Abstract
Introduction
Nearly a decade ago, researchers identified a potential interaction between tamoxifen and strong CYP2D6 inhibitors, including several frequently used antidepressants. Based on evidence available at that time, a United States Food and Drug Administration advisory committee recommended tamoxifen’s label be changed in October 2006, noting that postmenopausal women with estrogen receptor-positive breast cancer who are poor CYP2D6 metabolizers by genotype or drug interactions may be at increased risk of cancer recurrence. The impact of accumulating drug risk information on antidepressant use is unknown.
Methods
Retrospective, longitudinal cohort study of 13,205 women aged 50–95 with breast cancer initiating tamoxifen between July 2004–December 2009. We evaluate trends in strong, moderate, and weak CYP2D6-inhibitor antidepressants and tamoxifen co-prescribing and factors associated with ongoing strong inhibitor use. A propensity-score matched control group (aromatase inhibitor initiators) was used to estimate changes in co-prescribing, accounting for secular trends.
Results
In each month, approximately 24% of tamoxifen and aromatase inhibitor users were prescribed antidepressants. Among women using tamoxifen and antidepressants, 34% used strong inhibitors between 2004 and 2006 versus 15% in 2010. Strong inhibitor use decreased more among tamoxifen users than aromatase inhibitor users (Difference-in-Differences, [DD]: −0.09, 95% Confidence Interval, [CI]: −0.15, −0.03). Weak inhibitor use increased among tamoxifen users from 32% between 2004 and 2006 to 52% in 2010, more rapidly than among aromatase inhibitor users (DD:0.15, CI:0.08, 0.23). The factor most strongly associated with strong inhibitor and tamoxifen co-prescribing after 2006 was prior strong inhibitor use (RR:4.73, CI:3.62–6.18).
Conclusion
There were substantial declines in strong CYP2D6-inhibitor use among tamoxifen users following dissemination of information suggesting a potential for increased risk with co-prescribing. Whether patients and providers will continue to avoid strong inhibitor antidepressants is yet to be seen, but clinicians appear to be responsive to drug interaction risk information in this setting.
Keywords: Tamoxifen, CYP2D6, drug interaction, FDA Advisories, drug utilization
INTRODUCTION
An estimated 230,480 women were diagnosed with breast cancer in the U.S. in 2011, and approximately two-thirds of these cancers were hormone (estrogen and/or progesterone) receptor-positive [1–4]. For women with hormone receptor-positive breast cancers, adjuvant endocrine therapy with tamoxifen or aromatase inhibitors substantially decreases the risk of cancer recurrence [5]. Current guidelines recommend treatment with tamoxifen and/or an aromatase inhibitor for 5 years for postmenopausal women with hormone receptor-positive early-stage breast cancer [7, 8].
Over the last decade researchers have been investigating a potential link between CYP2D6 inhibition and breast cancer recurrence among women taking tamoxifen [9, 10]. Several studies over that timeframe have suggested that certain antidepressants inhibit the metabolism of tamoxifen, theoretically reducing tamoxifen’s effectiveness and thereby increasing the risk of breast cancer recurrence [11–15]. In response to concerns about CYP2D6 inhibition, in October 2006 a Food and Drug Administration (FDA) advisory committee recommended tamoxifen’s label be changed, noting that postmenopausal women with ER-positive breast cancer who are poor CYP2D6 metabolizers by genotype or due to drug interactions may be at increased risk of cancer recurrence.
Subsequent evidence of the impact of concurrent antidepressant and tamoxifen use on patient outcomes has been mixed [11, 17–20], including two large, recently published studies that suggest that CYP2D6 metabolism is not associated with breast cancer outcomes.[18, 19] Nevertheless, as researchers have worked to identify the role of CYP2D6 metabolism on outcomes among patients taking tamoxifen clinical guidelines and publications developed over recent years have generally encouraged the avoidance of strong inhibitors among tamoxifen users [21–24] given the number of treatment alternatives available [11, 24–26]. Since antidepressants are frequently used for treating depression, anxiety, and hot flashes among women with breast cancer, [26–29] understanding the impact of accumulating drug risk information on overall antidepressant use is important.
Our objectives were to examine trends in overall antidepressant-tamoxifen co-prescribing over time, with a focus on changes following the October 2006 FDA advisory meeting. We also assessed changes in co-prescribing for strong, moderate, and weak CYP2D6 inhibitors and evaluated patient and treatment characteristics associated with use of strong CYP2D6 inhibitors following the advisory committee meeting.
METHODS
Data Source
We used data from the Thompson Reuters MarketScan Commercial Claims and Encounters and Medicare Supplement databases for calendar years 2004–2010. Together these data represent the healthcare experience of retired and non-retired employees and their dependents enrolled in commercial health insurance plans sponsored by over 100 large or medium sized U.S.-based employers. The data include monthly enrollment data, inpatient and outpatient medical claims, and outpatient prescription drug claims. The Medicare Supplemental database includes Medicare beneficiaries with employer-sponsored supplemental insurance from a MarketScan employer. In 2007, over half of all Medicare beneficiaries with incomes over $30,000 received employer-sponsored supplemental coverage [30]. Overall, the MarketScan data represent over 50 million enrollees and is the largest convenience sample available in proprietary U.S. claims databases [31].
Design and Study Populations
We assessed changes in co-prescribing of antidepressants among women taking tamoxifen before and after the FDA advisory committee meeting compared with women taking aromatase inhibitors (adjuvant endocrine therapy for which there are no concerns about interactions with CYP2D6 inhibitors). We identified women aged 50 years or older with two or more diagnoses for breast cancer (ICD-9 codes 174.x, at least 30 days apart) during 2004–2010 who had prescription drug coverage data reported to MarketScan. Among these women, we identified women with a first prescription for tamoxifen or an aromatase inhibitor (anastrozole, exemestane, or letrozole) between July 1, 2004 and December 31, 2009. The first observed dispensing date for an endocrine therapy was considered the index drug date. Women without at least 6 months of continuous enrollment before their index drug date were excluded, as were women with other cancer diagnoses (ICD-9 codes 140–208.81, except 174.x [breast cancer], 173.x [non-melanoma skin cancers], and 196–199.1 [metastatic cancer]), those who did not have a breast cancer diagnosis from 6 months before through 1 year after the index date and those who switched from tamoxifen to aromatase inhibitors (or vice versa) within the index year. This resulted in 52,612 women eligible for analyses.
