Abstract
Dengue is a globally expanding disease caused by infection with dengue virus (DENV) that ranges from febrile illness to acute disease with serious complications. Secondary infection predisposes individuals to more severe disease, and B lymphocytes may play a role in this phenomenon through production of Ab that enhance infection. To better define the acute B cell response during dengue, we analyzed peripheral B cells from an adult Brazilian hospital cohort with primary and secondary DENV infections of varying clinical severity. Circulating B cells in dengue patients were proliferating, activated and apoptotic relative to individuals with other febrile illnesses. Severe secondary DENV infection was associated with extraordinary peak plasmablast frequencies between 4 and 7 days of illness, averaging 46% and reaching 87% of B cells, significantly greater than those seen in mild illness or primary infections. On average more than 70% of IgG-secreting cells in individuals with severe secondary DENV infection were DENV-specific. Plasmablasts produced Ab that cross-reacted with heterotypic DENV serotypes but with a 3-fold greater reactivity to DENV-3, the infecting serotype. Plasmablast frequency did not correlate with acute serum neutralizing Ab titers to any DENV serotype regardless of severity of disease. These findings indicate that massive expansion of DENV-specific and serotype cross-reactive plasmablasts occurs in acute secondary DENV infection of adults in Brazil which is associated with increasing disease severity.
Introduction
Dengue is a mosquito-borne disease caused by infection with one of four different serotypes of dengue virus (DENV 1–4). Over the last five decades the incidence of dengue has increased dramatically around the world, with an estimated 50 million infections occurring annually (1). While most DENV infected individuals either do not show symptoms or recover following an acute febrile illness, a small proportion of infected individuals develop severe disease characterized by plasma leakage, thrombocytopenia and organ dysfunction with or without hemorrhage (2). The factors that contribute to dengue pathogenesis and disease severity are not fully understood. Neutralizing Ab raised in response to a primary infection provide long-term protection against reinfection with the same serotype but only transient protection against a different serotype (3). Classical epidemiological studies indicate that individuals with a secondary infection of a heterotypic DENV serotype are at increased risk of developing severe dengue, as are infants infected at the time of waning levels of maternally-acquired Ab (4–8). The leading hypothesis proposed to explain this phenomenon is Ab-dependent enhancement of infection (9, 10). In this process, pre-existing cross-reactive Ab form immune complexes with the infecting virus leading to increased virus uptake and infection of Fc receptor-bearing cells such as monocytes/macrophages (10–14).
Analysis of human sera indicates that during the natural course of DENV infection Ab are generated that are broadly reactive with DENV serotypes, but the Ab repertoires in primary and secondary infections appear to be distinct, both in acute infection and in long-term immune sera (15–17). Studies of mAb panels derived from immortalized B cells indicate that immune individuals have a large repertoire of DENV-reactive B cells, even years after infection, with specificities for both structural envelope (E) and precursor-membrane (PrM) proteins that are reactive across serotypes (18, 19). Similarly, memory B cells with specificity for E are highly serotype-specific in the convalescent phase of primary DENV infection, but are serotype cross-reactive following secondary infection (20).
While these studies have helped define the Ab response to DENV, we know surprisingly little about the B cell response itself, especially during acute infection when disease is manifest. Data are emerging to indicate that the magnitude of the plasmablast response in acute DENV infection may be substantially greater than that induced by vaccination or other febrile illness (OFI) (21–23). Whether this response correlates with disease severity is unknown and is a critical issue given the proposed relationship between Ab and disease in this infection. In addition, it is unknown whether B cells undergo the marked activation and apoptosis that is well-described for T cells in acute DENV infection (24). To address this gap in knowledge, in the current study we characterized the acute B cell response in dengue patients in a hospital cohort in Brazil, comparing both primary and secondary infections and mild and severe disease.
Materials and Methods
Subjects, laboratory diagnosis and clinical samples
The study was reviewed and approved by the Institutional Review Board of the University of Pittsburgh. Blood samples were obtained from patients ≥ 5 years of age enrolled in a hospital-based dengue cohort in Recife, northeast Brazil, between 2004 and 2006. The cohort included 354 cases of laboratory-confirmed dengue based on virus cDNA amplification, virus isolation, and/or serum Ab analysis (25). RT-PCR and virus isolation were performed on serum samples obtained at admission; all subsequent samples were analyzed serologically. DENV was isolated using serum inoculation onto monolayers of C6/36 insect cells followed by Ab staining using serotype-specific anti-DENV mAb and immunofluorescence analysis (25). For detection of viral RNA, two-step nested RT-PCR was performed using consensus primers against all four DENV serotypes followed by second-round amplification with serotype-specific primers. Serologic Ab analyses used anti-DENV IgM or anti-DENV IgG ELISA on acute and convalescent samples (25). Clinical pathologic analyses included automated WBC, platelet and differential leukocyte counts and measurement of serum aspartate aminotransferase and alanine aminotransferase concentrations as indicators of liver function. Primary infection was characterized by the absence of DENV-specific IgG and the presence of DENV-specific IgM, virus isolation and/or viral RNA in the acute stage, followed by the presence of DENV-specific IgG in the convalescent stage. Secondary infection was characterized by DENV-specific IgG, virus isolation and/or viral RNA with low to absent DENV-specific IgM in the acute stage, followed by a rise in DENV-specific IgG titer and presence of DENV-specific IgM in the convalescent stage (25, 26). Clinical disease was classified as dengue fever (DF) based on standard World Health Organization guidelines (27), or as a more severe condition called complicated dengue fever (DFC) based on a classification scheme adopted by the Brazilian Ministry of Health (25, 28). DFC cases do not meet all the criteria necessary for a diagnosis of dengue hemorrhagic fever, but must present with at least one of the following complications: neurological signs, cardiopulmonary dysfunction, liver insufficiency, gastrointestinal bleeding, plasma leakage, thrombocytopenia, leukopenia, or death (28). DFC represents 1% of all notified dengue cases in Brazil and accounted for 370 deaths in 2010 (29).
