Abstract
Objectives
To identify the most effective sedation regimen for bone marrow aspiration and lumbar puncture procedures with a prospective trial of 3 combinations of sedation/analgesia.
Study design
In this double-blind crossover study, we randomly assigned 162 children with acute lymphoblastic leukemia or lymphoblastic lymphoma to receive fentanyl 1 mcg/kg, fentanyl 0.5 mcg/kg, or placebo, in addition to propofol and topical anesthetic for 355 procedures.
Results
We found no significant differences among the three regimens in the frequency of pain (pain score >0) or severe pain (PS ≥5) during recovery, or a >20% increase in hemodynamic/respiratory variables during anesthesia. Treatment with fentanyl 1 mcg/kg was associated with a lower frequency of movement during procedure as compared with treatment with fentanyl 0.5mcg/kg (P = 0.0476) or treatment with placebo (P = 0.0545). The placebo group required longer time to recover (median, 18 minutes) as compared with the fentanyl 0.5 mcg/kg group (median, 9 minutes) (median difference 2.0, P = 0.007) and the fentanyl 1 mcg/kg (median 8 minutes), (median difference 2.0, P = 0.15). The placebo group also required larger total dose of propofol (median 5 mg/kg) as compared with that of the fentanyl 1 mcg/kg group (median, 3.5 mg/kg) and the fentanyl 0.5 mcg/kg group (median 3.5 mg/kg) (median differences 1.5, P <0.00005, in both comparisons).
Conclusion
The addition of fentanyl 1 mcg/kg to propofol for brief painful procedures reduces movement, propofol dose, and recovery time.
Keywords: pain, sedation, procedures, bone marrow aspiration, pediatric oncology
Bone marrow aspiration and lumbar puncture are brief procedures, but they are associated with pain and anxiety. The repeated need of these procedures during treatment for childhood cancer constitutes a significant burden and the experience is often described as traumatic for patients and their parents [1]. Treatment for pediatric acute lymphoblastic leukemia at our institution requires 15 to 30 lumbar punctures for intrathecal chemotherapy with or without bone marrow aspiration, depending on leukemia risk category; treatment of other diseases also involves frequent painful procedures.
Various pharmacological regimens have been used to control procedure-related pain in pediatric oncology [2–5]. A recent review of management of painful procedures in children with cancer emphasizes the distinction between sedation and anesthetic regimens and their respective risks and benefits[6].
Propofol-based total intravenous anesthesia (TIVA) has been studied retrospectively [7, 8] and prospectively [2, 9–11], and offers the advantages of rapid onset, titratable level of sedation, rapid recovery, and a good safety profile when administered by trained personnel such as anesthesiologists and pediatric intensivists [8].
We evaluated three propofol-based anesthetic regimens for pediatric oncology procedures using fentanyl 1 mcg/kg, 0.5 mcg/kg, or placebo, and we compared the frequency of postoperative pain and of intraoperative movement and hemodynamic/respiratory instability, the total propofol dose required, and the time to recovery among the three groups.
METHODS
St. Jude Children’s Research Hospital is a tertiary-care institution for children with cancer and other life-threatening diseases. The facility has multiple outpatient clinics and 60 inpatient beds. Patients range from newborns to young adults at the time of diagnosis.
This prospective study included patients aged 2 to 17 years of age who were undergoing treatment for acute lymphoblastic leukemia or lymphoblastic lymphoma and who were expected to undergo at least three combined unilateral bone marrow aspiration and lumbar puncture procedures for intrathecal chemotherapy. Eligible patients were in complete remission, had a platelet count >50,000/mm3, and had not received daily opioids for pain management during the two weeks before the procedures. Patients with neurological impairment or Down syndrome were excluded, as were patients for whom general anesthesia or any of the anesthetic agents used in the three regimens were contraindicated. This study was approved by the St. Jude Institutional Review Board, and written informed consent was obtained from parents or guardians, and assent was obtained from the patients, as appropriate. The trial was registered with ClinicalTrials.gov (NCT00187135).