Ascertainment of Medication Use
Pharmacy claims were used to assess monthly utilization of tamoxifen, aromatase inhibitors and antidepressants. Each woman was considered a medication user in a month if they had at least 15 days of medication available during the month. Available medication was calculated using the fill date and days of supply.
We classified antidepressants by their cytochrome p450 inhibitor status based on the most recent guidelines from the American Psychiatric Association [22]. We considered bupropion, fluoxetine and paroxetine to be strong inhibitors; desipramine, duloxetine, escitalopram, norfluoxetine and sertraline to be moderate inhibitors; and citalopram, imipramine, nortriptyline, selegiline, desmethylsertraline and venlafaxine to be weak inhibitors.
Covariates
We adjusted for the following control variables measured in the month of the patient’s index endocrine therapy: patient age, insurance type (commercial or Medicare), region, and the enrollees’ relationship to employee (employee or spouse) and quarter during which the endocrine therapy was prescribed. We used administrative data to characterize other diagnoses and health care utilization in the 6 months preceding the index prescription fill date. We assessed antidepressant need by identifying diagnoses of major depressive disorder (ICD-9 codes: 296.2, 296.3), other depression diagnoses (ICD-9 codes: 300.4x, 311.x), and anxiety disorder (ICD-9 codes: 293.84, 300.0x, 300.2x, 300.3, 308.3, 309.21, 309.81). We documented hospitalizations with a breast cancer diagnosis code and inpatient or outpatient diagnosis codes for metastatic breast cancer (ICD-9 codes: 196.x–199.1), and we characterized comorbidity using the Klabunde modification of the Charlson score [32]. We characterized health service utilization using measures of the number of medication classes [33, 34](excluding antineoplastic and psychotherapeutic agents), the number of outpatient visits, receipt of treatment from a psychiatrist, and care by primary care physicians and oncologists, categorized as primary care physicians only, oncologists only, or both primary care physicians and oncologists. We adjusted for differences in antidepressant need and health care services use over time by controlling for depression diagnoses, prior antidepressant use (separate indicators for strong, moderate or weak inhibitors) and frequency of outpatient visits during the 6-month period preceding each endocrine fill.
Propensity Score Match
We created a propensity-score matched cohort in which patients initiating treatment with tamoxifen were matched to those initiating treatment with aromatase inhibitors [35]. Using women taking aromatase inhibitors as the control group allowed us to estimate changes in co-prescribing among tamoxifen users while controlling for changes in antidepressant use over the study period in a group of patients that would not be subjected to the same drug interaction risk concerns (i.e., aromatase inhibitors are not metabolized via the CYP2D6 pathway). To perform the propensity score match, we modeled the probability of receiving tamoxifen (vs. aromatase inhibitor) as a function of all of the pre-treatment covariates described above, with separate matching within calendar year to account for changes in adoption of endocrine therapy and interactions between covariates and time. Next, using the resulting propensity score, we matched patients initiating treatment with tamoxifen 1:1 to those initiating treatment with aromatase inhibitors.
Analytic Strategy
Using the propensity score matched cohort, we estimated multivariate adjusted changes in the proportion of women receiving an antidepressant for each person-month of endocrine therapy use. Next, we estimated changes in the use of strong, moderate and weak CYP2D6 inhibitors among antidepressant users for each person-month, thus the unit of analysis was the medication-fill. We used generalized estimating equations with a log link and Poisson distribution to control for repeated observations on individuals over time. Women were allowed to contribute up to 12 months of person-time to the analysis. For each outcome we compared average monthly prescribing before the FDA advisory committee meeting (from October 2004–October 2006) to prescribing in each subsequent year (from November 2006–October 2010). To isolate changes in antidepressant use among tamoxifen users that were due to concerns about CYP2D6 interaction risk, and not antidepressant market changes alone, we implemented a difference-in-differences modeling approach. We used SAS 9.2 (Cary, NC) for analyses.
Finally, we modeled patient and treatment characteristics related to use of strong inhibitors following the FDA advisory committee meeting. We restricted these analyses to women using tamoxifen and an antidepressant in the post-advisory period. Comparisons were between those co-prescribed strong inhibitors and those co-prescribed moderate, weak or unclassified inhibitors.
Sensitivity Analyses
To test the robustness of our study findings to the timeframe selected, we revised the timeframe to consider (1) an aggregate post-advisory period (from November 2006–October 2010), and (2) shorter and longer-term changes in co-prescribing by estimating use in the first year post-advisory period (from November 2006–October2007) separately from the remaining post-advisory period.
Second, we restricted our sample to women who had continuous enrollment for the 12-month period following their index prescription-fill to determine whether differences in follow-up might bias our estimates. Third, to account for delays in transitioning patients from strong inhibitors we also assessed patient and treatment characteristics of individuals receiving strong inhibitors two years or more following the advisory (from November 2008–October 2010). Finally, we excluded 16% of the patients in our sample who received carved-out mental health services since our ability to detect mental health diagnoses (but not prescriptions) may be limited for these individuals.
RESULTS
Patient Characteristics
Before matching, women receiving tamoxifen as their index treatment differed from women receiving aromatase inhibitors (Table 1). Women receiving tamoxifen were younger, less likely to be treated for cancer in an inpatient setting or to have metastatic breast cancer diagnoses, and had fewer outpatient visits and classes of medications prescribed in the 6 months before the index prescription date. We successfully matched 13,205 of 14,346 tamoxifen users to an aromatase inhibitor user. After matching, the two groups were well balanced on all baseline characteristics. Tamoxifen users who were unmatched (approximately 8% of the sample) were younger, but otherwise similar, to those in the matched cohort.
Table 1.
