Abstract
Ventilatory limitation to exercise remains an important unresolved clinical issue; as a result, many individuals misinterpret the effects of expiratory flow limitation as an all-or-nothing phenomenon. Expiratory flow limitation is not all-or-none; approaching maximal expiratory flow can have important effects not only on ventilatory capacity but also on breathing mechanics, ventilatory control, and possibly exertional dyspnea and exercise intolerance.
Keywords: dynamic compression of the airways, maximal expiratory flow, breathing mechanics, ventilatory limitations to exercise, ventilatory limitations, ventilatory constraints, cardiopulmonary exercise testing
INTRODUCTION
Exercise ventilatory limitation occurs when the ventilatory output (minute ventilation, V̇E L/min) closely approaches or matches ventilatory capacity. Currently, there is no universally accepted agreement on how to quantify ventilatory capacity. However, for many research investigators and clinical practitioners who study exercise ventilatory mechanics, the maximal flow-volume loop is used to define expiratory and inspiratory ventilatory limits (4;11). Overlaying an exercise tidal flow-volume loop within the maximal flow-volume loop visualizes the proportion of ventilatory capacity utilized and the amount of ventilatory reserve potentially remaining for increasing 1) expiratory and inspiratory flow (i.e., capacity for increasing breathing frequency, Fb) and 2) tidal volume (VT) (Figure 1A). Because the determinants of maximal expiratory flow (i.e., mechanical factors regulating flow through a collapsible tube) and maximal inspiratory flow (i.e., limited only by inspiratory force available to overcome airway resistance and respiratory system compliance) are so drastically different, the major concern during exercise has been with the restrictions imposed by maximal expiratory flow. Thus, when tidal expiratory flow closely approaches or impinges on maximal expiratory flow, this is referred to as expiratory flow limitation (EFL) (Figure 1B). Once tidal expiratory flow approaches maximal expiratory flow over a large portion of VT (i.e., > 30–50% of VT), and/or tidal inspiratory flow approaches maximal inspiratory flow, and/or VT approaches 50–60% of forced vital capacity (FVC), ventilatory output is usually considered to be close to ventilatory capacity and exercise ventilatory limitations may occur (4;11).
Figure 1.
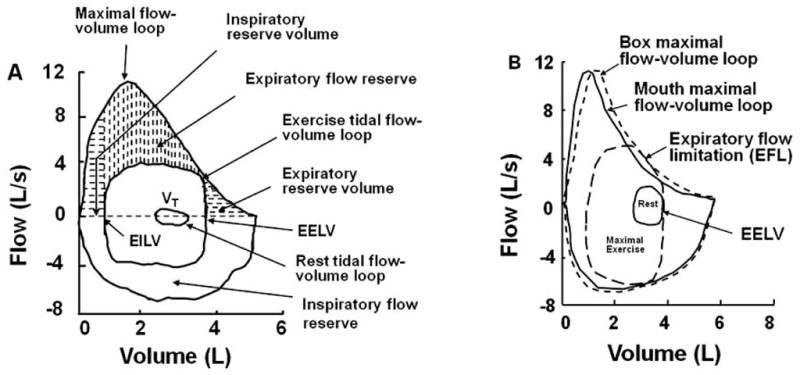
Maximal flow-volume loops and tidal flow-volume loops at rest and during exercise. A. Normal adult without expiratory flow limitation (EFL) showing expiratory flow, inspiratory flow, and tidal volume (VT) reserves. B. Maximal flow-volume loop determined from flow measured at the mouth (mouth loop = solid line) and maximal flow-volume loop determined from flow measured at the mouth and volume measured from a pressure corrected volume-displacement body plethysmograph (Box loop = large-dashed line). When tidal expiratory flow approaches or impinges on maximal expiratory flow, this is referred to as EFL. At this operational lung volume (EELV), ventilatory reserve is greater than ventilatory output, at least in absolute limits. However, the breathing consequences of approaching this absolute limit are under appreciated. Volume = expired volume below total lung capacity; VT = tidal volume; EELV = end-expiratory lung volume; EILV = end-inspiratory lung volume. [Adapted from (4). Copyright © 1999 Lippincott Williams & Wilkins. Used with permission.]
However, EFL is not an all-or-nothing phenomenon. Approaching maximal expiratory flow and/or with the onset of dynamic compression of the airways, airway mechanics begin to change, which continue to change until maximal expiratory flow is achieved. Based upon our studies of EFL and respiratory mechanics during exercise in healthy adults and respiratory patients, we hypothesize that approaching maximal expiratory flow, just like EFL, can have important effects not only on ventilatory capacity but also on breathing mechanics, ventilatory control, and possibly exertional dyspnea and exercise intolerance. Thus, determining the exact point and magnitude of impingement of tidal expiratory flow on maximal expiratory flow (i.e., EFL) may not be the only important concern. Approaching maximal expiratory flow and/or minimal EFL may play a larger role in changing breathing mechanics (e.g., operational lung volumes, breathing pattern, mean flow rates) and ventilatory control (e.g., exercise ventilatory response, breath timing), and possibly contributing to breathing discomfort and exercise intolerance than the limitations it imposes on expiratory ventilatory capacity as conventionally thought. This manuscript will focus on understanding EFL and the potential role it plays on ventilatory output, breathing mechanics, and ventilatory control, and possibly exertional dyspnea and exercise intolerance. Recognizing the impact of these effects is important to properly interpret ventilatory limitations that occur during exercise and their potential for imposing ventilatory limitations to exercise.
