Abstract
The measurement of the absolute CD4 T-cell count is critical in the initial evaluation and staging of HIV-infected persons, yet access to this technology remains limited in many low resource settings where disease burden is highest. Here we evaluate the performance of a prototype point-of-care device (POC)1 to quantify CD4 T cells from MBio Diagnostics, Inc. Whole blood samples, both venous and capillary (finger stick), were collected from known HIV-infected participants at the University of California, San Diego Antiviral Research Center, and tested using the MBio system and conventional flow cytometry. A total of 94 venipuncture and 52 capillary samples were processed and statistical analyses included comparison to flow cytometry results. For the venipuncture samples, Bland-Altman analysis resulted in a mean bias of −10 cells/μl (−23 to +3 cells/μl, 95% CI), and limits of agreement (LOA) of −132 and +112 cells/μl. For the capillary samples, Bland-Altman resulted in a mean bias of −4 cells/μl (−31 to +23 cells/μl, 95% CL), and LOA of −195 and +186 cells/μl. For the San Diego study cohort, the prototype MBio system showed negligible quantitative bias relative to flow cytometry. Higher variability was observed in the capillary samples relative to venipuncture, but system precision for both capillary and venipuncture samples was good. There was also close agreement between results from the same participant when tested with two different systems, different operators and different locations. This preliminary evaluation suggests that the MBio CD4 device holds promise as a POC system for quantitation of CD4 T cells in limited-resource settings.
Keywords: HIV, CD4, diagnostic, point-of-care
1. INTRODUCTION
Destruction of CD4 helper T cells is the hallmark of HIV infection. Thus, the CD4 T cell count is an important measurement used for disease staging, management of prophylaxis for opportunistic infections, and together with HIV viral load testing, determining need for and monitoring of antiretroviral therapy. Flow cytometry provides accurate measurements of CD4 T cells and is the current standard-of-care in most settings. While there are examples of implementation of effective flow cytometry solutions at the national level(Glencross et al., 2008a; Glencross et al., 2008b), access to timely, cost-effective CD4 counts is still limited in many high disease burden, low resource settings.(Peter et al., 2008; Taiwo and Murphy, 2008; WHO, 2012).
Despite significant advances in HIV care, adequate laboratory infrastructure for HIV diagnosis and monitoring remains a major global health challenge, particularly in resource-limited areas(Vitoria et al., 2009; WHO, 2011). With each step in the HIV testing and treatment process, loss to follow up rates increase. Pre-treatment loss to follow up rates can exceed 50% in some areas and are a major challenge in HIV care(Djomand et al., 2003; Amuron et al., 2009; Micek et al., 2009; Losina et al., 2010; Rosen and Fox, 2011). Recent studies show that access to point-of-care (POC) CD4 T cell counts improve patient retention and initiation of antiretroviral therapy in health clinics in resource-limited settings(Jani et al., 2011a). The increasing prevalence of HIV infection worldwide, along with changing criteria for treatment will further increase the demand for more available, reliable, and cost-effective methods for T-cell enumeration. Point-of-care T cell quantitation is an important step in the decentralization and integration of HIV treatment, and thus is a major priority in the next phase of HIV care(WHO, 2011). While advances have been made in POC diagnostics, many still have disadvantages that potentially limit their usefulness. These limitations include cost, requirements for technical expertise, quality assurance, and throughput, and vary between different technologies(Zachariah et al., 2011; Boyle et al., 2012).
MBio Diagnostics, Inc. is developing a simple and cost-effective CD4 T-cell counting system that would allow for decentralization of testing and treatment in resource-limited settings. The system is designed to be compatible with batch operation, such that a single operator using a single system could process 60 to 80 samples in a day (~10 / hour). There are two overall purposes for the current study. First, by placing the prototype system in a clinical setting, development engineers obtained operational feedback from users in an intended use setting. This pre-marketing evaluation provided input for design improvements. Second, the study provided a system performance assessment relative to flow cytometry using fresh whole blood samples from a cohort of HIV-infected participants. The assay protocol used in this preliminary study is a laboratory protocol requiring a skilled operator. MBio Diagnostics anticipates simplification of that assay protocol as the product moves toward final format.
2. METHODS
2.1 System Description
The MBio Diagnostics CD4 quantification system, referred to here as the MBio SnapCount™ System, combines single-use, disposable cartridges with a simple reader instrument. Based on the principle of static imaging cytometry with fluorescent immunostaining, the system utilizes a novel, laser-based illumination approach combined with MBio's proprietary planar waveguide technology. A detailed description of the optical system is beyond the scope of this manuscript. A related system based on the MBio planar waveguide technology has been previously described for multiplexed immunoassay applications (Lochhead et al., 2011).