Ab staining and flow cytometric analysis
All Ab used were from BD Biosciences unless noted. PBMC were thawed and incubated with various combinations of fluorochrome-labeled Ab against cell surface Ag CD19 (clone HIB19), CD20 (2H7), CD10 (HI10a), CD27 (M-T271), CD69 (L78), CD95 (DX-2), CD3 (SP34-2), CD21 (B-ly4) and CD38 (AT-1, Stem Cell Technologies). Following staining, cells were incubated with LIVE/DEAD (Invitrogen), an amine-reactive fluorescent dye used to distinguish live from dead cells. For intracellular staining, cells were first labeled with Ab to cell surface Ag then fixed and permeabilized using the Cytofix/Cytoperm kit (BD Bioscience) followed by staining with Ab to active caspase-3 (C92-605) and Ki-67 (B56). All samples were acquired on an LSRII flow cytometer (Becton Dickinson) and data analyzed using FlowJo software (Tree Star). The proportion of lymphocyte subsets in PBMC based on flow cytometric analysis was converted to absolute count by multiplying by the number of mononuclear cells/μL blood calculated from the automated hematologic analysis.
Elispot assay
The Elispot assay was adapted from a published protocol (30). 96-well Elispot plates (Millipore) were coated overnight at 4°C with goat anti-human IgG (10 μg/ml; Bethyl Laboratories) to detect IgG-secreting cells. To detect cells secreting Ab specific for DENV we coated plates with UV-inactivated DENV-1 (Hawaii strain), DENV-2 (16681) or DENV-3 (H87) that had been propagated on C6/36 insect cells and concentrated by centrifugation at 100,000 × g for 4 h over a 20% glycerol cushion. Virus was diluted in PBS and 1×105 plaque forming units added to each well. As irrelevant virus controls wells were coated with influenza vaccine consisting of three different inactivated influenza virus strains (6μg/ml, Fluarix, GlaxoSmithKline) or 2% BSA in PBS. Plates were washed with PBS and blocked with RPMI 1640 with 10% FBS for 2 h. Serial dilutions of PBMC were added to the plates and incubated 18–20 h at 37°C. After incubations cells were discarded and wells were washed with PBS plus 0.1% Tween20. Plates were then incubated with biotinylated goat anti-human IgG-Fc (Bethyl laboratories) for 1 h at room temperature. Streptavidin-conjugated alkaline phosphatase (BioRad) was then added and plates were developed using an alkaline phosphatase conjugate substrate kit (BioRad). Spots were counted using a CTL ImmunoSpot reader and counting software (Cellular technologies Ltd).
Plaque reduction neutralization test
The primary virus strains DENV-1 (PE/97-42735), DENV-2 (PE/95-3808), DENV-3 (PE/02-95016) and DENV-4 (IEC) isolated in Brazil were expanded on African green monkey kidney cells and used in a standard plaque reduction neutralization test with the same cell line and heat-inactivated patient sera essentially as described (31). Sera were used at two-fold dilutions ranging from 1/20 to 1/2560. The 50% end-point dilution of each serum, corresponding to the dilution at which 50% of the wells were completely protected from infection, was determined according to standard methods. Fifty percent plaque neutralization titers (PRNT50) were calculated as the highest dilution of Ab reducing virus plaques by 50% and were depicted on a log10 scale.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc). For clinical laboratory measurements, comparisons were made between groups using a non-parametric one-way analysis of variance followed by a Dunn’s post test analysis. For all other comparisons we used a non-parametric Mann-Whitney two-tailed U-test. Comparisons were made with the OFI group to control for the effect of febrile illness on lymphocyte populations. In some analyses comparisons were also made between secondary DFC and other groups. Correlations were calculated using the non-parametric Spearman rank test. P values less than 0.05 were considered significant.
Results
Characteristics of the dengue patient cohort
PBMC taken 1 to 9 days after onset of symptoms were analyzed from 84 patients in the cohort with laboratory-confirmed primary or secondary DENV-infections and either DF or DFC (Table I) (25). Also studied were individuals with OFI, who presented to the same hospitals with suspected dengue that was ruled out by subsequent laboratory analysis. As additional controls we included healthy local residents that were DENV seronegative (healthy naive) or had recovered from DENV infection encountered more than 250 days previously (healthy recovered) (Table I). The demographic characteristics of individuals studied were comparable across groups except for median age, as primary DF was mainly noted in children, whereas individuals in the other groups consisted mainly of adults, reflecting the epidemiology of dengue in Brazil (Table I) (25, 29). Viral RNA was detected at admission in 43 to 61% of all laboratory-confirmed cases, all of which corresponded to DENV-3 (Table I) (25). Individuals in the DFC group had significantly lower platelet and WBC counts and significantly higher serum concentrations of aspartate aminotransferase and alanine aminotransferase than patients with DF and OFI, reflecting a severe clinical condition, whereas patients with DF did not differ in any of these indices from individuals with OFI (Table I).
Table I.
Clinical and demographic characteristics of subjects
| Healthy Naive | Other Febrile Illness | Dengue Fever | Complicated Dengue Fever | Healthy Recovered | |||
|---|---|---|---|---|---|---|---|
| Type of infection | N/A | N/A | Primary | Secondary | Primary | Secondary | N/A |
| Number (n) | 10 | 15 | 15 | 23 | 18 | 28 | 13 |
| Male, number (%) | 5 (50) | 6 (40) | 5 (33) | 15 (65) | 8 (44) | 17 (61) | 5 (38) |
| Age, years, median (range) | 32 (21–54) | 21 (5–64) | 7 (5–37) | 33 (6–62) | 22 (4–65) | 37 (6–76) | 28 (8–49) |
| ≤18 years, number | 0 | 6 | 9 | 9 | 8 | 3 | 4 |
| >18 years, number | 10 | 9 | 6 | 14 | 10 | 25 | 9 |
| Day of symptoms, median (range) | N/A | 5 (2–8) | 6 (3–7) | 5 (2–9) | 6 (3–8) | 6 (1–8) | 308 (253–398) |
| DENV-PCR+, number (%)1 | N/A | N/A | 8 (53) | 10 (43) | 11 (61) | 11 (54) | N/A |
| Platelet nadir, ×103/μL, mean (SD) | N/A | 209 (69) | 199 (55) | 93 (26)*** ### | N/A | ||
| WBC, cells/μL, mean (SD) | N/A | 6114 (1910) | 6250 (3849) | 4144 (2218)** ## | N/A | ||
| Neutrophils, cells/μL, mean (SD) | N/A | 3197 (1746) | 3051 (2946) | 1682 (1557)* ## | N/A | ||
| AST, IU/L, mean (SD) | N/A | 54 (78) | 73 (87) | 172 (155)*** ### | N/A | ||
| ALT, IU/L, mean (SD) | N/A | 46 (46) | 68 (105) | 161 (168)*** ## | N/A | ||
N/A, not applicable; AST, aspartate aminotransferase; ALT, alanine aminotransferase; WBC, white blood cells
RT-PCR performed using primers specific for DENV-1, DENV-2, DENV-3 and DENV-4. Positive samples were all DENV-3.