MAXIMAL EXPIRATORY FLOW AND EXPIRATORY FLOW LIMITATION (EFL)
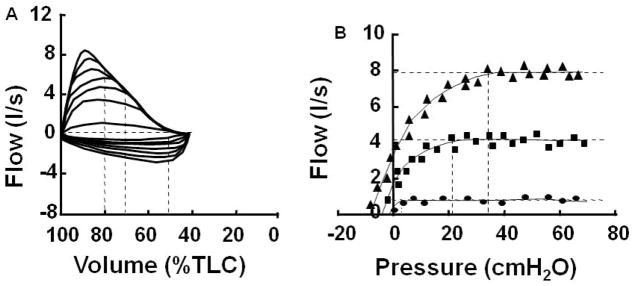
To appreciate the significance of approaching and/or obtaining maximal expiratory flow (i.e., EFL), it is important to understand briefly the determinants of maximal expiratory flow as illustrated by the pressure-flow relationship at a given lung volume (i.e., isovolume pressure-flow relationship). This relationship can be demonstrated by plotting isovolume pressure-flow curves (IVPF curves), which are used to define maximal expiratory flow (12)(Figure 2). Below 80% of the FVC, expiratory flow reaches a maximum regardless of how hard one forcibly expires (i.e., effort independent). The mechanism of this limit, while important, is not necessary for understanding the effects of approaching maximal expiratory flow during exercise and will not be discussed here. There are many good reviews available on this topic by Robert E. Hyatt or Jerry Mead.
Figure 2.
Isovolume pressure flow curves. Left panel: graded flow-volume loops used to construct isovolume pressure flow curves (right panel). Vertical dashed lines in the left panel indicate the flows and pressures measured at 80, 70, and 50% of total lung capacity (TLC). Vertical dashed lines in the right panel indicate the minimal critical pressure for maximal flow (Pcrit) and the horizontal dashed lines indicate maximal expiratory flow. [Adapted from (12). Copyright © 2000 The American Physiological Society. Used with permission.]
In the IVPF plot (Figure 2B), pleural pressure rises as flow increases until the onset of dynamic compression of the airways (i.e., up until the onset of compression, the relation between flow and pressure can be modeled by P=K1V̇+K2V̇2). After the onset of dynamic compression of the airways, pressure increases at a rate greater than that predicted by the above equation and much faster than flow. This is shown for an example IVPF curve produced at 65% of FVC in Figure 3 (22). As a result, airway resistance (i.e., flow/pressure) can reach a value up to 6-fold greater by the time expiratory flow becomes fixed and the diameter of the flow-limiting segment of the airway is fixed (see Appendix 1, Figure 12 in Mead et. al., 1967) (22).
Figure 3.
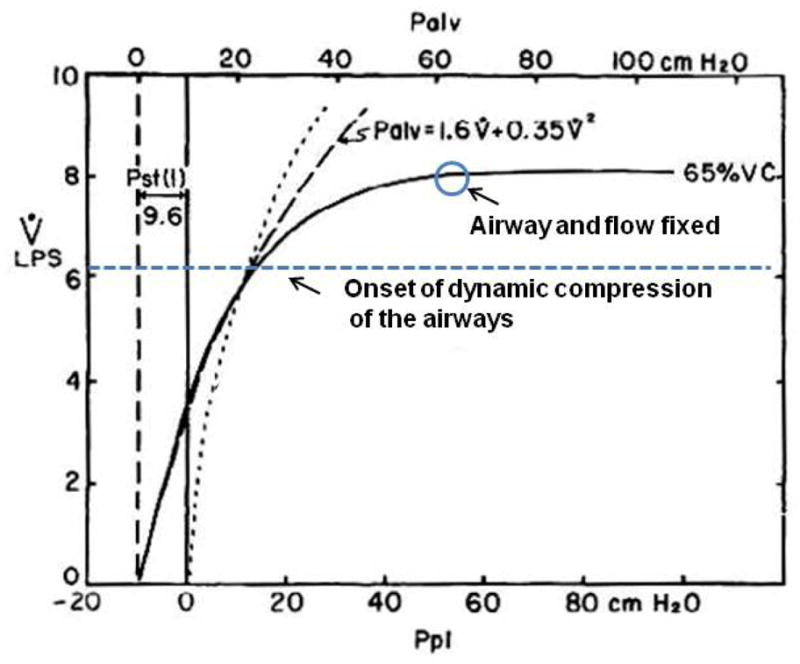
Mean Isovolume pressure flow curve at 65% vital capacity. Note onset of dynamic compression of the airways at divergence of Palv and isovolume pressure flow curve. Palv = alveolar pressure, Ppl = pleural pressure, VC = vital capacity, Pst(l) = static recoil pressure of the lung. [Adapted from (22). Copyright © 1967 The American Physiological Society. Used with permission.]
The important concept to recognize here is that the onset of dynamic airway compression and subsequent increase in airway resistance start long before expiratory flow becomes limited. In Figure 3 for example, at 65% of total lung capacity (TLC), the onset of dynamic compression starts at an expiratory flow 2 L/s below maximal expiratory flow (22). This means that when tidal expiratory flow approaches maximal expiratory flow and/or reaches the onset of dynamic compression of the airways, airway mechanics begin to change, which continue to change until maximal expiratory flow is achieved. Depending on lung volume this could give a wide threshold of 1 to 2 L/s of expiratory flow where airway mechanics could influence breathing. Thus, determining the exact point of impingement of tidal expiratory flow on maximal expiratory flow (i.e., EFL) is not the only major concern. Marked changes in airway mechanics have already started to occur much before EFL is definitively obtained and/or before ventilatory capacity is truly limited (i.e., tidal expiratory flow matches maximal expiratory flow over a large portion of VT – see below).
Furthermore, in many cases the slope of the tidal expiratory flow curve can be similar to that of maximal expiratory flow (i.e., parallel but lower than maximal expiratory flow) as tidal expiratory flow approaches maximal expiratory flow. This is because the time constant for airflow out of the lung is determined by many of the same factors as those that determine maximal expiratory flow (i.e., airway resistance, and lung compliance) (22). Therefore, establishing the exact point where tidal expiratory flow matches maximal expiratory flow (i.e., EFL) or the exact amount of EFL are mainly academic questions. Clinically and physiologically, getting close to maximal expiratory flow may be just as important as EFL. Respiratory constraints and/or ventilatory limitations have already been initiated as maximal expiratory flow is approached and long before EFL actually limits expiratory ventilatory capacity. Thus, the onset of dynamic airway compression of the airways and the large increases in airway resistance could initiate subsequent changes in breathing mechanics (i.e., changes in operational lung volumes and respiratory muscle function), ventilatory control (i.e., including ventilatory output), and possibly exertional dyspnea and exercise intolerance, not absolute limitations to ventilatory capacity (i.e., EFL). Other possibilities could include changes in cardiovascular function resulting from swings in thorax pressures, but these changes are outside the scope of this manuscript (31). Also, there are different techniques for the determination of EFL, some having a more all-or-none view, but these will not be discussed since they deal with only pin-pointing the exact point and magnitude of EFL (1).