Baseline Characteristicsa of Enrollees Initiating Endocrine Therapy
| Unmatched Sample | Propensity Score Matched Sample | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Tamoxifen (N = 14,346) | Aromatase Inhibitors (N = 38,266) | P-valueb | Tamoxifen (N = 13,205) | Aromatase Inhibitors (N = 13,205) | P-valueb | |
| Age – Mean (standard deviation [SD]) | 60.5 (10.3) | 64.2 (9.7) | < 0.001 | 61.3 (10.3) | 61.3 (10.2) | 0.89 |
| Age in years - % | < 0.001 | 0.73 | ||||
| 50–59 | 57.2 | 37.9 | 53.7 | 54.0 | ||
| 60–69 | 22.1 | 34.1 | 24.0 | 23.8 | ||
| 70–79 | 13.6 | 18.6 | 14.7 | 14.9 | ||
| 80+ | 7.1 | 9.4 | 7.7 | 7.3 | ||
| Insurance - % | < 0.001 | 0.61 | ||||
| Commercial | 72.5 | 61.9 | 70.3 | 70.6 | ||
| Medicare | 27.5 | 38.1 | 29.7 | 29.4 | ||
| Region - % | 0.005 | 0.72 | ||||
| Northeast | 9.8 | 9.0 | 9.3 | 9.4 | ||
| North Central | 31.3 | 32.2 | 31.5 | 31.4 | ||
| South | 36.2 | 36.7 | 36.3 | 36.8 | ||
| West | 22.7 | 22.2 | 22.9 | 22.4 | ||
| Relationship to Employee – % | 0.66 | |||||
| Employee | 62.4 | 60.8 | 0.001 | 62.7 | 62.4 | |
| Spouse | 37.6 | 39.2 | 37.3 | 37.6 | ||
| Klabunde Modification of Charlson Index – % | < 0.001 | 0.11 | ||||
| 0 | 89.2 | 85.3 | 88.5 | 88.5 | ||
| 1 | 8.9 | 11.6 | 9.4 | 9.1 | ||
| 2+ | 1.9 | 3.1 | 2.0 | 2.4 | ||
| Prior Inpatient Cancer Visits – % | < 0.001 | 0.10 | ||||
| Any in 6-month pre-index date | 13.1 | 20.0 | 13.7 | 13.0 | ||
| Prior Metastatic Breast Cancer Diagnoses – % | < 0.001 | 0.04 | ||||
| Any in 6-month pre-index date | 8.6 | 19.3 | 9.3 | 8.6 | ||
| Prior Mental Health Inpatient Treatment – % | 0.35 | 0.84 | ||||
| Any in 6-month pre-index date | 0.4 | 0.4 | 0.4 | 0.3 | ||
| Prior Major Depressive Disorder – % | 0.27 | 0.36 | ||||
| Any in 6-month pre-index date | 2.5 | 2.3 | 2.4 | 2.6 | ||
| Other Prior Depressive Diagnoses – % | 0.05 | 0.57 | ||||
| Any in 6-month pre-index date | 3.6 | 3.2 | 3.5 | 3.6 | ||
| Prior Anxiety – % | 0.02 | 0.51 | ||||
| Any in 6-month pre-index date | 1.2 | 0.9 | 1.0 | 1.1 | ||
| Prior Antidepressant Use - % | ||||||
| Prior strong inhibitor use | 8.2 | 8.0 | 0.52 | 8.2 | 8.0 | 0.64 |
| Prior moderate inhibitor use | 8.4 | 9.0 | 0.02 | 8.4 | 8.8 | 0.21 |
| Prior weak inhibitor use | 8.2 | 8.0 | 0.48 | 8.2 | 8.3 | 0.84 |
| Received Treatment from a Psychiatrist | ||||||
| Any in 6-month pre-index date | 2.7 | 2.8 | 0.60 | 2.7 | 2.8 | 0.88 |
| Outpatient Visits in 6-Month Pre-Period – Mean (SD) | 26.6 (17.4) | 29.1 (18.2) | < 0.001 | 26.7 (17.7) | 26.5 (18.0) | 0.33 |
| Medication Classesc in 6-Month Pre-Period – Mean(SD) | 5.9 (3.9) | 6.9 (4.3) | < 0.001 | 6.1 (3.9) | 6.1 (4.1) | 0.91 |
| Types of Providers in Prior 6 Months – % | ||||||
| Oncology only | 9.3 | 9.5 | 0.53 | 9.4 | 9.2 | 0.57 |
| Primary care only | 36.6 | 36.4 | 0.61 | 37.1 | 37.2 | 0.77 |
| Oncology or primary care | 42.1 | 43.2 | 0.03 | 41.8 | 41.6 | 0.74 |
Characteristics measured at the index prescription fill date unless otherwise indicated.
Statistical significance tested using t-tests for continuous variables and chi-squared tests for categorical variables.
Medication class count variable excludes antineoplastic and psychotherapeutic agents. Count is based on filled prescriptions.
Endocrine Therapy and Antidepressant Co-Prescribing
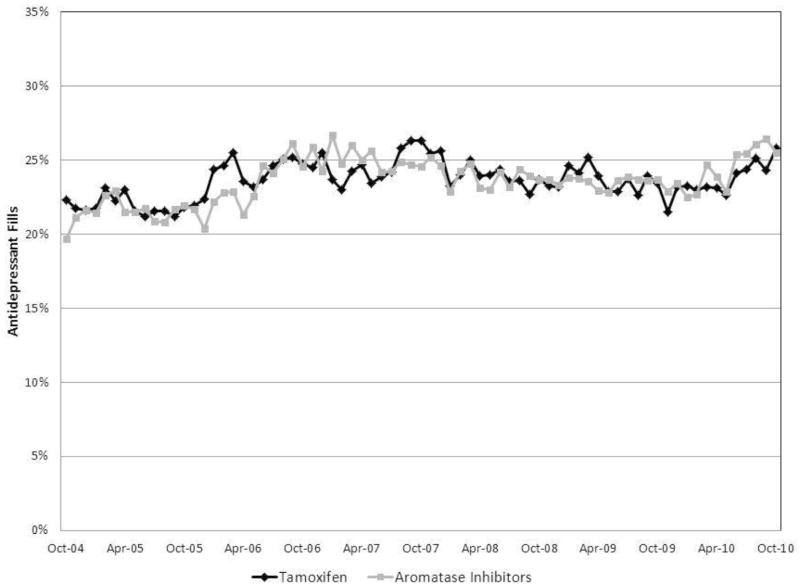
Approximately 24% of women using either tamoxifen or aromatase inhibitors in any given month also received a prescription for an antidepressant within that same month. Co-prescribing of antidepressant therapies remained constant over the study period among women taking tamoxifen and women taking aromatase inhibitors (Figure 1).
