Abstract
Objective
Cervical cancer is a preventable disease which causes significant morbidity and mortality, particularly in developing countries. While technology for early detection continues to improve, prevention programs suffer from significant barriers. Community Based Participatory Research is an approach to research which focuses on collaboration with the community to surmount these barriers. The objective of this study was to evaluate the utility of Community Based Participatory Research techniques in a mother-child screen/treat and vaccinate program for cervical cancer prevention in Manchay, Peru.
Methods/materials
HPV self-sampling and cryotherapy were utilized for the screen/treat intervention, and the Gardasil vaccine was utilized for the vaccine intervention. Community health workers from Manchay participated in a 3-day educational course, designed by the research team. The community health workers then decided how to implement the interventions in their community. The success of the program was measured by: 1) the ability of the community health workers to determine an implementation plan, 2) the successful use of research forms provided, 3) participation and retention rates, and 4) satisfaction of the participants.
Results
1) The community health workers used a door-to-door approach through which participants were successfully registered and both interventions were successfully carried out; 2) registration forms, consent forms, and result forms were utilized correctly with minimal error; 3) screen/treat intervention: 97% of registered participants gave an HPV sample, 94% of HPV positive women were treated, and 90% returned for 6-month follow-up; vaccine intervention: 95% of registered girls received the 1st vaccine, 97% of those received the 2nd vaccine, and 93% the 3rd; 4) 96% of participants in the screen/treat intervention reported high satisfaction.
Conclusion
Community Based Participatory Research techniques successfully helped to implement a screen/treat and vaccinate cervical cancer prevention program in Manchay, Peru. These techniques may help overcome barriers to large-scale preventive health-care interventions.
Keywords: cervical cancer, prevention, community based participatory research
I. INTRO
Community-Based Participatory Research (CBPR) is a collaborative approach to research used to help address barriers of health-care delivery in the developed and developing world (1–3). CBPR is a partnership between researchers, a community, and local community healthcare workers (CHW). Together they develop a system to function within the community to address important needs in a particular population (4). If this partnership can raise community awareness and gain its trust, it may be especially useful for delivery of preventive services where reaching a large proportion of the population is optimal.
Cervical cancer represents the only human cancer for which we know the necessary cause (5) and which can successfully be prevented. Nonetheless, there were 275,000 deaths from cervical cancer in 2008, 88% of which occurred in developing countries (6). In Peru, the incidence of cervical cancer is in GLOBOCAN’s highest category (34.5/100,000), and there is an extremely high mortality rate (16.3/100,000) compared with other countries (7). Manchay is a shanty town close to the city of Lima, with a population of 60 – 80,000, 53% of whom live in conditions of extreme poverty (8). Prevention services such as cervical cancer screening are often unavailable in Manchay.
While technology to prevent cervical cancer has improved throughout recent years, prevention programs in the developing world continue to suffer from cost constraints, poor participation, lost to follow-up, and concerns about sustainability. For example, the TATI* project in Peru recruited only 32.3% of their target population and 50% of test-positive patients were lost to follow-up (9, 10). Surmounting the barriers of cost and poor participation and follow-up require that prevention programs use an effective intervention and deliver it in a manner compatible with the community’s values, beliefs, and economic realities.
While the technology to eradicate cervical cancer currently exists, the strategies to implement large-scale screening are lacking. The objective of this study therefore was to embrace concepts of community based participatory research (CBPR) in order to develop a reliable model for preventive healthcare interventions. We incorporated CBPR techniques in a mother/child screen, treat, and vaccinate program for cervical cancer prevention in Manchay, Peru. This manuscript discusses the CBPR strategies utilized and results obtained in the process of developing a model for large-scale screening.
II. MATERIALS AND METHODS
International Review Board approval was achieved through the Instituto Nacional de Enfermedades Neoplásicas (INEN)**, the Peruvian NIH, and the Cleveland Clinic. The project is registered with the US National Institutes of Health as number NCT01338051. Funding was obtained through the Merck Inc. “Investigator Initiated Studies Program”.