POTENTIAL CONSEQUENCES WITH THE ONSET OF EFL – VENTILATORY CAPACITY
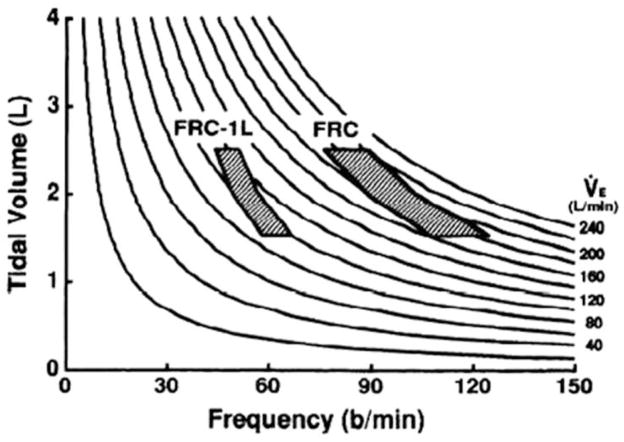
In an extensive analysis of the maximal flow-volume loop, we demonstrated the influence of maximal expiratory flow, end-expiratory lung volume (EELV), and breathing pattern on theoretical ventilatory capacity (4;6;11) (Figure 4). In the schematic representation in Figure 4, maximal V̇E is calculated for different combinations of functional residual capacity (FRC, functionally equivalent to EELV), VT, and Fb for a given flow-volume loop and plotted against ventilatory isopleths. This plot shows that for a VT that originates at a higher FRC ventilatory capacity is increased significantly vs. a VT that originates at a lower FRC (e.g., FRC vs. FRC-1L). In a comparison of these theoretical results with actual exercise data, we established that through subtle physiological adjustments in breathing mechanics (e.g., increase in EELV, decrease in expiratory timing, and increase or decrease in VT) ventilatory capacity is usually greater than ventilatory demand throughout submaximal exercise (6;10;11).
Figure 4.
Schematic representation of the effect of functional residual capacity (FRC, functionally equivalent to end-expiratory lung volume [EELV]) on estimated maximal ventilation for given tidal volume and breathing pattern. Values were calculated from maximal expiratory flow-volume curve. Note how a greater theoretical range of minute ventilation (V̇E, 160–220 L/min) can be produced at the higher FRC. The calculated range ventilatory range is much lower at FRC-1 L. However, the theoretical ventilatory frequencies calculated are much higher than ever expected during exercise. [Adapted from (11). Copyright © 1993. The American Physiological Society. Used with permission.]
The reason ventilatory capacity is usually greater than V̇E is that seldom does EFL occur over the entire range of VT. Generating maximal expiratory flow at the beginning of expiration (i.e., early during expiration) would take a great deal of effort and would increase V̇E marginally. Plus, greater effort during the beginning of expiration would increase the magnitude of EFL (i.e., greater dynamic compression of the airways) during the later portion of expiration (i.e., end of VT near EELV). We found that healthy individuals and patients with chronic airflow limitation alike do not usually utilize the upper-portion of the maximal expiratory flow-volume curve due to these reasons (2;3;5;10;13;16). The explanation for this could involve other ventilatory control mechanisms as will be discussed later.
Thus, tidal expiratory flow-volume curves are relatively rounded or have a slope similar to the maximal expiratory flow-volume curve for the first 50% of VT with EFL occurring over the last 0–50% of VT (Figure 5). Nevertheless, tidal expiratory flow in the first 50% of the VT can be near or above the onset of dynamic compression of the airways (i.e., partially collapsed but not yet flow limited). This typical tidal expiratory profile is usually not altered even when EELV is increased, except in extreme cases of respiratory disease, ventilatory stress, or brief voluntary breathing maneuvers. Thus, ventilatory output is almost always less than the absolute theoretical or calculated maximal ventilatory capacity for a given EELV. This concept is extremely important. When an exercise practitioner appraises whether maximal mechanical ventilatory limitation is obtained or approached during exercise, a tidal expiratory curve with EFL over only a portion of the expiratory curve, as shown in Figure 5, is what should be considered as ventilatory constraint or limitation, in contrast to a tidal expiratory flow-volume curve with EFL over the entire range of VT.
Figure 5.
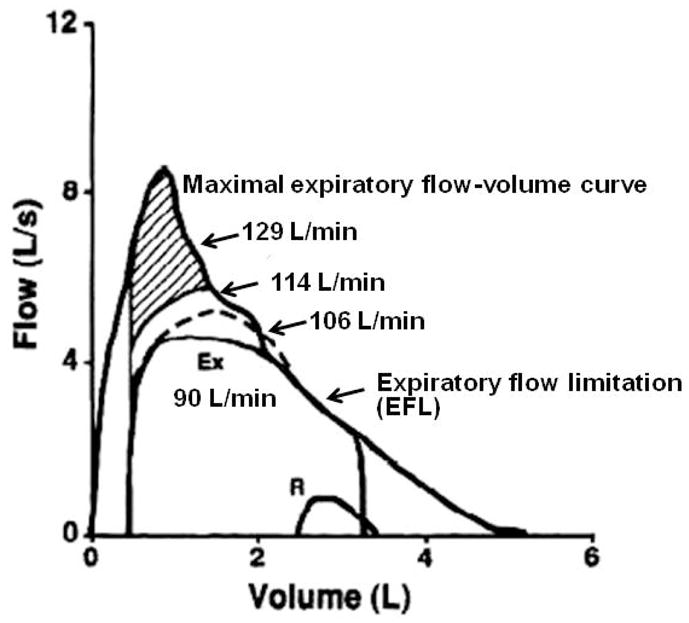
Maximal and tidal expiratory flow-volume curves. Calculated maximal minute ventilation (V̇EL/min) with utilization of greater portions of the maximal expiratory flow curve. If all expiratory reserve was utilized a maximum V̇E of 129 L/min could be obtained. However, except for extreme circumstances or voluntary breathing maneuvers such as the maximal voluntary ventilation (MVV), this is never observed. The curves yielding 114 or 106 L/min are more realistic. Thus, at 90 L/min, ventilatory output is very close to ventilatory capacity. Ex = exercise, R = rest. [Adapted from (11). Copyright © 1993. The American Physiological Society. Used with permission.]