Figure 1. Proportion of Women Receiving Antidepressants and Endocrine Therapy by Month – Propensity Score Matched Sample.
The figure demonstrates the proportion of women taking tamoxifen (black line) and aromatase inhibitors (gray line) who were also prescribed an antidepressant in each month of observation.
Strong Inhibitor Co-Prescribing
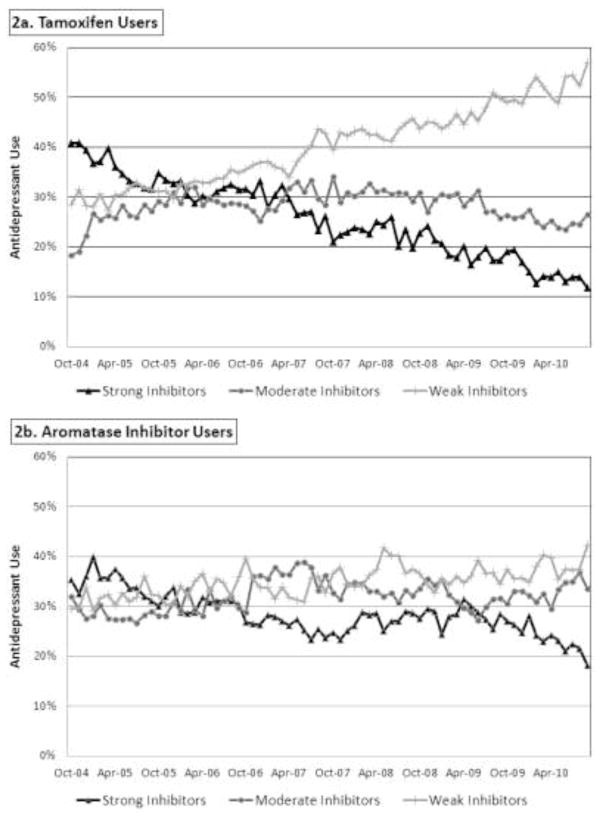
For women taking both tamoxifen and antidepressants, 34% were prescribed strong inhibitors during the first two years of observation (Figure 2). Strong inhibitor use decreased among all endocrine therapy users following the FDA advisory committee meeting in October 2006. However, comparing use between the two groups, co-prescribing decreased more among tamoxifen users than aromatase inhibitor users from the pre-advisory period to the most recent year (Difference-in-Differences [DD]: −0.09, 95% Confidence Interval[CI]: −0.15, −0.03) (Table 2). By 2010, strong inhibitors were used in approximately 15% of women co-prescribed tamoxifen and antidepressants versus 24% of women co-prescribed aromatase inhibitors and antidepressants.
Figure 2. Antidepressant Co-Prescribing by CYP2D6 Status – Propensity Score Matched Sample.
The figure demonstrates the proportion of medication fills for strong (black line), moderate (gray line), and weak (light gray line) inhibitors among women taking tamoxifen (Panel 2a) and aromatase inhibitors (Panel 2b).
Table 2.
Trends in Co-Prescribing Overall and by Inhibitor Groupa, Propensity Score Matched Cohort
| Tamoxifen | Aromatase Inhibitors | Tamoxifen vs. Aromatase Inhibitors Over Time | ||
|---|---|---|---|---|
|
| ||||
| Multivariate Adjusted Modeled Outcomeb | Adjusted Risk | Adjusted Risk | Difference-in- Differences | 95% Confidence Interval |
| Any Antidepressant Co-Prescribing | ||||
| Pre-Advisory Committee Meeting (Oct 2004–Oct 2006) | 0.21 | 0.21 | 0.00 | REF |
| One Year Post (Nov 2006–Oct 2007) | 0.23 | 0.23 | −0.00 | −0.02, 0.02 |
| Two Years Post (Nov 2007–Oct 2008) | 0.23 | 0.22 | 0.01 | −0.02, 0.03 |
| Three Years Post (Nov 2008–Oct 2009) | 0.22 | 0.21 | 0.01 | −0.01, 0.03 |
| Four Years Post (Nov 2009 – Oct 2010) | 0.22 | 0.21 | 0.00 | −0.02, 0.03 |
| Strong Inhibitor Use Among Antidepressant Users | ||||
| Pre-Advisory Committee Meeting (Oct 2004–Oct 2006) | 0.31 | 0.30 | 0.00 | REF |
| One Year Post (Nov 2006–Oct 2007) | 0.25 | 0.23 | 0.01 | −0.04, 0.07 |
| Two Years Post (Nov 2007–Oct 2008) | 0.20 | 0.25 | −0.05 | −0.11, 0.00 |
| Three Years Post (Nov 2008–Oct 2009) | 0.17 | 0.25 | −0.10 | −0.15, −0.05 |
| Four Years Post (Nov 2009 – Oct 2010) | 0.13 | 0.21 | −0.09 | −0.15, −0.03 |
| Moderate Inhibitor Use Among Antidepressant Users | ||||
| Pre-Advisory Committee Meeting (Oct 2004–Oct 2006) | 0.24 | 0.26 | 0.00 | REF |
| One Year Post (Nov 2006–Oct 2007) | 0.26 | 0.33 | −0.05 | −0.10, 0.01 |
| Two Years Post (Nov 2007–Oct 2008) | 0.28 | 0.30 | −0.00 | −0.06, 0.05 |
| Three Years Post (Nov 2008–Oct 2009) | 0.25 | 0.28 | −0.01 | −0.06, 0.04 |
| Four Years Post (Nov 2009 – Oct 2010) | 0.22 | 0.29 | −0.06 | −0.12, 0.01 |
| Weak Inhibitor Use Among Antidepressant Users | ||||
| Pre-Advisory Committee Meeting (Oct 2004–Oct 2006) | 0.36 | 0.37 | 0.00 | REF |
| One Year Post (Nov 2006–Oct 2007) | 0.42 | 0.38 | 0.05 | −0.00, 0.11 |
| Two Years Post (Nov 2007–Oct 2008) | 0.47 | 0.42 | 0.07 | 0.01, 0.12 |
| Three Years Post (Nov 2008–Oct 2009) | 0.50 | 0.40 | 0.12 | 0.07, 0.18 |
| Four Years Post (Nov 2009 – Oct 2010) | 0.55 | 0.41 | 0.15 | 0.08, 0.23 |
Classified as strong (bupropion, fluoxetine and paroxetine), moderate (desipramine, duloxetine, escitalopram, norfluoxetine and sertraline) or weak (citalopram, imipramine, nortriptyline, selegiline, desmethylsertraline and venlafaxine)
Generalized estimating equations with a Poisson distribution were used to account for the repeated measures on each subject.
