Abstract
Much of the extant literature addressing the psychosocial aspects of BRCA1/2 mutation testing and risk management aggregates mutation carriers of all ages in study recruitment, data analysis, and interpretation. This analytic strategy does not adequately address the needs of the youngest genetic testing consumers, i.e., women aged 18–25. Despite low absolute cancer risk estimates before age 30, BRCA1/2 mutation-positive women aged 18–25 feel vulnerable to a cancer diagnosis but find themselves in a management quandary because the clinical utility of screening and prevention options are not yet well defined for such young carriers.
We present three cases, selected from a larger study of 32 BRCA1/2 mutation-positive women who completed or considered genetic testing before age 25, to demonstrate the unique developmental, relational and temporal influences, as well as the challenges, experienced by very young BRCA mutation-positive women as they complete genetic testing and initiate cancer risk management. The first case describes the maturation of a young woman whose family participated in a national cancer registry. The second addresses the experiences and expectations of a young woman who completed genetic testing after learning that her unaffected father was a mutation carrier. The third case highlights the experiences of a young woman parentally bereaved in childhood, who presented for genetic counseling and testing due to intense family pressure.
Together, these cases suggest that BRCA1/2-positive women aged 18–25 are challenged to reconcile their burgeoning independence from their families with risk-related support needs. Loved ones acting in ways meant to care for these young women may inadvertently apply pressure, convoluting family support dynamics and autonomous decision-making. Ongoing support from competent healthcare professionals will enable these young women to remain informed and receive objective counsel about their risk-management decisions.
Keywords: BRCA1/2 genetic mutations, hereditary cancer, family relations, human development, genetic testing stress, family influence on genetic testing
INTRODUCTION
Hereditary breast and ovarian cancer risk and prevention for women aged 18–25
By age 70, an estimated 60–70% of BRCA1 mutation carriers and 45–55% of BRCA2 mutation carriers will have developed breast cancer, and 40% of BRCA1 mutation carriers and 20% of BRCA2 mutation carriers will have developed ovarian cancer (Clark & Domchek, 2011). Results of a large clinic- and population-based study and a meta-analysis suggest that by age 30, 3.4% of BRCA1 mutation carriers and 1.5% of BRCA2 mutation carriers will have developed breast cancer and between 1–2% of BRCA1 and BRCA2 mutation carriers will have developed ovarian cancer (Chen & Parmigiani, 2007; Chen, Iverson, Friebel et al., 2006; Evans, Skrzynia, Susswein & Harlan, 2005/2006).
Despite these relatively low absolute cancer risk estimates before age 30, BRCA1/2 mutation carriers aged 18–25 often feel extremely vulnerable to a cancer diagnosis, behaving as if their lifetime risks were likely to be experienced in the short run (Werner-Lin, Hoskins, Doyle, and Greene, 2012), rather than over many decades. Protocols for early detection and prevention among women from hereditary breast/ovarian cancer (HBOC) families may be ineffective for women aged 18–25, and no evidence-based, widely accepted management plan developed specifically for these youngest mutation carriers currently exists. Various expert recommendations present conflicting information (Samuel & Ollila, 2005/2006); as a result, women aged 18–25 pursuing genetic testing to identify a BRCA1/2 mutation may receive emotionally charged cancer risk information with ambiguous management implications (Werner-Lin et al., 2012). In addition, the typical primary care provider is not likely equipped to bring a thoughtful, balanced, authoritative perspective to the extraordinary life problems faced by young mutation carriers. As a result, these young women may experience significant challenges in adjusting to their mutation status, leading to greater HBOC-related distress (Lodder, Fret, Trijsburg, et al., 2002; van Oostrom, Meijers-Heijboer, Lodder et al., 2003; Watson, Foster, Eeles et al., 2004) than older mutation-positive women.
The aim of this study is to provide a rich description of the experiences of women undergoing BRCA1/2 mutation testing and initiating risk management during a developmental period in which readiness to autonomously elect genetic testing, to fully understand and act on genetic information, and to confidently make decisions with life-long implications are all evolving processes.
Two conceptual pillars: Ecological Systems Theory and the Theory of Emerging Adulthood
This study drew on two theoretical frameworks: ecological systems theory and the theory of emerging adulthood. Ecological systems theory argues that development occurs within important familial and environmental contexts that support or constrain growth and change. The theory addresses the reciprocal influence of dynamic, interacting systems (e.g., family, community, work, society) on human development through the life course (Bronfenbrenner, 1977, 1979, 2006). For BRCA1/2 mutation carriers of reproductive age, individual and social factors [i.e. risk perceptions, social support, exposure to family illness] intersect with the larger contexts and environments [illness and gender norms, medical and reproductive technologies, public policy] over time to predispose them to varied risk constructions, as well as risk management and family life trajectories (Hoskins & Greene, in press; Hoskins, Roy, & Greene, 2012; Werner-Lin, 2007; 2008).
Arnett (2000) first described individuals aged approximately 18–25 as emerging adults. He argues emerging adulthood is theoretically distinct from adolescence, marked by the rapid physiological and relational changes of the high school years, and from early adulthood, marked by durable responsibilities to family and work. Emerging adulthood, rather, is characterized by instability, exploration, change, and possibility. Emerging adults are less constrained by normative roles and responsibilities of older adults; as a cohort, they are delaying marriage and childbearing (Census, 2009), they may experience residential variability, inconsistency in education and career paths (Arnett & Galambos, 2003), and potent concerns about body image (Tiggemann & Pennington, 1990). Exploration during emerging adulthood supports identity development and continued socialization into and preparation for adult roles in work and family life. Exploration also involves experimentation with risk behaviors at higher rates than adolescents and adults.
By the end of the emerging adult years, most individuals have made significant decisions that will have lifelong ramifications. Yet these decisions may be quite complicated when families experience early illness and cancer death, not uncommon in HBOC families (Werner-Lin & Gardner, 2009). Exposure to family experiences with cancer, either directly or indirectly, may significantly shape how young adults create expectations about their futures (McDaniel, Rolland, Feetham & Miller, 2006). As a result, emerging adults from families with confirmed BRCA1/2 mutations may contemplate normative life cycle transitions, such as partnering and family planning (McGoldrick & Carter, 2011) against the backdrop of an expected illness timeline, disease related anxiety, or chronic grief (Werner Lin, 2007). For example, emerging adults may struggle to move away from home if a parent is ill or has died, or if a younger sibling is still there. Parents and emerging adult children may struggle with renegotiating dependence and autonomy in the face of illness. Thus, relationships with parents may not change as rapidly as those of peers. By integrating ecological systems and emerging adulthood theories, this project investigated the critical developmental, familial, cultural, and medical influences on service delivery to these young women, and implications of genetic testing in emerging adulthood.
METHOD
Case studies allow documentation of unique, bounded systems with specific historical, relational and environmental contexts (Feagin, Orum, & Sjoberg, 1991; Stake, 2000; Yin, 1988). Case studies trace change (trajectories) over time, are holistic and multi-faceted, and capture the “insider” perspective on life experience (Anderson, Crabtree, Benjamin et al., 2005). The cases in this paper were identified from a larger data synthesis across three separate qualitative studies. They were selected from a sample of 32 BRCA1/2 mutation-positive women who completed or considered genetic testing before age 25 (Werner-Lin et al., 2012). Patient names and selected details have been changed to protect privacy.