Hence, we believe reaching the absolute theoretical or volitional maximal expiratory flow-volume curve may not be necessary for obtaining significant or important ventilatory limitations. Moreover, our work has shown that approaching the onset of dynamic compression could be just as important as EFL in evoking adjustments in breathing mechanics and minimizing the extent of EFL during exercise (4;11;13).
POTENTIAL CONSEQUENCES WITH THE ONSET OF EFL – BREATHING MECHANICS
When maximal expiratory flow is approached significantly or EFL is achieved over some fraction of VT, there are now well recognized responses in breathing mechanics. These can be seen in patients with chronic airflow limitation (5;10;13), elderly adults (3), obese adults (8;17), young men with hyperbaric-imposed flow limitation (27), and in younger and older athletes (7;19). Ourfindings suggest that the responses to EFL are the same regardless of the cause of EFL (i.e., decrease in maximal expiratory flow due to disease, aging, or environmental exposure, or increase in ventilatory demand). However, the magnitude of EFL or frequency of occurrence of EFL may differ among different populations and even genders. The clinical use and consequences of these changes in breathing mechanics was recently reviewed in determining ventilatory limitations to exercise (4).
Briefly, EELV usually decreases with the initiation of exercise due to recruitment of expiratory muscles. This decrease in EELV may be responsible for a large proportion of the increase in VT initially (e.g., up to 100% in some cases) with end-inspiratory lung volume (EILV) accounting for the remaining increase in VT (9). This partitioning of the increase in VT over both the expiratory reserve volume and the inspiratory reserve volume also partitions the increase in the work of breathing between the expiratory and inspiratory muscles. Over most of the exercise range, V̇E is increased by increasing both VT and Fb but predominately by increasing VT, especially at lower intensity exercise while Fb increases steeply at higher intensity exercise. The magnitude of decrease in EELV during exercise is thought to be presumably limited by nonlinearities of the chest wall pressure-volume relationship in individuals who never reach EFL or the onset of dynamic compression of the airways. However, we found in obese adults that the decrease in EELV during exercise is less than that obtained when they lie supine, which suggests some active control may be involved in the determination of exercise EELV (8;17). EILV usually increases until it approaches 80–90% of TLC and is usually thought to be limited by elastic work on the chest wall and inspiratory muscle strength.
Approaching maximal expiratory flow (i.e., the onset of dynamic compression of the airways) or EFL is associated with an increase in EELV (i.e., hyperinflation of EELV) (28). We have established that when exercise EELV and EILV are increased relative to resting EELV and TLC respectively, they indicate hyperinflation of these dynamically determined lung volumes (i.e., in contrast to lung volumes statically determined at rest like FRC) (4;11;13). It is important to remember that the increase in EELV is both an indication of approaching ventilatory limitation and an increase in ventilatory capacity as mentioned earlier. Our work showed that the two move in tandem except in extreme cases of maximal expiratory flow limitation (6;11). Since both demand and capacity are dynamic, it makes it very difficult to determine when ventilatory capacity is obtained and/or when V̇E is truly limited. Nevertheless, it is imperative to recognize when the process occurs (i.e., rise in EELV and changes in breathing mechanics) in contrast to focusing on the exact magnitude of EFL (i.e., %VT). As explained above, it is possible that the absolute limitation to ventilatory capacity is less important than the process of approaching EFL and changes in EELV. In part, this is because we do not yet understand fully the mechanism by which EELV rises.
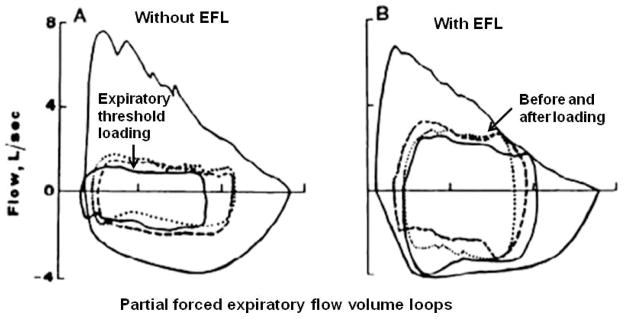
Originally, it was thought that as tidal expiratory flow was limited expiratory time (TE) took longer than the respiratory controller allowed (i.e., flow limitation-prolonged TE) and the rise in EELV increased passively. However, the onset of dynamic compression of the airways could signal an early termination of expiration, thus leading to an increase in EELV (27;28). To investigate this possibility, we applied an expiratory threshold load in individuals with and without EFL during exercise (28). In subjects without EFL, expiratory flow was decreased significantly and TE increased without a change in VT, and EELV increased slightly when the load was applied (Figure 6). When the expiratory load was imposed in subjects with EFL, expiratory flow decreased, and the subjects did not obtain EFL until a lower lung volume (i.e., a decrease in EELV). The decrease in expiratory flow allowed TE to increase. These findings suggested that compression of the airways downstream of the flow-limiting segment mightelicit a reflex response that could alter breathing mechanics, specifically expiratory time thereby increasing EELV.
Figure 6.
Composite flow-volume curves without and with tidal expiratory flow limitation (EFL). Large flow-volume loops represent partial forced expiratory maneuvers. Dashed, continuous, and dotted loops recorded before, during, and after imposed expiratory threshold loading, respectively. [Adapted from (28). Copyright © 1999. The American Physiological Society. Used with permission.]