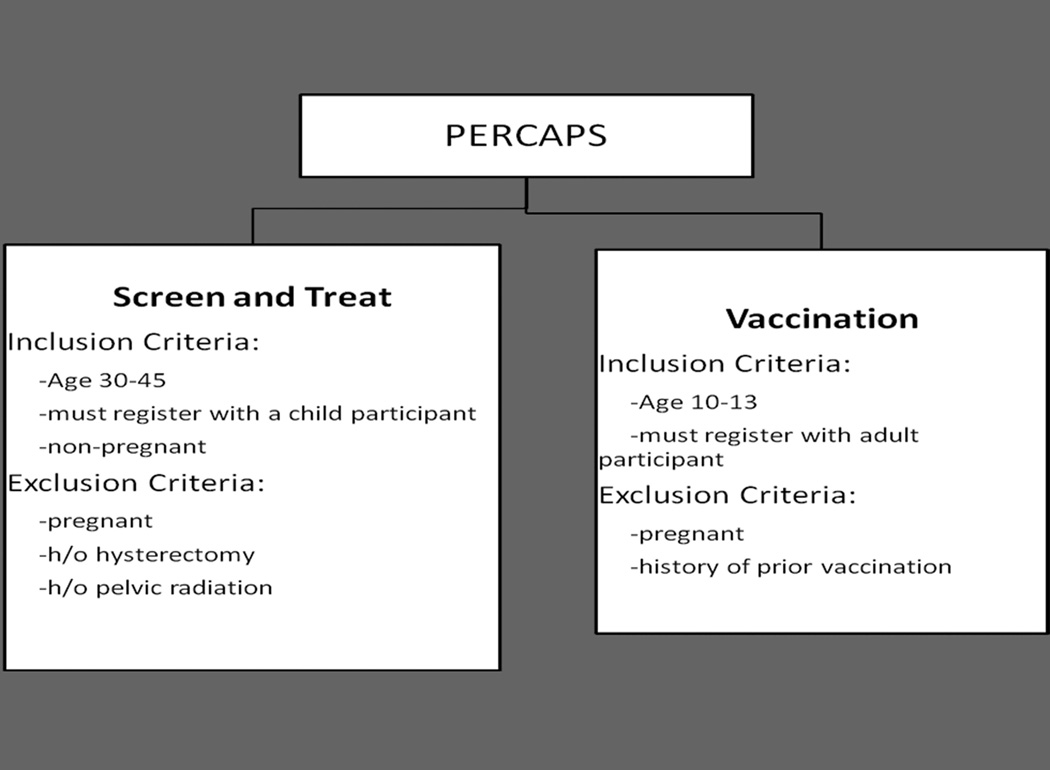
The medical technologies utilized were: 1) cervical HPV self-sampling [technology developed over the past 14 years by Preventive Oncology International (“POI”) and The Cleveland Clinic (11,12)] followed by treatment with cryotherapy for HPV positive participants, and 2) the Gardasil vaccine by Merck (scheduled in three doses at 0,2, and 6 months). Inclusion and exclusion criteria are listed in Figure 1.
Figure 1.
Inclusion and Exclusion Criteria for Screen/Treat and Vaccine Interventions
a. Pre-Study Preparation
Cervical cancer was pre-selected as the focus of this intervention rather than leaving this decision to the community. Cervical cancer was chosen because there is a high incidence in Peru, there are proven preventive measures, and because it is the area of expertise and interest of the investigators. With this focus in mind, in 2009 a site visit to Peru identified Manchay, in the district of Pachamac, as an area of great need without complex geography adding difficulty in reaching the population, and therefore an ideal location for the study. POI presented the Manchay community with the concept of participating in a cervical cancer prevention program and formed a partnership with Manchay’s community leaders, the community health center, the neighborhood “promotoras”*** who participated as CHWs, and INEN in Lima.
At the outset of the project, the research team designed a three-day program for the CHWs, and initially, a large group of CHWs were recruited to allow for flexibility in the CBPR strategies. Therefore, if the chosen strategy required more CHWs, they would have the necessary background for the project. The primary educator and facilitator was one of the authors of this study (JS). The program focused on 1) education about HPV, cervical cancer, and the interventions for the study, 2) the design of the implementation plan by the CHWs (how to advertise, recruit, register, collect and report results), and 3) introduction to the standardized study forms created by the research team. One day of training was dedicated to each of these tasks (Table 1).
