Abstract
Induction of c-Jun and c-Fos, partners that comprise the AP1 transcription factor, is critical for GnRH regulation of FSHβ gene expression. The signaling pathways that are necessary for regulation of AP1 in the gonadotrope cell are not known. Here, we investigate the mechanism of c-Jun induction by GnRH, the sole regulator of c-Jun in the gonadotrope. We identify that GnRH phosphorylates ATF2 via p38 and JNK, the same pathways responsible for GnRH induction of c-Jun. Upon phosphorylation, ATF2 binds the CRE element within the c-Jun proximal promoter and interacts with NFY. Functional ATF2 is necessary for both GnRH induction of c-Jun and FSHβ. Taken together, these studies elucidate the specificity of c-Jun induction by GnRH in the gonadotrope by demonstrating GnRH activation of the p38 and JNK signaling pathways that lead to phosphorylation of ATF2, providing critical insight into GnRH regulation of its target gene, the gonadotropin subunit FSHβ.
Keywords: c-Jun, ATF2, ATF3, p38, JNK, GnRH
1. Introduction
c-Jun is an immediate early gene that homodimerizes or heterodimerizes with c-Fos to form the AP1 transcription factor. AP1 is induced by gonadotropin-releasing hormone (GnRH) both in vivo (Padmanabhan et al., 1995) and in immortalized gonadotrope cells (Cesnjaj et al., 1994, Wurmbach et al., 2001), to stimulate the transcription of the follicle-stimulating hormone (FSH) β gene (Strahl et al., 1997, Coss et al., 2004, Wang et al., 2008). FSHβ then heterodimerizes with a common α-subunit to create the mature FSH glycohormone that stimulates folliculogenesis in the gonads (Pierce and Parsons, 1981). Regulation of FSHβ transcription is the limiting component in the synthesis of the mature FSH and is therefore a key step in the control of FSH concentration in circulation (Papavasiliou et al., 1986, Kaiser et al., 1997). Thus, elucidating the regulation of major players in FSH synthesis, such as c-Jun, is necessary to understand the etiology of infertility.
Although GnRH signaling has been studied extensively over the last 10 years since the advent of the immortalized gonadotrope cell lines, αT3 and LβT2, a careful dissection of the signaling pathways that lead to transcription factor activation remains a gap in our knowledge. The pathways whereby GnRH signal specifically induces gonadotropin genes, compared to other hormones that may utilize the same intracellular pathways but do not result in gonadotropin gene regulation, are not known. AP1 is the only known mediator of GnRH induction of the mouse, ovine and human FSHβ genes, while CREB mediates rat FSHβ induction (Strahl et al., 1998, Coss et al., 2004, Ciccone et al., 2008, Wang et al., 2008). Both AP1 and CREB bind the FSHβ promoter at the conserved -72 site that contains a sequence identical to both half-TRE (TPA-response element; 7 bp consensus AP1 site) and half-CRE (cyclic-AMP response element; 8 bp consensus CREB site). Thus, to elucidate signaling pathways necessary for FSHβ induction by GnRH, there is a need to determine the regulation of AP1 transcription factor. We analyzed the induction of c-Fos (Ely et al., 2011) and determined that GnRH induces c-Fos primarily through an increase in intracellular calcium concentration and activation of calcium calmodulin kinase II (CamKII), which then leads to phosphorylation of the SRF transcription factor. Although receptors for several growth factors and hormones that induce c-Fos in other cell lines are expressed in the gonadotrope, these factors are not sufficient to induce c-Fos in the gonadotrope and therefore are not involved in the regulation of FSHβ, likely because they do not cause an increase in intracellular calcium.
The mechanism of induction of c-Jun, a c-Fos binding partner, has been analyzed in several other tissues, although less extensively than c-Fos. Three sites within the proximal promoter that play a role in c-Jun induction have been identified: CRE at -70, originally named Jun1TRE, is bound by ATF1 following stimulation by EGF; TRE at -191 plays a role in induction by IL1; and MADS box at -58 is bound by MEF2 following EGR treatment (Muegge et al., 1993, Clarke et al., 1998). Thus, it is possible that GnRH may use any or all of these sites to induce c-Jun, since it activates FSHβ through the CRE / TRE-related site and it induces c-Fos through the SRF site that is similar to the MADS box. c-Jun protein induction by GnRH is dependent on calcium from intracellular stores and JNK, but not p38, in immature gonadotrope cells, αT3, which do not express FSHβ (Cesnjaj et al., 1994, Mulvaney et al., 1999, Roberson et al., 1999, Mulvaney and Roberson, 2000).
Here, we examine GnRH induction of c-Jun, a c-Fos binding partner which comprises the AP1 transcription factor in the mature LβT2 gonadotrope cell line, that endogenously expresses FSHβ. We determine the specificity of GnRH signaling to the c-Jun promoter, since c-Jun is induced in other cell types by a multitude of stimuli that do not induce c-Jun in the gonadotrope. We find that induction of the c-Jun gene by GnRH is achieved by GnRH activation of the ATF2 transcription factor that binds the -70 CRE element within the proximal c-Jun promoter. Surprisingly, other CRE site binding proteins are not involved in this activation despite the induction of ATF3 and the phosphorylation of ATF1, likely because ATF2 interacts with NFY, that binds CCAAT that is critical for basal expression. Furthermore, we determined that ATF2 activation by phosphorylation and c-Jun induction both rely on p38 activity that is activated by GnRH.
2. Materials and Methods
2.1 Plasmids and Materials
The 1000 base pairs of the murine c-Jun proximal promoter; 4 tandem copies of AP1 / TRE site (TGAGTCA; TRE multimer) or 4 tandem copies of CRE site (TGACGTCA; CRE multimer) were cloned into KpnI and HindIII sites into the pGL3 luciferase reporter. Expression vectors for wild type ATF2, constitutively active ATF2 and dominant-negative ATF2 were kindly provided by Dr. Gerald Thiel (University of Saarland, Homberg, Germany). ATF3 expression vector was kindly provided by Dr. Chunhong Yan and Dr. Tsonwin Hai (Ohio State University, OH). Dr. Richard Goodman kindly provided the expression vectors for ATF1, constitutively active CREB DIEL and wild type CREB (Oregon Health and Science University, Portland, Oregon). The Glutathione S-Transferase (GST)- NF-YA in the pGEX vector was kindly provided by Dr. Sankar Maity (MD Anderson, TX). The c-Jun expression vector was obtained from Dr. Michael Birrer (NIH, MD) and the c-Fos expression vector was obtained from Dr. Eugene Tulchinsky (Leicester, UK). Constitutively active p38 and MEK expression vectors were kindly provided by Jiahuai Han (Scripps Research Institute, La Jolla, CA). GnRH was purchased from Sigma-Aldrich (St. Louis, MO). An immortalized LβT2 cell line, kindly provided by Pamela Mellon (UCSD, La Jolla, CA) was cultured in 10 cm plates in DMEM (Dulbecco's Modification of Eagles Medium from Mediatech Inc., Herndon, VA) with 10% FBS (Fetal Bovine Serum from Gemini Bio-Products, West Sacramento, CA) and penicillin/streptomycin antibiotics (Gibco/Invitrogen, Grand Island, NY) at 37°C. Cells were passaged using 1X Trypsin- EDTA (Sigma-Aldrich, St. Louis, MO).
2.2. Mutagenesis
5’ truncations of the c-Jun promoter were created by PCR sub-cloning with Platinum Taq HiFi (Invitrogen, Carlsbad, CA) using primers that contained KpnI and HindIII restriction sites. The products were phenol/chloroform extracted and ethanol precipitated. Gel-purified samples and the pGL3 vector were double digested with KpnI and HindIII for 2 hours at 37°C and ligated overnight with Amersham's Ready-To-Go T4 DNA Ligase (Piscataway, NJ). Ligations were transformed into DH5α Supercompetent cells (Invitrogen, Carlsbad, CA) and DNA was analyzed for the insert by digestion, prior to sequencing through the UCSD Cancer Center.
Mutagenesis was performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) following the manufacturer's instructions. The mutagenesis of the murine c-Jun-luciferase reporter was performed with PCR (95°C for 30 sec., 95°C for 30 sec., 55°C for 1 min., 68°C for 14 min.) for 18 cycles followed by 37°C for 1 hour of Dpn treatment, and products were transformed into XL1 Supercompetent Cells (Stratagene, La Jolla, CA). 5′-TGAGAACGACGCAAGgggATGGGAA-3′ primer was used to mutate NFY site (CCAAT, mutations in lower case letters); and 5′-AAGCCTCGGGGTccCAaaATGGGCT-3′ primer to mutate CRE site (TGACATCA, mutations in lower case letters). Mutations were confirmed by dideoxyribonucleotide sequencing performed by the DNA Sequencing Shared Resources, UCSD Cancer Center.