The disposable cartridges for the CD4 quantification system are simple, single channel devices with passive fluidics (no pumps or valves). All flow in the device is by capillary action. The sample preparation protocol (described in detail below) includes addition of whole blood to a proprietary reagent prior to transfer to the assay cartridge. Cartridges are processed on the bench top, independent of the reader instrument. As a result, multiple samples can be processed in parallel; a single operator can process approximately 15 cartridges per hour with the system described here.
2.2 Study Participants
HIV-infected male and female individuals were recruited for this study from the Antiviral Research Center (AVRC) and the Owen Clinic at the University of California, San Diego (UCSD) Medical Center between May 2011 and October 2011. All participants provided written informed consent. Inclusion criteria included documented HIV infection. Exclusion criteria included anemia or other contraindication to venipuncture. No participants were excluded on the basis of gender, race or ethnicity, or socioeconomic or treatment status. The Institutional Review Board at UCSD approved this study.
2.3 Sample Collection
After enrollment, participants provided 3 separate 3mL whole blood specimens, collected via venipuncture into evacuated K2EDTA BD Vacutainer® tubes. One tube was used to process the specimen on the MBio SnapCount™ device at the AVRC. A second tube was sent to the reference flow cytometry laboratory. A third tube was sent to MBio Diagnostics in Boulder, Colorado via next day shipping to be tested on a second SnapCount™ device, for device and operator comparisons. Participants also provided a finger stick (capillary) sample when an operator was available for immediate MBio system testing. Capillary samples were collected using CAPIJECT® safety lancets (1.5mm width blade, 2.0 mm depth; Terumo Medical Corporation, New Jersey, USA), and 10μL MicroSafe capillary tubes (SafeTec, Pennsylvania, USA).
2.4 Flow Cytometry
Reference flow cytometry CD4 counts for each sample were generated at the Immunogenetics Laboratory at the Veteran's Health Administration of San Diego using one of the three Vacutainer® tubes. The Immunogenetics Lab uses a dual platform approach with a Becton Dickinson FACSCalibur flow cytometer. Flow cytometric results were reported after completion of the SnapCount™ analysis.
2.5 SnapCount CD4 T cell Enumeration
The laboratory assay protocol used in this study was as follows. All steps were performed at ambient temperature, which for this study was between 20°C and 25°C. For venous samples, 10μL of whole blood was transferred from the Vacutainer® tube to a microtube containing pre-measured proprietary liquid stain reagent using an adjustable pipetter. For the capillary blood collection, a disposable plastic capillary tube (MicroSafe) was used to transfer 10 μL of whole blood from the study participant's finger to a microtube containing the pre-measured proprietary liquid stain reagent.
Once venous or capillary samples were added to the microtubes for staining, they were briefly mixed by either aspirate-dispense with a pipetter or by vortexing. Immediately after mixing, 35μL of the diluted blood sample was transferred to the inlet port of an MBio cartridge using an adjustable pipetter. Each cartridge was incubated on the bench top for 20 minutes, a fixative was added, and then the cartridges were inserted into the SnapCount™ Reader for analysis within 1 hour. The cartridge read time was approximately 3 minutes in the instrument, which reports an absolute CD4 count in cells/μL. The results were recorded after each test and both counts and images were stored electronically.
2.6 Statistical Analysis>
Statistical analysis comparing the SnapCount™ system to flow cytometry was performed using Bland-Altman analysis and the percentage similarity model (%SIM) (Scott et al., 2003). The percentage similarity of SnapCount™ and flow cytometry is conveyed as the average percentage similarity ± relative standard deviation. In the %SIM model, mean values ranging between 95% and 105% are considered adequate for method agreement, and precision (%SIM coefficient of variation [CV]) is adequate when less than 8%(Scott et al., 2003). For a subset of samples, both venous and capillary samples were performed in triplicate (3 separate samples from one finger stick), and the %CV, defined as the ratio of sample standard deviation to the mean value, was calculated.
3. RESULTS
3.1 Study Participants
A total of 94 participants provided venous whole blood samples and 52 capillary whole blood specimens. Study participants were HIV-infected individuals enrolled through the Antiviral Research Center (AVRC) and the Owen Clinic, a large primary HIV care program, at the University of California, San Diego. Participants were predominantly male (95%), of Caucasian and Hispanic race/ethnicity, with a median age of 44 years. The median CD4 T cell count measured by FACSCalibur was 541 cells/μL. Of the 94 participants, antiretroviral therapy (ART) status and HIV viral load measurements were available for 92 participants. Of these, 90 (97.8%) were on antiretroviral therapy. Of these, all but 12 (13.3%) had plasma HIV-1 RNA levels that were less than 40 copies/mL. For those not on antiretroviral therapy (n=2), CD4 T cell counts were 19 and 148 cells/μL and the mean plasma HIV RNA level was 67,796 copies/ml. (Table 1).