P<0.05,
P <0.01,
P <0.001 vs. dengue fever;
P<0.01,
P<0.001 vs. other febrile illness by one-way ANOVA
Increased turnover and activation of B cells during acute DENV infection
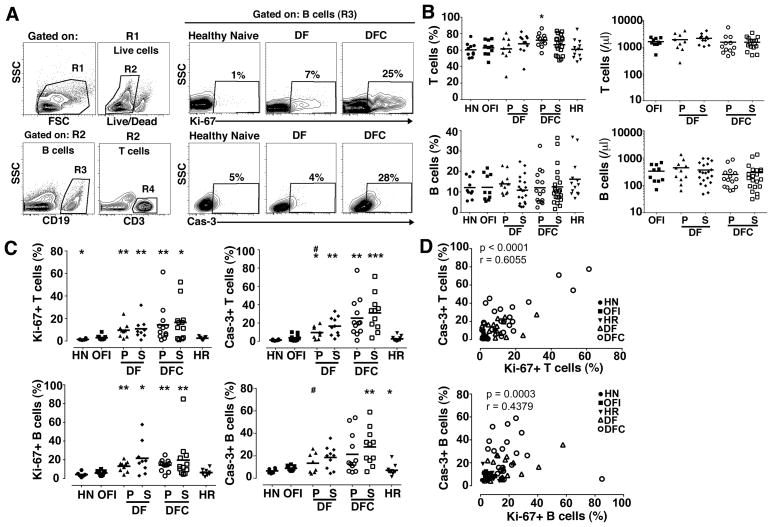
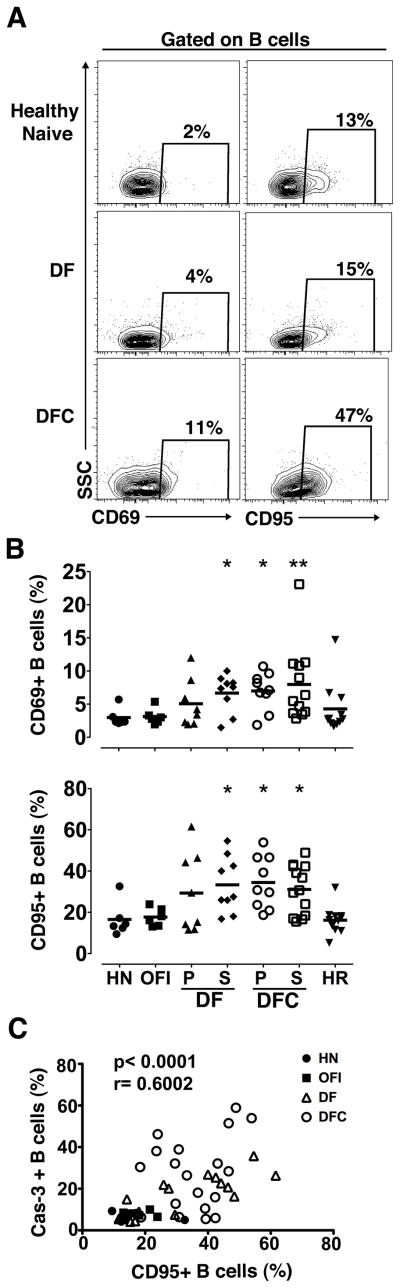
To evaluate the effect of DENV infection on the number of B cells and their turnover in vivo, we performed flow cytometric analysis on PBMC. Cells were initially analyzed by forward and side scatter parameters and dead cells were then identified and excluded by gating on viable cells with low-level staining by the amine-reactive LIVE/DEAD dye. Viability varied between 80 to 90% on all samples with this analysis. B and T cells were identified within the viable population using Ab to CD19 and CD3, respectively (Figure 1A). Ab to Ki-67 and active caspase-3 were used as markers of cell proliferation and early apoptosis, respectively (Figure 1A). The proportion of B and T cells within total PBMC did not differ between OFI and other groups with the exception of T cells in the primary DFC group (Figure 1B). The absolute number of B and T cells in blood also was not affected by DENV infection, despite the leukopenia in individuals with DFC (Figure 1B, Table I). This may reflect the fact that the major contributing factor to leukopenia was loss of granulocytes (data not shown). Significant proliferation and apoptosis were noted in T cells of DENV-infected individuals relative to OFI, as has previously been reported (Figure 1C) (32, 33). We observed a similar effect in the B cell population, with an increase in the proportion of proliferating and to a lesser extent apoptotic B cells in patients with dengue relative to individuals with OFI (Figure 1C). The proportion of apoptotic B cells in secondary DFC approached 60% in some individuals and was significantly greater than that noted in OFI as well as primary DF (Figure 1C). We found a positive correlation between proliferation and apoptosis for both T and B cells, indicating that cell division was offset by cell death (Figure 1D). We also found a significant increase in the proportion of B cells expressing the activation marker CD69 in secondary DF and both types of DFC relative to OFI individuals, reflecting dengue-related B cell activation (Figure 2A, B). Similarly, B cells in the same dengue groups had increased expression of the pro-apoptotic marker CD95 relative to OFI, which was positively correlated with active caspase-3 (Figure 2A–C).
Figure 1. Increased turnover of B and T cells in acute DENV infection.