Table 1.
Overview of Course for the Community Health Workers
| Day 1 |
|
| Day 2 |
|
| Day 3 |
|
Three weeks were dedicated to raising community awareness for the screening and for registration of participants. Registration forms requiring name, DOB, and baseline (non-personal) characteristics of participants were provided, and at the time of registration, each participant was given a unique identification code. This study was designed as a mother/child study, where each participant was registered with her daughters or granddaughters age 10–13. Each participant signed a consent form for herself and her daughter(s). Assent forms were signed by the daughters participating.
For the screen and treat intervention, an instruction form for collection of the HPV self-sample was provided to each participant (Fig 1). Once collected, specimens were recorded on a de-identified Excel spreadsheet which was printed and shipped with the samples [using solid transfer media iFTA-elute (GE Healthcare, Piscataway, NJ)] to BGI Shenzhen, in Shenzhen, China. Specimens were tested for high-risk HPV (type specific) and results were returned to a research team member in Peru within one week. Results were then matched with the identities of participants for notification by the CHWs. Treatment day for HPV positive participants was planned 4 days after results were distributed. HPV positive women were triaged with VIA (unmagnified visual inspection using acetic acid) to determine appropriateness of immediate cryotherapy (performed at the health clinic in Manchay) or referral to INEN. Patients received verbal post treatment instructions from the research team. Women treated with cryotherapy were re-contacted after 6 months for follow-up colposcopy, biopsies, and repeat HPV testing.
For the vaccine intervention, the guardian (i.e. mother, grandmother) of each participant was given a written record of each vaccination. Additionally, each CHW filled out a vaccination record sheet that was kept by the research team.
An evaluation of the study was conducted face to face with each adult participant four weeks after the treatment of HPV positive patients, and after administration of the first vaccine. A research physician, fluent in the local language, conducted all interviews. All participants were given the opportunity to ask additional questions about the study, HPV, cervical cancer, or other health concerns.
b. CBPR Decisions made by the CHWs
In accordance with CBPR principles, the procedures for advertising, subject recruitment, registration, consent, delivery of interventions, and delivery of results were established collaboratively between the research team and the Manchay CHWs, during the 3-day training. Ten CHWs were ultimately decided upon as the appropriate number for the study, and these individuals were the primary decision makers for recruitment methods, distribution and collection of samples, and reporting of results.
The CHWs decided to each recruit and register 30–33 women in a manner chosen by themselves, depending on the characteristics of their neighborhood. A few CHWs developed systematic techniques, going door-to-door, starting centrally within a sector and moving outward; other CHWs first asked friends, family members, and neighbors; others went to public places, such as the market, to recruit women. Specimen collection from adults and the first vaccination of children were planned for the same day.
The CHWs located each HPV positive woman and accompanied them for their treatment and 6 month follow-up appointments. They also planned to visit each participant’s home or workplace for delivery of results.
c. Analysis
Much of the analyses for this study are descriptive in nature. The outcomes evaluated included: 1) success of the training course, measured by the ability of the CHW’s to determine an implementation plan, 2) use and understanding of the forms provided by the research team, 3) program participation and retention rates for both interventions, and 4) satisfaction of the participants. All quantitative analysis was performed using STATA 10.0 software (College Station, TX).
III.RESULTS
a. CBPR planning
The CHWs worked together during the 3-day training course and determined an implementation strategy for their community. While the CHWs were challenged to develop a plan for a large-scale screening trial, they ultimately decided upon a door-to-door strategy. Utilizing this approach, the speed and success of collection and vaccination depended on each CHW's organizational skills. The most efficient CHWs were those who were systematic during recruitment. At the end of the project, several CHWs recognized that a door-to-door model might be difficult on a larger scale; however, they continued to believe that this approach would obtain the best participation rates in their community.
b. Utilization of forms
Registration of participants proceeded smoothly with forms filled out correctly in the majority of cases. Two women were enrolled who did not qualify for the study by inclusion and exclusion criteria, and one woman was registered twice. These errors were recognized quickly, prior to sample collection, and these participants were excluded.