3.2 Venous Samples
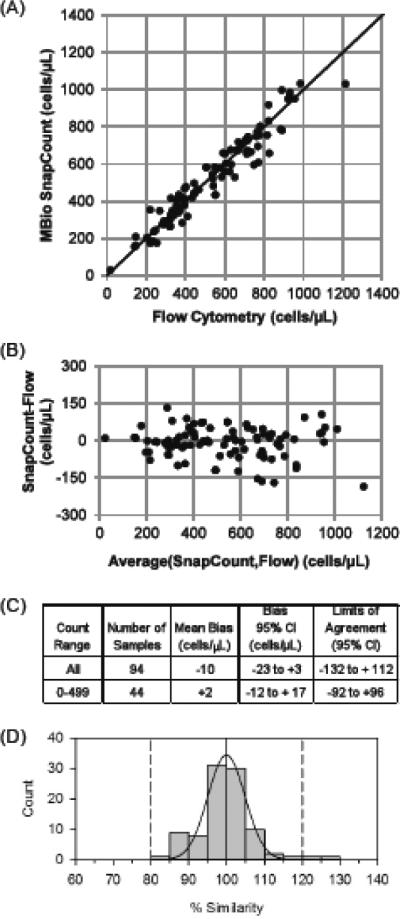
Correlation between the SnapCount™ System and reference FACSCalibur was generally very good. Method comparison results for the venous whole blood samples are presented in Figure 1. For all venous samples, Bland-Altman analysis resulted in a mean bias of −10 cells/μl (−23 to +3 cells/μl at 95% confidence). Limits of agreement (LOA) were −132 and +112 cells/μl. Of the 94 samples, 44 were in the most clinically relevant range of < 500 cells/μL. For these, Bland-Altman analysis resulted in a mean bias of −2 cells/μl (−12 to +17 cells/μl at 95% confidence). Limits of agreement (LOA) for these samples were −92 and +96 cells/μl. Percentage similarity calculations for the venous samples showed good accuracy (mean %SIM 99.8%) and good precision (%SIM SD 7.3%), with overall good agreement (%SIM CV 7.3%). A histogram demonstrating percentage similarity data plotted against the FACSCalibur CD4 T cell counts is shown in Figure 1D.
FIGURE 1. SnapCount™ performance with venous blood samples.
(A) Scatter plot of SnapCount™ versus FACSCalibur CD4 cell counts. The solid line is the identity line. (B) Bland-Altman plot comparing SnapCount™ and FACSCalibur. (C) Bland-Altman parameters. (D) Histogram for percentage similarity (%SIM) of SnapCount™ and FACSCalibur. The solid curve is a normal distribution fitted to the histogram, which yields a %SIM of 99.8% ± 7.3% and a 7.3%SIM CV.
The World Health Organization (WHO) recommended CD4 threshold for initiation of antiretroviral treatment for HIV-infected individuals of less than 350 cells/μL(WHO, 2010b) was used to determine misclassification by the SnapCount™ System. Of the 94 venous samples, 5 (5.3%) were misclassified. Two samples (2.1%) were misclassified above this threshold. Both high misclassifications were close to the WHO threshold. One of the samples had a SnapCount™ result of 353 cells/μL (versus 221 cells/μL for FACSCalibur), and the other was 415 cells/μL (versus 326 for FACSCalibur). Three samples (3.2%) were misclassified below the 350 cells/μL threshold. Again, all misclassified samples were very close to the threshold: 338, 282, and 317 for SnapCount™, versus 366, 383, and 411 for FACSCalibur. Reproducibility of the SnapCount™ on venous whole blood samples was also good; both when examined within the same participant sample as well as between 2 separate sites, prototypes and operators. Triplicate measurements on the same participant sample show a coefficient of variation (CV) of 7% for samples with more than 350 cells/μL (n = 85) and 10% for samples with less than 350 cells/μL (n = 33). There was also close agreement between results from the same participant when tested with two different SnapCount™ devices, different operators and different locations (Figure 2).
FIGURE 2. Comparison of SnapCount™ Systems.
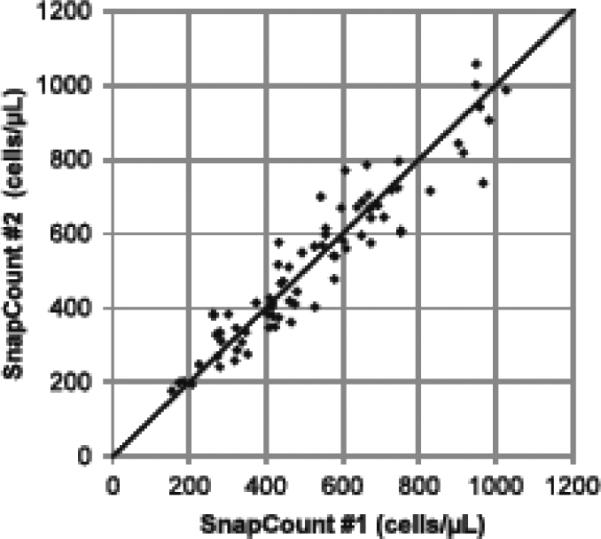
Scatter plot of SnapCount™ #1 versus SnapCount™ #2 using the same blood samples. SnapCount™ #1 was operated in San Diego, CA, and SnapCount™ #2 was operated at MBio Diagnostics, in Boulder, Colorado. Blood tubes were shipped overnight from San Diego to Colorado. The solid line is the identity line.