Procedures
The authors independently recruited national samples of English-speaking BRCA1/2 mutation-positive women aged 18–35 from urban medical centers, and nationally through online organizations. Participants across both data sets underwent genetic counseling and testing over a thirteen-year period (1997–2010), and all data were collected over a six-year period (2004–2010). Interview guides from both studies elicited extensive and data-rich reports of personal and family experiences with cancer and genetic testing; the impact of these experiences on relationships with families of origin, peers, and romantic partners; beliefs about how cancer risk influences individual development; family formation decisions; and attitudes towards risk reduction. The second author co-created genograms with each participant during the interview, and the first author created genograms after each interview. In both studies, genograms captured family medical histories in addition to data about family relationships and dynamics around illness and care giving.
Participants from the authors’ independent investigations were eligible for secondary analysis if, at the time of data collection, they were (a) mutation-positive and aged 18–24, or (b) mutation-positive and aged 25–27, but considered or completed genetic testing prior to their 25th birthdays. Alphanumeric identifiers linking each transcript to the original study were given to 26 eligible transcripts. All eligible participants were unaffected with breast and ovarian cancer.
Investigators then used the same eligibility criteria to recruit participants for a focus group convened during the annual meeting of a national consumer group. Institutional review boards at both investigators’ institutions approved the focus group protocol. The two investigators developed a focus group interview guide based on preliminary findings from the secondary data analysis ([Author 1, Author 2], 2011). The interview guide addressed: perspectives on the benefits and challenges of learning about cancer risk before age 25; attitudes and intentions regarding cancer risk management; and the impact of genetic testing on identity, sexuality, family and social life, and family formation. Six women participated in a focus group session lasting approximately 90 minutes. Within eight weeks of the conference, the first author completed a 25–90 minute follow-up phone call with each of the six participants during which she constructed a genogram to capture the participant’s family medical history and relational patterns. The focus groups and follow-up phone interviews were audio recorded and transcribed verbatim by a professional transcription service.
One case was selected from each contributing data set. The cases are thematically representative, but not exhaustive, of the trajectories through cancer risk counseling and management observed in our research. Together, they are intended to convey examples of the types of decisions, contextual influences, risk management decisions and possible outcomes that may occur when completing genetic testing during these years. The first case details the growth into maturity of a young woman whose family participated in a national family cancer registry (Theresa). The second addresses the experiences and expectations of a young woman who completed genetic testing with little direct knowledge of her family’s cancer history (Josie). The third case highlights a woman, parentally bereaved in childhood, who presented for genetic counseling and testing due to intense pressure from her half-sister and maternal aunt (Emily).
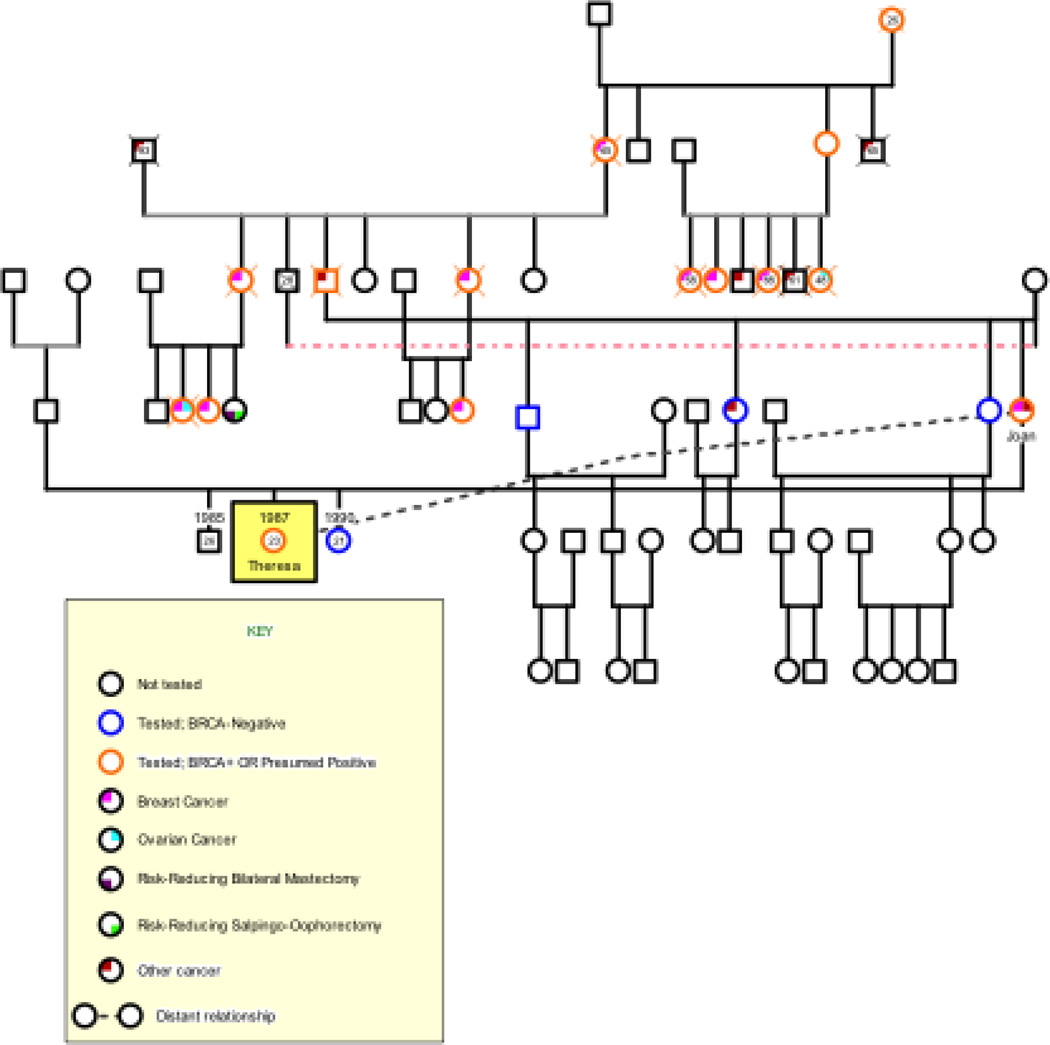
THE CASE OF THERESA
Theresa, 23, grew up with an extended maternal family that was recruited for a large, multi-site cancer screening study. When Theresa was five, her mother Joan was diagnosed with breast cancer. Since Joan was screened regularly through the study, the cancer was caught early and treated via mastectomy, but without the need for chemotherapy or radiation. From Theresa’s perspective, that process was relatively quick; the memory of her mother’s illness and its treatment was neither traumatic nor painful. She “thought the hospital was kind of cool because [her mother] had all these weird tubes, and it looked like a giant science experiment.” While Joan was recovering from surgery, Theresa “thought it was fun because I got to go to all these people’s houses and be babysat by all these people, and they gave me candy.” Two years later, researchers identified the BRCA1 mutation, due in part to the research contributions of families like Theresa’s. Theresa’s prolonged exposure to the cancer screening study left her unable to remember a time when she was not aware that she had a 50% chance of inheriting the mutation. She remembers developing breasts during puberty and feeling uneasy about them. She recalled, “I don’t remember when I chose to be tested. I know for sure it was before my 14th birthday.”