Moderate Inhibitor Co-Prescribing
Use of moderate CYP2D6 inhibitors increased somewhat among all endocrine therapy users during the first two years following the FDA advisory committee meeting. Use among women taking tamoxifen returned to pre-advisory levels in the third and fourth year following the advisory. There were no statistically significant differences in moderate inhibitor use over time between tamoxifen users and aromatase inhibitor users (Table 2).
Weak Inhibitor Co-Prescribing
Use of weak CYP2D6 inhibitors increased significantly among tamoxifen users following the FDA advisory committee meeting. For women taking tamoxifen, weak inhibitors made up 32% of antidepressant fills before the FDA advisory, and 52% by 2010. From the pre-advisory period to the most recent year, weak inhibitor use grew more among tamoxifen users than aromatase inhibitor users (DD:0.15, CI:0.08, 0.23, Table 2).
Characteristics Associated with Strong Inhibitor Use
Several characteristics were associated with co-prescribing of strong inhibitors and tamoxifen following the FDA advisory committee meeting (Table 3). Women using strong inhibitors before the advisory were more likely to use strong inhibitors after the advisory period, compared with women using moderate, weak or unclassified inhibitors (RR:4.73, CI:3.62, 6.18). The year during which tamoxifen treatment was initiated was also associated with strong inhibitor use, with individuals initiating tamoxifen prior to 2009 being more likely to receive strong inhibitors. Finally, having more comorbid conditions (measured using the Klabunde modification of the Charlson index) and having a higher than average number of outpatient visits were associated with a greater likelihood of strong inhibitor use.
Table 3.
Characteristics of Enrollees Co-Prescribed Tamoxifen and Strong Inhibitors in the Post-Advisory Period
| Unadjusted Risk Ratio | 95% Confidence Interval | P-value | Adjusted Risk Ratio | 95% Confidence Interval | P-value | |
|---|---|---|---|---|---|---|
|
Demographic Characteristics
| ||||||
| Age | 0.99 | 0.98, 1.00 | 0. 13 | 0.99 | 0.98, 1.00 | 0.07 |
| Region | ||||||
| Northeast | 1.02 | 0.77, 1.36 | 0.88 | 1.01 | 0.76, 1.33 | 0.96 |
| North Central | 0.89 | 0.71, 1.11 | 0.29 | 0.91 | 0.73, 1.13 | 0.40 |
| South | 0.85 | 0.69, 1.04 | 0.12 | 0.90 | 0.74, 1.10 | 0.30 |
| West | 1.00 | REF | 1.00 | REF | ||
| Relationship to Employee | ||||||
| Employee | 1.02 | 0.88, 1.19 | 0.78 | 1.00 | 0.86,1.16 | 0.99 |
| Spouse | 1.00 | REF | 1.00 | REF | ||
|
| ||||||
|
Year Treatment Initiated
| ||||||
| 2005 | 1.88 | 1.19, 2.99 | 0.007 | 1.35 | 0.90, 2.02 | 0.15 |
| 2006 | 1.76 | 1.41, 2.20 | < 0.001 | 1.25 | 0.91, 1.70 | 0.17 |
| 2007 | 1.37 | 1.09, 1.72 | 0.007 | 1.34 | 1.07, 1.67 | 0.01 |
| 2008 | 1.22 | 0.99, 1.51 | 0.06 | 1.23 | 0.99, 1.51 | 0.06 |
| 2009 | 1.00 | REF | 1.00 | REF | ||
|
| ||||||
|
Disease Severity Measures
| ||||||
| Klabunde Modification of Charlson Index | 1.31 | 1.13, 1.52 | <0.001 | 1.34 | 1.16, 1.56 | <0.001 |
| Prior Inpatient Cancer Visits | 1.06 | 0.86, 1.30 | 0.57 | 0.98 | 0.80, 1.21 | 0.89 |
| Prior Metastatic Breast Cancer Diagnoses | 1.03 | 0.79, 1.34 | 0.83 | 1.04 | 0.80, 1.36 | 0.76 |
|
| ||||||
|
Mental Health Indicators
| ||||||
| Prior Antidepressant Use | ||||||
| Prior strong inhibitor use | 4.52 | 4.08, 5.02 | < 0.001 | 4.73 | 3.62, 6.18 | < 0.001 |
| Prior moderate inhibitor use | 0.75 | 0.45, 1.23 | 0.25 | 0.53 | 0.35, 0.80 | 0.002 |
| Prior weak inhibitor use | 0.26 | 0.12, 0.54 | < 0.001 | 0.23 | 0.11, 0.48 | < 0.001 |
| Prior Major Depressive Disorder | 1.33 | 1.06, 1.68 | 0.01 | 1.20 | 0.93, 1.55 | 0.16 |
| Other Prior Depressive Diagnoses | 1.26 | 1.05, 1.53 | 0.01 | 1.21 | 1.00, 1.47 | 0.05 |
| Prior Anxiety | 1.12 | 0.75, 1.68 | 0.57 | 0.93 | 0.62, 1.38 | 0.72 |
| Prior Treatment from a Psychiatrist | 1. 33 | 1.06, 1.68 | 0.01 | 1.18 | 0.93, 1.50 | 0.73 |
|
| ||||||
|
Health Services Utilization Measures
| ||||||
| Prior Outpatient Services Use | ||||||
| Lower than Average Number of Outpatient Visits | 0.99 | 0.84, 1.18 | 0.97 | 1.04 | 0.90, 1.22 | 0.57 |
| Higher than Average Number of Outpatient Visits | 1.24 | 1.10, 1.39 | <0.001 | 1.19 | 1.07, 1.34 | 0.002 |
| Average Number of Outpatient Visits | 1.00 | REF | 1.00 | REF | ||
| Prior Medication Use | ||||||
| Lower than Average Number of Medications | 1.01 | 0.86, 1.17 | 0.93 | 1.04 | 0.89, 1.20 | 0.64 |
| Higher than Average Number of Medications | 1.04 | 0.91, 1.20 | 0.56 | 0.74 | 0.84, 1.20 | 0.62 |
| Average Number of Medications | 1.00 | REF | 1.00 | REF | ||
| Types of Providers in Prior 6 Months | ||||||
| Oncologists Only | 0.71 | 0.56, 0.90 | 0.004 | 0.86 | 0.64, 1.17 | 0.33 |
| Primary Care Only | 1.00 | 0.86, 1.16 | 0.99 | 1.10 | 0.87, 1.40 | 0.41 |
| Oncologists and Primary Care | 1.19 | 1.04, 1.36 | 0.01 | 1.22 | 0.97, 1.54 | 0.09 |