With this in mind, the following scenario appears to prevail in our understanding. When approaching EFL, or with EFL itself, EELV can rise until EILV approaches TLC, which could increase the work of breathing and/or the perception of breathing (i.e., shortness of breath). Once EELV starts to increase, VT often plateaus or declines as Fb is increased. If respiratory timing (inspiratory time divided by total time, TI/Ttot) is preserved, the increase in VE requires an increase in mean expiratory flow rate (VT/TE). When tidal expiratory flow nears or reaches maximal expiratory flow, mean lung volume must be increased to increase mean expiratory flow (i.e., hyperinflation to utilize greater expiratory flow rates). Once EILV approaches TLC, mean lung volume can only be increased by increasing EELV. Therefore, at high levels of V̇E, VT plateaus and then decreases. At this point, V̇E increases only by increasing Fb (i.e., greater flow rates due to increased activation of expiratory and inspiratory muscles). Although the exact mechanisms of this response are not known, it appears that the respiratory controller is programmed to maintain normal timing if possible and that the onset of dynamic compression of the airways or EFL is a powerful stimulus to terminate expiration and initiate the next breath (27;28).
With age-related reductions in maximal expiratory flow and/or reductions in maximal expiratory flow due to chronic airflow limitation (12), we found that increasing V̇E during exercise often leads to small decreases in EELV that soon produce the onset of dynamic compression and/or EFL (2;3;6;10;13). As detailed above, once this occurs, further increases in V̇E are produced by preserving the normal relationship between mean expiratory and inspiratory flow rates and increasing EELV. As stated earlier, this is in contrast to increasing expiratory effort to utilize maximal flows throughout expiration, which means that complete EFL is rarely observed except in extreme cases (6;11). While the magnitude of EFL affects ventilatory capacity to some basic extent, the onset of dynamic compression of the airways and even minimal EFL affects breathing mechanics and ventilatory regulation including ventilatory output itself (see section on Potential Consequences with the Onset of EFL – Ventilatory Control).
POTENTIAL CONSEQUENCES WITH THE ONSET OF EFL – VENTILATORY CONTROL
The normal ventilatory response to exercise is linear up to approximately 50% of peak exercise. Beyond this, V̇E becomes nonlinear with work (e.g., oxygen uptake, VO2 or work rate). In general, a low ventilatory response could indicate mechanical ventilatory constraints. Likewise, an excess ventilatory response to exercise could indicate increased ventilatory demand (i.e., increased dead space or ventilatory inefficiency). The “break point” in the ventilatory response to exercise is referred to as the ventilatory threshold (VTh), although the mechanism of VTh remains controversial. Nevertheless, locating a VTh is helpful to indentify submaximal from heavy exercise. What is much less known is how approaching EFL may alter the exercise ventilatory response from rest to exercise (i.e., change in V̇E divided by the change in expired carbon dioxide, ΔV̇E/ΔV̇CO2).
Approaching maximal expiratory flow and/or the onset of dynamic compression of the airways could influence ventilatory output itself. As stated above, we found that approaching or reaching maximal expiratory flow appears to influence the termination of expiration and the initiation of the next breath (28). This was demonstrated when an expiratory threshold load was applied during exercise, which decreased expiratory flow, decreased the amount of EFL, and prolonged expiration thereby decreasing EELV and increasing VT slightly in patients who had EFL. In the patients without EFL, the opposite EELV response was observed. Others have found a similar effect by adding an expiratory load such as pursed lip breathing, which decreases the magnitude of dynamic compression of the airways, during rest and exercise (30).
In response to pursed lip breathing, healthy adults maintain EELV, prolong expiration, increase VT, and decrease Fb. It is unknown how close tidal expiratory flow was to maximal expiratory flow in these adults when the pursed lip breathing was applied; but patients with chronic airflow limitation have similar responses as to expiratory threshold breathing, and experience less breathing symptoms supposedly due to the decreased dynamic compression of the airways and decreased EELV (24). Adding a very small expiratory resistive load when EFL is not present actually shifts EELV to a higher lung volume and increases ventilatory capacity and V̇E at rest and during exercise (18;30). Therefore, it is not just the change in expiratory resistance that is responsible for these changes with pursed lipped breathing but possibly the change in airway mechanics. The important concept here is that an increase or decrease in airway dynamic compression can alter respiratory control, especially expiratory and inspiratory timing, which affects mean flow rates, VT, and EELV.
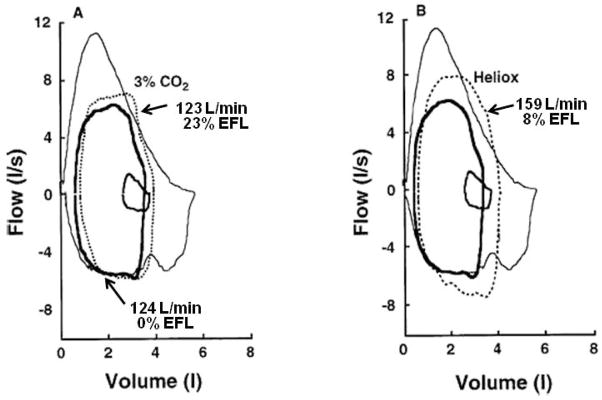
There are also numerous examples where the exercise ventilatory response may become fixed when EFL is approached or partially achieved over even a small portion of VT, despite the addition of external chemical respiratory stimuli (6;15;19;21). We reported this in older adults at peak exercise when maximal V̇E could not be increased with breathing inspired carbon dioxide, CO2 (3). These volunteers had EFL typical of older adults unlike that of younger sedentary subjects who could increase ventilatory output at peak exercise while breathing inspired CO2 (Figure 7) (2). It was concluded that once maximal expiratory flow was approached, even a minimal level of EFL, V̇E cannot be increased even when respiratory drive is increased (3). It was not that ventilatory capacity was limited, but rather the onset of dynamic compression of the airways was great enough to alter ventilatory control and more-or-less fix the ventilatory response to exercise. In the same study, maximal expiratory flow was increased by breathing a heliox mixture (HeO2 breathing), which increased exercise V̇E but only until the same level of EFL was obtained. We established the same findings in older athletes (7) and chronic airflow limitation patients (5). In a study on normal healthy subjects during exercise at 3 atmospheres (ATA) where maximal expiratory flow is remarkably decreased, we confirmed that the occurrence of EFL increased EELV during exercise and decreased the slope of the ventilatory response to exercise just as in lung patients and older adults (27). In fact to obtain the same level of V̇E measured during sea level exercise the work rate had to be increased significantly.