When Theresa turned 18, she decided she was ready for genetic testing. Since she had “been filling out questionnaires for hereditary cancer studies since before [she] had breasts,” Theresa elected not to have genetic counseling, a decision she regretted afterwards. Months later, when her results were available, she received them “over the phone, alone, in a dorm room” many miles from home. When Theresa called her mother to disclose her results, Joan became upset and quickly hung up the phone crying. Theresa felt an acute absence of support, a loss that continued to the time of her interview five years later. Joan is the only one of four siblings to inherit the BRCA1 mutation, so none of Theresa’s cousins are mutation carriers. Theresa’s younger sister tested negative and her older brother has not yet been tested; therefore, she is the sole BRCA pre-vivor in her family.
Theresa was aware that several distant cousins with BRCA1 mutations had developed breast cancer in their twenties and, as a result, Theresa actively pursued screening and chemoprevention. Although she was younger than the age at which regular breast screening is recommended (i.e., age 25, Narod, 2010; Pruthi, Gostout, & Lindor, 2010), Theresa was screened regularly through the cancer study in which her family was enrolled. Four years into that experience, she said:
“The screening actually gave me a lot of peace because they were checking me out every couple of months and they were like, ‘Yeah, no cancer.’ And I’m like, all right. I can go the next six months without worrying.”
Theresa was also taking tamoxifen as a strategy for reducing her breast cancer risk. She saw this as a way to extend her healthy years, hopefully allowing her to experience pregnancy and breastfeeding before she made the more definitive decision to remove her breasts. Yet, she wondered whether this was a sound decision, saying, “I have heard a lot of negative things about tamoxifen. I think I am going to go research it a little bit more than I initially did.” As a result of what she learned about potential tamoxifen-related side effects (e.g., hot flashes, amenorrhea, deep venous thrombosis, stroke, endometrial cancer), Theresa started to consider undergoing risk-reducing bilateral mastectomy (RRBM) in the near term, rather than waiting until after childbearing was complete. She stated: “I feel a lot of pressure from the medical community to get the surgeries,” a pressure she also feels from the BRCA advocacy community. Another major factor in her consideration was her approaching ineligibility for coverage by her parents’ robust insurance policy. Six months before her 24th birthday at the time of her interview, Theresa stated that:
My biggest pressure is like if I got this done before my 24th birthday, I could get the rock star awesome mastectomy with the reconstruction exactly the way I want it and [my parents’ insurance] would pay for everything. And I wouldn’t have to worry about the money side of things. And then the rest of me is like, but money is really not the reason to make this decision.
When she imagined facing an RRBM later in her reproductive years, Theresa feared that she would not be able to afford good health insurance and therefore would not have access to the quality healthcare she would need to manage her mutation, precluding her ability to complete either risk-reducing surgery.
In discussing RRBM with her parents, Theresa felt they “put up huge roadblocks. They’re completely against it.” Theresa’s father shared with her his belief that mastectomy had somehow changed her mother, Joan. He told Theresa that he did not want her to have the surgery because “that’ll change me, and he likes me the way I am.” Though this statement was clearly said in love, Theresa perceived it as unsupportive because it moved her a step away from the surgery. As a result, she felt “completely alone,” saying, “I had no one, there was no one” for support. Theresa anticipated extensive physical and emotional support needs following RRBM and, in the absence of parental support, she worried about where she could turn. Unable to find “insider” emotional support in her family of origin, Theresa worked to form an online support network comprised of other young BRCA mutation carriers. She also relied heavily on her “Bomb Squad,” a group of breast cancer survivors in their 40s and 50s that Theresa met at a breast cancer support meeting near her former home. Like other asymptomatic mutation-positive women in their reproductive years, Theresa referred to her mutation as “a ticking time bomb” (Werner-Lin, 2007). Members of the Bomb Squad had served as surrogate parents to Theresa during difficult risk management decisions and in other aspects of her daily life.
Theresa grappled with the decision about whether and when to undergo RRBM; she wanted to have the experience of breastfeeding. Further, the idea that she would someday also need to pursue risk-reducing salpingo-oophorectomy (RRSO) loomed large in her mind. Theresa was aware that ovarian cancer is both harder to detect and more difficult to treat than breast cancer, and she felt frustrated by the lack of effective ovarian cancer screening that made surgery seem necessary. Theresa’s doctors advised her to remove her ovaries by age 40. Even at 23, that deadline seemed constricting. Describing the pressure to choose between her desire to have a family and the need to have her ovaries removed, she stated,
If I had to choose a body part to get rid of, I would probably hang onto my ovaries until my dying day. I’m super attached. I want as many kids as I can possibly have… but I hate that BRCA has taken that dream away, and I feel like it has.
The immediate effect of all of these decisions on Theresa’s life as a 23-year-old was an intense sense of pressure to meet a partner and get married. She felt as though she needed to “…find Prince Charming really quickly and then immediately have children and then to have them really quickly, like one right after the other. And I’m single. I don’t have any immediate prospects.”
Theresa worried whether her mutation status and impending surgeries would constrain her potential for finding a life partner and bearing children. Theresa was committed to being open about her HBOC risk with friends and potential romantic partners; years of participating in the family cancer study primed her to consider cancer risk as core to her identity, “it is just part of who I am.” She admitted, “I probably make people a little uncomfortable because I am always talking about breasts and ovaries and cancer, and my 22 and 23 year old guy friends are like, ‘Too much information’!” But she went on to say, “This gene is not my fault. You can deal with it, or let’s not even talk about this relationship. I don’t want someone who’s so shallow that a genetic issue is going to turn them off.” Yet this confidence was undermined by her worry that a potential romantic partner might be intimidated by her timeline for family formation and riskreducing surgery.
In addition to her BRCA mutation status, Theresa suffers from fibromyalgia, which she reports, “stops me from living my life on a daily basis.” She contrasts fibromyalgia with carrying a BRCA1 mutation; the latter, she says, is “ruining my lifelong dream of having kids. That one’s just heartbreaking.”
Case analysis
Theresa’s early and ongoing exposure to the family cancer study powerfully shaped her identity, an identity reinforced by receiving positive genetic testing results. She considered cancer risk as a core feature of her family’s daily life and never questioned the decision to pursue genetic testing. Theresa’s experience speaks to the need for genetic counseling, even when BRCA-related cancer risk is familiar to the patient and to her family. Genetic counseling might have enabled Theresa to make a more autonomous, informed choice about the timing of learning her cancer mutation status.