In a crossover design, each patient was randomly assigned to a schedule that included all three treatment regimens in different sequences, and each patient was expected to receive each regimen once. Randomization was stratified by age group (2–4, 5–12, ≥13 years), to ensure that the treatment arms were balanced with respect to age at randomization. A program for conducting randomization was provided by the St. Jude Department of Biostatistics. Study medications were prepared in the pharmacy, labeled “study drug” and delivered to the procedure area. Therefore, clinicians who administered the anesthetics or performed the procedures, the data collection and data analysis teams, and the patients and families were blinded to the assigned regimens. The regimens differed only in the use and dose of fentanyl during induction of anesthesia (1 mcg/kg fentanyl, 0.5 mcg/kg fentanyl, or placebo [normal saline]; treatment arms 1, 2, and 3, respectively). All regimens included topical anesthetic (eutectic mixture of 2.5% lidocaine/prilocaine or 4% liposomal lidocaine) or infiltration of local anesthetic (lidocaine 1%) at the puncture sites and titration of intravenous propofol to immobility and loss of consciousness. All patients reporting pain on waking from anesthesia received 0.5 mcg/kg IV fentanyl as needed (maximum three doses).
Description of monitoring and anesthetic technique
Standard monitoring during the anesthetic included intermittent blood pressure measuring, and continuous pulse oxymetry, electrocardiogram, respiratory rate and end tidal carbon dioxide monitoring. Oxygen was administered by face mask for at least 1 minute before the administration of the study drug and continued throughout the anesthetic. Propofol was administered in increments of 1 mg/kg until loss of consciousness, followed by doses of 0.5 mg/kg as needed for any movement during the procedure. Ondansetron was given with the induction of anesthesia as part of the routine clinical care before BMA/LPIT, in order to minimize nausea and vomiting.
Outcome Measures
The primary study outcomes were the frequency of pain (pain score [PS] >0) and the frequency of severe pain (PS ≥5) during recovery from anesthesia. Pain was measured on an 11-point scale, using the FLACC, FACES, or numerical rating system as appropriate for age and cognitive ability [12–14]. Pain was assessed throughout the recovery period (defined as the time from the end of the procedure until an Aldrete score of 8 was reached), and the highest pain score during each recovery period was used in comparisons (before administration of fentanyl as needed for pain).
The secondary study outcomes were the frequency of movement during the procedure and the frequency of respiratory or hemodynamic instability (>20% increase in respiratory rate, heart rate, or blood pressure, as indirect measures for inadequate analgesia) during anesthesia. We also compared the time to recovery, the total dose of propofol, and the frequency with which patients required fentanyl for pain during recovery. All the study data were collected by a research associate. All primary and secondary outcome measures were compared across the three groups.
Statistical Analyses
This double-blind, randomized crossover trial was designed with 80% power to detect pairwise differences of 15% in the frequency of post-procedural pain (PS >0) among the three treatment arms with an overall type-I error probability of 0.05. The calculated sample sizes needed were 127, 70, and 92 patients for comparison pairs A, B and C, respectively.
McNemar test was used for pairwise comparisons of the frequency of pain (PS >0), severe pain (PS ≥5), movement, change in vital signs, and fentanyl administration for pain during recovery. The Wilcoxon signed-rank test was used for pairwise comparisons of the median time to recovery and the median total dose of propofol required. In the analysis of the primary outcomes P-values <0.0167 were considered statistically significant based on the Bonferroni adjustment for multiple testing to maintain an overall type I error rate of 0.05. P-values <0.05 were considered statistically significant for secondary outcomes. All analyses were conducted using the StatXact Version 8 (Cytel Inc.) or SAS Version 9.2 (Cary, NC) software.