3.3 Capillary Samples
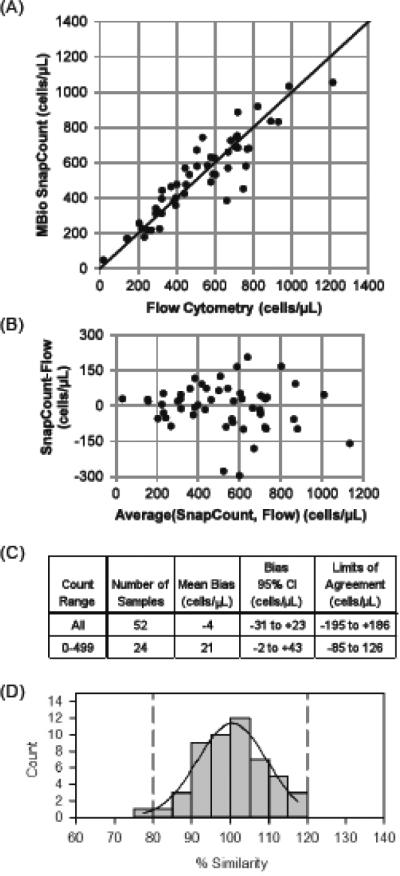
Agreement of capillary SnapCount™ sample counts with FACSCalibur was good (Figure 3). Bland-Altman mean bias of the SnapCount™ device was −4 cells/μL (−31 to 23 cells/μL at 95% confidence) as compared to FACSCalibur, with limits of agreement (LOA) of −195 and +186 cells/μL.
FIGURE 3. SnapCount™ performance with capillary blood samples.
(A) Scatter plot of SnapCount™ versus FACSCalibur CD4 cell counts. The identity line is indicated with the solid line. (B) Bland-Altman plot comparing SnapCount™ and FACSCalibur. (C) Bland-Altman parameters. (D) Histogram for percentage similarity (%SIM) to FACSCalibur CD4 cell counts. One sample with CD4 count < 50 cells/ μl has been excluded from the %SIM analysis, as discussed in the text. The solid curve is a normal distribution fitted to the histogram, which yields a mean %SIM of 100.7%, with %SIM SD of 9.0%, and %SIM CV of 9.0%.
Percentage similarity calculations for capillary samples show good agreement. We first note that one sample had a very low CD4 count on both FACSCalibur and SnapCountTM (19 and 48 cells/μL, respectively). This difference is clinically irrelevant and small in terms of absolute count difference (+29 cells/μL). Because the percentage deviation is large at very low count, this sample disproportionately impacts the %SIM parameters. %SIM results are therefore reported both with and without this sample. When the single low count sample is excluded, mean %SIM is 100.7%, with %SIM SD of 9.0%, and %SIM CV of 9.0% (Fig 3D). This is slightly worse than the %SIM CV of the venous samples (7.3%). If the low CD4 count sample is included in the dataset, the mean %SIM is 102% with a %SIM SD of 13.7% and %SIM CV of 13.4%.
For misclassification analysis using capillary samples, a treatment initiation threshold of 350 cells/μL was again used. Of the 52 finger stick samples, two (3.8%) were misclassified. Both samples were misclassified above the treatment threshold. As with venous measurements, both were close to the threshold, with counts of 396 and 442 cells/μL for SnapCount™, versus 324 and 326 cells/μL, respectively by FACSCalibur. Increased variability among triplicate testing with finger stick samples was noted when compared with venous sampling. Triplicate measurements from the same finger stick showed a CV of 13% (n=30) for samples with more than 350 cells/μL and 11% for samples below the 350 threshold (n=11).
4. DISCUSSION
While access to HIV testing and counseling services, as well as antiretroviral therapy, have increased in recent years, timely access to CD4 testing remains a bottleneck in HIV treatment services(Fredlund and Nash, 2007; Micek et al., 2009; WHO, 2010a; WHO, 2011; Zachariah et al., 2011). With more aggressive testing and counseling strategies and increasing availability of antiretroviral therapy, the number of patients in need of CD4 monitoring continues to rise. While there has been a large investment in technology for CD4 quantitation and expansion of central laboratory services in developing countries, these are not capable of meeting the high clinical demands, particularly in rural settings. Sustainability of new equipment even in central referral laboratories is challenged by maintenance difficulties, poor retention of human resources with technical training, environmental factors, including temperature, dust and power outages, as well as intermittent shortages of reagent supplies. In rural clinics and health posts, CD4 testing is even more limited, hindering timely initiation and management of antiretroviral therapy and preventive services for opportunistic complications of HIV (Fredlund and Nash, 2007; Larsen, 2008; Peter et al., 2008; Taiwo and Murphy, 2008).