2.3. Transient Transfections
Transfection was performed one day following the plating into 12-well plates in DMEM with 10% FBS with FuGENE 6 (Roche Molecular Biochemicals, Indianapolis, IN) transfection reagent following the manufacturer's instructions. Each well was transfected with 0.5 μg of a luciferase-reporter plasmid and 0.1 μg of Herpes virus thymidine kinase (TK) promoter driven β-galactosidase, as a control for transfection efficiency. The cells were starved overnight in serum-free DMEM with 0.1% BSA and penicillin/streptomycin antibiotics, prior to five-hour treatment with 100 nM GnRH or vehicle. Forty-eight hours after transfection, cells were washed with 1X PBS and lysed with 100 nM K-PO4 buffer containing 0.2% Triton X-100. Ninety-six-well black plates were loaded with 20 μl of each lysate and luciferase activity was measured on a luminometer (Veritas Microplate luminometer from Turner Biosystems) by injecting 100 μl of buffer containing 25 mM Tris pH 7.8, 15 mM MgSO4, 10 mM ATP, and 65 μM luciferin per well. The Tropix Galacto-light β-galactosidase assay (Applied Biosystems, Foster City, CA) was used to measure β-galactosidase activity on the luminometer following the manufacturer's instructions. Transfections were performed in triplicate and repeated 3 times. Transfection efficiency was controlled by dividing the luciferase values by β-galactosidase and individual experiments were normalized by dividing the luciferase/β-galactosidase ratio by control vector pGL3-luciferase/β–galactosidase ratio. Normalized luciferase/β–galactosidase values from three experiments were averaged and ANOVA followed by the Tukey's posthoc test was performed using the JMP program with significance set at p≤0.05.
2.4. Nuclear Extract and Electrophoretic Mobility Shift Assay (EMSA)
LβT2 cells were starved in serum free DMEM with 0.1% BSA and treated with 100 nM GnRH. The cells were rinsed with 1X PBS and hypotonic buffer (20 mM Tris pH 7.4, 10 mM NaCl, 1 mM MgCl2, 1 mM PMSF, protease inhibitor cocktail from Sigma (Sigma-Aldrich), 10 mM NaF, 0.5 mM EDTA, 0.1 mM EGTA) was added. After swelling the cells were passed 3 times through a 25 G needle. The nuclear material was spun down and the pellet was re-suspended using hypertonic buffer (20 mM Hepes pH 7.8, 20% glycerol, 420 mM KCl, 1.5 mM MgCl2, 1 mM PMSF, protease inhibitor cocktail (Sigma-Aldrich), 10 mM NaF, 0.5 mM EDTA, 0.1 mM EGTA). After incubating for 20 minutes, the samples were centrifuged and supernatant was aliquoted. The Bradford reagent (Bio-Rad Laboratories, Inc., Hercules, CA) was used for protein determination that was calculated on a basis of a standard curve performed each time. Oligonucleotides for probes were obtained from Integrated DNA Technologies, annealed, labeled with γ32P ATP using T4 Polynucleotide Kinase (New England Biolabs, Inc., Beverly, MA) and column purified using Micro Bio-Spin Chromatography Columns (Bio-Rad Laboratories, Inc., Hercules, CA). 20 μl binding reactions contained 2 μg of nuclear extract, 10 mM Hepes pH 7.8, 50 mM KCl, 0.5 mM MgCl2, 10% glycerol, 0.1% NP-40, 0.25 μg dIdC, 5 mM DTT, and 5 fmol of probe. In competition or antibody shift assays, 1 nM of unlabeled oligonucleotide or 1 μg antibody was added to the binding reaction. Electrophoresis was carried out on a 5% nondenaturing polyacrylamide gel. Gels were run at 250 V/cm2, dried and autoradiography was performed to identify complexes.
2.5. Whole Cell Extract and Western Blotting
The LβT2 cells were starved overnight in serum-free DMEM with 0.1% BSA. The cells were treated with 100 nM GnRH for times indicated and then rinsed with 1x PBS and lysed with a buffer containing: 20 mM Tris pH 7.4, 140 mM NaCl, protease inhibitors (Sigma), 1 mM PMSF, 10 mM NaF, 1% NP-40, 0.5 mM EDTA, and 1 mM EGTA. Bradford reagent was used for determining protein concentrations and the concentrations were calculated using a standard curve. Equal amounts of protein from whole cell extracts were loaded with 4x sample buffer, resolved in the gel using electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane with blocked with 10% milk in wash buffer (20 mM Tris 7.4, .1% tween, 150 mM NaCl, and 0.5% BSA) and then probed with antibodies to: ATF2 (Santa Cruz/sc-242), phospho-ATF2 (Santa Cruz, sc-7982), CREB (Santa Cruz, sc-271), c-Jun (Santa Cruz, sc-1694), ATF3 (Santa Cruz, sc-188), phospho-CREB, p38, phospho-p38, JNK, p-JNK, ERK1/2, phospho-ERK1/2 (Upstate biotechnology, now Millipore), TBP, β-tubulin (Abcam, 63766-100), NFY (Rockland Immunochemical, Philadelphia, PA). Proteins were detected with a secondary antibody to rabbit or mouse IgG linked to horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA.) and Enhanced Chemiluminescence (ECL) Western Blotting Detection Reagent (GE Healthcare). The GeneGnome Bio Imaging Chemiluminescence (Syngene, Frederick, MD) reader was used to quantify the bands. For each experiment the results of the specific blot were normalized to the β-tubulin amount and the average of all experiments was presented in the graphs. Statistical significance was determined by using ANOVA and Tukey's posthoc test and indicated with an * in the figure.
2.6. mRNA Extraction and Quantitative PCR
RNA was obtained with Trizol reagent (Invitrogen/Gibco, Carlsbad, CA) according to the manufacturer's instructions following GnRH treatment for the times indicated. Contaminating DNA was removed with DNA-free reagent (Ambion, Austin, TX) and 2 μg RNA was reverse transcribed using Superscript III First-strand Synthesis System (Invitrogen, Carlsbad, CA). Quantitative Real-Time PCR was performed in an iCycler from Bio-Rad (Hercules, CA), using QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA) and the following primers: c-Jun forward-GTCCCCTATCGACATGGAGTCT, reverse-GAGTTTTGCGCTTTCAAGGTTT; GAPDH forward-TGCACCACCAACTGCTTAG, reverse-GGATGCAGGGATGATGTTC under the following conditions: 95 C for 15 min, followed by 40 cycles at 95 C for 15 sec, 56 C for 30 sec and 72 C for 30 sec. Each sample was assayed in triplicate and the experiment was repeated 3 times. A standard curve with dilutions of 10 pg/well, 1 pg/well, 100 fg/well and 10 fg/well of a plasmid containing c-Jun cDNA was generated in each run with the samples. In each experiment, the amount of c-Jun or GAPDH was calculated by comparing a threshold cycle obtained for each sample with the standard curve generated in the same run. Replicates were averaged and divided by the mean value of GAPDH in the same sample. After each run, a melting curve analysis was performed to confirm that a single amplicon was generated in each reaction.
2.7. GST Interaction Assay
35S-labeled proteins were produced using the TnT® T7 Coupled Reticulocyte Lysate System (Promega Corporation, Madison, WI). Bacteria transformed with the pGEX vectors were grown to an OD of 0.6, upon which protein expression was induced by addition of 0.25 mM isopropyl-β-D-thiogalactosidase (IPTG). Bacterial pellets were sonicated in PBS with 5 mM EDTA and 0.1% Triton X-100, centrifuged and the supernatant was bound to glutathione sepharose beads (GE Healthcare / Amersham, NY). Beads were washed 4 times with sonication buffer followed by equilibration in the binding buffer (below), and split equally between different samples and the control. 35S-labeled proteins were added to the beads and bound for 1 h at 4°C in 20 mM Hepes (pH 7.8), with 50 mM NaCl, 10 mg/ml BSA, 0.1% NP-40, and 5 mM DTT. After extensive washing, samples were eluted from the beads by boiling in Leammli sample buffer and subjected to SDS-PAGE. Afterwards, the gels were dried and autoradiographed.