It became clear throughout the evaluation process that some girls included in the study were not in fact biological children of the adult participants, but rather were cared for by the family or “children of the community”. The relationships to the registered adult included daughter (89%), niece (5.9%), neighbor (0.8%); additionally, 1 child was the step-daughter of a registrant, 1 was the granddaughter, and 1 was the sister. In 1.7% of cases, the registered adult was the child’s guardian or caretaker.
c. Participation rates
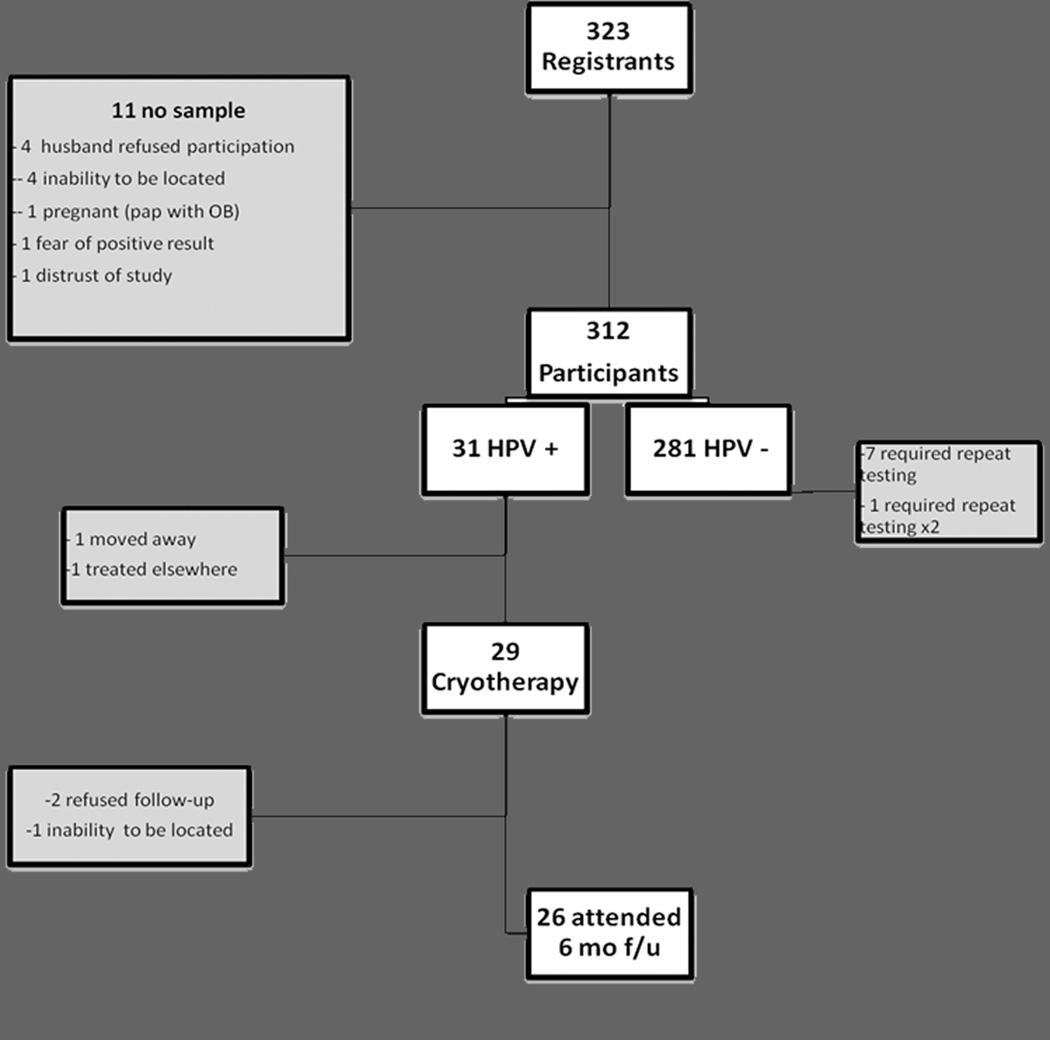
Ultimately, 323 adults were registered, and 312 specimens (96.6%) were collected. Reasons for not providing a self-sample included: their husband did not want them to participate (4), inability to be located (4), positive pregnancy test (1), fear of a positive result (1), and distrust of the study (1) (Fig. 2).