The current healthcare delivery system in many rural settings requires multiple visits for testing and staging of HIV disease, and are associated with long travel distances and financial burdens that prevent patient retention in care(Fredlund and Nash, 2007; Amuron et al., 2009; Bassett et al., 2009; Micek et al., 2009). Hence, there is a great need for POC CD4 testing that can be coupled with HIV testing services to rapidly diagnose and stage disease in one visit. This may allow earlier initiation of ART, appropriate and timely prophylaxis for opportunistic infections, ability to identify those in need of closer follow-up, and potentially decrease loss to follow-up rates(Taiwo and Murphy, 2008; Amuron et al., 2009; Bassett et al., 2009; Nakanjako et al., 2009; Jani et al., 2011a). Increasing access to POC diagnostics and monitoring tools and decentralization of HIV care are major priorities for HIV care in the coming decade(WHO, 2011).
In this study, we demonstrated that CD4 testing performed with the MBio SnapCount™ produces results similar to laboratory-based flow cytometry. The Bland-Altman analysis showed a minimal downward bias of −10 cells per microliter with venous samples, and a minimal downward bias of only −4 cells per microliter with capillary samples, which is comparable to conventional technologies, as well as other POC technologies available or in development. The percentage similarity analysis confirmed adequate agreement between the methods, although with decreased precision observed for capillary samples. The misclassification of participants at the CD4 T cell threshold for ART initiation of 350 cells/μL was also minimal, with both upward and downward misclassification of less than 5% for venous and capillary measurements. Reproducibility was good between triplicate venous samples, and no difference was seen between the separate devices, different locations and different operators.
While there is no established standard threshold for acceptable misclassification, the SnapCount™ appears to match or improve upon results of other technologies(Landay et al., 1993; Gernow et al., 1995; Balakrishnan et al., 2006; Idigbe et al., 2006; Karcher et al., 2006; Spacek et al., 2006; Thakar et al., 2006; Lutwama et al., 2008; Mtapuri-Zinyowera et al., 2010; Diaw et al., 2011; Jani et al., 2011b; Herbert et al., 2012; Manabe et al., 2012; Mnyani et al., 2012). There was no overall trend in the direction of misclassification, and 3 of 7 were misclassified in favor of antiretroviral therapy initiation. In addition, all of the misclassified samples were within 100 cells/μL of the target threshold and most were within 100 cell/μL of the FACSCalibur result. Variation of counts with conventional flow cytometry technology, as well as physiologic variability has been previously observed. Combined with an evolving treatment threshold, one might conclude that discordances are unlikely to drastically change patient management, especially at the current threshold of 350 cell/μL. Patients who have CD4 count measurements near the threshold could experience premature or delayed initiation of therapy based on their POC results, however, might also be scheduled for closer follow up and monitoring. For those in whom therapy was delayed, subsequent testing or clinical staging would likely indicate need for treatment
The slight discordance between the capillary sample SnapCount™ results and flow cytometry results is most likely due to errors in pipetting and finger stick specimen collection using the current assay. Training on finger stick protocol and care was taken to ensure proper specimen collection, however, occasional difficulties were encountered, such as slow bleeding and occasional clotting. Preliminary studies at MBio, as well as with other POC technologies that utilize capillary sampling strategies, have demonstrated increased variability with incorrect collection methods(Yang et al., 2001; Mtapuri-Zinyowera et al., 2010; Diaw et al., 2011; Jani et al., 2011b; Glencross et al., 2012; Manabe et al., 2012). The final product version of the MBio CD4 system will incorporate design features that minimize capillary collection variability, but those features were not implemented at the time of this study.
The SnapCount™ has been developed to provide many features that are likely to make it practical for use in resource-limited settings. These include: 1) minimal blood volume for testing (10μL), which can be obtained by finger stick, an advantage when phlebotomy is unavailable; 2) all fluids stay in the cartridges, minimizing biohazard; and 3) cartridges use passive fluidics, so there are no moving parts, simplifying manufacturing, minimizing cost, and improving reliability.
Each test takes approximately 25 minutes to run; however, the instrument itself is only utilized during the actual imaging process (currently ~3 minutes). Since the instrument is only utilized for a short period of time, batch processing is possible, and ~10 tests can be completed per hour by one operator using one system. In a typical busy health clinic, throughput of the SnapCount™ system is estimated at 80 tests per day.