Theresa’s decision to decline genetic counseling prior to undergoing genetic testing left her without clarity about how to optimally manage her risk and without an informed health care provider ally. Our research participants repeatedly bemoan the dearth of recommendations for screening and prevention that respect developmental needs and challenges while also providing a sense of safety (Hoskins, 2010; Werner-Lin, 2010; Werner-Lin, Hoskins & Rubin, 2011). Without clear medical guidelines, Theresa felt pressure to complete RRBM earlier than she might otherwise choose; she was also motivated by the impending loss of her parents’ insurance coverage as a loss of opportunity to have surgery, and as a result, was pursuing surgical consultations for purely financial reasons. This particular challenge may be increasingly common since the Affordable Care Act, signed into law in March of 2010 as part of broad health care reform, requires group health plans and health insurance companies to provide coverage of dependent children until their 26th birthday.
Like older mutation-positive women of reproductive age (Werner-Lin et al., 2011), Theresa believes her unwavering desire to bear biological children is in direct conflict with provider recommendations for RRSO before her 40th birthday. Although this recommendation leaves Theresa, currently age 23, with 17 years of fertility ahead, she feels that her time is constrained. She articulates an urgency to find “Prince Charming,” emblematic of the cultural directives and pressures on young women to marry and procreate. This pressure separated her socially from her peers. Combined with the lack of support she feels from her parents, Theresa needed, and successfully sought out, other forms of emotional and instrumental support.
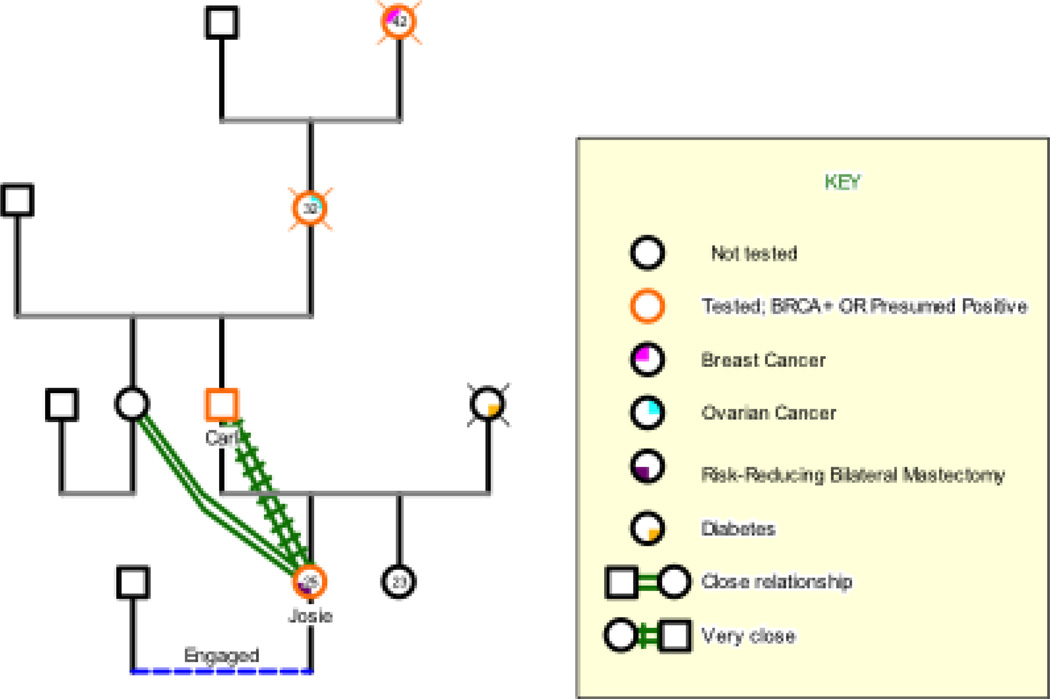
THE CASE OF JOSIE
Josie was interviewed the night before her 26th birthday in her Midwestern home. Josie tested positive for a BRCA2 mutation in February, and decided to complete RRBM and simultaneous breast reconstruction in July of the same year. At the time of her interview, she was three weeks past the surgery and still actively recovering. Unlike most of the other women in our collective sample (Werner-Lin et al., 2012), Josie did not grow up familiar with cancer, yet parental illness was not novel to her. Josie’s mother had systemic lupus erythematosus, which Josie describes as “a completely different beast,” and she died of lupus-related heart complications seven years prior to the interview, when Josie was 18.
Josie inherited the BRCA2 mutation from her father, Carl, a practicing family physician who was personally unaffected and in good health. His mother, Josie’s paternal grandmother, died of ovarian cancer when she was 32 and Carl was four, and her mother, Josie’s paternal great-grandmother, died of breast cancer in her early 40s. Carl had only vague memories of his mother, but told Josie, as other family members did that, “she was a wonderful, lively person.” Josie went on to report, “They say I look a lot like her.” As a result of his family history, Carl decided to pursue BRCA genetic testing when Josie turned 25, an age at which he perceived her to be old enough to cope with this information, yet young enough to still be unaffected by cancer.
Josie believed her understanding of cancer, and her approach to risk management, were unique within the context of the BRCA mutation-positive community since she did not have first-hand experience with cancer growing up. She told the interviewer, “It didn’t really register with me that much because I never knew anyone personally that died of breast cancer.” Before testing, Josie felt what she described as a “false security with having good health, which I guess is pretty common for people in their twenties.” She went on to say, “I really thought of myself as having invincible health. I was twenty-five, never went to the doctor, never even got a cold, never had allergies, nothing.” At the time she consulted her genetic counselor, Josie was completing a Master’s degree program. In her spare time before genetic testing, she would troll the internet for jobs, “making a beeline for the career path.”
Genetic testing changed all that.
Josie had a positive experience with genetic counseling. She consulted an urban clinic with a “strict protocol” where, despite her protests, providers would not permit her to have her blood drawn on the first visit. She says, “The second time was very quick –they drew the blood, and that was it. And the third time, of course, you come back for the results.” Josie anticipated her results without much fanfare. She “knew it was a 50–50 chance, and I had a logical plan in mind, so I didn’t worry about it so much.” She was confident in her plan to pursue RRBM quickly should she test positive.
Josie reported that receiving positive genetic testing results “shatters your image of being healthy forever.” She looked back to her remote family history with cancer, with which she was previously unfamiliar, and began to feel vulnerable to a cancer diagnosis. To cope with this new vulnerability, Josie shifted her focus online, searching for medical resources: “I was spending all of my time on the Internet looking for surgeons.” Josie had started breast cancer screening prior to RRBM, and reported feeling like “a hypochondriac. I had my first mammogram and breast MRI back in March. And I was sweating bullets because my grandmother was diagnosed at thirty-one, so I could very easily have it now.”
Josie had difficulty communicating her results to, and therefore in seeking support from, her loved ones and friends. A favorite aunt was consumed with her husband’s illness and Josie did not want to upset her further. Josie found that with close friends, “you have to explain what is BRCA, what is a mastectomy, that there are reconstruction options, and people don’t generally know about it or think about it.” She found these conversations cumbersome, and friends rarely reacted in ways she experienced as supportive. Josie also had trouble discussing her fears and concerns with her father. Carl’s reaction to Josie’s mutation status was intense and grief-stricken. She described this saying:
After the testing he was in tears almost, and he was, like, ‘I lost my mother to this, I didn’t know my grandmother [because of] this, and I’ll be damned if I lose my daughter to it.’ So my dad was willing to have the surgery done.