(A) Representative flow cytometry contour plots illustrating the gating strategy to define B cell and T cell subsets (left), and expression of Ki-67 and active caspase-3 (Cas-3) on B cells from a healthy naïve individual and individuals with DF and DFC (right). Boxes are defined based on fluorescence with isotype control Ab. (B) Percentage (left) and absolute number (right) of T cells and B cells within the PBMC of each group. Symbols represent individual subjects and horizontal lines represent means. (C) Percentage of T cells and B cells expressing Ki-67 and active caspase-3 in the different groups. (D) Correlation between the percent of T cells and B cells expressing Ki-67 and active caspase-3. HN=healthy naïve, OFI=other febrile illness, DF=dengue fever, DFC=complicated dengue fever, HR=healthy recovered, P=primary infection, S=secondary infection. *P<.05, ** P< .01 and *** P<.001 vs. OFI; # P<.05 vs. secondary DFC (dengue groups only).
Figure 2. Increased activation of B cells in acute DENV infection.
(A) Representative flow cytometry contour plots illustrating the gating strategy to determine expression of CD69 and CD95 on B cells in a healthy naïve individual and individuals with DF and DFC. Boxes are defined based on fluorescence with relevant isotype control Ab. (B) Percentage of B cells expressing CD69 and CD95 in the different groups. Symbols represent individual subjects and horizontal lines represent means. (C) Correlation between the percent of B cells expressing CD95 and active caspase-3. HN=healthy naïve, OFI=other febrile illness, DF=dengue fever, DFC=complicated dengue fever, HR=healthy recovered, P=primary infection, S=secondary infection. * P<.05 vs. OFI.
Marked plasmablast response in severe acute secondary DENV infection
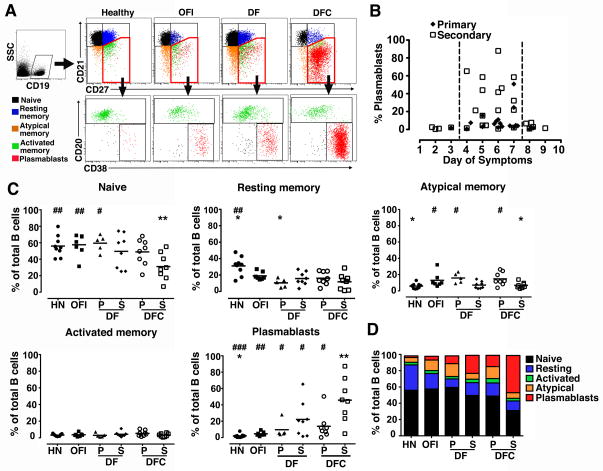
To further dissect the B cell response in acute DENV infection we used a panel of Ab to define specific B cell subsets in peripheral blood. We gated on CD19+CD10− mature B cells and then used staining with Ab to CD27 and CD21 to identify naïve (CD27−CD21+), resting memory (CD27+CD21+) and atypical memory B cells (CD27−CD21−). The CD27+CD21− fraction was further analyzed for expression of CD20 and CD38 to distinguish plasmablasts (CD20−CD38+) from activated memory B cells (CD20+CD38−/low) (Figure 3A) (34, 35). We then used this panel to define the period of peak plasmablast expansion relative to the onset of symptoms in DENV-infected individuals in a cross-section of 45 patients. These data revealed that in both primary and secondary infections plasmablasts expanded over a narrow period of time, from days 4 to 7 of symptoms, and that the magnitude of the response in secondary infections was substantially greater, consistent with an anamnestic response (Figure 3B). Using this limited period of days 4 to 7 of symptoms, we then characterized the B cell fractions in a subset of patients with primary and secondary DF and DFC and compared them to healthy individuals and to those with OFI at the same stage of illness. Naive B cells made up roughly half of the circulating B cells on average in HN individuals, as well as individuals with OFI, DF, and primary DFC. However, in patients with secondary DFC the proportion of naive B cells fell to 30% on average, significantly lower than HN and OFI as well as primary DF (Figure 3C, D). The low frequency of naive B cells in secondary DFC patients reflected a very high proportion of plasmablasts which averaged 46%, reaching a remarkable 87% in one individual (Figure 3A, C). By comparison, individuals with OFI had average plasmablast frequencies of 5%. Moreover, while the mean proportions of plasmablasts in DF and primary DFC were elevated relative to health they were not significantly greater than the OFI group (Figure 3C). Notably, plasmablast frequencies in secondary DFC were significantly greater than each of the other dengue groups (Figure 3C). The magnitude of the plasmablast response did not correlate with the detection of virus in patient serum at admission in any group (data not shown). There also was no difference in the percentage of activated memory B cells and minor although in some cases significant differences in the proportion of resting and atypical memory B cells between the groups (Figure 3C, D).
Figure 3. Marked expansion of plasmablasts in severe secondary DENV infection.
(A) Representative flow cytometry contour plots illustrating the gating strategy to define naïve B cells (black), resting memory B cells (blue), atypical memory B cells (orange), activated memory B cells (green) and plasmablasts (red) in a healthy individual and individuals with OFI, DF and DFC. (B) Percentage of B cells that are plasmablasts in 45 patient samples with either primary (closed diamonds) or secondary (open squares) acute DENV infection analyzed at different intervals after the onset of symptoms. The interval between the dotted lines indicates the peak response. (C) Percentage of naïve B cells, resting memory B cells, atypical memory B cells, activated memory B cells and plasmablasts at 4 to 7 days of symptoms in the different groups. Symbols represent individual subjects and horizontal lines represent means. (D) The relative proportion of B cell subsets in each group shown as stacked bars. HN=healthy naïve, OFI=other febrile illness, DF=dengue fever, DFC=complicated dengue fever, P=primary infection, S=secondary infection. * P<.05, ** P< .01 vs. OFI; # P<.05, ## P<.01, ### P<.001 vs. secondary DFC.