3. Results
3.1. The CCAAT Element is Necessary for Basal Expression, while the CRE Site is Necessary for GnRH Induction of c-Jun
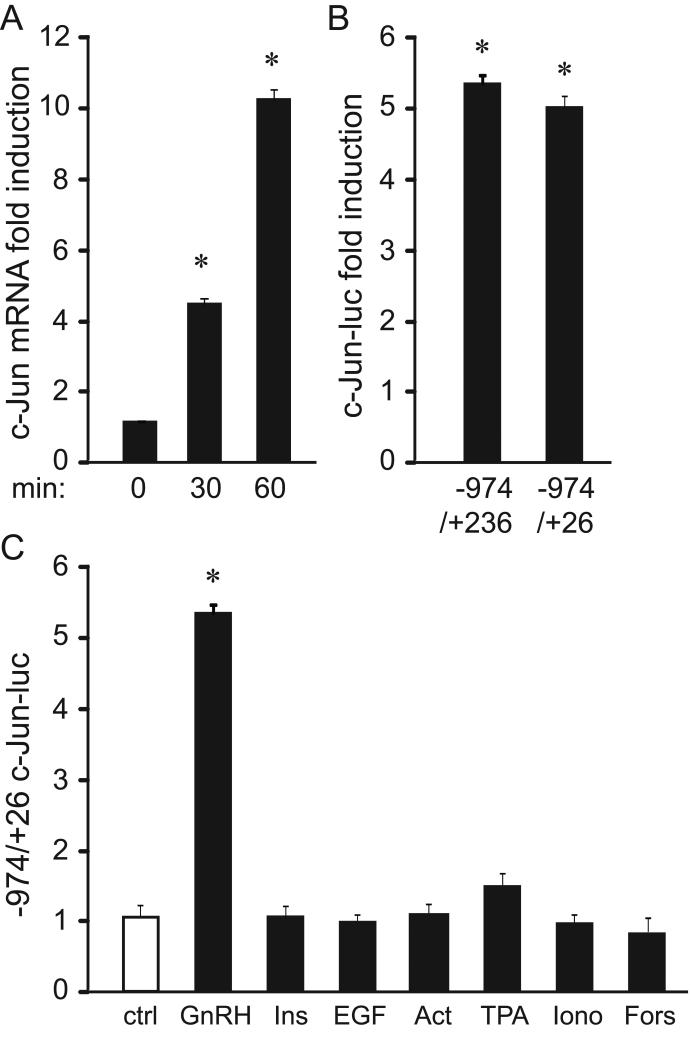
Since the AP1 transcription factor is the critical mediator of GnRH induction of the FSHβ subunit gene, we set out to identify the mechanism of GnRH regulation of c-Jun induction, which comprises AP1, as a homodimer or a heterodimer with c-Fos. Understanding proper regulation of intermediates will contribute to comprehending regulation of FSH and thus, molecular causes of fertility disorders. To determine the timing of c-Jun mRNA induction by GnRH we performed quantitative PCR following LβT2 cell treatment with GnRH. GnRH induces c-Jun mRNA, reaching 4-fold at 30 minutes and 9.2-fold at 1 hour of treatment (Fig. 1A). To investigate whether the proximal c-Jun promoter is responsive to GnRH, LβT2 cells were transfected with -974/+236 and -974/+26 of the murine c-Jun promoter fused to a pGL3 luciferase reporter together with thymidine kinase-β-galactosidase (β-gal), and then treated with GnRH for 5 hours (Fig. 1B). Given that we concentrate on specificity of GnRH induction in the gonadotrope, samples treated with GnRH were normalized to the vehicle-treated samples for each reporter and results represented as fold induction for easier observation of the GnRH effect. Both reporters were induced by GnRH at the same level (Fig. 1B), suggesting that the proximal promoter is sufficient for responsiveness to GnRH, and that the coding region does not contribute to induction by GnRH. Moreover, responsiveness of the c-Jun promoter to other stimuli was compared to GnRH (Fig. 1C). In contrast to the effect of GnRH, insulin does not induces c-Jun, although insulin induces another immediate-early gene, Egr-1, in the gonadotrope (Buggs et al., 2006). In addition, EGF and activin fail to induce c-Jun. Furthermore, activators of selective signaling pathways do not induce c-Jun including: TPA that activates PKC, ionomycin that increases intracellular calcium and forskolin that activates PKA. Thus, based on these findings we conclude that GnRH can solely induce c-Jun in LβT2 gonadotrope cell model.
Figure 1.
c-Jun is induced rapidly by GnRH. A, LβT2 cells were treated with 100 nM GnRH and total RNA was obtained after indicated time. Following reverse transcription, real-time quantitative PCR was carried out and the amount calculated according to the standard curve performed with c-Jun cDNA. The amount of c-Jun was normalized to the amount of GAPDH in each sample and the results were presented as fold induction for vehicle treated samples. B, -974/+236 and -974/+26 of the c-Jun promoter in pGL3 backbone were transfected into LβT2 cells with Herpes virus thymidine kinase-driven β-galactosidase gene as an internal control for transfection efficiency. After overnight starvation, cells were treated with vehicle or 100 nM GnRH and the amount of luciferase over β-galactosidase in GnRH treated samples were normalized to the ratio in vehicle treated samples for each reporter to determine GnRH fold induction. C, -974/+26 of the c-Jun promoter was transfected and after overnight starvation, cells were treated with vehicle or 100 nM GnRH, 50 nM insulin, 50 ng/ml EGF, 100 nM TPA, 1 μM forskolin, or 0.5 μM ionomycin for 5 hours, after which the luciferase and β-galactosidase values were obtained. * indicates significant induction by GnRH.
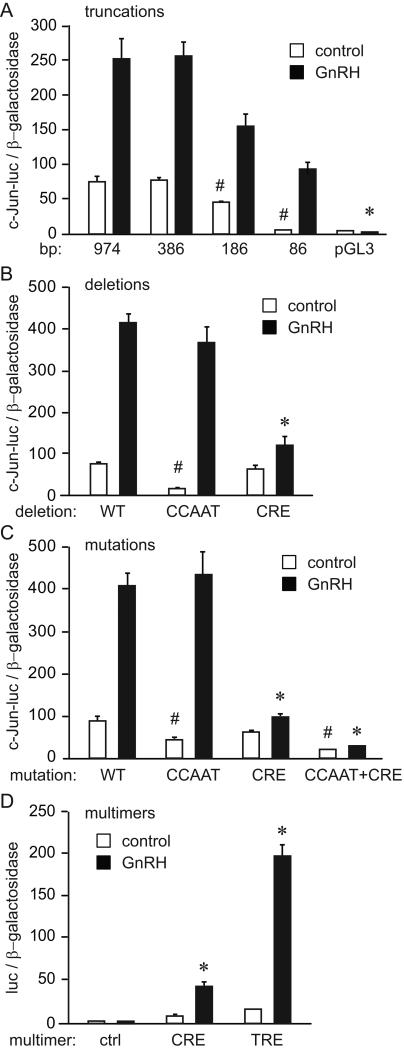
Using transient transfection assays in LβT2 cells, we analyzed the molecular mechanism of GnRH induction of c-Jun. To narrow the area of the promoter that may contain elements for basal expression and for the responsiveness to GnRH, truncation deletion analysis was performed using different lengths of the c-Jun promoter (Fig. 2A). Basal expression of the promoter was lowered by 42% when the promoter was truncated from -386 to -186 base pairs from the transcriptional start site and by 91% when the promoter was truncated to -86 base pairs. However, GnRH induction was maintained in this most proximal region containing -86 base pairs. When normalized to fold induction for each truncation, fold induction by GnRH was not significantly reduced with any truncation, indicating that the critical area for GnRH responsiveness is within the proximal -86 bases.
Figure 2.
The CCAAT box is important for basal expression and the CRE site is critical for GnRH induction of c-Jun. A, Different lengths of the c-Jun promoter linked to luciferase reporter in pGL3 backbone were transfected into LβT2 cells with Herpes virus thymidine kinase-driven β-galactosidase gene as an internal control for transfection efficiency. After overnight starvation, cells were treated with vehicle or GnRH for 5 hours, after which the luciferase and β-galactosidase values were obtained. # indicates a significant drop in luciferase expression from the previous truncation, while * indicates significant decrease in GnRH fold induction. B, 10 base pair internal deletions that encompass the CCAAT box and the CRE site were created in the -1000 c-Jun-luciferase reporter (WT) and the experiment was performed as above. C, Three and four base pair mutations were created in the residues that were previously determined to be critical for CCATT and CRE sites function, respectively. # indicates a significant decline in luciferase expression from the wild type control (WT), while * indicates significant decrease in GnRH fold induction. D, Four tandem copies of CRE (TGACGTCA) or TRE (TGAGTCA) sites were linked to the minimal heterologous promoter (ctrl) and luciferase reporter, and co-transfected with β-galactosidase internal control. * indicates significant induction by GnRH.
Sequence analysis of the c-Jun promoter proximal regions that convey basal and GnRH responsiveness revealed a CCAAT box at -90 position and a CRE element at -70. We created 10 base pair internal deletions that encompassed these elements. Although ten base pairs may affect the spacing, it is equivalent to one turn of the DNA helix and, thus, does not affect the topology of the transcription factor binding sites. Deletion of the -90/-81 sequence that eliminated the CCAAT element reduced basal expression by 68% (Fig. 2B, CCAAT). In contrast, deletion of -70/-61 that disrupted the CRE element diminished GnRH induction by 80% (Fig. 2B, CRE). Point mutations in these elements confirm their necessity (Fig. 2C). Mutation of the CCAAT element reduced basal expression by 71%, while mutation of the CRE element reduced fold induction by GnRH by 61% (Fig. 2C). Interestingly, double mutation further reduced basal expression by 85% from the wild type promoter, and completely abrogated GnRH induction, indicating a functional interaction between these two elements.
Since the CRE site is necessary for GnRH induction, we analyzed the sufficiency of the CRE element to convey GnRH responsiveness to a heterologous promoter. We have previously shown that tandem copies of the element found at -76 of the FSHβ promoter, which consists of half-TRE or half-CRE (GTCA) with the adjacent NFY site, is sufficient for GnRH induction (Coss et al., 2004). We ligated 4 tandem copies of the 8 bp consensus CRE (TGACGTCA) element to the minimal thymidine kinase promoter driving luciferase (control, ctrl in Fig. 2D) and compared its expression with and without GnRH to the expression of the very similar 7 bp consensus TRE (TGAGTCA) multimer, which has 4 tandem copies of the TRE element. The CRE multimer was induced 6.3 fold by GnRH (Fig. 2D, CRE), while the TRE multimer was induced 12.7 fold (Fig. 2D, TRE); thus both elements were sufficient to convey GnRH responsiveness. Collectively, results in figure 2 support the hypothesis that the CRE site is sufficient and necessary for GnRH induction of the c-Jun promoter.