RESULTS
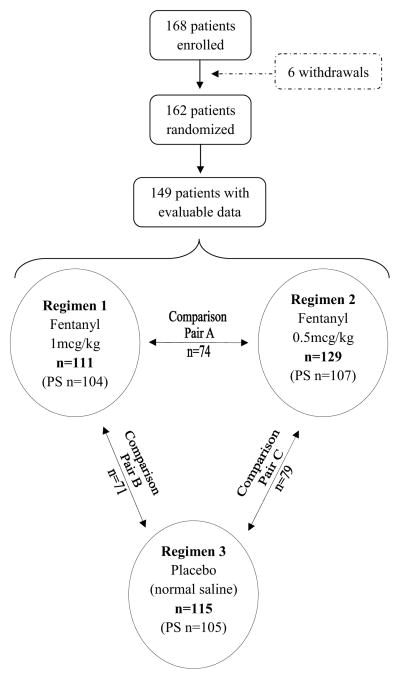
Between March 2002 and August 2007, 168 patients were enrolled. Six patients withdrew for various reasons before randomization; 162 were randomized and underwent at least one anesthetic regimen (Figure). Patients’ demographic characteristics, diagnoses, and anesthetic regimens are shown in Table I. Data from 149 patients (355 procedures) were evaluable for movement and hemodynamic instability. Data from 110 patients (316 procedures) were evaluable for pairwise comparisons of pain during recovery, propofol dose, time to recovery, and use of fentanyl during recovery; 39 patients underwent only one regimen and were excluded from the pairwise comparisons. Regimens 1, 2, and 3 were completed by 111, 129, and 115 patients, respectively. Each patient who underwent at least two regimens contributed to the analysis of three comparison pairs; 110 and 57 patients completed two and three regimens, respectively, and comparison pairs A, B, and C included 74, 71, and 79 patients, respectively.
Figure.
Flow Chart of Patient Enrollment and Randomization to Three Treatment Arms.
PS: Pain score (n = number of procedures with evaluable pain scores during recovery).
Note: Not all 162 randomized patients completed all 3 regimens
TABLE 1.
Demographics, Diagnoses, and Anesthetic Regimens of 162 Pediatric Oncology Patients Randomly Assigned to Three Treatment Arms
| Arm 1 | Arm 2 | Arm 3 | ||
|---|---|---|---|---|
| Total | Fentanyl 1.0 mcg/kg | Fentanyl 0.5 mcg/kg | Placebo (normal saline) | |
| Total | 162 | 104 | 107 | 105 |
| Age (years) | ||||
| 2–4 | 72 | 48 | 50 | 49 |
| 5–12 | 69 | 45 | 45 | 49 |
| 13–17 | 21 | 11 | 12 | 7 |
| Sex | ||||
| Male | 92 | 61 | 61 | 51 |
| Female | 70 | 43 | 46 | 54 |
| Oncology diagnosis | ||||
| ALL | 161 | 104 | 107 | 104 |
| LL | 1 | 0 | 0 | 1 |
| Race | ||||
| White | 121 | 78 | 81 | 79 |
| Black | 32 | 19 | 22 | 22 |
| Hispanic | 0 | 0 | 0 | 0 |
| Asian/other | 9 | 7 | 4 | 4 |
ALL: acute lymphoblastic leukemia; LL: lymphoblastic leukemia
Study Outcomes
We found no significant difference in the frequency of pain after treatment with fentanyl 1 mcg/kg vs. placebo (comparison pair B, Table II); this comparison was adequately powered to detect a 15% difference in the proportion of patients with pain. Comparison pairs A and C also showed no significant difference in the frequency of pain, but the comparisons were not adequately powered to detect a 15% difference. Due to the design parameters of our study, these comparisons required a larger sample size to yield 80% power which was not obtained because of slow accrual that necessitated early termination of the study.
TABLE 2.