Plasmablasts in secondary DFC are DENV-specific and preferentially react with the infecting serotype
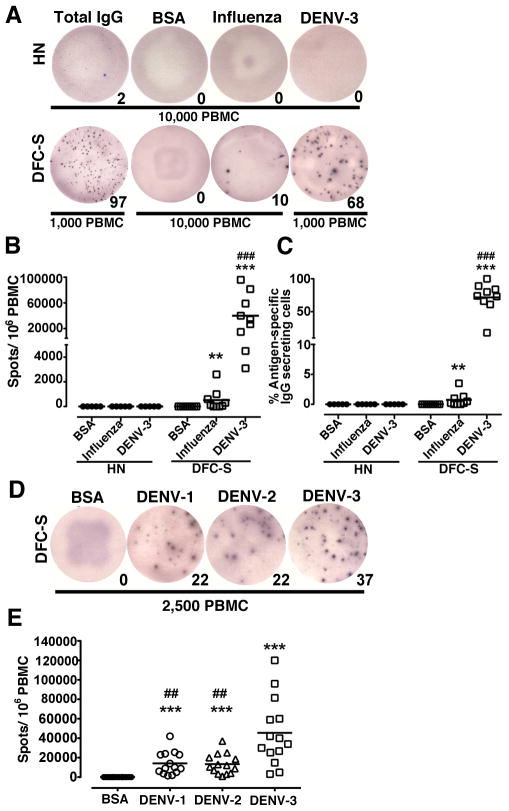
To determine the Ag specificity of plasmablasts we performed Elispot assays, focusing on DFC cases with secondary infections given the predominance of plasmablasts in this group. Cryopreserved plasmablasts have comparable function to freshly isolated cells in this assay, indicating that analysis of archived samples accurately reflects plasmablast specificity in blood (36). As an initial test of virus specificity we used DENV-3 virus particles as the capture Ag given the prevalence of DENV-3 infections in the cohort (Table 1) (25). Samples taken at days 4 to 7 of symptoms from a subset of 9 patients with secondary DFC were used for this analysis. PBMC were plated without prior stimulation to identify cells spontaneously releasing Ab. PBMC from 5 healthy naïve individuals had negligible numbers of cells secreting IgG and no detectable cells secreting Ab reactive with DENV-3, as expected. In contrast, PBMC from the individuals with secondary DFC had an average of around 40,000 DENV-reactive plasmablasts per million PBMC based on binding to DENV-3 (Figure 4A, B). This represented an average frequency of 72% of all IgG-secreting cells (Figure 4C). The frequency of DENV-specific plasmablasts was not correlated with the magnitude of the peak plasmablast response, as the individuals with the lowest (4%) and highest (87%) proportion of plasmablasts had >80% DENV-specific IgG secreting cells. There was also a minor although significant number of IgG secreting cells that reacted with influenza virus in these patients, averaging 520 plasmablasts per million PBMC and representing an average frequency of 0.7% of all IgG secreting cells (Figure 4B, C). Given that these individuals had secondary infections and therefore had immunity to a previously encountered DENV serotype, we next asked whether the expanded plasmablasts reacted with DENV serotypes other than the infecting virus. Using samples from 14 individuals with secondary DFC, we repeated the Elispot assays using DENV-1, DENV-2 and DENV-3 as capture Ag. In all cases plasmablasts reacted with all 3 DENV serotypes, but not BSA. However, on average there was a 3-fold greater reactivity of IgG-secreting cells with DENV-3 than with either DENV-1 or 2, a difference that was statistically significant (Figure 4D, E).
Figure 4. Plasmablasts in severe secondary DENV infection are DENV-specific and serotype cross-reactive but preferentially react with the infecting serotype.
(A) Representative Elispot assay depicting plasmablasts secreting Ab reactive with BSA, influenza virus or DENV-3 compared with the total number of IgG-secreting cells from a healthy naïve individual (HN) and a patient with secondary DFC (DFC-S). Numerals represent number of spots. 10,000 cells were used for each condition except for total IgG and DENV-3 in the DFC-S individual where 1,000 cells were used. (B, C) Graphical representation of the number of Ab-secreting cells per 1 million PBMC (B) and the percent of all Ab-secreting cells (C) that are reactive with BSA, influenza virus and DENV-3 in healthy naïve individuals compared with individuals with secondary DFC. **P<.01, ***P<.001 vs. BSA; ### P<.001 vs. influenza virus. **P<.01, ***P<.001 vs. BSA control; ### P<.001 vs. influenza virus. (D) Representative Elispot assay depicting plasmablasts secreting Ab reactive with BSA, DENV-1, DENV-2 or DENV-3 in a patient with secondary DFC. Numerals represent number of spots. 2,500 cells were used per well. (E) Graphical representation of the number of Ab-secreting cells per 1 million PBMC that are reactive with BSA or each of the 3 DENV serotypes in individuals with secondary DFC. ***P<.001 vs. BSA; ## P<.01 vs. DENV-3.
Lack of correlation between plasmablast frequency and serum neutralizing Ab
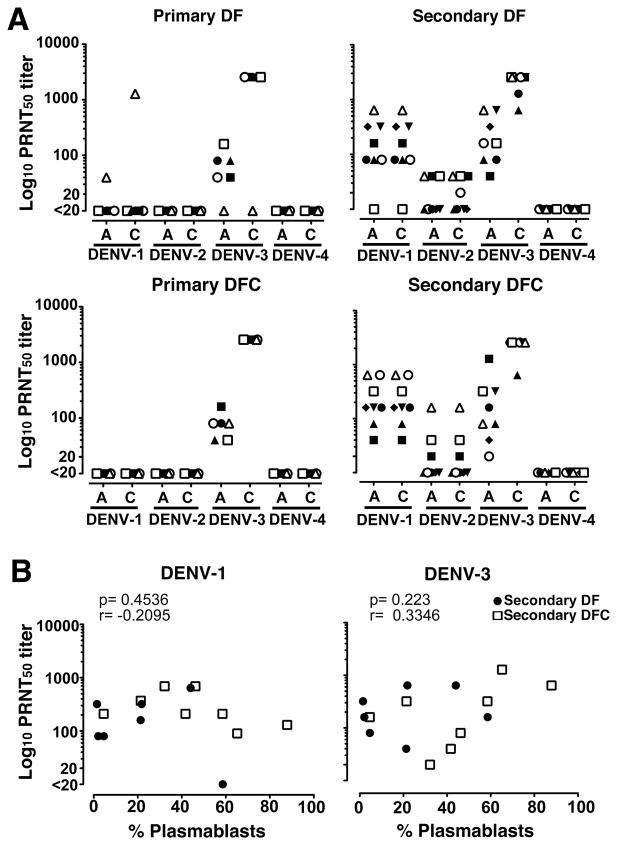
We next analyzed patient sera to determine if there was a relationship between the magnitude of the plasmablast response and the presence of DENV-specific Ab in the circulation. We focused on neutralizing Ab as these provide the most information regarding the identity of the infecting virus and the history of infection (7, 37). We limited our investigations to individuals with serum samples taken on the same day of symptoms as the plasmablast analysis to provide a relevant comparison, and analyzed paired sera in each patient. All individuals with primary infections had low titers of neutralizing Ab to only one serotype at acute infection that increased in titer at convalescence, reflecting the specific infecting virus. All primary infections were with DENV-3 except for one primary DF case infected with DENV-1 (open triangle, Figure 5A). In all secondary infections the infecting virus was DENV-3, based on increasing DENV-3-specific neutralizing Ab over time. Secondary infections likely followed earlier primary infections with DENV-1, based on the presence of significant DENV-1-specific neutralizing Ab of a constant titer (Figure 5A). One third of individuals with secondary infections also had low and constant titers of neutralizing Ab to DENV-2, reflecting either cross-reactive neutralizing Ab or potentially a prior infection with DENV-2, which circulates in Brazil along with DENV-1 and DENV-3 (Figure 5A) (38). All sera analyzed had undetectable neutralizing Ab to DENV-4 (Figure 5A). To determine if the magnitude of the plasmablast response was reflected in differences in the corresponding neutralizing Ab titer during acute infection we did a simple linear correlation. There was no correlation between the percentage of plasmablasts in PBMC at days 4 to 7 after onset of symptoms and the titer of serum neutralizing Ab against DENV-1, DENV-2, or DENV-3 in individuals with secondary infections regardless of severity of disease (Figure 5B and data not shown). Similarly, plasmablast frequency was not correlated with neutralizing Ab titer against any virus serotype in acute primary infections (data not shown).