Figure 2.
Participation for Screen/Treat Intervention
Thirty-one women (9.9%) tested positive for high-risk HPV, 29 (93.5%) of whom returned for evaluation and treatment. One of the participants who declined to return elected to go to INEN to talk with an oncologist. The other moved away from Manchay and could not be contacted for her results (Fig. 2).
Due to policy changes at INEN, transportation of colposcopy equipment to Manchay was not possible; therefore, the 6-month follow-up for HPV positive patients was conducted by physicians at INEN rather than at the health clinic in Manchay. Each CHW traveled with the participants to INEN and assisted in facilitation of the follow-up visits. The cost of transportation to and from INEN was covered by the study, and visits occurred on several dates to accommodate participants’ schedules. Several participants required two follow-up visits because, with the change of location, biopsies were not initially collected. Out of 29 women initially treated, 26 (90%) were seen and evaluated for the 6 month follow-up. The other 3 participants either refused follow-up (2) or were not able to be re-located (1). Additionally, 1 patient was pregnant, therefore, her evaluation was delayed (Fig. 2).
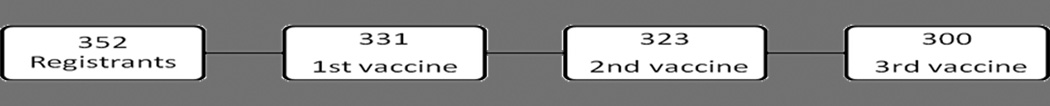
For the vaccination intervention, 331 (94.0%) out of 352 girls who were initially registered received the 1st dose of vaccine. While most girls received the vaccine on the day of specimen collection, 5 girls were not available and therefore received the 1st vaccination later in the week. An additional 12 participants were identified during the evaluation that desired the vaccination, but had not initially received it because they were away or unavailable. The CHWs arranged for these girls to come to the health clinic to obtain the first vaccine. For the 21 girls who registered but did not receive the vaccine, reasons included: moving away from Manchay (2), subsequent refusal by caregivers (18), and 1 girl received the vaccine in school. Reasons given for refusal included fear that the vaccine might lead to sterilization, and belief that their daughters “did not need” the vaccine because they were not sexually-active. Three women also reported that their husbands prohibited their daughter from receiving the vaccine.
Three-hundred and twenty three girls (97.6%) who received the 1st vaccine received the 2nd vaccine (4 refused, 4 moved or were unable to be located), and 300 (92.9% of those who received the 2nd vaccine) received the 3rd vaccine. Therefore, 92.9% of girls who initiated the vaccine series received all 3 shots (Fig. 3). In order to achieve this participation rate, CHWs were encouraged by study staff weekly to continue to pursue a 100% completion rate.
Figure 3.
Participation for Vaccine Intervention
Due to difficulties with coordinating and shipping the Gardasil vaccine to the site, the shipment of the second vaccine did not arrive in Manchay in accordance with the original schedule. Therefore, the research team was unable to obtain sufficient quantity of vaccine until 3 months after the 1st vaccination, and girls were relocated 3 months after the 1st vaccination rather than the intended 2 month interval. The CHWs were consistently updated by the research team on the status of the 2nd vaccination, and the CHWs then updated participants so that expectations were changed and met. The third vaccine was given, as intended, 4 months after the second vaccination.
d. Satisfaction
Two hundred ninety-nine of the 312 participants who collected an HPV sample were interviewed by the medical staff, including all HPV positive women other than the woman who was not able to be located for delivery of her results. Both HPV positive and HPV negative participants reported high levels of study satisfaction, with 96.4% reporting that they would participate in this type of program in the future. Patients were satisfied with the door-to door model developed by the CHWs and reported that combining this model with self-sampling was convenient for them with their work and family responsibilities. The only change suggested by a large number of participants was that they should be given a written record of their results in addition to the face-to-face visit from the CHW. Thirty-one percent reported that they would like to have their results on paper; therefore, after the evaluation period concluded, each patient was provided with a record of her results with the appropriate recommendation for follow-up.