Sensitivity Analyses
Results of sensitivity analyses using alternative definitions of the post-advisory periods and those restricted to women with 12 months of continuous enrollment were consistent with the primary analysis. Similarly, for modeling patient and provider characteristics associated with post-advisory strong inhibitor use, results from our primary analysis were identical to those restricted to patients using strong inhibitors during 2009 and 2010. Finally, there were no differences in our primary analysis results and models excluding individuals with possible missing mental health services data.
CONCLUSIONS
Among women with breast cancer initiating tamoxifen therapy, we found significant reductions in strong CYP2D6 inhibitor use following reports of potential drug-drug interactions. There were also large increases in weak inhibitor use and no changes in overall antidepressant use, suggesting shifts from stronger to weaker CYP2D6 inhibitors. By 2010, strong CYP2D6 inhibitors were co-prescribed with tamoxifen in approximately 15% of patients using antidepressants. These declines in strong CYP2D6 inhibitor co-prescribing suggest that providers treating patients with breast cancer were responsive to information regarding possible drug interaction risks.
Until recently, publications and guidelines encouraged providers to avoid strong inhibitors in patients who were using tamoxifen. In our sample, we found that patients with previous use of a strong inhibitor, more comorbidities, and a higher than average number of outpatient visits were more likely to be co-prescribed a strong CYP2D6 inhibitor with tamoxifen following the FDA advisory meeting.
Recent evidence raises questions about whether poor metabolizers of CYP2D6 have a higher risk of disease recurrence [18, 19]. In fact, these studies add to a larger body of literature that has produced contradictory data on the importance of CYP2D6 metabolism for patients using tamoxifen.[20] Moreover, while these studies were based on large clinical trials and were generally viewed as providing an overall answer to the debate regarding CYP2D6 metabolism and outcomes, [36] they have also received significant criticism.[37–39] Given the currently mixed data regarding the impact of strong inhibitors on outcomes, the routine use of antidepressants among breast cancer patients and the large number of treatment alternatives available, the benefits of reducing co-prescribing of strong CYP2D6 inhibitors and tamoxifen may outweigh any downsides.
When evaluating characteristics of patients who were co-prescribed antidepressants and tamoxifen following the FDA Advisory Committee’s recommendation to change tamoxifen’s label to warn of drug interaction risks, we observed that past strong inhibitor use was associated with future use, and we also found that individuals with more comorbid conditions and those using more outpatient care were more likely than others to be co-prescribed a strong rather than a moderate or weak CYP2D6 inhibitor with tamoxifen. There are several explanations that may account for this. Patients with more frequent office visits and more comorbid illness may see multiple providers who prescribe different medications and may not be aware of other prescribed therapies. Additionally, clinicians may hesitate to change a patient’s medication prescribed by another specialist. Patients may also fail to disclose information about their ongoing medication use to clinicians, impairing the clinician’s ability to recognize a potential drug-drug interaction.
With regard to the impact of prior strong inhibitor use on later use, clinicians might have hesitated to switch antidepressants for patients who were previously stabilized on a strong inhibitor antidepressant. In fact, clinicians and patients must carefully weigh the benefits and risks of changing therapies for patients who have responded well to a particular medication. Given more recent evidence suggesting a lack of impact on CYP2D6 metabolism on outcome, risks of switching for current antidepressant users may outweigh potential benefits.
It is important to note that clinicians who continued to prescribe strong inhibitors and tamoxifen concurrently following the FDA advisory committee’s decision may have done so for multiple reasons. For example, clinicians might have been unaware of, or disagreed with, the data available at that time regarding potential interaction risk between tamoxifen and CYP2D6 inhibitors. Additionally, they might have lacked information on medication regimens currently used by the patient. These issues may be particularly relevant if primary care providers or psychiatrists are managing a patients’ depression or anxiety, especially if those providers do not share a medical record with the tamoxifen prescriber.