He said of her breasts, “They’re comin’ off!” Josie knew her father was trying to protect her, and she was committed to completing RRBM, yet she lamented that Carl never asked how she felt about her plan to pursue RRBM since learning her genetic testing results.
Josie’s fiancé, a medical resident, was also of the opinion that Josie should have RRBM, telling her, “I love you more than your boobs! They’re not worth it, and I want to have you around for a while.” Although these two most important people in her life felt strongly about Josie pursuing RRBM, Josie reported that her decision was predicated on her interpretation of her mutation status “as a cancer diagnosis like, stage zero-zero-zero, or stage minus two cancer,” and she qualified this by saying, “what is it, eighty-seven is the official number? That’s really kind of when and not if, in my opinion.” She looked at the legacy of early maternal death on both sides of her family, adding,
The way I was gearing myself up for it is, well, get it over with now, or do it later and then have to have chemotherapy, radiation, and balance having kids and work and whatever else is going on in my life at the time.
As a result, Josie agreed with Carl and her fiancé, and decided to complete surgery sooner rather than later: “considering my family history, this seems to be hitting my family younger rather than later.” She said, projecting forward to a hypothetical experience of repeated and ongoing screening, “Every time I turn around I’d be nervous about going in and finding out the results, and it wasn’t worth it.” As she consulted breast surgeons, she said, “I am going to bring a picture of my wedding gown and I’m gonna say, ‘Make me into that. I want to fit into that dress.’”
After earning her Master’s degree in June, Josie’s focus turned towards preparing for her upcoming surgery, scheduled for July. Although she believed surgery was the best course of action, anticipating the surgery was distressing. She remembered:
When July first hit, I was, uh-oh, surgery’s at the end of July. So then I was nervous again about surgery and preparing and second-guessing myself if I should have it done, all those kinds of things. Cold feet. But that’s kind of a process since the diagnosis.
Her fiancé also experienced “cold feet” leading into the surgery: “It hit him like a ton of bricks about a month before the surgery. He was saying, ‘Are you sure we should do this? Is this [the] right decision?’” Previously, Josie relied on her father and her fiancé to provide strength and steadfast guidance, and his vacillation left her unmoored.
During the time leading up to surgery, she described her approach to the stress by saying, “My pragmatic side and my emotional side don’t coincide necessarily.” Josie felt the need for resources beyond her father and fiancé to shore her up emotionally before heading into surgery. Looking back into her family history again, she felt protected by the spirits of her mother and grandmother, women who did not have the opportunities for longevity that Josie had. She stated, “(G)oing into surgery, I was like, my mom’s going to be looking over me, my grandmother’s going to be looking over me, they’ll make sure everything’s okay.” She was proud of her decision to have RRBM, saying. “I took steps to hopefully prevent myself from having the same kind of destiny.”
With RRBM behind her, Josie looked forward to her upcoming wedding and to building a family with her fiancé. Josie lamented that she would not be able to breast feed, and as a result, hoped to experience other “natural” parts of conception and childbirth. Yet she reported her fiancé was “pretty determined that he wants my eggs to be tested prior to us getting pregnant.” She saw this as unnecessary, saying, “Part of me’s like, well, by the time they would have to worry about it, twenty, thirty years from now, who knows what’s going to be available. We may have a vaccine by then.” Yet, Josie anticipated conceding to his preference, saying, “We’ll probably end up going that route just to make sure this gene dies with my generation.”
At the time of her interview, Josie was committed to supporting her younger sister, age 23, through genetic testing. Although her sister was still untested, Josie insisted, “She will be tested. She’s got some time to play with. She’s in medical school right now, so we’re kind of waiting for a lull in her schedule.”
Case Analysis
The BRCA1 gene mutation was masked in Josie’s father’s generation because both Carl and Carl’s sister were unaffected; therefore, Josie had no immediate exposure to cancer or cancer-related death. The multigenerational legacy of loss in Carl’s family, though unfamiliar to Josie, led Carl to experience increased anxiety about Josie’s risk that was expressed as pressure on Josie to make tough choices quickly. We selected this case for presentation because it provides an example of a recently-identified BRCA mutation carrier making surgical decisions quite early in the process of understanding and living with hereditary cancer risk. Although mutation-positive women may eventually arrive at the same risk management decisions given more time to adjust, research demonstrates that risk perceptions spike immediately following genetic testing and then normalize after a period of time (van Oostrom, Meijers-Heijboer, Lodder, et al., 2003). As a result, decisions made by asymptomatic emerging adult women in the immediate aftermath of genetic testing may not be autonomous and calculated, but rather based on fear or family pressure.
Josie had difficulty sharing worries and discussing decisions with her father and her aunt due to her desire to minimize the emotional impact of her mutation status and of her distress. BRCA1/2 mutation carriers of reproductive age employ what has been called “protective buffering” with partners (Manne, Dougherty, Veach, & Kless, 1999) and with parents (Werner-Lin, 2008). As a result, many highlight the need for auxiliary emotional support beyond genetic testing and throughout risk management (Werner-Lin et al., 2011), either to support surveillance fatigue (Hoskins & Greene, in press) or decision-making about surgical risk reduction. Josie understood Carl’s commitment to her RRBM was connected to his grief over the untimely loss of both his mother and his wife, complicating honest and forthcoming communication with him (Sobel & Cowan, 2003). Josie likely would have benefitted from an examination of how her family’s medical history, as well as her family’s relationships and prolonged grief (Daly, Farmer, & Harrop-Stein, 1999) impact her ability to autonomously consider genetic testing and navigate risk management.
Josie’s interview was one of the very first in our sample to be collected, before the public’s growing, yet still nascent, familiarity with pre-implantation genetic diagnosis (PGD). Since PGD tests embryos and not eggs, her fiancé’s plan to “have her eggs tested” indicates that the couple has not fully researched or understood this option. The couple might have different moral or emotional reactions to dealing with embryos, rather than eggs; additionally, assisted reproductive technologies will continue to evolve as this couple moves through their childbearing years. Each of these could shift their individual and collective perspective on uptake of PGD. Further, the pragmatic and financial demands of PGD might complicate uptake. Josie presents her fiancé as more committed to the idea of reproductive intervention than she, a relationship dynamic that should be attended to during genetic counseling, and potentially referred out to a medical family therapist for further exploration.