Figure 5. Lack of correlation between plasmablast response and serum neutralizing Ab titers.
(A) Reciprocal 50% neutralizing Ab (PRNT50) titers to each of the four DENV serotypes in sera of patients with primary and secondary DENV infections and either DF or DFC, measured at 4 to 7 days after onset of symptoms (A) and at convalescence (C), as determined by plaque reduction neutralization test. Convalescent samples were taken 15 to 54 days after onset of symptoms except for two secondary DFC patients (closed triangle and closed square) taken 2 and 3 days after the acute sample, respectively. Each symbol represents the response of an individual patient across each of the four DENV serotypes. Individuals with secondary DFC correspond to those with the same symbols in Figure 5 where applicable. (B) Correlation between percent plasmablasts at day 4 to 7 after onset of symptoms and the corresponding PRNT50 titer of serum Ab against DENV-1 and DEN-3 in patients with secondary DF (closed circles) and DFC (open squares).
Discussion
It has previously been shown that activation and apoptosis of T cells increase with severity of disease in young DENV-infected patients, a process that is widely considered to be involved in the pathogenesis of dengue (32, 33, 39–43). Similarly, we found that one third of live B cells in individuals with severe secondary DENV infection were undergoing apoptosis which was positively correlated with B cell proliferation and activation. These data suggest that DENV infection promotes activation-induced B cell death and increased B cell turnover, which is consistent with our finding of homeostatic levels of B cells in the circulation despite severe disease. Activated B cells expressed CD95 which may contribute to apoptosis through engagement of the CD95/CD95L pathway; a similar mechanism has been proposed to account for apoptosis of CD8+ T cells during severe DENV infection (32, 33).
Our finding of massive expansion of plasmablasts during days 4 to 7 of dengue symptoms in adults in Brazil is in accordance with recent reports of adult dengue patients in Singapore and Vietnam, where significant plasmablast responses were also associated with secondary DENV infections that exceeded those of individuals with non dengue-related acute illness (21, 44). A recent report in pediatric patients in Nicaragua also documented significant increases in the frequency of circulating plasmablasts/plasma cells in dengue relative to OFI, although there was no difference between primary and secondary DENV infections (23). Similarly, massive plasmablast responses were noted in adult dengue patients in Bangkok, Thailand, that were significantly greater than those induced by either inactivated influenza virus or live attenuated yellow fever virus vaccination of healthy individuals (22). Previous clinical reports have noted that extreme plasmacytosis to the levels seen in plasma cell leukemia or myeloma is a common hematologic finding in DENV infection (45, 46). Our data substantially extend these findings by showing that the magnitude of the plasmablast response in patients with dengue increases both with secondary infection and with disease severity. Extraordinary plasmablast frequencies averaging 46% of B cells were documented in patients with secondary DFC, and these frequencies were significantly greater than those in individuals with primary DENV infection, regardless of disease severity, as well as secondary DF.
A key question relating to the function of plasmablasts in dengue is their specificity and capacity to cross-react with different DENV serotypes. We found that on average more than 70% of plasmablasts in the individuals tested with secondary DFC produced DENV-specific IgG, a finding that corresponds to other recently published data (22, 23). There was also minor reactivity of plasmablasts against influenza virus in a subset of patients that could reflect concurrent influenza virus infection, although this remains to be determined. DENV-specific Ab produced by plasmablasts reacted with several DENV serotypes reflecting cross-reactivity of the B cell response in acute secondary infection, as noted in other dengue patient cohorts (21, 23). However, in DENV-3-infected patients with severe disease in our study, plasmablasts had 3-fold higher reactivity with the infecting serotype than with two other heterotypic serotypes. In contrast, studies of DENV-3-infected patients in Nicaragua revealed significantly higher frequencies of cross-reactive plasmablasts/plasma cells that reacted with a previous infecting serotype (23), consistent with the concept of original antigenic sin in dengue pathogenesis (47, 48). There are several related factors that could potentially account for this difference in B-cell cross-reactivity. The median age of the patients with secondary DFC for which detailed plasmablast analyses were done in our cohort was 37 years, and the primary DENV infection in these patients could extend up to 20 years earlier with the introduction of DENV-1 into Brazil in 1986 (25, 49). In addition, DENV serotypes circulate in Brazil for extended periods of time; DENV-2 was the predominant serotype for 10 years prior to emergence of DENV-3 in 2000 (49). In contrast, patients in the Nicaraguan cohort with secondary DENV-3 infections were children with a median age of 10.5 years who may have experienced a primary DENV infection considerably more recently than individuals in our study. DENV serotypes in Nicaragua also have a short circulation time relative to Brazil, with the dominant serotype switching every 3 to 4 years since 1999 (50–53). These complex interactions between age and the period of primary immunity on cross-reactivity of DENV-specific plasmablasts during secondary infections may be important in dengue immunopathogenesis and deserve further attention.