Figure 7.
Maximal and tidal flow-volume loops measured at rest (small solid loop) and during peak exercise breathing room air (thicker solid line), inspired 3% CO2 (A), and inspired Heliox mixture (HeO2) (B). Volume = total lung volume. [Adapted from (3). Copyright © 1997. The American Physiological Society. Used with permission.]
Thus our hyperbaric study, and studies by others (19;21), support the concept that approaching maximal expiratory flow, the onset of dynamic compression of the airways, and/or EFL or impending EFL can alter the control of EELV, breathing pattern, respiratory motor output, and the ventilatory response to exercise. It is possible that the onset of dynamic compression of the airways could be enough to reflexively terminate expiratory effort (3;13;21;28). The mechanism of this proprioceptive feedback on ventilatory control is unknown and has not been tested directly. However, it was thought that experimental airway anesthesia during exercise might address this issue (20) but no attempts have been made yet to study ventilatory responses with and without EFL, before and after anesthesia including simultaneous measurements of flow-volume loops, EELV, V̇E, and breathing pattern.
The possibility of EFL altering ventilatory control during exercise is compatible with our recent findings regarding the short-term modulation of the exercise ventilatory response (32–34). Short-term modulation of the exercise ventilatory response is defined as a reversible experimental augmentation of the exercise ventilatory response with added external dead space, which has been proposed as a general mechanism linking the exercise ventilatory response with resting ventilatory drive (14). Although the current understanding of ventilatory control of exercise hyperpnea remains unclear, the potential of adaptive control strategies such as short-term modulation in the regulation of breathing during exercise has become clearer (14;23).
In the case of EFL or the onset of dynamic compression of the airways, it is potentially possible that the neural system controlling breathing is modulated (i.e., neurochemically-induced alteration in cellular properties, or synaptic strength that adjusts neural network function) and once the stimulus for modulation is removed (i.e., EFL or dynamic compression of the airways), functional alterations in the neural system rapidly reverse, restoring ventilatory function to normal (14). This process appears similar to our findings on the effect of expiratory threshold loading, where the response of EELV depends on the presence or absence of EFL. In the experimental example of short-term modulation, the exercise ventilatory response is increased, but modulation of the exercise ventilatory response could be relevant to a decreased ventilatory response due to neural alterations that rapidly reverse after expiratory flow limitations are removed. Alterations in the ventilatory response to exercise or inspired CO2 due to resistive loading or imposed mechanical limitations have been reported by others (15;29). However, the mechanism of these alterations is unknown but could be related to mechanical ventilatory effort or the work of breathing or dynamic compression of the airways that so often accompanies these situations or experiments of imposed ventilatory limitations. Further prospective studies are needed to investigate mechanisms of human ventilatory modulation and whether aged and/or diseased populations may utilize this strategy as a means by which exercise ventilatory response does not outpace ventilatory capacity.
POTENTIAL CONSEQUENCES WITH THE ONSET OF EFL – DYSPNEA
It has been suggested that the onset of dynamic compression of the airways is also associated with dyspnea on exertion. When airflow limitation was imposed by increasing ambient pressure (i.e., hyperbaric conditions), normal healthy subjects experienced a severe choking dyspnea during exercise (35). In this experiment, the onset of dynamic compression was determined by the measurement of IVPF curves at sea level and at 4 and 10 ATA. With increasing ambient pressures, the slopes of the IVPF curves were significantly reduced, as was maximal expiratory flow. At the onset of dynamic compression of the airways and/or EFL, the subjects complained of a choking sensation. The authors suggested dynamic airway compression of the airways as a likely mechanism of the dyspnea, however the subsequent exercise intolerance could have been related to ventilatory limitation as well (35). How dynamic compression of the airways stimulated dyspnea was not addressed.
A mechanistically similar approach was taken to investigate this mechanism by increasing the magnitude of compression below the flow limiting segment with expiratory assist (i.e., generation of flow-proportional negative pressure at the mouth). When mouth pressure became more negative, severe chronic obstructive pulmonary disease (COPD) patients at rest complained of increased breathing sensation and a change in breathing pattern with increased airway compression (25). The authors suggested that the onset or increased dynamic compression alters ventilatory control possibly due to upper airway mechanoreceptor activity. This unpleasant respiratory sensation could be related to upper airway mechanoreceptors, which could alter ventilatory control and contribute to dyspnea (25).
The opposite approach was taken to decrease the magnitude of airway compression below the flow limiting segment with continuous positive airway pressure during submaximal exercise in control subjects and patients with COPD (26). This experimental approach is theoretically similar to pursed lipped breathing. However, application of positive pressure during expiration only resulted in inconsistent and insignificant changes in the sense of breathing in the patients. A decrease in sensation could have been dependent upon whether or not the patients were approaching maximal expiratory flow or had significant EFL before the positive pressure was applied. Unfortunately, respiratory mechanics were not measured in this study. This could be why some patients had a decrease in effort while others had an increase in effort similar to that observed in normal control subjects who supposedly were not approaching EFL. Thus, a limitation to the study was that esophageal pressures and flow-volume loops were not measured to determine the level of EFL before applying the expiratory load during exercise. However, this novel and innovative study supports our hypothesis and leaves us with the possibility that dynamic airway compression may play a role in exertional dyspnea along with other mechanical respiratory limitations.