IV. DISCUSSION
There are multiple interventions available which prevent cervical cancer. However, a major issue that remains is how to make this technology available to those most at risk for this disease. This challenge applies not only to cervical cancer screening, but also on a broader scale to many screening and vaccination technologies available and on the horizon.
This study was implemented to begin the process of designing a model for large-scale delivery of preventive healthcare, with cervical cancer screening and vaccination as the target interventions. To this end, significant progress was made. While many CBPR results are specific to this community, significant lessons can be learned with regards to implementation of the CBPR strategies. In examining the results of this study, the CHWs were able to determine an implementation strategy for the interventions in their community, all forms were filled out effectively, there was minimal loss to follow-up and high rates of satisfaction. We were encouraged by these results and would expect replication of these results outside of the research environment. Ultimately, we believe a core model can be designed.
In this study, several unexpected challenges occurred. The first was the delay in arrival of the 2nd vaccination. Partially due to this delay and partially due to the long time period over which vaccinations occurred, significant influence and participation of study staff was necessary to achieve a high completion rate. This influence was not needed for the HPV intervention, and this may be a reason to de-link these interventions and provide vaccines in school, which has historically been successful (13, 14). Another challenge in this study was the change in location for 6 month follow-up of HPV positive participants. Frequent adjustments are often necessary in resource poor settings, especially when physicians and equipment are needed. Despite these unanticipated changes, participation rates remained high, largely due to the dedication of the CHWs who facilitated the changes and maintained the trust of the participants. Although we viewed this follow-up as an important safety factor for the study participants, it likely would not be included in many large public health programs.
There are several limitations of this study. As this was the first in a series of studies aimed at developing the screening model, it is difficult to determine fixed endpoints. In order to adequately evaluate the value of the CBPR techniques, it would be necessary to determine what percentage of the eligible population can be screened through the strategies utilized, an evaluation which was beyond the scope of this initial study. Furthermore, although the quantitative outcomes of participation, lost-to-follow-up, and satisfaction are promising, it is difficult to distinguish the influence of the unique technology (the HPV self-sampling and Gardasil vaccine) from CBPR techniques employed. Also, this research team had limited experience with CBPR, and this study is therefore part of the learning process of how to effectively apply key CBPR principles and balance the goals of the research with the decisions of the CHWs. A cost analysis is an important aspect of public health prevention interventions, However, in order to do a proper cost comparison an analysis of this model would need to be compared to the usual caregiver directed screening and assays. Our model is directed towards the medically underserved, and when fully developed will be at its cost effective best when the volumes become very large. The type of analysis needed is far beyond the scope of this work at the present time. However, a proper cost analysis will be a requisite component of the final preventive healthcare model. In terms of the “charges” for the “Just for Me” self-sampling kit, processing the sample, and reporting the result, we anticipate this should not exceed $5.00 per sample. We hope as the chip sizes increase with new sequencing technologies, the cost per case will be pushed even lower.
One of the key strengths of this study were that decisions made by the CHWs were implemented regardless of cost or efficiency. While the approach chosen by the CHWs in Manchay was a less time-efficient process, the goal of CBPR techniques is to partner with the CHWs, trusting their insights about the community to determine the optimal strategic processes. While long-term data regarding sustainability is not available, this study does show that CBPR techniques were successful in achieving the high participation and satisfaction rates accomplished in this community at this time. Another strength of this study is that it made key steps towards creating a model within which concepts of CBPR can be utilized. Finally, 95% of the participants in this study were interviewed, allowing for a comprehensive understanding of the successes and challenges.
Although this study is an important step in developing a model for preventive screening, the next steps will be to further refine this model in culturally distinct and geographically complex settings and to test it in a large-scale screening program. If the screening program can be accomplished up to the point of the identification of HPV positive women without significant involvement of the existing healthcare infrastructure, resources can be directed more efficiently toward those who need them the most.