Although this system has many advantages, more effort is needed before widespread implementation in busy health clinics. The mean CD4 count of the population for this study is well above the clinically relevant treatment threshold of 350 cells/μL. Combined with the low sample size, the findings of this study cannot be extrapolated to a typical population in a resource-limited setting. Similar to other POC CD4 quantitation technologies, the SnapCount™ does not provide CD4% (Moodley et al., 1997; Embree et al., 2001), although future versions of the system could be configured for CD4% and CD4/CD8 ratio. Finally, the system described here requires pipettes for sample preparation and a laptop computer for software analysis. Developmental efforts are underway on a cartridge that incorporates lyophilized reagents and allows direct whole blood addition, combined with a reader device that includes an integrated touchscreen, internal and external quality control features, data management software and network connectivity. The “one-step” system will require additional validation studies.
ACKNOWLEGEMENTS
This work was supported by Public Health Service grant AI070052 from the National Institute of Allergy and Infectious Disease (NIAID) and award number 70NANB7H7053 under the National Institute of Standards and Technology (NIST) Advanced Technology Program through the U.S. Department of Commerce. The authors thank all the participants who made this study possible, as well as the staff at the Antiviral Research Center and Owen Clinic at the University of California, San Diego for their assistance with conducting this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: POC, Point-of-care; LOA, limits of agreement; AVRC, Antiviral Research Center; UCSD, University of California San Diego; %SIM, percentage similarity; CV, coefficient of variation; ART, antiretroviral therapy; WHO, World Health Organization
7. REFERENCES
- Amuron B, Namara G, Birungi J, Nabiryo C, Levin J, Grosskurth H, Coutinho A, Jaffar S. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC public health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan P, Solomon S, Mohanakrishnan J, Cecelia AJ, Kumarasamy N, Murugavel KG, Venkatakrishnan B, Solomon SS, Crowe SM, Ganesh AK, Thyagarajan SP, Flanigan T, Mayer KH. A reliable and inexpensive EasyCD4 assay for monitoring HIV-infected individuals in resource-limited settings. Journal of acquired immune deficiency syndromes. 2006;43:23–6. doi: 10.1097/01.qai.0000227791.92203.d5. 1999. [DOI] [PubMed] [Google Scholar]
- Bassett IV, Wang B, Chetty S, Mazibuko M, Bearnot B, Giddy J, Lu Z, Losina E, Walensky RP, Freedberg KA. Loss to care and death before antiretroviral therapy in Durban, South Africa. Journal of acquired immune deficiency syndromes. 2009;51:135–9. doi: 10.1097/qai.0b013e3181a44ef2. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle DS, Hawkins KR, Steele MS, Singhal M, Cheng X. Emerging technologies for point-of-care CD4 T-lymphocyte counting. Trends in biotechnology. 2012;30:45–54. doi: 10.1016/j.tibtech.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaw PA, Daneau G, Coly AA, Ndiaye BP, Wade D, Camara M, Mboup S, Kestens L, Dieye TN. Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the PIMA CD4. Journal of acquired immune deficiency syndromes. 2011;58:e103–11. doi: 10.1097/QAI.0b013e318235b378. 1999. [DOI] [PubMed] [Google Scholar]
- Djomand G, Roels T, Ellerbrock T, Hanson D, Diomande F, Monga B, Maurice C, Nkengasong J, Konan-Koko R, Kadio A, Wiktor S, Lackritz E, Saba J, Chorba T. Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d'Ivoire. AIDS. 2003;17(Suppl 3):S5–15. doi: 10.1097/00002030-200317003-00002. [DOI] [PubMed] [Google Scholar]
- Embree J, Bwayo J, Nagelkerke N, Njenga S, Nyange P, Ndinya-Achola J, Pamba H, Plummer F. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. The Pediatric infectious disease journal. 2001;20:397–403. doi: 10.1097/00006454-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Fredlund VG, Nash J. How far should they walk? Increasing antiretroviral therapy access in a rural community in northern KwaZulu-Natal, South Africa. The Journal of infectious diseases. 2007;196(Suppl 3):S469–73. doi: 10.1086/521115. [DOI] [PubMed] [Google Scholar]