Because treatment-related side effects of endocrine therapies may impact adherence [40], and untreated depression is associated with treatment non-adherence, poorer quality of life and worse health outcomes for patients with cancer [27, 41, 42], it is important for patients to continue to receive pharmacologic and non-pharmacologic therapies. For women currently co-prescribed strong inhibitors and tamoxifen who remain concerned about a potential drug interaction risk, there are many alternative treatments or treatment strategies to consider. These include switching antidepressants (e.g., to weak inhibitors), switching to a non-antidepressant treatment (e.g., to psychotherapy), or switching from tamoxifen to an aromatase inhibitor (for post-menopausal women) if there are concerns that the patient would experience adverse outcomes due to switching antidepressant therapies. As mentioned previously, benefits and risks of therapeutic switching should be carefully weighed by the patient and their provider.
When initiating therapy with antidepressants among women who are currently taking tamoxifen, a prudent approach might be to avoid strong inhibitors as a first choice of therapy. Although patients vary in their response to antidepressant treatments, these drugs are similarly effective in treating depression or anxiety disorders [43, 44] and venlafaxine (a weak inhibitor) may be effective in treating hot flashes among some breast cancer survivors [45]. Moreover, among the known weak inhibitors, there are agents from each antidepressant category and most are available in generic forms, suggesting that barriers to initiating weak inhibitors over strong inhibitors (e.g., formulary restrictions, patient or clinician preference for a specific category of antidepressants) are likely to be minimal.
This study has some limitations. First, we excluded women <50 years because including pre-menopausal women would not allow for a well-matched control population. Furthermore, we used age as an indicator of menopausal status but were unable to measure this directly, so some women included may not have been candidates for aromatase inhibitors. Second, using propensity score matched controls only accounts for measured confounders, thus unmeasured confounders may still influence our results. Third, we lacked information on antidepressant treatment preferences and reasons for treatment selection, including the primary indication (e.g., depression, anxiety, or hot flashes). Fourth, our cohort included women with employer-based insurance and/or supplemental Medicare coverage and our results may not generalize to publicly insured (Medicaid or Medicare without employer supplemental coverage) or uninsured individuals. Fifth, although we could identify the specialty of physicians seen, we were unable to identify prescribing physicians. Sixth, new data regarding management of hot flashes with medications such as venlafaxine (a weak inhibitor) became available during our study period. This might account for some of the increase in weak inhibitor use, particularly if there are large imbalances between tamoxifen users and aromatase inhibitor users in the proportion receiving antidepressants for hot flashes management. Seventh, we do not have information on patient genotype which might have played a role in the decision to continue to use strong inhibitors among ongoing users. Finally, declines in drug utilization are likely the result of a combination of factors including media coverage, pharmaceutical promotion, regulatory actions and clinical communications. While we use the FDA’s advisory committee meeting as reference point for our analysis, this should not be construed to indicate that the committee’s recommendation was solely responsible for the decline in co-prescribing observed in our study.
In conclusion, use of strong CYP2D6 inhibitors declined substantially among tamoxifen users following dissemination of information suggesting a potential for increased risk with co-prescribing. The evidence related to the impact of strong CYP2D6 inhibitors on breast cancer recurrence remains controversial. Whether patients and providers will continue to avoid strong inhibitor antidepressants is yet to be seen, but clinicians appear to be responsive to drug interaction risk information in this setting.
Acknowledgments
Author Funding
This work was supported by the Agency for Healthcare Research and Quality (RO1 HS0189960). The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript for publication.
Footnotes
Potential Conflicts of Interest:
Additional author funding and potential conflicts of interest are noted below:
Stacie B. Dusetzina, PhD: Dr. Dusetzina currently receives funding through a Ruth L. Kirschstein-National Research Service Award Post-Doctoral Traineeship sponsored by NIMH and Harvard Medical School, Department of Health Care Policy, Grant No. T32MH019733-17. Dr. Dusetzina has no conflicts of interest.
G. Caleb Alexander, MD, MS: Dr. Alexander has served as an ad hoc member of the FDA Drug Safety and Risk Management (DSaRM) Advisory Committee and is a consultant for IMS Health.
Rachel A. Freedman, MD, MPH: Dr. Freedman’s effort was supported by the Susan G. Komen for the Cure Foundation. Dr. Freedman has no conflicts of interest.
Haiden A. Huskamp, PhD: Dr. Huskamp serves as an uncompensated steering committee member of the IMS Health Services Research Network.
Nancy L. Keating, MD, MPH: Dr. Keating’s effort was supported by the Susan G. Komen for the Cure Foundation. Dr. Keating has no conflicts of interest.
References
- 1.Breast Cancer Key Statistics. [ http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-key-statistics]
- 2.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103(18):1397–1402. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76(1):27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. version 2.2011 [ http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site]
- 7.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23(3):619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 8.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 9.Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 10.Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, Fritz P, Simon W, Suman VJ, Ames MM, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. Jama. 2009;302(13):1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry. 2009;70(12):1688–1697. doi: 10.4088/JCP.08r04856blu. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97(1):30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 13.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 14.Stearns V, Rae JM. Pharmacogenetics and breast cancer endocrine therapy: CYP2D6 as a predictive factor for tamoxifen metabolism and drug response? Expert Rev Mol Med. 2008;10:e34. doi: 10.1017/S1462399408000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 16.CDER. Advisory Committee Meeting Documents.2006. [Google Scholar]