Other studies of the B cell response in acute DENV infection have shown that the titer of DENV-specific serum Ab rapidly increases within days of the onset of symptoms and correlates with the frequency of plasmablasts (21, 22). Our data now show that there is no apparent relationship between the neutralization capacities of serum Ab to the infecting serotype or heterotypic serotypes in acute secondary infection and the magnitude of the peak plasmablast response, consistent with a previous report (23). This is perhaps not surprising, as serum neutralizing Ab titers against the newly encountered virus strain are expected to be low in the acute phase. In addition to neutralization capacity, the avidity of DENV-specific Ab is emerging as an important factor in understanding the immune response to primary and secondary DENV infections (23, 54). Given that plasmablasts and plasma cells that rapidly expand in acute infection are the source of virus-specific Ab, it is likely that further detailed analysis of the specificity and reactivity of Ab produced by plasmablasts will provide key insights into dengue immunity.
The mechanisms responsible for this very large expansion of plasmablasts in severe secondary DENV infection remain to be determined and are likely to be complex. IL-10 and TNF-α drive plasma cell development and are elevated in dengue patients with increasing disease severity (39, 55–57). However, in our patient cohort there was no apparent correlation between the serum concentration of these cytokines and frequency of circulating plasmablasts in acute disease (data not shown). Similarly, preliminary serologic analysis of other candidate cytokines that drive B-cell differentiation including IL-6, IL-21, BAFF and APRIL did not reveal any association with the magnitude of the plasmablast response (data not shown). Further in-depth analysis of factors known to regulate B-cell differentiation may provide insight into the plasmablast response to DENV infection and its relationship to disease severity.
Acknowledgments
The authors thank Charles R. Rinaldo Jr. for critical reading of the manuscript; Donald S. Burke for continued advice and support; members of the Center for Vaccine Research dengue working group for helpful discussions; and Laura Gil, Clintiano Curvêlo, Verônica Gomes, Lamartine Silva and Anderson Silva for sample collection, preparation and shipment.
These studies were supported by training grant AI049820 to TMGB and research grant AI118297 from the US National Institutes of Health.
References
- 1.Simmons CP, Farrar JJ, Nguyenv V, Wills B. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 5.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 6.Graham RR, Juffrie M, Tan R, Hayes CG, Laksono I, Ma’roef C, Erlin, Sutaryo, Porter KR, Halstead SB. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 7.Guzman MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vazques S, Delgado I, Halstead SB. Epidemiologic studies on Dengue in Santiago de Cuba, 1997. Am J Epidemiol. 2000;152:793–799. doi: 10.1093/aje/152.9.793. discussion 804. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, Endy TP. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–1479. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halstead SB. Observations related to pathogensis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970;42:350–362. [PMC free article] [PubMed] [Google Scholar]
- 10.Halstead SB, O’Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, Dung NT, Lienle B, Quy NT, Hieu NT, Hieu LT, Hien TT, Hung NT, Farrar J, Simmons CP. Dengue in Vietnamese infants--results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198:516–524. doi: 10.1086/590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 13.Littaua R, Kurane I, Ennis FA. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol. 1990;144:3183–3186. [PubMed] [Google Scholar]
- 14.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 15.Crill WD, Hughes HR, Delorey MJ, Chang GJ. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One. 2009;4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai CY, Tsai WY, Lin SR, Kao CL, Hu HP, King CC, Wu HC, Chang GJ, Wang WK. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol. 2008;82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392:103–113. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, Michael SF, Robinson JE. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J. 7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew A, West K, Kalayanarooj S, Gibbons RV, Srikiatkhachorn A, Green S, Libraty D, Jaiswal S, Rothman AL. B-cell responses during primary and secondary dengue virus infections in humans. J Infect Dis. 2011;204:1514–1522. doi: 10.1093/infdis/jir607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balakrishnan T, Bela-Ong DB, Toh YX, Flamand M, Devi S, Koh MB, Hibberd ML, Ooi EE, Low JG, Leo YS, Gu F, Fink K. Dengue virus activates polyreactive, natural IgG B cells after primary and secondary infection. PLoS One. 2011;6:e29430. doi: 10.1371/journal.pone.0029430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson PC, Wittawatmongkol O, Yoksan S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Ahmed R. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J Virol. 2011;86:2911–2918. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zompi S, Montoya M, Pohl MO, Balmaseda A, Harris E. Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Negl Trop Dis. 2012;6:e1568. doi: 10.1371/journal.pntd.0001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nature reviews Immunology. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 25.Cordeiro MT, Silva AM, Brito CA, Nascimento EJ, Magalhaes MC, Guimaraes GF, Lucena-Silva N, de Carvalho EM, Marques ET., Jr Characterization of a dengue patient cohort in Recife, Brazil. Am J Trop Med Hyg. 2007;77:1128–1134. [PubMed] [Google Scholar]
- 26.Cordeiro MT, Braga-Neto U, Nogueira RM, Marques ET., Jr Reliable classifier to differentiate primary and secondary acute dengue infection based on IgG ELISA. PLoS One. 2009;4:e4945. doi: 10.1371/journal.pone.0004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. Dengue haemorrhagic fever: Diagnosis, treatment, prevention and control. 2. World Health Organization; Geneva: 1997. [Google Scholar]
- 28.Brazil Ministry of Health. Dengue: Diagnostico e Manejo Clinico. 2. Ministerio da Saude (Brasil); Brasilia: 2005. [Google Scholar]
- 29.Siqueira JB, Vinhal LC, Said RFC, Hoffmann JL, Martins J, Barbiratto SB, Coelho GE. Saude Brasil 2010. Ministerio da Saude (Brasil); Brasilia: 2011. Dengue no Brasil: Tendencias e madancas na epidemiologia, com enfase nas epidemias de 2008 e 2010; pp. 157–171. [Google Scholar]