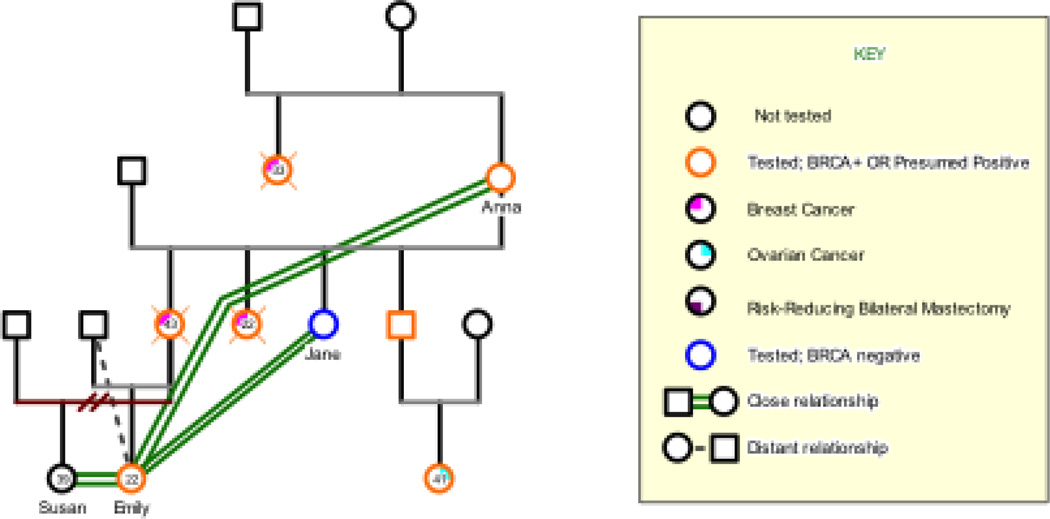
THE CASE OF EMILY
Emily is a 22-year-old of Ashkenazi Jewish descent who tested positive for a BRCA1 founder mutation three years prior to her interview. She had much to share about her experience in a family afflicted by HBOC; her maternal aunt died of breast cancer at the age of 22, before Emily’s birth, and Emily’s mother died of breast cancer at the age of 43, when Emily was just starting kindergarten at age five. Her father raised Emily, with significant emotional and instrumental support from Emily’s much older half-sister Susan, who was a product of her mother’s first marriage. At the time of the interview Emily continued to live with her father and, since his retirement, to support him financially. She maintained close relationships with her mother’s only surviving sister, Jane, who was BRCA1 mutation-negative; and her maternal grandmother, Anna, a presumed mutation carrier who was never tested. She says of her early childhood, “that was the hardest point or part of my life I guess. Growing up with only a father. But my grandmother was like a mother figure for me.” When asked what she knew about her mother’s illness, she said, “Just what my older sister told me. ‘Cause its such a young age, I think I kind of blocked it out.” With few memories of her own, Emily relied on her family for information. But, she says, “My father and I weren’t really talking about it. So I’d only hear from my grandmother and my sister, and they really didn’t want to talk about it, because they thought I would get upset.” Although she lives with her father, she reported that they are not very close.
When Emily turned 18, Susan, who functioned as a surrogate mother to Emily during her childhood and adolescence, sat Emily down to tell her about the family’s BRCA1 mutation and to urge Emily to get genetic testing. With additional pressure from her aunt Jane, Emily went begrudgingly to meet Jane’s genetic counselor. She recounted:
I really didn’t want to, but then again my sister pushed me, and my aunt, and then one of my cousins has ovarian cancer. Basically all three pushed me, even though I was young, they wanted me to be aware if I did have the gene.
Emily conceded to genetic testing primarily because her aunt died of breast cancer in her early 20’s, and her determined family, “wanted to make sure I got checked early on.” Susan made an appointment for Emily to see the genetic counselor Jane had consulted. Emily had only vague memories of genetic counseling, but she remembered receiving her results: “I just basically was in shock, I didn’t have any questions or any -- like I was just like oh, my god.” She went on to say of the team of providers:
They were so concerned because of my age, of telling me something like this. They were like, ‘A lot of people get depressed after this, want to even kill themselves.’ I think they brought psychologist in, a few doctors, just to make sure that I was okay with the news.
Since receiving her genetic testing results, Emily says she is “constantly worried. Because you don’t know when something’s going to hit.” She continues to struggle with recommendations from providers. She says, “It was really hard for them to even tell me what to do, because of the young age. They were like there’s [sic] not enough studies out.” As a result, she participates regularly in research, when she finds access, hoping these studies might provide information about and access to novel technologies.
Emily hoped to stave off surgery for as long as possible, saying: “A lot of people in my family want me to have surgery, and I don’t know if I’m ready. You know what I mean? If I have a kid, breastfeeding and stuff, you know? So that’s the hardest decision.” Although Emily believed her family meant well, she recognized that she was in a unique position, because, “no one had [genetic testing] at my age. A lot of people in my family are older. They’re all married. It’s different.” She was relieved that, for the moment, her doctors recommend against surgery because of her age. Regarding RRBM, she said “I think I’ll feel less stressed with one. But I really don’t want one until I’m like married and settled.” In the meantime, she received clinical breast exams every three months, mammogram and breast MRI once each year separated by six months, and CA-125 serum marker every year. She still felt unprotected, however, and wanted to “learn more and more about what I should be doing prevention wise.” When she began screening, she struggled with poor insurance coverage and could not afford primary breast screening. She was relieved to join a resource-rich breast imaging study to receive these screening tests free of charge. She was aware that she was the youngest participant enrolled in the breast imaging study and found this disquieting.
Frequent visits to the high risk clinic, regular conversations with family about risk reduction, and her own research into BRCA1 mutations lead Emily to question her identity, especially when friends and romantic partners have reacted poorly to her disclosure. She wondered, “Just because I have it, am I different? I don’t want them to think differently of me. I just don’t even like talking about it.” Emily was in a bind as far as social support; while her sister and aunt “would understand it better, because they went through stuff like this,” their counsel was far from impartial. Her friends, she reports, are “too immature to understand.”
At the time she completed genetic testing, Emily was dating casually. She said of that partner, “I don’t think he understood. That’s I think the biggest thing, like you don’t want to explain this whole history and then like oh, my god, you know? Scare people away.” She worried regularly about disclosing her mutation status to romantic partners, and she was frustrated thinking about when and how best to disclose. She stated, “I think when the time comes, I’ll just have to do it, I’ll have no choice.” She felt pressure to identify a life partner quickly in order to marry and begin childbearing.
When asked to consider how best to communicate cancer risk information to future children, Emily hesitated. She recognized her children might start asking about the absence of their maternal grandmother (Emily’s mother) “as early as five or six” years old. She intended to “make sure they know at an early age” about her mother’s life and about the risks associated with a BRCA1 mutation. Yet, she wanted to approach her children differently than her family approached her. Although she first identified age 12 or 13 for initial conversations with her children about BRCA, she says: “I don’t want to scare them, but I want them to be informed and able to talk to me about it.”
Case Analysis
Emily’s sense of isolation and burden were striking during her interview. She was highly responsive to the pressure exerted by her surviving relatives. This responsiveness was intensified by a loyalty towards Susan and Jane, a loyalty born out of early maternal loss and their role as surrogate mothers. She understood their motivation, and shared their interest in keeping her safe. However, without a mutation-positive sibling or a cousin her age also traversing the same core life cycle tasks, Emily felt alone and emotionally unsupported. She refused to share her doubts and fears with either Susan or Jane, especially the most emotionally debilitating parts of coping with HBOC risk. As a result, she felt relieved when her physicians recommended she postpone RRBM despite her family’s insistence otherwise.