- 17.Seruga B, Amir E. Cytochrome P450 2D6 and outcomes of adjuvant tamoxifen therapy: results of a meta-analysis. Breast Cancer Res Treat. 2010;122(3):609–617. doi: 10.1007/s10549-010-0902-3. [DOI] [PubMed] [Google Scholar]
- 18.Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, Dell’orto P, Biasi MO, Thurlimann B, Lyng MB, et al. CYP2D6 Genotype and Tamoxifen Response in Postmenopausal Women with Endocrine-Responsive Breast Cancer: The Breast International Group 1-98 Trial. J Natl Cancer Inst. 2012;104(6):441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP, Salter J, Sestak I, Cuzick J, Dowsett M, et al. CYP2D6 and UGT2B7 Genotype and Risk of Recurrence in Tamoxifen-Treated Breast Cancer Patients. J Natl Cancer Inst. 2012;104(6):452–460. doi: 10.1093/jnci/djs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertz DL, McLeod HL, Irvin WJ., Jr Tamoxifen and CYP2D6: a contradiction of data. Oncologist. 2012;17(5):620–630. doi: 10.1634/theoncologist.2011-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferraldeschi R, Howell SJ, Thompson AM, Newman WG. Avoidance of CYP2D6 inhibitors in patients receiving tamoxifen. J Clin Oncol. 2010;28(29):e584–585. doi: 10.1200/JCO.2010.30.1887. author reply e586. [DOI] [PubMed] [Google Scholar]
- 22.Gelenberg A, Freeman M, Markowitz J, Rosenbaum J, Thase M, Trivedi M, Van Rhoads R, Reus V, DePaulo J, Jr, Fawcett J, et al. American Psychiatric Association Practice Guidelines for the Treatment of Psychiatric Disorders. 3. 2010. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. [Google Scholar]
- 23.Higgins MJ, Stearns V. CYP2D6 polymorphisms and tamoxifen metabolism: clinical relevance. Curr Oncol Rep. 2010;12(1):7–15. doi: 10.1007/s11912-009-0076-5. [DOI] [PubMed] [Google Scholar]
- 24.Sideras K, Ingle JN, Ames MM, Loprinzi CL, Mrazek DP, Black JL, Weinshilboum RM, Hawse JR, Spelsberg TC, Goetz MP. Coprescription of tamoxifen and medications that inhibit CYP2D6. J Clin Oncol. 2010;28(16):2768–2776. doi: 10.1200/JCO.2009.23.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry NL, Stearns V, Flockhart DA, Hayes DF, Riba M. Drug interactions and pharmacogenomics in the treatment of breast cancer and depression. Am J Psychiatry. 2008;165(10):1251–1255. doi: 10.1176/appi.ajp.2008.08040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desmarais JE, Looper KJ. Managing menopausal symptoms and depression in tamoxifen users: implications of drug and medicinal interactions. Maturitas. 2010;67(4):296–308. doi: 10.1016/j.maturitas.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA, Gralow J. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30(2):112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Hunter MS, Grunfeld EA, Mittal S, Sikka P, Ramirez AJ, Fentiman I, Hamed H. Menopausal symptoms in women with breast cancer: prevalence and treatment preferences. Psychooncology. 2004;13(11):769–778. doi: 10.1002/pon.793. [DOI] [PubMed] [Google Scholar]
- 29.Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, et al. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30(3):208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Cubanski J, Neuman T, Damico A, Huang J. Examining Sources of Supplemental Insurance and Prescription Drug Coverage Among Medicare Beneficiaries: Findings from the Medicare Current Beneficiary Survey, 2007. The Henry J. Kaiser Family Foundation; 2009. [Google Scholar]
- 31.Thompson Reuters. Comparative Effectiveness Research - Research and Analytics. [ http://thomsonreuters.com/products_services/healthcare/healthcare_products/a-z/comparative_effectiveness_research/]
- 32.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154(9):854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 34.Farley JF, Harley CR, Devine JW. A comparison of comorbidity measurements to predict healthcare expenditures. The American journal of managed care. 2006;12(2):110–119. [PubMed] [Google Scholar]
- 35.Parsons LS. Reducing bias in propensity score matched-pair sample using greedy matching techniques. Poster Presented at the twenty-sixth annual SAS users group international conference; April 22–25, 2001; Long Beach, CA. [Google Scholar]
- 36.Kelly CM, Pritchard KI. CYP2D6 genotype as a marker for benefit of adjuvant tamoxifen in postmenopausal women: lessons learned. J Natl Cancer Inst. 2012;104(6):427–428. doi: 10.1093/jnci/djs139. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Ratain MJ, Cox NJ, McLeod HL, Kroetz DL, Flockhart DA. Re: CYP2D6 Genotype and Tamoxifen Response in Postmenopausal Women With Endocrine-Responsive Breast Cancer: The Breast International Group 1-98 Trial. J Natl Cancer Inst. 2012;104(16):1264. doi: 10.1093/jnci/djs304. [DOI] [PubMed] [Google Scholar]
- 38.Pharoah PD, Abraham J, Caldas C. Re: CYP2D6 Genotype and Tamoxifen Response in Postmenopausal Women With Endocrine-Responsive Breast Cancer: The Breast International Group 1-98 Trial and Re: CYP2D6 and UGT2B7 Genotype and Risk of Recurrence in Tamoxifen-Treated Breast Cancer Patients. J Natl Cancer Inst. 2012;104(16):1263–1264. doi: 10.1093/jnci/djs312. [DOI] [PubMed] [Google Scholar]
- 39.Stanton V., Jr Re: CYP2D6 Genotype and Tamoxifen Response in Postmenopausal Women With Endocrine-Responsive Breast Cancer: The Breast International Group 1-98 Trial. J Natl Cancer Inst. 2012;104(16):1265–1266. doi: 10.1093/jnci/djs305. [DOI] [PubMed] [Google Scholar]
- 40.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107(2):167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52(1):106–111. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356(9238):1326–1327. doi: 10.1016/S0140-6736(00)02821-X. [DOI] [PubMed] [Google Scholar]
- 43.Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, Van Noord M, Mager U, Thieda P, Gaynes BN, Wilkins T, et al. Comparative Benefits and Harms of Second-Generation Antidepressants for Treating Major Depressive Disorder: An Updated Meta-analysis. Ann Intern Med. 2011;155(11):772–785. doi: 10.7326/0003-4819-155-11-201112060-00009. [DOI] [PubMed] [Google Scholar]
- 44.Gartlehner G, Hansen RA, Reichenpfader U, Kaminski A, Kien C, Strobelberger M, Van Noord M, Thieda P, Thaler K, Gaynes B. Drug Class Review: Second-Generation Antidepressants: Final Update 5 Report edn. Portland (OR): 2011. [PubMed] [Google Scholar]
- 45.Bordeleau L, Pritchard KI, Loprinzi CL, Ennis M, Jugovic O, Warr D, Haq R, Goodwin PJ. Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28(35):5147–5152. doi: 10.1200/JCO.2010.29.9230. [DOI] [PubMed] [Google Scholar]