- 30.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 31.de Melo AB, da Silva MD, Magalhaes MC, Gonzales Gil LH, Freese de Carvalho EM, Braga-Neto UM, Bertani GR, Marques ET, Jr, Cordeiro MT. Description of a Prospective 17DD Yellow Fever Vaccine Cohort in Recife, Brazil. Am J Trop Med Hyg. 2011;85:739–747. doi: 10.4269/ajtmh.2011.10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myint KS, Endy TP, Mongkolsirichaikul D, Manomuth C, Kalayanarooj S, Vaughn DW, Nisalak A, Green S, Rothman AL, Ennis FA, Libraty DH. Cellular immune activation in children with acute dengue virus infections is modulated by apoptosis. J Infect Dis. 2006;194:600–607. doi: 10.1086/506451. [DOI] [PubMed] [Google Scholar]
- 33.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 34.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian Y, Wei C, Eun-Hyung Lee F, Campbell J, Halliley J, Lee JA, Cai J, Kong YM, Sadat E, Thomson E, Dunn P, Seegmiller AC, Karandikar NJ, Tipton CM, Mosmann T, Sanz I, Scheuermann RH. Elucidation of seventeen human peripheral blood B-cell subsets and quantification of the tetanus response using a density-based method for the automated identification of cell populations in multidimensional flow cytometry data. Cytometry B Clin Cytom. 2010;78(Suppl 1):S69–82. doi: 10.1002/cyto.b.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyu SY, Kobie J, Yang H, Zand MS, Topham DJ, Quataert SA, Sanz I, Lee FE. Frequencies of human influenza-specific antibody secreting cells or plasmablasts post vaccination from fresh and frozen peripheral blood mononuclear cells. J Immunol Methods. 2009;340:42–47. doi: 10.1016/j.jim.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Barraquer I, Cordeiro MT, Braga C, de Souza WV, Marques ET, Cummings DA. From re-emergence to hyperendemicity: the natural history of the dengue epidemic in Brazil. PLoS Negl Trop Dis. 2011;5:e935. doi: 10.1371/journal.pntd.0000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis BL, Kurane I, Rothman AL, Ennis FA. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 40.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev. 2008;225:300–313. doi: 10.1111/j.1600-065X.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 41.Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, Jairungsri A, Kanlaya R, Tangthawornchaikul N, Puttikhunt C, Pattanakitsakul SN, Yenchitsomanus PT, Mongkolsapaya J, Kasinrerk W, Sittisombut N, Husmann M, Blettner M, Vasanawathana S, Bhakdi S, Malasit P. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193:1078–1088. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- 42.Zivna I, Green S, Vaughn DW, Kalayanarooj S, Stephens HA, Chandanayingyong D, Nisalak A, Ennis FA, Rothman AL. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J Immunol. 2002;168:5959–5965. doi: 10.4049/jimmunol.168.11.5959. [DOI] [PubMed] [Google Scholar]
- 43.Kurane I, Innis BL, Nimmannitya S, Nisalak A, Meager A, Janus J, Ennis FA. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J Clin Invest. 1991;88:1473–1480. doi: 10.1172/JCI115457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmons CP, Popper S, Dolocek C, Chau TN, Griffiths M, Dung NT, Long TH, Hoang DM, Chau NV, Thaole TT, Hien TT, Relman DA, Farrar J. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J Infect Dis. 2007;195:1097–1107. doi: 10.1086/512162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thai KT, Wismeijer JA, Zumpolle C, de Jong MD, Kersten MJ, de Vries PJ. High incidence of peripheral blood plasmacytosis in patients with dengue virus infection. Clin Microbiol Infect. 2010 doi: 10.1111/j.1469-0691.2010.03434.x. [DOI] [PubMed] [Google Scholar]
- 46.Gawoski JM, Ooi WW. Dengue fever mimicking plasma cell leukemia. Arch Pathol Lab Med. 2003;127:1026–1027. doi: 10.5858/2003-127-1026-DFMPCL. [DOI] [PubMed] [Google Scholar]
- 47.Halstead SB, Rojanasuphot S, Sangkawibha N. Original antigenic sin in dengue. Am J Trop Med Hyg. 1983;32:154–156. doi: 10.4269/ajtmh.1983.32.154. [DOI] [PubMed] [Google Scholar]
- 48.Midgley CM, Bajwa-Joseph M, Vasanawathana S, Limpitikul W, Wills B, Flanagan A, Waiyaiya E, Tran HB, Cowper AE, Chotiyarnwong P, Grimes JM, Yoksan S, Malasit P, Simmons CP, Mongkolsapaya J, Screaton GR. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2011;85:410–421. doi: 10.1128/JVI.01826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siqueira JB, Jr, Martelli CM, Coelho GE, Simplicio AC, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerg Infect Dis. 2005;11:48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, Saborio SI, Mercado JC, Perez L, Videa E, Almanza E, Kuan G, Reyes M, Saenz L, Amador JJ, Harris E. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11:935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 51.Balmaseda A, Standish K, Mercado JC, Matute JC, Tellez Y, Saborio S, Hammond SN, Nunez A, Aviles W, Henn MR, Holmes EC, Gordon A, Coloma J, Kuan G, Harris E. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis. 201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, Videa E, Rodriguez Y, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Gonzalez A, Harris E. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 53.Kuan G, Gordon A, Aviles W, Ortega O, Hammond SN, Elizondo D, Nunez A, Coloma J, Balmaseda A, Harris E. The Nicaraguan pediatric dengue cohort study: study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol. 2009;170:120–129. doi: 10.1093/aje/kwp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zompi S, Santich BH, Beatty PR, Harris E. Protection from secondary dengue virus infection in a mouse model reveals the role of serotype cross-reactive B and T cells. J Immunol. 2011;188:404–416. doi: 10.4049/jimmunol.1102124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Rothman AL, Ennis FA. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J Med Virol. 1999;59:329–334. [PubMed] [Google Scholar]
- 56.Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, Le BL, Lin CF, Yeh TM, Do QH, Vu TQ, Chen LC, Huang JH, Lam TM, Liu CC, Halstead SB. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–232. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- 57.Devignot S, Sapet C, Duong V, Bergon A, Rihet P, Ong S, Lorn PT, Chroeung N, Ngeav S, Tolou HJ, Buchy P, Couissinier-Paris P. Genome-wide expression profiling deciphers host responses altered during dengue shock syndrome and reveals the role of innate immunity in severe dengue. PLoS One. 2010;5:e11671. doi: 10.1371/journal.pone.0011671. [DOI] [PMC free article] [PubMed] [Google Scholar]