Emily reported feeling different from peers and other mutation-positive family members, a feeling intensified by (1) her peers’ lack of understanding or capacity for mature and measured responsiveness, (2) her knowledge that she was the youngest participant in the breast imaging study, and (3) the mystique around her family’s experiences with cancer and her mother’s death. Her sense of isolation and alienation led her to worry about whether she would be accepted by a future partner after RRBM. As a result, she hoped to stave off surgery until she is “married and settled.”
Emily’s case speaks to the need for improved family communication between 18–25 year old mutation-positive women and their families-of-origin. A basic assessment of family dynamics around cancer and cancer-related loss would have illuminated the coercive pressures on Emily to pursue genetic testing and her relatives’ ongoing insistence on surgical risk reduction. By providing Emily with the resources, or with a forum, to approach her family, she could have been empowered to make an autonomous, informed decision about genetic testing whether or not her decision was consonant with her family’s interests.
DISCUSSION
The three cases presented here demonstrate the unique developmental, relational and temporal influences (Anaf, Drummond, & Sheppard, 2007), as well as the challenges, experienced by 18–25 year-old BRCA mutation-positive women as they complete genetic testing and initiate cancer risk management during this critical developmental period. In each case, the young adult’s family-of-origin provided the relational context for pursuing genetic testing. As a result, the same individuals who shaped the instrumental and emotional resources available for managing cancer risk were those most closely tied to family legacies of illness and loss (Werner-Lin & Gardner, 2009). Whether or not the emerging adult is aware of these dynamics, primary relationships with parents may be intensified due to the parent’s experience with cancer or cancer-related death, as in the case of Josie’s father. For each set of surviving relatives, the emerging adult woman became a vessel for her family’s grief and fears. During data analysis, we quickly collapsed the codes “family support” and “family pressure” as they emerged as inseparable and perspective-dependent: what a parent intended as support, the emerging adult experienced as pressure. This pressure is especially potent when perceived risk is high and tolerance for ambiguity is low (Hoskins, 2010). As a result, family processes silence the emerging adult’s needs and limit the possibility of autonomy in decision-making. Further, the physical and emotional presence of the parent in the genetic testing process inhibited these women from asking critical questions about genetic testing and cancer risk management, compromising truly informed consent. As long as the emerging adult remains pragmatically and emotionally dependent on parents for resources, her ability to act independently may be constrained. Theresa, Josie, and Emily all experienced this dynamic as pressure towards genetic testing and specific risk management trajectories. As these three women struggled to negotiate independence from their families, a natural aspect of the emerging adult years, they were, ironically, still in need of expert guidance (Hamilton, Williams, Bowers, & Calzone, 2009) to facilitate informed decision-making. Thus, access to competent and compassionate genetic care providers is essential. As women approach the age of thirty, form life-long partnerships, or in other ways move beyond the immediate influence of the family-of-origin and move into families of choice (Hoskins, Roy, Peters et al., 2008), the intensity of these pressures may diminish.
Theresa, Josie, and Emily’s experiences demonstrate the need for ongoing support to undergird medical decision-making and coping due to the complexities of relationships with parents. Kenen and colleagues (2006) defined “social separation” experienced by BRCA mutation-positive women under age 40 as “relational dissonance,” in which mutation-positive women feel new or increased isolation and estrangement in previously healthy relationships, a change marked by disruption in patterns of communication and interaction. For women aged 18–25 in our sample, as exemplified by those whose cases are presented herein, this isolation occurs at a moment of developmentally normative separation from families of origin. As described above, this moment of individuation, theoretically normative in emerging adulthood, is compromised by intense family relationships and legacies of loss. Thus, Theresa’s and Emily’s awareness of life-threatening illness risk was out of sync with the experiences of their peers who are more likely to be pursuing developmentally-normative tasks such as exploratory partnering, developing educational or job prospects, and building an identity through experimentation. The experiences of peers diverge further as these three women anticipated risk-reducing surgery, periods of illness and convalescence, dramatic changes in body image, and the need to provide care for ill parents.
Theresa’s anticipation of losing her parent’s insurance coverage, Josie’s anticipation of surveillance fatigue (Hoskins et al., 2012), and Emily’s relief at her physician’s recommendations that she delay RRBM all speak to the need for prolonged partnerships between providers and the youngest consumers of genetic testing. In the current model of genetic counseling, which has been optimized for older (>30 years) mutation carriers, it is typical for the patient to undergo a limited number of visits in the course of genetic risk assessment, testing and results disclosure, with little or no ongoing follow-up or contact with the counselor. Our findings suggest that ongoing relationships between providers and 18–25 year-old patients are essential. Such a model would mirror the cognitive and relational changes that are a hallmark of this developmental period, and permit the provider/patient relationship to evolve with the patient’s ability to make sound, autonomous decisions. Periodic contact is critical as BRCA mutation-positive women “come of age” so that they have the most current information with which to make decisions about whether and how to initiate regular breast and ovarian cancer screening, and risk-reducing surgery.
Figure 1.
Theresa’s Genogram
Figure 2.
Josie’s Genogram
Figure 3.
Emily’s Genogram
Contributor Information
Lindsey M. Hoskins, Division of Cancer Epidemiology and Genetics, National Cancer Institute.
Allison Werner-Lin, Silver School of Social Work, New York University.