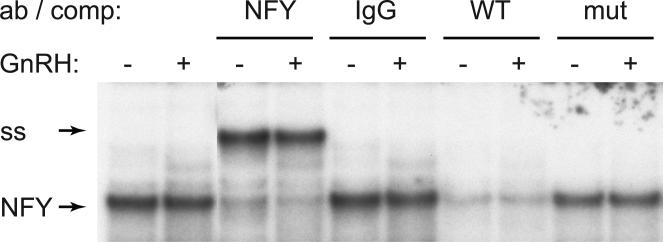
To determine the identity of proteins that bind the -90 site, we analyzed the CCAAT element that is critical for basal expression by electrophoretic mobility shift assay (EMSA). We included NFY antibodies in the binding reaction, since NFY can potentially bind the CCAAT box and is involved in basal expression of gonadotrope-specific genes (Keri et al., 2000, Jacobs et al., 2003). We determined that NFY bound the -105/-76 probe that encompassed CCAAT, since the complex labeled “NFY” (Fig. 3) was supershifted (ss) with antibodies to the NFY-A isoform. We confirmed that this complex was specific and bound the CCAAT site, since it was competed with excess wild type competitor (WT lanes), but not with the competitor that contained mutations in the CCAAT site (mut). Binding of NFY did not change with GnRH treatment consistent with its role in basal regulation. Thus, NFY binds the c-Jun promoter at the CCAAT box, which is necessary for basal expression.
Figure 3.
NFY binds to the CCAAT site. 30 bp probe (-105/-86) encompassing the CCAAT box was incubated with LβT2 nuclear extracts. Antibodies to NFY-A subunit or non-immune IgG control were included in the binding reaction in corresponding lanes to identify the protein complex that contains NFY (arrow), since they cause a supershift (ss). 200 fold excess of non-labeled cold probe (WT) or oligonucleotides that contain mutation in the CCAAT site (the same 3 bp mutation used in the transfection assay) were used as competitors to identify the sequence needed for binding.
3.2. ATF2 is Phosphorylated and ATF3 is Induced by GnRH to Bind the c-Jun Promoter
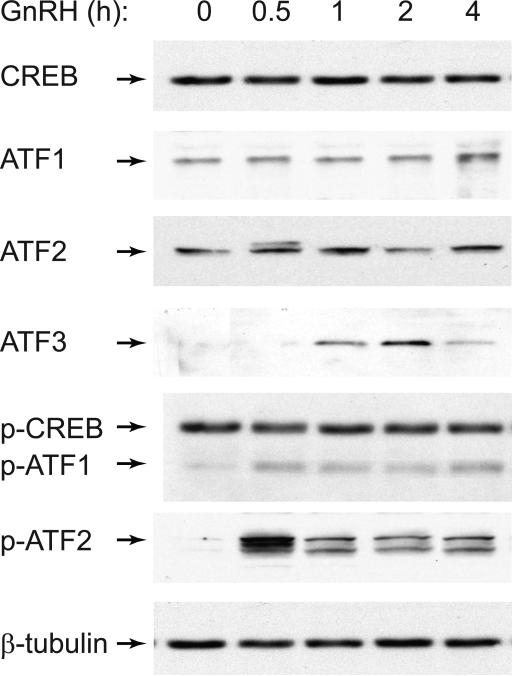
Since the CRE element was necessary for GnRH induction of c-Jun, we determined whether GnRH regulates proteins that are known to bind to this site. The CRE site is bound by CREB protein, as well as members of the ATF family (Hai and Hartman, 2001). We analyzed whether the amount of any of these proteins changed following GnRH treatment of immortalized gonadotrope cells, using western blotting. There was no change in the amount of CREB, ATF1 or ATF2 at any time point up to 4 hours of GnRH treatment; however, ATF3 protein was induced starting at one hour, with the maximal induction at 2 hours of treatment. Because CREB, ATF1 and ATF2, as opposed to ATF3, are expressed at a high basal level and regulated by post-translational modification in other cell types, we analyzed whether GnRH treatment leads to phosphorylation of these proteins,. Surprisingly, we did not detect a change in the phosphorylation level of CREB protein, since CREB was already phosphorylated at the basal state. Due to sequence similarity, phospho-CREB antibody also recognizes phospho-ATF1 and phospho-CREM (information from Upstate biotechnology / Millipore), and the molecular weight of the band that changed following GnRH treatment corresponded to the molecular weight of ATF1, and not of CREB. GnRH treatment at 30 minutes led to a strong phosphorylation of ATF2 which was maintained for 4 hours, albeit at a lower level. Thus, GnRH treatment causes the phosphorylation of ATF1 and ATF2 transcription factors in immortalized gonadotropes, LβT2.
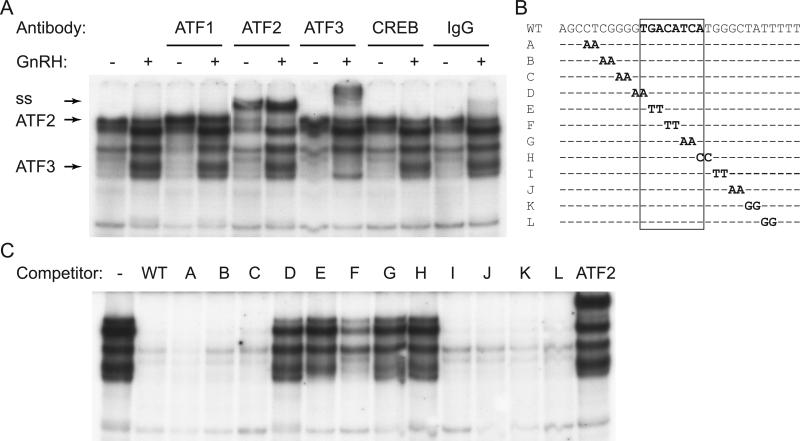
Given that ATF1 and ATF2 were phosphorylated, while ATF3 was induced following GnRH treatment, we then determined which of these proteins can bind the CRE element in the c-Jun promoter using EMSA. We treated LβT2 cells with vehicle or GnRH for 1 hour and extracted nuclear proteins that were used in the binding reaction (Fig. 5A). GnRH treatment caused several complexes to change binding to the -80/-51 probe. Antibodies to ATF1 did not cause a supershift, suggesting that ATF1, although phosphorylated, does not bind this CRE element. Antibodies to ATF2 supershifted a complex of higher molecular weight, indicated with an arrow (Fig. 5A, ss). This complex is present in both vehicle and GnRH-treated lanes, consistent with previous reports that transcription factors that regulate immediate early genes, such as c-Jun, bind the promoter in the basal state and are activated by phosphorylation, which induces transcription (Lin et al, 2008). Antibodies to ATF3 supershifted a complex present in the GnRH treated extract only, which is consistent with ATF3 being induced by GnRH treatment. Neither inclusion of a CREB antibody, nor antibody to phosphorylated CREB (which interacts with phospho-ATF1 and phospho-CREB, data not shown), nor control IgG, cause supershifts. Together, these results indicate that ATF2 and ATF3 bind the c-Jun promoter in the area that encompasses the -70 CRE element.
Figure 5.
ATF2 binds the CRE site. A, Nuclear extract from LβT2 cells treated with vehicle or GnRH for 1 hour were incubated with a probe that encompasses the CRE site (sequence listed in B) and antibodies to CRE binding proteins. Complexes containing ATF2 and ATF3, and supershift (ss) are indicated with arrows. B, Probe sequence is listed above the mutations that were used in 200 fold excess as competitors in the binding reaction to determine residues needed for binding. Scanning competitors', A-L, sequence was the same except for the two base pairs that are listed. The 8 bp CRE site is designated with a rectangle. C, Mutations listed in B were used as competitors 200 fold in excess of labeled probe in a binding reaction with GnRH treated nuclear extracts. ATF2 lane represents a lane that contains ATF2 antibody instead of competitor.
To evaluate the necessity of base pairs for complex interaction with the c-Jun promoter, we used 2 base pair scanning mutations, made within the -80/-51 wild-type c-Jun promoter sequence, as competitors in EMSA (A-L, Fig. 5B). Nuclear extracts were collected from GnRH-treated LβT2 cells and incubated in the binding reaction with the non-labeled scanning mutation competitors in 200-fold excess and the 32P-labeled wild-type probe. WT competitor competed for binding of all the complexes, indicating that the binding is specific. When the core CRE sequence was mutated, as in lanes containing competitors D-H, the oligonucleotides were not able to compete for the GnRH-induced complexes (Fig. 5C). In the last lane, we included antibodies to ATF2 instead of the competitor. These experiments illustrate that each of these complexes require the CRE site for binding to DNA, since mutation of the core residues eliminates binding to the competitor and retains binding to the probe. The data also indicate that GnRH activates several proteins that can interact with the same sequence on the c-Jun promoter.