- Gernow A, Lisse IM, Bottiger B, Christensen L, Brattegaard K. Determination of CD4+ and CD8+ lymphocytes with the cytosphere assay: a comparative study with flow cytometry and the immunoalkaline phosphatase method. Clinical immunology and immunopathology. 1995;76:135–41. doi: 10.1006/clin.1995.1107. [DOI] [PubMed] [Google Scholar]
- Glencross DK, Aggett HM, Stevens WS, Mandy F. African regional external quality assessment for CD4 T-cell enumeration: development, outcomes, and performance of laboratories. Cytometry. Part B, Clinical cytometry. 2008a;74(Suppl 1):S69–79. doi: 10.1002/cyto.b.20397. [DOI] [PubMed] [Google Scholar]
- Glencross DK, Coetzee LM, Faal M, Masango M, Stevens WS, Venter WF, Osih R. Performance evaluation of the point-of-care CD4 analyser Pima using capillary blood sampling in field tests in South Africa. Journal of the International AIDS Society. 2012;15:3. doi: 10.1186/1758-2652-15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glencross DK, Janossy G, Coetzee LM, Lawrie D, Aggett HM, Scott LE, Sanne I, McIntyre JA, Stevens W. Large-scale affordable PanLeucogated CD4+ testing with proactive internal and external quality assessment: in support of the South African national comprehensive care, treatment and management programme for HIV and AIDS. Cytometry. Part B, Clinical cytometry. 2008b;74(Suppl 1):S40–51. doi: 10.1002/cyto.b.20384. [DOI] [PubMed] [Google Scholar]
- Herbert S, Edwards S, Carrick G, Copas A, Sandford C, Amphlett M, Benn P. Evaluation of PIMA point-of-care CD4 testing in a large UK HIV service. Sexually transmitted infections. 2012 doi: 10.1136/sextrans-2012-050507. [DOI] [PubMed] [Google Scholar]
- Idigbe EO, Audu RA, Oparaugo CT, Onwujekwe D, Onubogu CC, Adedoyin J, Mafe AG, Okoye R, Onyewuche JI, Funso-Adebayo EO, Meshack E. Comparison of Dynabeads and Capcellia methods with FACScount for the estimation of CD4 T-lymphocyte levels in HIV/AIDS patients in Lagos, Nigeria. East African medical journal. 2006;83:105–11. doi: 10.4314/eamj.v83i4.9424. [DOI] [PubMed] [Google Scholar]
- Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, Lehe JD, Peter TF. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011a;378:1572–9. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- Jani IV, Sitoe NE, Chongo PL, Alfai ER, Quevedo JI, Tobaiwa O, Lehe JD, Peter TF. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011b;25:807–12. doi: 10.1097/QAD.0b013e328344f424. [DOI] [PubMed] [Google Scholar]
- Karcher H, Bohning D, Downing R, Mashate S, Harms G. Comparison of two alternative methods for CD4+ T-cell determination (Coulter manual CD4 count and CyFlow) against standard dual platform flow cytometry in Uganda. Cytometry. Part B, Clinical cytometry. 2006;70:163–9. doi: 10.1002/cyto.b.20093. [DOI] [PubMed] [Google Scholar]
- Landay A, Ho JL, Hom D, Russell T, Zwerner R, Minuty JG, Kataaha P, Mmiro F, Jackson B. A rapid manual method for CD4+ T-cell quantitation for use in developing countries. AIDS. 1993;7:1565–8. doi: 10.1097/00002030-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Larsen CH. The fragile environments of inexpensive CD4+ T-cell enumeration in the least developed countries: strategies for accessible support. Cytometry. Part B, Clinical cytometry. 2008;74(Suppl 1):S107–16. doi: 10.1002/cyto.b.20386. [DOI] [PubMed] [Google Scholar]
- Lochhead MJ, Todorof K, Delaney M, Ives JT, Greef C, Moll K, Rowley K, Vogel K, Myatt C, Zhang XQ, Logan C, Benson C, Reed S, Schooley RT. Rapid multiplexed immunoassay for simultaneous serodiagnosis of HIV-1 and coinfections. Journal of clinical microbiology. 2011;49:3584–90. doi: 10.1128/JCM.00970-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP, Ross D, Scott CA, Uhler LM, Katz JN, Holst H, Freedberg KA. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PloS one. 2010;5:e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutwama F, Serwadda R, Mayanja-Kizza H, Shihab HM, Ronald A, Kamya MR, Thomas D, Johnson E, Quinn TC, Moore RD, Spacek LA. Evaluation of Dynabeads and Cytospheres compared with flow cytometry to enumerate CD4+ T cells in HIV-infected Ugandans on antiretroviral therapy. Journal of acquired immune deficiency syndromes. 2008;48:297–303. doi: 10.1097/QAI.0b013e31817bbc3a. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe YC, Wang Y, Elbireer A, Auerbach B, Castelnuovo B. Evaluation of portable point-of-care CD4 counter with high sensitivity for detecting patients eligible for antiretroviral therapy. PloS one. 2012;7:e34319. doi: 10.1371/journal.pone.0034319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micek MA, Gimbel-Sherr K, Baptista AJ, Matediana E, Montoya P, Pfeiffer J, Melo A, Gimbel-Sherr S, Johnson W, Gloyd S. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. Journal of acquired immune deficiency syndromes. 2009;52:397–405. doi: 10.1097/QAI.0b013e3181ab73e2. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyani CN, McIntyre JA, Myer L. The Reliability of Point-of-Care CD4 Testing in Identifying HIV-Infected Pregnant Women Eligible for Antiretroviral Therapy. Journal of acquired immune deficiency syndromes. 2012;60:260–4. doi: 10.1097/QAI.0b013e318256b651. 1999. [DOI] [PubMed] [Google Scholar]