Pain Scores during Recovery from Anesthesia
| Comparison Pairs (N) | Agents compared | Frequency of pain >0 | Frequency of pain 5–10 | Frequency of Movement |
|---|---|---|---|---|
| A (74) | Fentanyl 1 mcg/kg | 5 (6.8%) | 2 (2.7%) | 29 (39.2%) |
| Fentanyl 0.5 mcg/kg | 7 (9.5%) | 4 (5.4%) | 37 (50%) | |
| B (71) | Fentanyl1 mcg/kg | 7 (9.9%) | 3 (4.2%) | 30 (42.3%) |
| Placebo (NS) | 10 (14.1%) | 6 (8.5%) | 37 (52.1%) | |
| C (79) | Fentanyl0.5 mcg/kg | 14 (17.7%) | 9 (11.4%) | 41 (51.9%) |
| Placebo (NS) | 11 (13.9%) | 6 (7.6%) | 42 (53.2%) |
NS = normal saline
P values for pain frequency ranged from 0.5314 to 0.5350 (McNemar test)
P values for movement were 0.0476 in pair A, 0.0545 in pair B, and 0.87 in pair C. (McNemar’s test)
Of all 316 procedures analyzed, 11.4%, 6.7%, and 20.6% had >20% increases in blood pressure, heart rate, and respiratory rate, respectively. There were no significant differences in the frequency of increase >20% in blood pressure, heart rate, and respiratory rate during anesthesia between the three treatment arms (all P-values =0.25). The frequency of movement differed significantly between the fentanyl 0.5 mcg/kg group and fentanyl 1 mcg group (P = 0.0476) and marginally between placebo and fentanyl 1 mcg/kg groups (P = 0.0545). There was no significant difference between placebo and fentanyl 0.5mcg/kg groups (P = 0.87) (Table II).
Table III compares the propofol dose and time to recovery in the three treatment arms and the three comparison pairs. The median time to recover was longer in the placebo group (18 minutes) than that of the fentanyl 0.5 mcg/kg group (9 minutes) (median difference 2.0, P = 0.007). The placebo group also tended to require longer time to recover than the fentanyl 1 mcg/kg group (median difference 2.0, P = 0.15). The placebo group required higher total dose of propofol than the fentanyl 1 mcg/kg and the fentanyl 0.5 mcg/kg groups (median differences 1.5 and 1.5, respectively; P <0.00005 for both comparisons). There was no significant difference in receiving additional fentanyl among the treatment groups when analyzed in pairwise comparisons. There were no deaths or serious adverse events attributed to the study, and the rate of other study-related adverse events did not exceed the threshold of 5% in any treatment group. Specifically, the most common adverse event was nausea and vomiting, found in 2.9% and 2.8% in the fentanyl 1 mcg/kg and 0.5 mcg/kg groups, respectively (3 cases in each group).
TABLE 3.
Propofol Dose and Time to Recovery in the Three Treatment Groups and Three Comparison Pairs
| Treatment Arm | n | Total propofol dose (mg/kg) Mean (SD) Median (IQR) |
Minutes to recovery Mean (SD) Median (IQR) | ||
|---|---|---|---|---|---|
| Fentanyl 1 mcg/kg | 104 | 3.6 (1.3) 3.5 (1.3) |
13.8 (13.8) 8 (22) |
||
| Fentanyl 0.5 mcg/kg | 107 | 3.9 (1.6) 3.5 (1.5) |
13.6 (12.8) 9 (23) |
||
| Placebo (NS) | 105 | 5.2 (2.1) 5.0 (2.5) |
17.6 (12.2) 18.0 (19) |
||
| Comparison Pairs | n |
Difference in propofol dose (mg/kg) Mean (SD) Median (IQR) |
P value* |
Difference in minutes to recovery Difference (min) Mean (SD) Median (IQR) |
P value* |
| Pair A 0.5 mcg/kg – 1 mcg/kg fentanyl |
74 | 0.4 (2.0) 0.0 (2.0) |
0.10 | 0.4 (14.7) 1.0 (16) |
0.68 |
| Pair B Placebo – 1 mcg/kg fentanyl |
71 | 1.6 (2.2) 1.5 (3.5) |
<0.00005 | 3.0 (17.8) 2.0 (23) |
0.15 |
| Pair C Placebo - 0.5 mcg/kg fentanyl |
79 | 1.4 (1.9) 1.5 (3.0) |
<0.00005 | 4.2 (14.2) 2.0 (13) |
0.007 |
Wilcoxon signed-rank test
IQR = interquartile range
DISCUSSION
Our data show that addition of fentanyl 1 mcg/kg to propofol for brief painful procedures, compared with placebo, tended to reduce the frequency of movement during procedures, the dose of propofol needed, and the time to recovery, while not affecting post-procedure pain control or intra-procedure hemodynamic/respiratory variability significantly.