3.3. ATF2 is Sufficient for c-Jun Induction
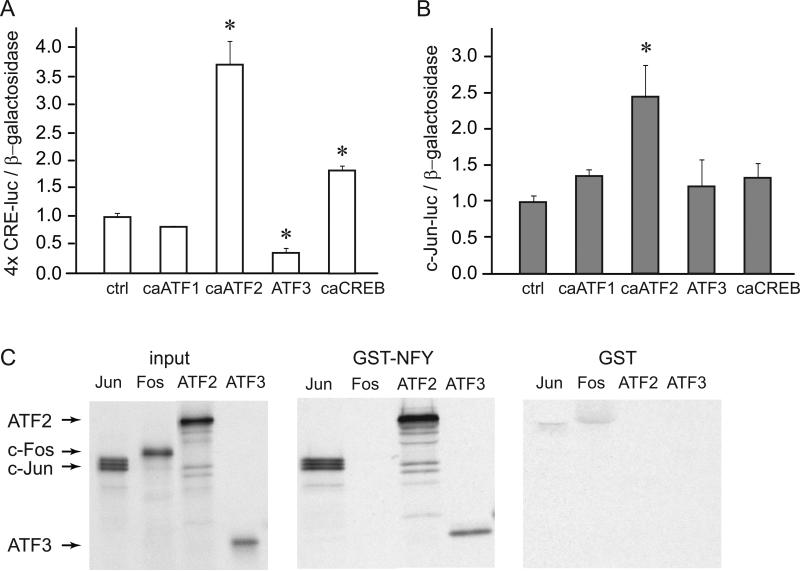
After establishing that ATF2 is phosphorylated and ATF3 protein is induced by GnRH treatment, we determined whether these CRE site binding proteins have functional significance in the induction of the isolated CRE element (4 × CRE-luc multimer) or c-Jun promoter reporter (c-Jun-luc) by overexpression of selective CRE binding proteins in the gonadotrope cell model, LβT2. Overexpression of wild-type ATF3 mimics GnRH induction, since ATF3 is induced by GnRH. However, ATF1, ATF2 and CREB are present at high basal levels and are activated by post-translational modification, usually phosphorylation following hormone treatment in various cells. To analyze their roles in the CRE-multimer or the c-Jun promoter induction in the gonadotrope, we obtained expression vectors that contain cDNAs with mutated sequences to mimic constitutively active (ca) forms of these transcription factors and confirmed that they overexpress the protein of interest.
The CRE multimer, containing 4 tandem copies of the 8-basepair CRE site, linked to the luciferase reporter was used in transient transfections to determine if the proteins in question work solely through the CRE element (Fig. 6A), since the CRE site was sufficient for induction by GnRH. LβT2 cells were co-transfected with multimer reporter and caATF1, caATF2, ATF3, or caCREB expression vector as indicted under the corresponding bars. The luciferase to β-gal ratio was normalized to empty vector control for each expression vector. caATF1 did not significantly induce the CRE multimer, while over-expression of caATF2 caused significant induction. ATF3 repressed the CRE site by 65%, consistent with the previously reported role of ATF3 as a repressor (Hai et al., 1989, Allen-Jennings et al., 2001, Hai and Hartman, 2001), while caCREB induced the CRE multimer 2 fold over the induction of the empty pGL3 vector. Thus, activated CREB and ATF2 can induce the CRE site, while ATF3 represses it.
Figure 6.
ATF2 induces c-Jun. Expression vectors for constitutively active (ca) ATF1, ATF2 and CREB or expression vector for ATF3 were co-expressed with CRE multimer (A) or c-Jun luciferase (B) and β-galactosidase internal control. * designates statistically significant change in luciferase expression compared to the empty vector control. C, GST-pull-down assays demonstrate that NFY-A can interact directly with ATF2 and ATF3. S35 labeled proteins, indicated above each panel, were used in the binding assay with GST-fusion proteins, labeled above each panel. GST-fusion proteins were induced with IPTG overnight and the bacterial pellets were sonicated. These proteins were bound to Glutathione Sepharose beads and in vitro transcribed/translated proteins were added. After extensive washing the precipitates were run on a gel and subjected to autoradiography. In the input panel, 1/10 of the in vitro transcribed and translated labeled proteins that were used in the binding reaction were run on the gel as control for their expression and labeling.
We then used the same expression vectors to analyze their role in c-Jun promoter expression. Surprisingly, overexpression of ATF3 did not repress the c-Jun promoter, and caATF1 and caCREB did not significantly induce c-Jun promoter in LβT2 cells (Fig. 6B). caATF2, however, increased expression of the c-Jun driven luciferase. Thus, caATF2 induced both CRE multimer and c-Jun gene, consistent with its phosphorylation by GnRH and binding to the promoter that we observed in EMSA.
3.4. ATF2 and ATF3 Interact with NFY In Vitro
Since double mutation established that there is functional interaction between the CRE site and the CCAAT site, we evaluated whether proteins that bind them, ATF2, ATF3 and NFY, can interact. Indeed, using GST pulldowns, S35-labeled ATF2 and ATF3 are retained in the precipitate with glutathione beads following interaction with GST-NFY, but not with GST alone control (Fig. 6C). NFY does not interact with c-Fos, a negative control, while it interacts with c-Jun used as a positive control (Coss et al., 2004). ATF2 interaction with NFY may explain why a site involved in basal expression, such as CCAAT contributes to induction by GnRH. Additionally, interaction of ATF3 with NFY provides an explanation of the differential effect of ATF3 on the CRE multimer that does not contain CCAAT site and the c-Jun promoter that does. These finding establish that ATF2 and ATF3 interact with NFY, likely contributing to the functional interaction between their respective binding sites.
3.5. p38 activity is necessary for c-Jun induction and ATF2 phosphorylation
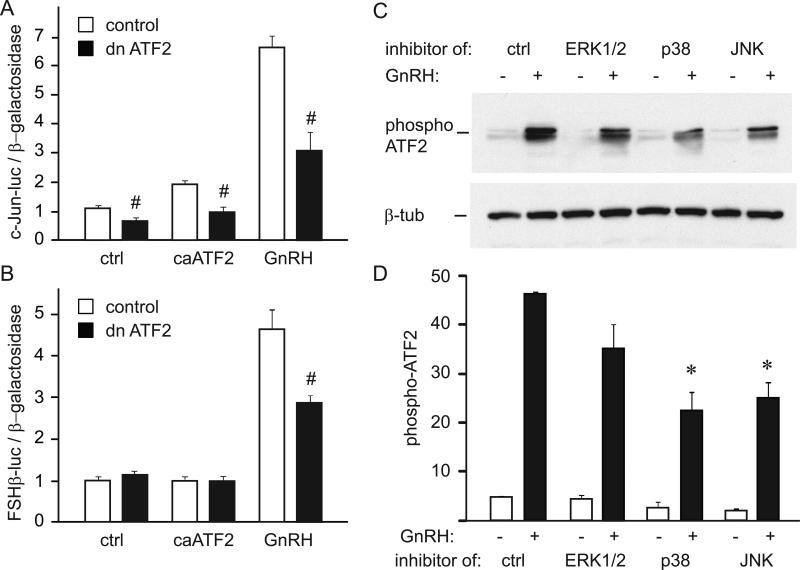
After establishing that activated ATF2 is sufficient for induction of the c-Jun promoter, we analyzed whether functional ATF2 is necessary, by overexpressing a dominant-negative ATF2 (dn ATF2), in which the DNA-binding domain is mutated. The dominant-negative ATF2 reduced basal expression of c-Jun by 35% (Fig. 7A, ctrl). Additionally, dn ATF2 abolished induction of the c-Jun promoter by caATF2 and reduced induction by GnRH treatment by 53%. Fold induction by GnRH also decreased from 6.1 to 4.5 fold, indicating that ATF2 is necessary for GnRH induction of the c-Jun promoter.
Figure 7.
Functional ATF2 is necessary for c-Jun and FSHβ induction by GnRH, and is phosphorylated through p38 and JNK pathways. A, c-Jun luciferase was induced with constitutively active (ca) ATF2 or GnRH and co-transfected with empty vector control (white bars) or dominant-negative ATF2 (dn ATF2, black bars) to assess necessity for a functional ATF2 in induction. # indicates significant decrease in luciferase expression with dominant-negative ATF2 overexpression. B, FSHβ luciferase was co-transfected with constitutively active (ca) ATF2 or induced by GnRH and co-transfected with empty vector control (white bars) or dominant-negative ATF2 (dn ATF2, black bars) to assess necessity for a functional ATF2 in induction. # indicates significant decrease in induction by GnRH with dominant-negative ATF2 overexpression. C, 5 μM UO126 (labeled ERK1/2 above the lanes; to inhibit MEK1 and activation of ERK1/2), 20 μM SB 202190 (p38; to inhibit p38), or 10 μM SP 600125 (JNK; to inhibit JNK), were added to cells as indicated 20 minutes prior to hormone treatment. Whole cell extracts following GnRH treatment, with or without addition of signaling pathway inhibitors, were subjected to western blotting with antibodies specific for phosphorylated ATF2. Following quantification, western blots were stripped and re-probed with antibodies to β-tubulin. D, The amount of phosphorylated ATF2 in each sample treated with vehicle (white bars) or GnRH (black bars) was quantified. Three separate experiments were averaged and * represents statistically significant decrease in ATF2 phosphorylation caused by inhibition of p38 and JNK pathways.
To establish if functional ATF2 is necessary for induction of the c-Jun target gene, FSHβ, in the gonadotrope, we overexpressed dn ATF2 with FSHβ luciferase reporter (Coss et al., 2004). Neither dominant-negative ATF2 alone nor caATF2 had an effect on the basal expression of FSHβ, likely due to the fact that c-Jun requires induction of its binding partner c-Fos to induce FSHβ. On the other hand, GnRH induction of the FSHβ promoter is reduced from 4.8 fold to 3 fold in the presence of dominant negative ATF2 (Fig. 7B), confirming that ATF2 is required for GnRH induction of c-Jun and its target gene FSHβ.
We have shown using EMSA that ATF2 occupies the promoter at the basal state, which is in agreement with previous reports that determined that phosphorylation leads to transcriptional activation of the constitutively bound transcription factor (Lin, yet 2008). Seeing as GnRH induces phosphorylation of ATF2, we examined GnRH signaling pathways that lead to phosphorylation of ATF2. We used inhibitors of MAPK pathways, ERK1/2, p38 and JNK with GnRH treatment to determine which signaling pathways are necessary for GnRH phosphorylation of ATF2 (Fig. 7C). After quantification of western blots, we determined that inhibitors of p38 and JNK significantly reduced the level of ATF2 phosphorylation (Fig. 7D). Therefore, p38 and JNK pathways are necessary for GnRH phosphorylation of ATF2.
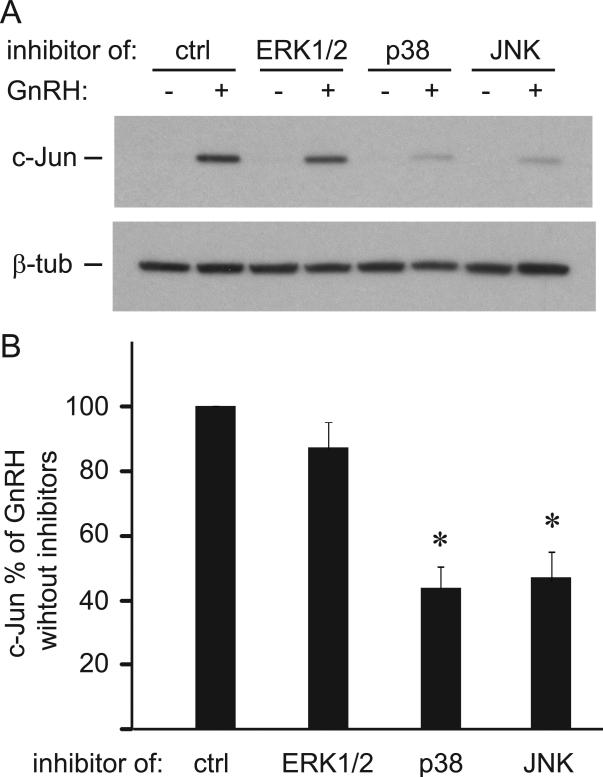
We used the same inhibitors to determine a role of these pathways in the GnRH induction of c-Jun (Fig. 8A). Inhibition of p38 and JNK reduced GnRH induction of c-Jun by 57% and 54%, respectively (Fig 8B). This results indicate that the same signaling pathways that GnRH activates to phosphorylate ATF2 are necessary for induction of c-Jun in the gonadotrope.
Figure 8.
c-Jun is induced by GnRH through p38 and JNK. A & B, Western blots (A) and quantification (B) of c-Jun normalized to β-tubulin in cells treated with GnRH and inhibitors. * represents statistically significant decrease in c-Jun expression with inhibition of p38 and JNK pathways.
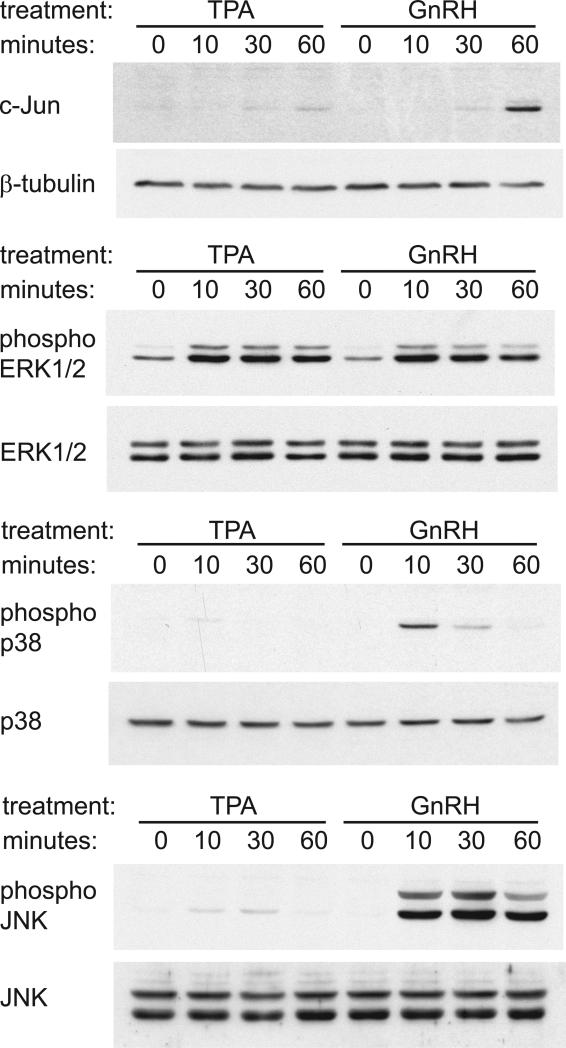
Since we reported that GnRH solely induces c-Jun in the gonadotrope (Fig. 1C) and p38 and JNK pathways are necessary for this induction, we analyzed whether GnRH and other stimuli, such as TPA, can activate p38 and JNK. Using western blots we confirmed the results of the reporter assays that TPA does not significantly induce c-Jun, while 60-minute treatment with GnRH causes induction of the c-Jun protein. While both TPA and GnRH can activate ERK1/2, GnRH activates p38 and JNK, while TPA does not (Fig. 9). Thus, specificity of GnRH induction of c-Jun in LβT2 gonadotrope cells is in its ability to activate p38 and JNK pathways that lead to ATF2 phosphorylation.
Figure 9.
A, Western blots were used to analyze the induction of c-Jun and the activation of MAPK pathways, ERK1/2, p38 and JNK, by TPA and GnRH. Whole cell extracts following treatment with 100 nM TPA or 100 nM GnRH for 10, 30, or 60 minutes, were run on the gel and after transfer, membranes were probed for c-Jun, or phosphorylated forms of ERK1/2, p38 and JNK as indication of activation. The membranes were stripped and re-probed with antibodies for β-tubulin or total, ERK1/2, p38 and JNK to indicate even loading.
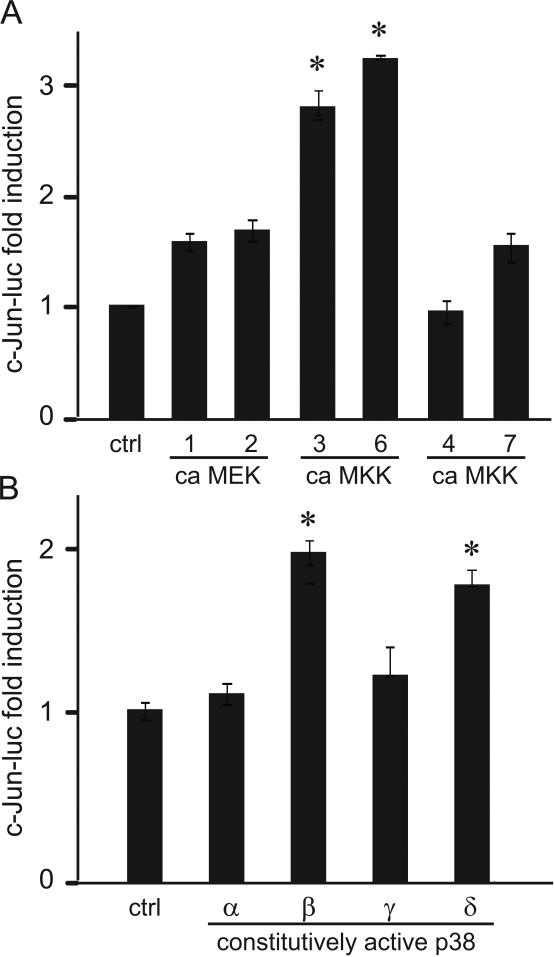
Moreover, we overexpressed constitutively active (ca) MEKK vectors that selectively activate ERK1/2 (MEK1 and MEK2), p38 (MKK3 and MKK6) and JNK (MKK4 and MKK7) (Fig. 10A). Overexpression of constitutively active MKK3 and MKK6 increased the expression of the c-Jun reporter, indicating that activation of the p38 pathway is sufficient to induce c-Jun. Thus, p38 activation is necessary and sufficient for c-Jun induction. Since p38 MAPK is comprised of 4 different isoforms α, β, γ, δ, we selectively overexpressed constitutively active p38 isoforms to determine which are sufficient for c-Jun induction (Fig. 10B). Overexpression of constitutively active β and δ isoforms induced c-Jun expression. Although necessity is not possible to determine due to the lack of specific inhibitors, with selective overexpression, we determined that p38β and p38δ isoforms are sufficient for c-Jun induction.
Figure 10.
A, Constitutively active (ca) expression vectors for activators of ERK1/2 (MEK1 and MEK2), p38 (MKK3 and MKK6) and JNK (MKK4 and MKK7) branches of MAPK pathways were overexpressed with c-Jun luciferase reporter to determine sufficiency of these pathways for c-Jun induction. * represents significant induction of c-Jun by constitutively active MKK3 and MKK6 that both activate p38. B, Constitutively active isoforms of p38 MAPK were overexpressed with c-Jun luciferase to analyze which isoform is sufficient for c-Jun expression. * represents significant induction comparing to the empty vector control.
4. Discussion
This study determined that GnRH induction of the c-Jun promoter occurs via phosphorylation of ATF2 by p38 MAPK, which is solely activated by GnRH in the gonadotrope. Furthermore, results identified a novel functional interaction between basal NFY factor and GnRH-regulated ATF2. As a component of the AP1 transcription factor, c-Jun is essential for proper regulation of FSHβ by GnRH and specificity of GnRH signaling and induction of FSHβ is critical for our understanding of regulation of female reproduction.
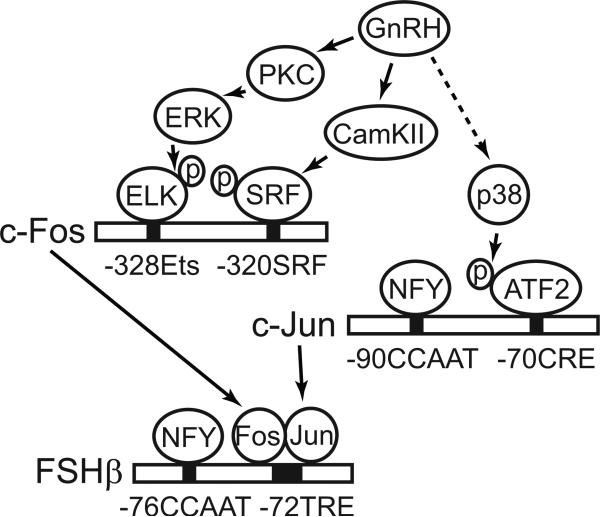
In our previous report investigating AP1, we determined that specificity of c-Fos induction by GnRH relies on SRF phosphorylation by CamKII, which is necessary and sufficient (Ely et al., 2011, Fig. 11). Induction of c-Fos is augmented via activation of pathways shared by various growth factors, which encompass ERK1/2 activation and phosphorylation of ELK1 (Fig. 11). ERK1/2 activation is dependent on PKC and mimicked by TPA. In this manuscript, we expand our knowledge by demonstrating that specific induction of c-Jun by GnRH occurs through activation of p38 and phosphorylation of ATF2, both of which are necessary and sufficient. Once induced, c-Fos and c-Jun bind the FSHβ promoter and activate its transcription as illustrated in Figure 11 (Coss et al., 2004).
Figure 11.
Illustration of GnRH signaling pathways necessary for FSHβ induction. c-Fos is rapidly induced by GnRH through activation of ERK1/2, which is dependent on PKC, and its phosphorylation of transcription factor ELK1, which is similar to c-Fos activation by growth factors. c-Fos induction however, also requires SRF phosphorylation by CamKII that allows prebound SRF to interact with ELK1 and activate transcription, which represents specificity of GnRH signaling pathway. GnRH induction of c-Jun occurs by activation of p38, through yet unknown mechanisms, and phosphorylation of prebound ATF2, which interacts with basal factor NFY. Once induced, c-Fos and c-Jun heterodimerize to form AP1, bind FSHβ promoter and interact with NFY to induce FSHβ transcription.
We identified two promoter elements important for c-Jun expression: a CCAAT site, important for basal expression, and a CRE site, critical for GnRH induction. Previous studies in other cell types have identified sites essential for basal c-Jun expression. First, a GC-rich sequence at -176 that is involved in basal expression and bound by the telomerase-associated factor Ku80 in HEK293 cells. Second, the SP-1 site at -120 plays a role in basal expression of c-Jun in HeLa. Third, the CCAAT box at -90 was identified as important in HepG2 cells (Angel et al., 1988, Han et al., 1992, Jiang et al., 2008). Our findings here suggest a major regulator of basal expression in the gonadotropes is the CCAAT box, which is bound by NFY. NFY has already been shown to be important for basal expression of other genes in the gonadotrope, including genes encoding rodent FSHβ (Jacobs et al., 2003) and bovine LHβ (Keri et al., 2000). Although there are likely additional elements important for basal expression in the upstream -386 and -186 region, the -90 CCAAT site, bound by NFY, integrates basal expression and GnRH induction of the c-Jun promoter. Our findings support this functional interaction with the CRE site to facilitate GnRH induction of c-Jun, since double mutation further reduced basal expression and abrogated induction. Previously, we reported that together the CCAAT box and half-TRE/CRE site contributed to GnRH induction of the FSHβ gene (Coss et al., 2004), providing evidence for such an interaction between these elements. Adjacent CRE and CCAAT sites have been reported to interact in several different genes (Alonso et al., 1996, Hirayama et al., 2001). Furthermore, we determine that NFY and ATF2 proteins interact and may contribute to the functional cooperation between their respective binding sites and induction of the target gene.
GnRH regulation of c-Jun occurs through the -70 CRE site. Although the highly similar TRE element is sufficient for GnRH induction, the TRE site at -191 is not involved in GnRH induction of c-Jun in the gonadotrope, since truncations eliminating this element retain full response to GnRH. CRE sites in different cell types are bound by either CREB or ATF family members. In the gonadotrope, ATF2 phosphorylated by GnRH binds -70 CRE site to induce c-Jun. In other cell types, c-Jun is induced by a variety of stimuli, hormones, growth factors, and cytokines by cell type specific mechanism. IL-1 regulation of c-Jun in HeLa cells phosphorylates ATF1 which binds the -70 CRE site (Muegge et al., 1993, Clarke et al., 1998). This is in contrast to our results which indicate that ATF1 fails to bind CRE in the gonadotrope despite its phosphorylation by GnRH. ATF3, which is induced by GnRH, binds this CRE element and can repress the CRE multimer, however it fails to modify c-Jun expression. The ability of ATF3 to change its role as an activator or a repressor is dependent on the context of the promoter and based on interaction with other proteins (Hai et al., 1989, Hai and Hartman, 2001). Therefore, the differential role of ATF3 on the isolated CRE element and c-Jun promoter which contains CCAAT, is likely due to ATF3 interaction with NFY.
An obvious candidate that might occupy the CRE element is the prototypical CRE binding protein, however, CREB does not change with GnRH treatment, nor does it bind the c-Jun promoter in EMSA. Furthermore, overexpression of constitutively active CREB does not induce the c-Jun promoter. Rather, we observe that overexpression of constitutively active CREB proteins activated empty pGL3 luciferase vector (data not shown). When induction of the promoter-luciferase was normalized to induction of the empty vector to analyze promoter-specific effects, c-Jun promoter was not induced by CREB, while the CRE multimer was. Although GnRH phosphorylation of CREB has been observed previously (Duan et al., 1999, Ciccone et al., 2008), we were not able to observe it under the conditions employed here, since CREB is already phosphorylated in the basal cell state. This result may explain the differential responses of the PKA pathway, as it is a pathway that phosphorylates CREB, previously reported in immature αT3 and mature LβT2 (Fowkes et al., 2003).
Rather than an action of CREB on the CRE site, active ATF2 is sufficient and necessary for c-Jun induction. Using western blots and EMSA, we determined that ATF2 is phosphorylated following GnRH treatment through p38 and JNK MAPK and that it binds CRE in the c-Jun promoter in LβT2 cells. Activation of p38 by its upstream kinases, MKK3 and MKK6, is sufficient for c-Jun induction. Although TPA, activator of PKC, induces c-Fos (Ely et al., 2011), it does not significantly increase c-Jun transcription in LβT2. PKC is upstream of ERK1/2 and activation of ERK1/2 is not sufficient for c-Jun induction, although ERK1/2 is involved in c-Fos transcription. Interestingly, TPA induces c-Jun in αT3 cells (Cesnjaj et al., 1994), and it is not clear whether ERK1/2 can activate c-Jun, or PKC can activate JNK in αT3 cells. In αT3 cells, c-Jun induction was dependent on JNK, but not p38 (Mulvaney et al., 1999, Roberson et al., 1999, Mulvaney and Roberson, 2000). p38 and JNK are activated by GnRH via alternative pathways to PKC in LβT2, likely by Raf interaction with the receptor in lipid rafts (Roberson et al., 2005). Of four p38 isoforms, p38β and p38δ are sufficient for c-Jun induction, indicating additional specificity of signaling in the gonadotrope.
4.2. Conclusion
The data presented here identify pathways and transcription factors activated by GnRH to induce c-Jun in LβT2 mature gonadotrope cells. c-Jun, a critical component of the AP1 transcription factor, is an immediate early gene with a significant role in FSHβ gene expression. GnRH phosphorylates ATF2, which binds to the CRE site in the c-Jun promoter, through p38 and JNK pathways. This would allow for timely regulation of this important transcription factor that is essential for proper regulation of FSHβ by GnRH.
Highlights.
GnRH specifically induces c-Jun in the gonadotrope through the CRE site
GnRH induces ATF3 and phosphorylates ATF1 and ATF2, but not CREB
ATF2 and ATF3, not ATF1 or CREB, bind the CRE site in the c-Jun promoter
ATF2 binds basal factor, NFY, causing a functional interaction between their sites
Activated by GnRH, p38 and JNK phosphorylate ATF2 and induce c-Jun
Figure 4.
GnRH phosphorylates ATF2 and induces ATF3. Western blots of the whole cell extracts from LβT2 cells treated with GnRH for the times indicated, were performed with antibodies for CRE site binding proteins and β-tubulin as a control.
Acknowledgements
The authors are grateful to Drs. Varykina G Thackray for thoughtful discussion and suggestions that improved the manuscript. The authors thank Dr. Pamela Mellon for LβT2 cells and Drs. Jiahuai Han, Gerald Thiel, Charles Vinson, Chunhong Yan, Tsonwin Hai, Richard Goodman, Marc Montminy, Eugene Tulchinsky and Michael Birrer for expression vectors.
This research was supported by NIH grants R01 HD057549, R21 HD058752 and R03 HD054595 (to DC); and NICHD/NIH cooperative agreement (U54 HD12303) as part of the Specialized Cooperative Centers Program in Reproduction Research. KMB was partially supported by NIH grant K99 HD060947. LLL and DMY were supported by the Howell Foundation and the Endocrine Society Summer Research Fellowships.
Abbreviations
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- ATF
activating transcription factor
- NFY
nuclear factor Y
- CRE
cyclic-AMP response element
- CREB
CRE-binding protein
- TRE
TPA-response element
- SRF
serum response factor
- GST
glutathione S-transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: The authors have nothing to disclose
References
- Allen-Jennings AE, Hartman MG, Kociba GJ, Hai T. The roles of ATF3 in glucose homeostasis. A transgenic mouse model with liver dysfunction and defects in endocrine pancreas. The Journal of biological chemistry. 2001;276:29507–29514. doi: 10.1074/jbc.M100986200. [DOI] [PubMed] [Google Scholar]
- Alonso CR, Pesce CG, Kornblihtt AR. The CCAAT-binding proteins CP1 and NF-I cooperate with ATF-2 in the transcription of the fibronectin gene. J Biol Chem. 1996;271:22271–22279. doi: 10.1074/jbc.271.36.22271. [DOI] [PubMed] [Google Scholar]
- Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Armstrong JL, Childs GV. Regulation of c-fos expression by EGF and GnRH in specific anterior pituitary cells from proestrous female rats. J Histochem Cytochem. 1998;46:935–944. doi: 10.1177/002215549804600807. [DOI] [PubMed] [Google Scholar]
- Buggs C, Weinberg F, Kim E, Wolfe A, Radovick S, Wondisford F. Insulin augments GnRH-stimulated LHbeta gene expression by Egr-1. Mol Cell Endocrinol. 2006;249:99–106. doi: 10.1016/j.mce.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesnjaj M, Catt KJ, Stojilkovic SS. Coordinate actions of calcium and protein kinase-C in the expression of primary response genes in pituitary gonadotrophs. Endocrinology. 1994;135:692–701. doi: 10.1210/endo.135.2.7518388. [DOI] [PubMed] [Google Scholar]
- Ciccone NA, Lacza CT, Hou MY, Gregory SJ, Kam KY, Xu S, Kaiser UB. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Molecular Endocrinology. 2008;22:1908–1923. doi: 10.1210/me.2007-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N, Arenzana N, Hai T, Minden A, Prywes R. Epidermal growth factor induction of the c-jun promoter by a Rac pathway. Mol Cell Biol. 1998;18:1065–1073. doi: 10.1128/mcb.18.2.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. The Journal of Biological Chemistry. 2004;279:152–162. doi: 10.1074/jbc.M304697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan WR, Shin JL, Jameson JL. Estradiol suppresses phosphorylation of cyclic adenosine 3',5'-monophosphate response element binding protein (CREB) in the pituitary: evidence for indirect action via gonadotropin-releasing hormone. Molecular Endocrinology. 1999;13:1338–1352. doi: 10.1210/mend.13.8.0322. [DOI] [PubMed] [Google Scholar]
- Ely HA, Mellon PL, Coss D. GnRH Induces the c-Fos gene via phosphorylation of SRF by the calcium/calmodulin kinase II pathway. Molecular Endocrinology. 2011;25:669–680. doi: 10.1210/me.2010-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes RC, Sidhu KK, Sosabowski JK, King P, Burrin JM. Absence of pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein alpha-subunit gene in LbetaT2 gonadotrophs reveals disrupted cAMP-mediated gene transcription. Journal of molecular endocrinology. 2003;31:263–278. doi: 10.1677/jme.0.0310263. [DOI] [PubMed] [Google Scholar]
- Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- Hai T, Liu F, Coukos WJ, Green MR. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes & Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Han TH, Lamph WW, Prywes R. Mapping of epidermal growth factor-, serum-, and phorbol ester-responsive sequence elements in the c-jun promoter. Mol Cell Biol. 1992;12:4472–4477. doi: 10.1128/mcb.12.10.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Angel P, Le Beau MM, Karin M. Structure and chromosomal localization of the functional intronless human JUN protooncogene. Proc Natl Acad Sci U S A. 1988;85:9148–9152. doi: 10.1073/pnas.85.23.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama K, Shimoji M, Swick L, Meyer A, Kapatos G. Characterization of GTP cyclohydrolase I gene expression in the human neuroblastoma SKN-BE(2)M17: enhanced transcription in response to cAMP is conferred by the proximal promoter. J Neurochem. 2001;79:576–587. doi: 10.1046/j.1471-4159.2001.00583.x. [DOI] [PubMed] [Google Scholar]
- Jacobs SB, Coss D, McGillivray SM, Mellon PL. Nuclear factor Y and steroidogenic factor 1 physically and functionally interact to contribute to cell-specific expression of the mouse Follicle-stimulating hormone-beta gene. Mol Endocrinol. 2003;17:1470–1483. doi: 10.1210/me.2002-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhou Y, Moxley RA, Jarrett HW. Purification and identification of positive regulators binding to a novel element in the c-Jun promoter. Biochemistry. 2008;47:9318–9334. doi: 10.1021/bi800285q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18:46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- Keri RA, Bachmann DJ, Behrooz A, Herr BD, Ameduri RK, Quirk CC, Nilson JH. An NF-Y binding site is important for basal, but not gonadotropin-releasing hormone-stimulated, expression of the luteinizing hormone beta subunit gene. The Journal of biological chemistry. 2000;275:13082–13088. doi: 10.1074/jbc.275.17.13082. [DOI] [PubMed] [Google Scholar]
- Lin DW, Chang IC, Tseng A, Wu ML, Chen CH, Patenaude CA, Layne MD, Yet SF. Transforming growth factor beta up-regulates cysteine-rich protein 2 in vascular smooth muscle cells via activating transcription factor 2. The Journal of biological chemistry. 2008;283:15003–14. doi: 10.1074/jbc.M801621200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge K, Vila M, Gusella GL, Musso T, Herrlich P, Stein B, Durum SK. Interleukin 1 induction of the c-jun promoter. Proc Natl Acad Sci USA. 1993;90:7054–7058. doi: 10.1073/pnas.90.15.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvaney JM, Roberson MS. Divergent signaling pathways requiring discrete calcium signals mediate concurrent activation of two mitogen-activated protein kinases by gonadotropin-releasing hormone. The Journal of biological chemistry. 2000;275:14182–14189. doi: 10.1074/jbc.275.19.14182. [DOI] [PubMed] [Google Scholar]
- Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. The Journal of biological chemistry. 1999;274:29796–29804. doi: 10.1074/jbc.274.42.29796. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Dalkin A, Yasin M, Haisenleder DJ, Marshall JC, Landefeld TD. Are immediate early genes involved in gonadotropin-releasing hormone receptor gene regulation? Characterization of changes in GnRH receptor (GnRH-R), c-fos, and c-jun messenger ribonucleic acids during the ovine estrous cycle. Biol Reprod. 1995;53:263–269. doi: 10.1095/biolreprod53.2.263. [DOI] [PubMed] [Google Scholar]
- Papavasiliou SS, Zmeili S, Khoury S, Landefeld TD, Chin WW, Marshall JC. Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone alpha and beta subunits in male rats. Proc Natl Acad Sci USA. 1986;83:4026–4029. doi: 10.1073/pnas.83.11.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Ann Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM. Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol. 2005;19:2412–2423. doi: 10.1210/me.2005-0094. [DOI] [PubMed] [Google Scholar]
- Roberson MS, Zhang T, Li HL, Mulvaney JM. Activation of the p38 mitogen-activated protein kinase pathway by gonadotropin-releasing hormone. Endocrinology. 1999;140:1310–1318. doi: 10.1210/endo.140.3.6579. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL. Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-beta gene. Endocrinology. 1997;138:2621–2631. doi: 10.1210/endo.138.6.5205. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Sebastian J, Ghosh BR, Miller WL. Transcriptional activation of the ovine follicle-stimulating hormone beta-subunit gene by gonadotropin-releasing hormone: involvement of two activating protein-1-binding sites and protein kinase C. Endocrinology. 1998;139:4455–4465. doi: 10.1210/endo.139.11.6281. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149:5577–5591. doi: 10.1210/en.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC. Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem. 2001;276:47195–47201. doi: 10.1074/jbc.M108716200. [DOI] [PubMed] [Google Scholar]
- Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS. Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Molecular Endocrinology. 2005;19:2624–2638. doi: 10.1210/me.2005-0056. [DOI] [PubMed] [Google Scholar]