- Moodley D, Bobat RA, Coovadia HM, Doorasamy T, Munsamy S, Gouws E. Lymphocyte subset changes between 3 and 15 months of age in infants born to HIV-seropositive women in South Africa. Tropical medicine & international health : TM & IH. 1997;2:415–21. [PubMed] [Google Scholar]
- Mtapuri-Zinyowera S, Chideme M, Mangwanya D, Mugurungi O, Gudukeya S, Hatzold K, Mangwiro A, Bhattacharya G, Lehe J, Peter T. Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. Journal of acquired immune deficiency syndromes. 2010;55:1–7. doi: 10.1097/QAI.0b013e3181e93071. 1999. [DOI] [PubMed] [Google Scholar]
- Nakanjako D, Colebunders R, Coutinho AG, Kamya MR. Strategies to optimize HIV treatment outcomes in resource-limited settings. AIDS reviews. 2009;11:179–89. [PubMed] [Google Scholar]
- Peter T, Badrichani A, Wu E, Freeman R, Ncube B, Ariki F, Daily J, Shimada Y, Murtagh M. Challenges in implementing CD4 testing in resource-limited settings. Cytometry. Part B, Clinical cytometry. 2008;74(Suppl 1):S123–30. doi: 10.1002/cyto.b.20416. [DOI] [PubMed] [Google Scholar]
- Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LE, Galpin JS, Glencross DK. Multiple method comparison: statistical model using percentage similarity. Cytometry. Part B, Clinical cytometry. 2003;54:46–53. doi: 10.1002/cyto.b.10016. [DOI] [PubMed] [Google Scholar]
- Spacek LA, Shihab HM, Lutwama F, Summerton J, Mayanja H, Kamya M, Ronald A, Margolick JB, Nilles TL, Quinn TC. Evaluation of a low-cost method, the Guava EasyCD4 assay, to enumerate CD4-positive lymphocyte counts in HIV-infected patients in the United States and Uganda. Journal of acquired immune deficiency syndromes. 2006;41:607–10. doi: 10.1097/01.qai.0000214807.98465.a2. 1999. [DOI] [PubMed] [Google Scholar]
- Taiwo BO, Murphy RL. Clinical applications and availability of CD4+ T cell count testing in sub-Saharan Africa. Cytometry. Part B, Clinical cytometry. 2008;74(Suppl 1):S11–8. doi: 10.1002/cyto.b.20383. [DOI] [PubMed] [Google Scholar]
- Thakar MR, Kumar BK, Mahajan BA, Mehendale SM, Paranjape RS. Comparison of capillary based microflurometric assay for CD4+ T cell count estimation with dual platform Flow cytometry. AIDS research and therapy. 2006;3:26. doi: 10.1186/1742-6405-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitoria M, Granich R, Gilks CF, Gunneberg C, Hosseini M, Were W, Raviglione M, De Cock KM. The global fight against HIV/AIDS, tuberculosis, and malaria: current status and future perspectives. American journal of clinical pathology. 2009;131:844–8. doi: 10.1309/AJCP5XHDB1PNAEYT. [DOI] [PubMed] [Google Scholar]
- WHO . Towards universal access: scaling up priority HIV/AIDS interventions in the health sector-Progress report 2009. 2010a. World Health Organization, UNAIDS, UNICEF. [Google Scholar]
- WHO . Recommendations for a public health approach. 2010b. World Health Organization. Antiretroviral Therapy for HIV infection in adults and adolescents. 2010 revision. [PubMed] [Google Scholar]
- WHO . The treatment 2.0 framework for action: catalysing the next phase of treatment, care and support. 2011. World Health Organization, UNAIDS. [Google Scholar]
- WHO . HIV/AIDS Diagnostic Technology Landscape. Second Edition 2012. [Google Scholar]
- Yang ZW, Yang SH, Chen L, Qu J, Zhu J, Tang Z. Comparison of blood counts in venous, fingertip and arterial blood and their measurement variation. Clinical and laboratory haematology. 2001;23:155–9. doi: 10.1046/j.1365-2257.2001.00388.x. [DOI] [PubMed] [Google Scholar]
- Zachariah R, Reid SD, Chaillet P, Massaquoi M, Schouten EJ, Harries AD. Viewpoint: Why do we need a point-of-care CD4 test for low-income countries? Tropical medicine & international health : TM & IH. 2011;16:37–41. doi: 10.1111/j.1365-3156.2010.02669.x. [DOI] [PubMed] [Google Scholar]