While some decisions based on the CBPR strategies may be specific to each community, our goal is to create a core model that will provide CHWs the tools to determine the best strategy for their community, and a framework upon which to implement that plan, with consistently low loss to follow-up across populations and diseases.
Acknowledgments
Funding:
Preventive Oncology International
Supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
This work was supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Instute of Dental and Craniofacial Research, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseaseses, and National Institutes of Health Office of Women’s Health and Research through the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Acronym for the Spanish term “tamizaje y tratamiento inmediato” translated as: triage and immediate treatment
English Translation : National Institute of Neoplastic Diseases
English Translation : promotor (in this setting, indicating “health promoter”)
References
- 1.Barbee L, Kobetz E, Menard J, et al. Assessing the acceptability of self-sampling for HPV among Haitian immigrant women: CBPR in action. Cancer Causes Control. 2010;21:421–431. doi: 10.1007/s10552-009-9474-0. [DOI] [PubMed] [Google Scholar]
- 2.Christopher S, Gidley A, Letiecq B, et al. A Cervical Cancer Community-Based Participatory Research Project in a Native American Community. Health Education and Behavior. 2008;35:821–834. doi: 10.1177/1090198107309457. [DOI] [PubMed] [Google Scholar]
- 3.Ma GX, Shive S, Tan Y, et al. Community-based colorectal cáncer intervention in underserved Korean Americans. Cancer Epidemiology. 2009;33:381–386. doi: 10.1016/j.canep.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Minkler M, Wallerstein N, editors. Community-Based Participatory Research for Health: From Process to Outcomes, Second Edition. San Francisco, CA: John Wiley and Sons; 2008. Part One: Introduction to Community-Based Participatory Research; pp. 5–19. [Google Scholar]
- 5.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Globocan 2008 Cancer Fact Sheet [Globocan (IARC) Web site] [Accessed May 23, 2012];2008 Available at: http://globocan.iarc.fr/factsheets/cancers/cervix.asp.
- 7.Globocan 2008 Fast Stats: Peru [Globocan (IARC) Web site] [Accessed May 23, 2012];2008 Available at: http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=604#WOMEN. [Google Scholar]
- 8.Local economic development as if people and the planet mattered: Peru (Manchay, Pachacamac, Lima) [Nef plugging the leaks Web site] [Accessed September 19, 2011];2008 Available at: http://www.pluggingtheleaks.org/communities_taking_action/peru.htm.
- 9.Luciani S, Winkler J. Cervical cancer prevention in Peru: lessons learned from the TATI demonstration project [Pan American Health Organization] [AccessedMay18, 2012];2006 Available at: http://www.paho.org/english/ad/dpc/nc/pcc-cc-tati-rpt.htm.
- 10.Robles SC, Ferreccio C, Tsu V, et al. Assessing participation of women in a cervical cancer screening program in Peru. Rev Panam Salud Publica. 2009;25:189–195. doi: 10.1590/s1020-49892009000300001. [DOI] [PubMed] [Google Scholar]
- 11.Belinson JL, Pretorius RG, Zhang WH, et al. Cervical cancer screening by simple visual inspection after acetic acid. Obstet Gynecol. 2001;98:441–444. doi: 10.1016/s0029-7844(01)01454-5. [DOI] [PubMed] [Google Scholar]
- 12.Belinson JL, Du H, Yang B, et al. Improved sensitivity of vaginal self-collection and high-risk human papillomavirus testing. Int J Cancer. 2012;130:1855–1860. doi: 10.1002/ijc.26202. [DOI] [PubMed] [Google Scholar]
- 13.Penny M, Bartolini R, Mosqueira NR, et al. Strategies to vaccinate against cancer of the cervix: feasibility of a school-based HPV vaccination program in Peru. Vaccine. 2011;29:5022–5030. doi: 10.1016/j.vaccine.2011.04.078. [DOI] [PubMed] [Google Scholar]
- 14.Kessels SJ, Marshall HS, Watson M, et al. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30:3546–3556. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]