References
- Anaf S, Drummond C, Sheppard LA. Combining case study research and systems theory as a heuristic model. Qualitative Health Research. 2007;17(10):1309–1315. doi: 10.1177/1049732307308946. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Crabtree, Benjamin F, Steele, David J, McDaniel, Reuben R., Jr Case study research: The view from complexity science. Qualitative Health Research. 2005;15(5):669–685. doi: 10.1177/1049732305275208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 55(5):469–480. [PubMed] [Google Scholar]
- Arnett JJ, Galambos NL. Culture and conceptions of adulthood. New Directions for Child and Adolescent Development. 2003;100:91–98. doi: 10.1002/cd.77. [DOI] [PubMed] [Google Scholar]
- Borysenko J. A woman’s book of life: The biology, psychology, and spirituality of the feminine life cycle. New York: Riverhead Books; 1996. [Google Scholar]
- Bronfenbrenner U. The ecology of human development: Experiments by nature and design. Cambridge: Harvard University Press; 1979. [Google Scholar]
- McGoldrick M, Carter B, Garcia-Preto N. Overview: The Life Cycle in its Changing Context: Individual, Family and Social Perspectives. In: McGoldrick M, Carter EA, Garcia-Preto N, editors. The expanded family life cycle: Individual, family, and social perspectives. 3rd Ed. Boston: Allyn & Bacon; 2011. [Google Scholar]
- Census, US. Estimated median age at first marriage, by sex: 1890 to 2009. 2009 [Google Scholar]
- Chen S, Iverson ES, Friebel T, Finkelstein D, Weber BL, Eisen A, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. Journal of Clinical Oncology. 2006;24(6):863–871. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Parmigiani G. Meta-Analysis of BRCA1 and BRCA2 penetrance. Journal of Clinical Oncology. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, Domchek SM. Clinical management of hereditary breast cancer syndromes. Journal of Mammary Gland Biology and Neoplasia. 2011;16(1):17–25. doi: 10.1007/s10911-011-9200-x. [DOI] [PubMed] [Google Scholar]
- Daly M, Farmer J, Harrop-Stein C, Montgomery S, Itzen M, Wagner Costalas J, Gillespie D. Exploring family relationships in cancer risk counseling using the genogram. Cancer Epidemiology, Biomarkers and Prevention. 1999;8:393–398. [PubMed] [Google Scholar]
- Evans JP, Skrzynia C, Susswein L, Harlan M. Genetics and the young woman with breast cancer. Breast Disease. 2005/2006;23:17–19. doi: 10.3233/bd-2006-23104. [DOI] [PubMed] [Google Scholar]
- Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nature Reviews Cancer. 2007;7:937–948. doi: 10.1038/nrc2054. [DOI] [PubMed] [Google Scholar]
- Feagin JR, Orum AM, Sjoberg G. A case for the case study. Chapel Hill, NC: University of North Carolina Press; 1991. [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. Journal of the National Cancer Institute. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Hamilton R, Williams JK, Bowers BJ, Calzone K. Life trajectories, genetic testing, and risk recution decisions in18–39 year old women at risk for hereditary breast and ovarian cancer. Journal of Genetic Counseling. 2009;18:147–59. doi: 10.1007/s10897-008-9200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins LM. Unpublished doctoral dissertation. College Park: University of Maryland; 2010. Negotiation of health risks and risk management for young adult BRCA1/2-positive women: Implications for partnering and family formation. [Google Scholar]
- Hoskins LM, Greene MH. Anticipatory loss and early mastectomy for young female BRCA1/2 mutation carriers. Qualitative Health Research. doi: 10.1177/1049732312458182. (in press). [DOI] [PubMed] [Google Scholar]
- Hoskins LM, Roy KM, Greene MH. Toward a new understanding of risk perception among young female BRCA1/2 “previvors.”. Families, Systems & Health. 2012;30(1):32–46. doi: 10.1037/a0027276. [DOI] [PubMed] [Google Scholar]
- Hoskins LM, Roy K, Peters JA, Loud J, Greene MH. Disclosure of positive BRCA 1/2-mutation status in young couples: The journey from uncertainty to bonding through partner support. Families, Systems & Health. 2008;26:296–316. doi: 10.1037/a0012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenen R, Ardern-Jones A, Eeles R. "Social separation" among women under 40 years of age diagnosed with breast cancer and carrying a BRCA1 or BRCA2 mutation. Journal of Genetic Counseling. 2006;15:149–162. doi: 10.1007/s10897-005-9015-2. [DOI] [PubMed] [Google Scholar]
- Lodder LN, Frets PG, Trijsburg RW, Meijers-Heijboer EJ, Klijn JGM, Seynaeve C, Niermeijer MF. One year follow-up of women opting for pre-symptomatic testing for BRCA1 and BRCA2: Emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery) Breast Cancer Research and Treatment. 2002;73:97–112. doi: 10.1023/a:1015269620265. [DOI] [PubMed] [Google Scholar]
- Manne S, Dougherty J, Veach S, Kless R. Hiding worries from one’s spouse: Protective buffering among cancer patients and their spouses. Cancer Research, Therapy and Control. 1999;8:175–188. [Google Scholar]
- Narod SA. BRCA mutation in the management of breast cancer: the state of the art. Nature Reviews Clinical Oncology. 2010;7(12):702–707. doi: 10.1038/nrclinonc.2010.166. [DOI] [PubMed] [Google Scholar]
- Pruthi S, Gostout BS, Lindor NM. Identification and management of women with BRCA mutations or hereditary predisposition for Breast and Ovarian Cancer. Mayo Clinic Proceedings. 2010;85(12):1111–1120. doi: 10.4065/mcp.2010.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel JC, Ollila DW. Prophylaxis and screening options: Recommendations for young women with BRCA mutations. Breast Disease. 2005–2006;23:31–35. doi: 10.3233/bd-2006-23105. [DOI] [PubMed] [Google Scholar]
- Sobel S, Cowan CB. Ambiguous loss and disenfranchised grief: The impact of DNA predictive testing on the family as a system. Family Process. 2003;42(1):47–57. doi: 10.1111/j.1545-5300.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Stake R. Case Studies. In: N. K. L. Denzin YS, editor. Handbook of qualitative research. Thousand Oaks, CA: Sage; 2000. pp. 435–454. [Google Scholar]
- Tiggemann M, Pennington B. The development of gender differences in body-size dissatisfaction. Australian Psychologist. 1990;25(3):306. [Google Scholar]
- van Oostrom I, Meijers-Heijboer H, Lodder LN, Duivenvoorden HJ, van Gool AR, Seynaeve C, Tibben A. Long-term psychological impact of carrying a BRCA1/2 mutation and prophylactic surgery: A 5-year follow-up study. Journal of Clinical Oncology. 2003;21:3867–3874. doi: 10.1200/JCO.2003.10.100. [DOI] [PubMed] [Google Scholar]
- Watson M, Foster C, Eeles R, Eccles D, Ashley S, Davidson R, et al. Psychosocial impact of breast/ovarian (BRCA1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. British Journal of Cancer. 2004;91(10):1787–1794. doi: 10.1038/sj.bjc.6602207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Lin A. Danger zones: Risk perceptions of young women from families with hereditary breast and ovarian cancer. Family Process. 2007;46(3):335–349. doi: 10.1111/j.1545-5300.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- Werner-Lin A. Formal and informal support needs of young women with BRCA mutations. Journal of Psychosocial Oncology. 2008;26(4):111–132. doi: 10.1080/07347330802359776. [DOI] [PubMed] [Google Scholar]
- Werner-Lin A. Building the cancer family: Family planning in the context of inherited breast and ovarian cancer risk. Journal of the Society for Social Work and Research. 2010;1(1):26–38. [Google Scholar]
- Werner-Lin A, Hoskins L, Doyle M, Greene M. “Cancer doesn’t have an age”: Genetic testing and cancer risk management in BRCA1/2 mutation-positive women aged 18–24. Health. 2012:1–19. doi: 10.1177/1363459312442420. [DOI] [PubMed] [Google Scholar]
- Werner-Lin A, Gardner DS. Family illness narratives of inherited cancer risk: Continuity and transformation. Families, Systems and Health. 2009;27(3):201–212. doi: 10.1037/a0016983. [DOI] [PubMed] [Google Scholar]
- Werner-Lin A, Hoskins L, Rubin L. Genetic counseling and cancer screening in BRCA1/2 mutation carriers aged 18–25. Oral presentation, 12th International Meeting on Psychosocial Aspects of Hereditary Cancer (IMPAHC); Amsterdam, Netherlands. 2011. [Google Scholar]
- Yin RK. Case study research: design and methods. Beverly Hills: Sage; 1988. [Google Scholar]