POTENTIAL CONSEQUENCES WITH THE ONSET OF EFL – EXERCISE INTOLERANCE
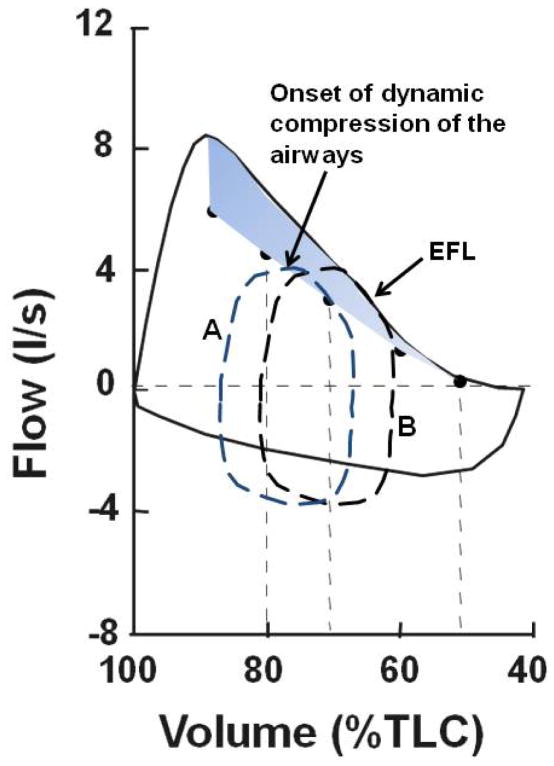
In extreme cases of EFL as in expiratory lung disease or increased ambient pressures, the association between EFL, ventilatory limitation, and exercise intolerance is strong. In mild cases of EFL, it is difficult to show whether ventilatory capacity is exhausted or that exercise is truly ventilatory limited (6;13). For those who measure respiratory mechanics routinely during exercise testing in normal adults and/or clinical patients, it is easy to document when and how mechanical ventilatory constraints and limitations occur during exercise. In addition, many of the patients may also experience unexplained dyspnea on exertion that is widely out of proportion to resting measurements of cardiopulmonary function. Most if not all of these patients will have the hallmark of impending EFL, hyperinflation, and/or possibly alterations in breath timing. However, ventilatory capacity will almost always be greater than ventilatory demand based on examination of flow-volume loop data (i.e., as explained above, as EELV increases so does ventilatory capacity). Furthermore, EFL is usually incomplete achieving approximately 0–50% of VT, and the exercise ventilatory response may be normal or altered. In these cases, it remains very difficult to determine if exercise tolerance is truly ventilatory limited, at least based on conventional wisdom. However, if the concepts regarding the importance of approaching maximal expiratory flow are kept in mind as outlined in this review, in contrast to the concept of absolute and complete EFL, more realistic interpretations of ventilatory limitation can be offered. In Figure 8, we show schematic tidal expiratory flow volume loops superimposed on the maximal flow-volume loop from Figure 2. In Figure 8, the onset of airway compression is shown by the shaded area. Once tidal expiratory flow reaches this area (e.g., tidal Loop A), changes in breathing mechanics, ventilatory control, and possibly exertional dyspnea and exercise intolerance may be encountered. Note how ventilatory capacity is not a concern at all in Loop A in contrast to Loop B, where there is limitation but ventilatory capacity is still larger theoretically. However, even in Loop A, the effects of flow limitation are already at play regarding the ventilatory response to exercise and breathing mechanics.
Figure 8.
Maximal and exercise tidal flow-volume loops. Shaded area denotes flow rates at which the onset of dynamic compression of the airways occur. In the left tidal loop, A, expiratory flow impinges on dynamic compression of the airways, and in the right loop, B, expiratory flow impinges on maximal expiratory flow (i.e., defined as expiratory flow limitation, EFL) demonstrating the conventional view of ventilatory limitation.
SUMMARY
Given all the individual and patient examples of mechanical ventilatory constraints and limitations to exercise, it is clear that the effects of EFL are not all-or-none and are extremely important physiologically and clinically. Approaching EFL can affect not only ventilatory capacity but also breathing mechanics, ventilatory control, and possibly exertional dyspnea and exercise intolerance. However, just comparing exercise tidal flow-volume loops with the maximal flow-volume loop measured at the mouth to determine the magnitude of EFL does not tell the whole story. Approaching EFL may evoke many changes to the exercise ventilatory response and all of these changes could play an important role in provoking ventilatory limitation and potentially exercise ventilatory limitation and exercise intolerance. Recognizing the impact of approaching maximal expiratory flow is important to properly understanding ventilatory limitations during exercise and their potential for imposing limitations to exercise.
Acknowledgments
Funding: NIH HL096782, King Charitable Foundation Trust, Cain Foundation, Texas Health Presbyterian Hospital Dallas
The author recognizes the contributions of many other researchers that could not be cited because of the reference limitations. This publication was supported in part by HL-096782, King Charitable Foundation Trust, Cain Foundation, and Texas Health Presbyterian Dallas.
Footnotes
The author has no conflicts of interest to report.
