant. 2012;12:1765. doi: 10.1111/j.1600-6143.2012.04031.x. Epub 2012 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Windt DJ, Bottino R, Kumar G, et al. Clinical islet xenotransplantation – how close are we? Diabetes. 2012 doi: 10.2337/db12-0033. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuetz P, Castro P, Shapiro NI. Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care. 2011;34:771. doi: 10.2337/dc10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva M, Jr, Marra AR, Pereira CA, et al. Bloodstream infection after kidney transplantation: Epidemiology, microbiology, associated risk factors, and outcome. Transplantation. 2010;90:581. doi: 10.1097/TP.0b013e3181e8a680. [DOI] [PubMed] [Google Scholar]
- 8.Echeverri GJ, McGrath K, Bottino R, et al. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant. 2009;9:2485. doi: 10.1111/j.1600-6143.2009.02815.x. [DOI] [PubMed] [Google Scholar]
- 9.van der Windt DJ, Smetanka C, Macedo C, et al. Investigation of lymphocyte depletion and repopulation using alemtuzumab (Campath-1H) in cynomolgus monkeys. Am J Transplant. 2010;10:773. doi: 10.1111/j.1600-6143.2010.03050.x. [DOI] [PubMed] [Google Scholar]
- 10.Osborne WL, Lennard AL. Acute renal failure and disseminated intravascular coagulation following an idiosyncratic reaction to Alemtuzumab (Campath 1H) or fludarabine. Haematologica. 2005;90:ECR05. [PubMed] [Google Scholar]
- 11.Ku G, Ting WC, Lim ST, Lee BT, Calne RY. Life-threatening coagulopathy associated with use of Campath (alemtuzumab) in maintenance steroid-free renal transplant given before surgery. Am J Transplant. 2008;8:884. doi: 10.1111/j.1600-6143.2008.02152.x. [DOI] [PubMed] [Google Scholar]
- 12.Sachdeva A, Matuschak GM. Diffuse alveolar hemorrhage following alemtuzumab. Chest. 2008;133:1476. doi: 10.1378/chest.07-2354. [DOI] [PubMed] [Google Scholar]
- 13.Haustein SV, Kolterman AJ, Sundblad JJ, Fechner JH, Knechtle SJ. Nonhuman primate infections after organ transplantation. ILAR J. 2008;49:209. doi: 10.1093/ilar.49.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85:217. doi: 10.4065/mcp.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med. 2010;38:1430. doi: 10.1097/CCM.0b013e3181de562c. [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, Egi M. Hypoglycemia in sepsis: biomarker, mediator, or both? Crit Care Med. 2011;39:2367. doi: 10.1097/CCM.0b013e31822a5be5. [DOI] [PubMed] [Google Scholar]
- 17.Schuetz P, Jones AE, Howell MD, et al. Diabetes is not associated with increased mortality in emergency department patients with sepsis. Ann Emerg Med. 2011;58:438. doi: 10.1016/j.annemergmed.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rood PPM, Bottino R, Balamurugan AN, et al. Induction of diabetes in cynomolgus monkeys with high-dose streptozotocin. Adverse effects and early responses. Pancreas. 2006;33:287. doi: 10.1097/01.mpa.0000235307.04110.a2. [DOI] [PubMed] [Google Scholar]
- 19.Rood PPM, Bottino R, Balamurugan AN, et al. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007;83:202. doi: 10.1097/01.tp.0000250680.36942.c6. [DOI] [PubMed] [Google Scholar]
- 20.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 21.Isenberg HD, D’Amato RF. Indigenous and pathogenic micro-organisms of humans. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. 6. Washington: ASM Press; 1995. p. 5. [Google Scholar]
- 22.Robin F, Paillard C, Marchandin H, Demeocq F, Bonnet R, Hennequin C. Lactobacillus rhamnosus meningitis following recurrent episodes of bacteremia in a child undergoing allogeneic hematopoietic stem cell transplantation. J Clin Microbiol. 2010;48:4317. doi: 10.1128/JCM.00250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dicks LM, Fraser T, ten Doeschate K, van Reenen CA. Lactic acid bacteria population in children diagnosed with human immunodeficiency virus. J Paediatr Child Health. 2009;45:567. doi: 10.1111/j.1440-1754.2009.01566.x. [DOI] [PubMed] [Google Scholar]
- 24.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115:178. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 25.Thompson C, McCarter YS, Krause PJ, Herson VC. Lactobacillus acidophilus sepsis in a neonate. J Perinatol. 2001;21:258. doi: 10.1038/sj.jp.7200509. [DOI] [PubMed] [Google Scholar]
- 26.Holdeman LV, Moore WEC. Anaerobe Laboratory Manual. Blacksburg: Southern Printing Company; 1973. Anaerobic gram-positive non-sporing rods; p. 61. [Google Scholar]
- 27.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dandona P, Aljada A, Mohanty P, et al. Insulin inhibits intranuclear Nuclear Factor kB and stimulates IkB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 29.Dandona P, Aljada A, Mohanty P. The anti-inflammatory and potential anti-atherogenic effect of insulin: a new paradigm. Diabetologia. 2002;45:924. doi: 10.1007/s00125-001-0766-5. [DOI] [PubMed] [Google Scholar]
- 30.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperclycemic crises. Diabetes. 2004;53:2079. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 31.Cooper DKC, Ye Y, Niekrasz M. Heart transplantation in primates. In: Makowka L, Cramer DV, Sher L, editors. Experimental Techniques in Transplantation. C.R.C. Press; Boca Raton: 1994. pp. 173–200. [Google Scholar]
- 32.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in α1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically-modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 34.Theriault BR, Thistlethwaite JR, Jr, Levisetti MG, et al. Induction, maintenance, and reversal of streptozotocin-induced insulin-dependent diabetes mellitus in the juvenile cynomolgus monkey (Macaca fascicularis) Transplantation. 1999;68:331. doi: 10.1097/00007890-199908150-00003. [DOI] [PubMed] [Google Scholar]