Several TIVA regimens for pediatric oncology procedures have been reported as combinations of propofol and ketamine or opioids (fentanyl [2, 5], remifentanil [15], alfentanil [4], and morphine [16]). The Pediatric Sedation Research Consortium analyzed 49,836 propofol sedation/anesthesia encounters outside of the operating room at 37 hospitals and found that propofol alone was most commonly used (80% of cases) and that opioids were the most common addition to TIVA with propofol (~10% of cases) [17]. The decision process between sedation and general anesthetic regimens has been explored [6], and it should consider the fact that a propofol based general anesthetic may have associated respiratory depression, apnea and loss of airway reflexes [18].
Two studies of propofol and fentanyl TIVA for procedures in children with cancer have been reported [2, 10]. One retrospective study compared four propofol-based regimens (propofol only, propofol plus midazolam, propofol plus fentanyl, and propofol plus fentanyl plus midazolam) in 52 children ages 2 to 15 years who underwent 335 sedations for intrathecal chemotherapy and/or bone marrow aspiration procedures[10]. The authors found no difference in the efficacy of sedation, but patients receiving propofol plus fentanyl plus midazolam required the least propofol (median dose, 210 mcg/kg/min vs. 420 mcg/kg/min for propofol alone, P = 0.0001) and the least recovery time (5 minutes, vs. 10 minutes in the propofol alone group, P = 0.03). In a randomized, placebo-controlled, double-blind, crossover study, 22 patients ages 2.2 to 17.2 years were sedated for 44 LPs with propofol plus placebo or propofol plus fentanyl 1 mcg/kg. The propofol/fentanyl regimen was associated with a lower median propofol dose (3 mg/kg vs. 5.05 mg/kg, P < 0.001) and a lower frequency of complications (18.2% vs. 50%, P = 0.02), the most common of which was hypotension [2]. The mean recovery time was 37 minutes for propofol/placebo vs. 26 minutes for propofol/fentanyl (P = 0.047), and 72.7% of families preferred propofol/fentanyl for future sedation for LP [11]. These findings are consistent with the propofol-sparing effect and shorter recovery time observed in our study with the addition of fentanyl.
This pediatric oncology study concurrently evaluates anesthetic regimens for both subjective analgesia measures (pain scores) and objective anesthesia measures (immobility, respiratory rate, and hemodynamic stability). We did find that additional fentanyl reduces the frequency of movement during procedures, the dose of propofol needed, and the time to recovery. Although the addition of fentanyl to propofol would reasonably be expected to enhance analgesia as well as sedation and hypnosis, we were unable to demonstrate a significant difference among our three regimens in the analgesia or respiratory/hemodynamic variables.