Reference List
- 1.American Thoracic Society. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med. 2003;167(2):211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 2.Babb TG. Ventilation and respiratory mechanics during exercise in younger subjects breathing CO2 or HeO2. Respir Physiol. 1997;109:15–28. doi: 10.1016/s0034-5687(97)84026-1. [DOI] [PubMed] [Google Scholar]
- 3.Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol. 1997;82:746–54. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- 4.Babb TG. Mechanical ventilatory constraints in aging, lung disease, and obesity: perspectives and brief review. Med Sci Sports Exerc. 1999;31(1):S12–S22. doi: 10.1097/00005768-199901001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Babb TG. Breathing He-O2 increases ventilation but does not decrease the work of breathing during exercise. Am J Respir Crit Care Med. 2001;163:1128–34. doi: 10.1164/ajrccm.163.5.9908025. [DOI] [PubMed] [Google Scholar]
- 6.Babb TG. Estimation of mechanical ventilatory limitation. Journal of Qinghai Medical College. 2005;26:145–52. [Google Scholar]
- 7.Babb TG, DeLorey DS, Wyrick BL. Ventilatory response to exercise in aged runners breathing He-O2 or inspired CO2. J Appl Physiol. 2003;94:685–93. doi: 10.1152/japplphysiol.00214.2002. [DOI] [PubMed] [Google Scholar]
- 8.Babb TG, DeLorey DS, Wyrick BL, Gardner PP. Mild obesity does not limit change in end-expiratory lung volume during cycling in young women. J Appl Physiol. 2002;92:2483–90. doi: 10.1152/japplphysiol.00235.2001. [DOI] [PubMed] [Google Scholar]
- 9.Babb TG, Rodarte JR. Lung volumes during low-intensity steady-state cycling. J Appl Physiol. 1991;70:934–7. doi: 10.1152/jappl.1991.70.2.934. [DOI] [PubMed] [Google Scholar]
- 10.Babb TG, Rodarte JR. Exercise capacity and breathing mechanics in patients with airflow limitation. Med Sci Sports Exerc. 1992;24:967–74. [PubMed] [Google Scholar]
- 11.Babb TG, Rodarte JR. Estimation of ventilatory capacity during submaximal exercise. J Appl Physiol. 1993;74:2016–22. doi: 10.1152/jappl.1993.74.4.2016. [DOI] [PubMed] [Google Scholar]
- 12.Babb TG, Rodarte JR. Mechanism of reduced maximal expiratory flow with aging. J Appl Physiol. 2000;89:505–11. doi: 10.1152/jappl.2000.89.2.505. [DOI] [PubMed] [Google Scholar]
- 13.Babb TG, Viggiano R, Hurley B, Staats BA, Rodarte JR. Effect of mild to moderate airflow limitation on exercise capacity. J Appl Physiol. 1991;70:223–30. doi: 10.1152/jappl.1991.70.1.223. [DOI] [PubMed] [Google Scholar]
- 14.Babb TG, Wood HE, Mitchell GS. Short- And Long-Term Modulation of the Exercise Ventilatory Response. Med Sci Sports Exerc. 2010;42(9):1681–7. doi: 10.1249/MSS.0b013e3181d7b212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark JM, Sinclair RD, Lenox JB. Chemical and nonchemical components of ventilation during hypercapnic exercise in man. J Appl Physiol. 1980;48:1065–76. doi: 10.1152/jappl.1980.48.6.1065. [DOI] [PubMed] [Google Scholar]
- 16.DeLorey DS, Babb TG. Progressive mechanical ventilatory constraints with aging. Am J Respir Crit Care Med. 1999;160:169–77. doi: 10.1164/ajrccm.160.1.9807045. [DOI] [PubMed] [Google Scholar]
- 17.DeLorey DS, Wyrick BL, Babb TG. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int J Obes. 2005;29:1039–47. doi: 10.1038/sj.ijo.0803003. [DOI] [PubMed] [Google Scholar]
- 18.Fee LL, Smith RM, English MB. Enhance ventilatory and exercise performance in athletes with slight expiratory resistive loading. J Appl Physiol. 1997;83:503–10. doi: 10.1152/jappl.1997.83.2.503. [DOI] [PubMed] [Google Scholar]
- 19.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol. 1992;73:874–86. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan B, Stockwell M, Clemens RE, Gallagher CG. Airway anesthesia and respiratory adaptations to dead space loading and exercise. Am J Respir Crit Care Med. 1997;155:459–65. doi: 10.1164/ajrccm.155.2.9032179. [DOI] [PubMed] [Google Scholar]
- 21.McClaran SR, Wetter TJ, Pegelow DF, Dempsey JA. Role of expiratory flow limitation in determining lung volumes and ventilation during exercise. J Appl Physiol. 1999;86:1357–66. doi: 10.1152/jappl.1999.86.4.1357. [DOI] [PubMed] [Google Scholar]
- 22.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell GS, Babb TG. Layers of exercise hyperpnea: modulation and plasticity. Respir Physiol Neurobiol. 2006;151:251–66. doi: 10.1016/j.resp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol. 1970;28(6):784–9. doi: 10.1152/jappl.1970.28.6.784. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell DE, Sanii R, Anthonisen NR, Younes M. Effect of dynamic airway compression on breathing pattern and respiratory sensation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987;135:912–8. doi: 10.1164/arrd.1987.135.4.912. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell DE, Sanii R, Giesbrecht GG, Younes M. Effect of continuous positive airway pressure on respiratory sensation in patients with chronic obstructive pulmonary disease during submaximal exercise. Am Rev Respir Dis. 1988;138:1185–91. doi: 10.1164/ajrccm/138.5.1185. [DOI] [PubMed] [Google Scholar]
- 27.O’Kroy JA, Lawler JM, Stone J, Babb TG. Airflow limitation and control of end expiratory lung volume during exercise. Respir Physiol. 2000;119:57–68. doi: 10.1016/s0034-5687(99)00094-8. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrino R, Brusasco V, Rodarte JR, Babb TG. Expiratory flow limitation and regulation of end-expiratory lung volume during exercise. J Appl Physiol. 1993;74:2552–8. doi: 10.1152/jappl.1993.74.5.2552. [DOI] [PubMed] [Google Scholar]
- 29.Poon CS. Effects of inspiratory resistive load on respiratory control in hypercapnia and exercise. J Appl Physiol. 1989;66:2391–9. doi: 10.1152/jappl.1989.66.5.2391. [DOI] [PubMed] [Google Scholar]
- 30.Spahija JA, Grassino A. Effects of pursed-lips breathing and expiratory resistive loading in healthy subjects. J Appl Physiol. 1996;80:1772–84. doi: 10.1152/jappl.1996.80.5.1772. [DOI] [PubMed] [Google Scholar]
- 31.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol. 2004;96:1920–7. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- 32.Wood HE, Mitchell GS, Babb TG. Short-term modulation of the exercise ventilatory response in young men. J Appl Physiol. 2008;104(1):244–52. doi: 10.1152/japplphysiol.00820.2007. [DOI] [PubMed] [Google Scholar]
- 33.Wood HE, Mitchell GS, Babb TG. Short-term modulation of the exercise ventilatory response in older men. Respir Physiol Neurobiol. 2010;173:37–46. doi: 10.1016/j.resp.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Wood HE, Mitchell GS, Babb TG. Short-term modulation of the exercise ventilatory response in younger and older women. Respir Physiol Neurobiol. 2011;179(2–3):235–47. doi: 10.1016/j.resp.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Wood LDH, Bryan AC. Exercise ventilatory mechanics at increased ambient pressure. J Appl Physiol. 1978;44:231–7. doi: 10.1152/jappl.1978.44.2.231. [DOI] [PubMed] [Google Scholar]