Hertzog et al [9] prospectively studied supplementation of propofol TIVA with topical eutectic mixture of lidocaine analgesia and subcutaneous lidocaine in 28 children undergoing 50 oncology procedures. They observed a mean propofol induction dose of 2 mg/kg (SD, 0.8 mg/kg) and a mean total dose of 6.6 mg/kg (SD, 2.3 mg/kg) for ambulatory patients and 7.9 mg/kg (SD 2.4 mg/kg) for hospitalized patients. The mean total propofol dose in our study was lower (5.2 mg/kg; SD, 2.1) when only a topical local anesthetic was used and lower still with the addition of fentanyl 0.5 mcg/kg (3.9 mg/kg; SD, 1.6 mg/kg) or fentanyl 1 mcg/kg (3.6 mg/kg; SD, 1.3 mg/kg). Keidan et al [15] reported a prospective randomized study of propofol and remifentanil vs. propofol alone for bone marrow aspiration in 80 children, using a propofol induction dose of 3 mg/kg followed by a 300 mcg/kg/min infusion and additional boluses as needed. The mean additional propofol used in the propofol-alone group was 3 mg/kg (SD, 1.2), resulting in a mean total dose >6 mg/kg. The mean recovery time for the propofol-alone group was significantly greater than that in the propofol-remifentanil group. The time to eye opening was 38 min (SD, 19 min) vs. 23 min (SD, 12 min), respectively, and the time to discharge readiness was 52 min (SD, 24 min) vs. 33 min (SD, 15 min), respectively. Hertzog et al [9] prospectively evaluated propofol anesthesia for 50 oncology procedures in 28 children and described a mean recovery time of 23.4 minutes. In our study, the mean time to recovery to an Aldrete score of 8 was 13.8 (SD, 13.8), 13.6 (SD, 12.8), and 17.6 (SD, 12.2) minutes in the fentanyl 1 mcg/kg, fentanyl 0.5 mcg/kg, and placebo groups, respectively.
Despite the strengths of our study, its complex design and slow accrual rate prevented our enrollment of sufficient patients for the full planned analysis; the planned sample size was achieved only for comparison arm B (fentanyl 1 mcg/kg vs. placebo). This comparison, which showed no significant difference in the frequency or severity of pain during recovery, was expected to show the largest difference if the addition of fentanyl was more effective than placebo. We also acknowledge that our patients underwent as many as three different procedures over time and that the intensity of their pain experience may have been compounded as cancer treatment progressed.
Our results showed that the addition of fentanyl 1mcg/kg to propofol for brief, painful procedures tended to reduce movement during procedures, the required dose of propofol, and the time to recovery, while not influencing respiratory/hemodynamic changes, pain scores, or the need for post-procedure pain control.
Acknowledgments
Supported by the Cancer Center Support Grant (5P30CA021765-32) and the American Lebanese Syrian Associated Charities, neither of which had any involvement in the planning or conduct of this study.
We thank Sharon Naron (funded by American Lebanese Syrian Associated Charities) for editing the manuscript.
Abbreviations
- PS
pain score
- TIVA
total intravenous anesthesia
- SD
standard deviation
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powers SW, Blount RL, Bachanas PJ, Cotter MW, Swan SC. Helping preschool leukemia patients and their parents cope during injections. J Pediatr Psychol. 1993;18:681–95. doi: 10.1093/jpepsy/18.6.681. [DOI] [PubMed] [Google Scholar]
- 2.Hollman GA, Schultz MM, Eickhoff JC, Christenson DK. Propofol-fentanyl versus propofol alone for lumbar puncture sedation in children with acute hematologic malignancies: propofol dosing and adverse events. Pediatr Crit Care Med. 2008;9:616–22. doi: 10.1097/PCC.0b013e31818e3ad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. 2006;367:766–80. doi: 10.1016/S0140-6736(06)68230-5. [DOI] [PubMed] [Google Scholar]
- 4.Von Heijne M, Bredlov B, Soderhall S, Olsson GL. Propofol or propofol--alfentanil anesthesia for painful procedures in the pediatric oncology ward. Paediatr Anaesth. 2004;14:670–5. doi: 10.1111/j.1460-9592.2004.01279.x. [DOI] [PubMed] [Google Scholar]
- 5.Holdsworth MT, Raisch DW, Winter SS, Frost JD, Moro MA, Doran NH, et al. Pain and distress from bone marrow aspirations and lumbar punctures. Ann Pharmacother. 2003;37:17–22. doi: 10.1345/aph.1C088. [DOI] [PubMed] [Google Scholar]
- 6.Hockenberry MJ, McCarthy K, Taylor O, Scarberry M, Franklin Q, Louis CU, et al. Managing painful procedures in children with cancer. Journal of pediatric hematology/oncology: official journal of the American Society of Pediatric Hematology/Oncology. 2011;33:119–27. doi: 10.1097/MPH.0b013e3181f46a65. [DOI] [PubMed] [Google Scholar]
- 7.McDowall RH, Scher CS, Barst SM. Total intravenous anesthesia for children undergoing brief diagnostic or therapeutic procedures. J Clin Anesth. 1995;7:273–80. doi: 10.1016/0952-8180(95)00017-c. [DOI] [PubMed] [Google Scholar]
- 8.Hertzog JH, Campbell JK, Dalton HJ, Hauser GJ. Propofol anesthesia for invasive procedures in ambulatory and hospitalized children: experience in the pediatric intensive care unit. Pediatrics. 1999;103:E30. doi: 10.1542/peds.103.3.e30. [DOI] [PubMed] [Google Scholar]
- 9.Hertzog JH, Dalton HJ, Anderson BD, Shad AT, Gootenberg JE, Hauser GJ. Prospective evaluation of propofol anesthesia in the pediatric intensive care unit for elective oncology procedures in ambulatory and hospitalized children. Pediatrics. 2000;106:742–7. doi: 10.1542/peds.106.4.742. [DOI] [PubMed] [Google Scholar]
- 10.Jayabose S, Levendoglu-Tugal O, Giamelli J, Grodin W, Cohn M, Sandoval C, et al. Intravenous anesthesia with propofol for painful procedures in children with cancer. J Pediatr Hematol Oncol. 2001;23:290–3. doi: 10.1097/00043426-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Cechvala MM, Christenson D, Eickhoff JC, Hollman GA. Sedative preference of families for lumbar punctures in children with acute leukemia: propofol alone or propofol and fentanyl. J Pediatr Hematol Oncol. 2008;30:142–7. doi: 10.1097/MPH.0b013e31815d8953. [DOI] [PubMed] [Google Scholar]
- 12.Hockenberry M, Wong’s Wilson D. Essentials of Pediatric Nursing. 8. St. Louis: Mosby; 2009. [Google Scholar]
- 13.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 14.von Baeyer CL. Children’s self-reports of pain intensity: Scale selection, limitations and interpretation. Pain Res Manag. 2006;11:157–62. doi: 10.1155/2006/197616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keidan I, Berkenstadt H, Sidi A, Perel A. Propofol/remifentanil versus propofol alone for bone marrow aspiration in paediatric haemato-oncological patients. J Pediatr Psychol. 2001;11:297–301. doi: 10.1046/j.1460-9592.2001.00662.x. [DOI] [PubMed] [Google Scholar]
- 16.Gottschling S, Meyer S, Krenn T, Reinhard H, Lothschuetz D, Nunold H, et al. Propofol versus midazolam/ketamine for procedural sedation in pediatric oncology. J Pediatr Hematol Oncol. 2005;27:471–6. doi: 10.1097/01.mph.0000179238.37647.91. [DOI] [PubMed] [Google Scholar]
- 17.Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108:795–804. doi: 10.1213/ane.0b013e31818fc334. [DOI] [PubMed] [Google Scholar]
- 18.Continuum of depth of sedation: Definition of general anesthesia and levels of sedation/analgesia. [Accessed April 4, 2012];Approved by the American Society of Anesthesiologists House of Delegates on October 13, 1999, and amended on October 21, 2009. http://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx.