Abstract
Abs to microbial capsules are critical for host defense against encapsulated pathogens, but very little is known about the effects of Ab binding on the capsule, apart from producing qualitative capsular reactions (“quellung” effects). A problem in studying Ab–capsule interactions is the lack of experimental methodology, given that capsules are fragile, highly hydrated structures. In this study, we pioneered the use of optical tweezers microscopy to study Ab–capsule interactions. Binding of protective mAbs to the capsule of the fungal pathogen Cryptococcus neoformans impaired yeast budding by trapping newly emerging buds inside the parental capsule. This effect is due to profound mAb-mediated changes in capsular mechanical properties, demonstrated by a concentration-dependent increase in capsule stiffness. This increase involved mAb-mediated cross-linking of capsular polysaccharide molecules. These results provide new insights into Ab-mediated immunity, while suggesting a new nonclassical mechanism of Ab function, which may apply to other encapsulated pathogens. Our findings add to the growing body of evidence that Abs have direct antimicrobial functions independent of other components of the immune system.
Introduction
Antibodies to microbial surfaces can promote host defense by mediating the effector functions of other components of the immune system, such as complement and phagocytic cells (1). However, binding of Abs can also mediate direct antimicrobial effects for the benefit of the host, even for encapsulated microbes, when binding occurs at a certain distance away from the cell. Examples of such direct antimicrobial effects include alterations of microbial metabolic activity, gene expression, quorum sensing, and susceptibility to drugs (2, 3). The physical mechanism(s) of such direct Ab-mediated effects upon capsule binding are poorly understood. The study of Ab–capsule interaction is important for understanding the mechanisms by which Ab-mediated immunity interacts with microbes, for identifying useful Abs for antimicrobial treatment (1), and for designing more effective vaccines.
One of the best-studied microbial capsules is that of the fungal pathogen Cryptococcus neoformans, which is a complex polysaccharide (PS) structure that enlarges during infection (∼8× the cell’s volume) and is essential for virulence (4, 5). The cryptococcal capsule exhibits strong antiphagocytic properties (6, 7), isolating the fungal cell from host immune factors and pattern recognition receptors on immune cells (8). Macromolecular analysis of extracted PSs suggests that the capsule is composed of various interconnected PS molecules with branch-like structural characteristics (9–11). This complex surface structure is considered the main virulence factor (4, 5) and remains a major target for the development of therapeutic strategies (12).
The mAbs to glucuronoxylomannan (GXM), the main PS constituent of the capsule, can mediate protection against C. neoformans infection by decreasing fungal burden and dissemination and, thus, increasing survival of lethally infected mice (13–16). One mAb, 18B7 (IgG1), was evaluated clinically as a therapeutic agent against cryptococcosis (17, 18). The mechanism of mAb-mediated protection against C. neoformans appears to be multifactorial, involving classical and nonclassical mechanisms of Ab function (1). Classical mechanisms of mAbs to GXM include enhancement of phagocytosis, complement activation, and recruitment of inflammatory cells (19–22). In addition, mAbs to GXM can function directly, affecting the normal function of C. neoformans upon binding to the PS capsule. mAbs can inhibit PS Ag release (23) and biofilm formation in vitro (24) and can increase drug susceptibility by somehow triggering changes in cryptococcal metabolism and gene expression (2, 25). The mechanism of such direct Ab-mediated effects on C. neoformans physiology remains poorly understood and requires new approaches for studying Ab–capsule interaction.
The protective efficacy of mAbs to GXM against experimental cryptococcosis depends greatly on their capacity to interact with the capsule (26). For instance, the capacity of mAbs to GXM to alter the optical properties of the capsule (i.e., “quellung” effect or capsular swelling) and their fluorescence-binding pattern (i.e., annular or punctate) correlated with protective efficacy (22, 26–28). Other important determinants of protective efficacy are Ab isotype and epitope specificity (29), as well as the concentration and localization of these epitopes in the capsule (22).
In this study, we examined the direct effect of mAbs to GXM on cellular replication and capsule mechanical properties, using light and optical tweezers microscopy analysis on intact C. neoformans yeast cells. Our data show that binding of protective, but not nonprotective, mAbs produces a concentration-dependent increase in the stiffness of the capsule. This binding translated into a situation whereby daughter cells are trapped in a saclike structure made from the parental capsule. The ability of mAbs to increase the capsule stiffness correlated with their capacity to cross-link PS molecules in solution. Our results show a new Ab-mediated effect on microbial function through the alteration of capsular mechanical properties.
Materials and Methods
Yeast culture
C. neoformans serotype A strain H99 (ATCC 208821) was grown under constant agitation at 30°C for 48 h in minimal medium (15 mM dextrose, 10 mM MgSO4, 29.3 mM KH2PO4, 13 mM glycine, 3 μM thiamine-HCl; adjusted to pH 5.5).
mAbs
The GXM-specific mAbs used in this study were 18B7 (IgG1), 13F1 (IgM), 2D10 (IgM), and the 3E5 family of switch variants (IgG1, IgG2, IgG2b, IgG3), all previously described (14, 30, 31). The mAbs were purified from hybridoma cell supernatants recovered after growing the cells for 2 wk. The different IgG isotypes were purified by protein A or G chromatography, and the IgM Abs were purified by mannan-binding protein chromatography. The resulting mAbs were dialyzed in PBS, and concentration was determined by ELISA relative to isotype-matched standards of known concentration.
Time-lapse microscopy
Approximately 1 × 106 C. neoformans cells were washed three times with PBS and incubated with 10 μg ml−1 of a mAb [18b7 (IgG1), 13F1 (IgM), or 2D10 (IgM)] for 1 h at 37°C under constant shaking. Then, 10 μl of this suspension, containing ∼104 Ab-coated cells, was diluted in a well chamber-slide containing 200 μl of fresh minimal media. The wells were previously coated with 10 μg ml−1 of each particular mAb to help immobilized cells to the bottom of the well and facilitate image acquisition. The untreated cells (control) were immobilized using mAb 18B7 to coat the bottom of the well. The chamber-slide was placed in a temperature-controlled microscope chamber set to 37°C. Time-lapse images of cells were taken at 4-min intervals with a 63× differential interference contrast objective using an Axiovert 200 M inverted microscope equipped with a Hamamatsu ORCA ER cooled charge-coupled device camera and controlled by AxioVision 4.6 software (Carl Zeiss Micro Imaging, New York, NY).
Young’s modulus measurements of the capsule
Approximately 106 C. neoformans cells, previously washed with PBS three times, were incubated for 1 h at 37°C in the presence of different concentrations of mAb (20, 10, 1, 0.5, 0.1, or 0.01 μg ml−1). Following incubation, the cells were washed (three times with PBS), and a suspension (104) of yeast cells in PBS was added to glass-bottom dishes previously coated with 10 μg ml−1 of mAb IgG1 18B7 for 1 h at 37°C. After 1 h incubation at room temperature, plates were washed with PBS to remove nonadherent cells. Uncoated polystyrene beads (radius, 1.52 ± 0.02 μm) (Polysciences, Warrington, PA) were added to the plate, and the samples were placed in an optical tweezers system composed of an infrared 1064-nm Nd:YAG laser (Quantronix, East Setauket, NY) attached to an inverted Nikon Eclipse TE300 microscope (Nikon, Melville, NY). The capsule–bead interaction is strong and likely mediated by van der Waals forces and/or differences in charge between both surfaces (32). Alternatively, it is possible that hydrophobic interactions contribute to this, given that the capsule has lipid-like domains (33). Measurements were performed, and Young’s modulus values were determined following the previously described procedures (9, 32, 34).
PS cross-linking
The ability of mAbs to mediate cross-linking of PS molecules was examined by monitoring the average hydrodynamic size of PS samples using dynamic light scattering (DLS) in a 90Plus/BI-MAS (Brookhaven Instruments, Hotsville, NY), as previously described (9). DLS measures the fluctuations in light intensity scattered by particles in solution. The frequencies of these intensity fluctuations are related to the diffusion (Brownian motion) of the particles in solution. The fluctuations of light-scattered intensity are correlated or compared as a function of time by the autocorrelation function, which decays exponentially until plateau (35). The intensity autocorrelation function is used to determine an average translational diffusion coefficient, which is related to particle size based on the Einstein–Stoke equation (the larger the particle, the slower its diffusion).
Cryptococcal PS was isolated from supernatant by ultrafiltration, as described (10), and dissolved in PBS (2 mg ml−1). PS solution was cleared of any dust particles by centrifugation at 7500 × g for 5 min and equilibrated to 25°C before measurements. Average hydrodynamic size values from PS solutions (1 mg ml−1) in the absence (control) or presence of mAb were obtained from 10 repeated measurements. Cross-linking studies were done by monitoring the changes in particle size distribution and autocorrelation function curves of a PS solution, before and after (20, 40, and 60 min) addition of mAb 18B7 (10 μg ml−1). The loss of light-scattering signal after 60 min of incubation was confirmed by three independent experiments. To study the effect of the different mAbs on the average hydrodynamic size, PS solutions (1 mg ml−1) containing 10 μg ml−1 of each mAb were incubated for 30 min at 37°C and analyzed by DLS.
Results
Protective mAbs prevent the release of newly budded cells
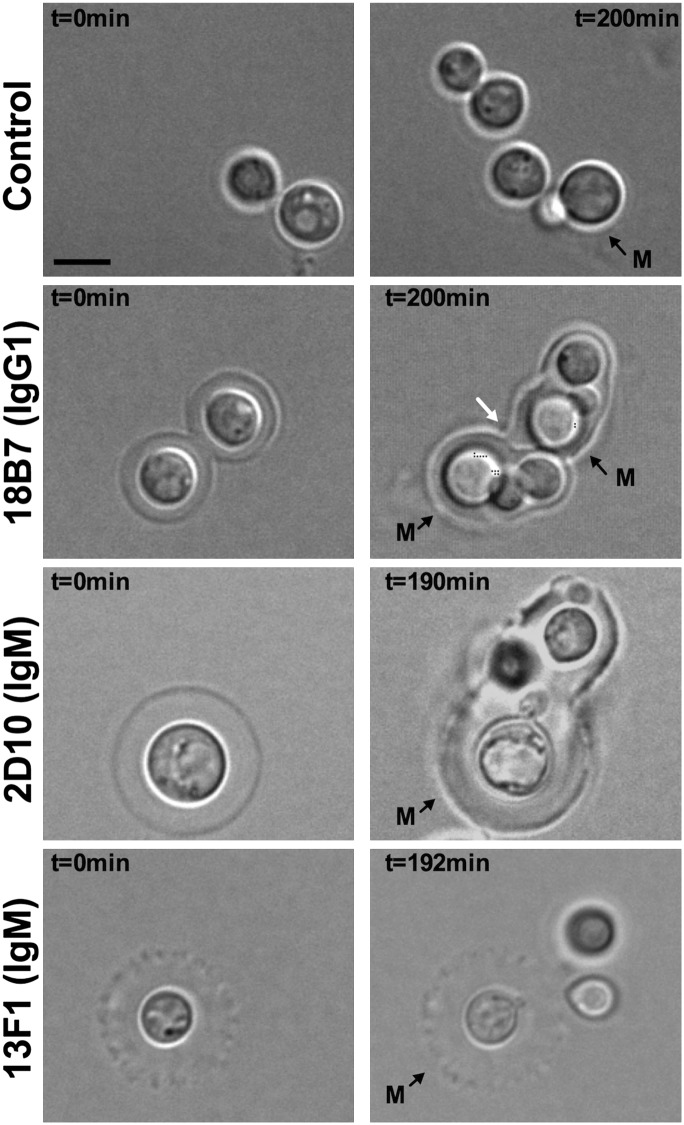
To examine the impact of mAbs to GXM on the normal function of C. neoformans, we analyzed the cryptococcal replication process, using time-lapse microscopy. We observed multiple budding events (>10× for some cells) through a single budding site, which explains the limitations of using bud scars on C. neoformans surface as markers for replicative life-span determination (36). In some instances, cells continued the replication process through a new cell site when a budding event was not readily completed. Analysis of the doubling time of individual cells revealed considerable cell-to-cell variation with a mean doubling time variance of 14.5 ± 2.1%, but the average doubling time was 2.1 ± 0.2 h, which was similar to a 2.2 ± 0.4 h doubling time of yeast cultures based on turbidity measurements. No significant change in average budding rate could be detected between mAb-treated and untreated cells by time-lapse microscopy (within a 10-min SE). Binding of the protective mAb 18B7 (IgG1), however, prevented the full release of newly budded cells, trapping them inside a saclike structure made from the parental capsule (Fig. 1, Supplemental Video 1). This phenomenon was also observed for protective mAb 2D10 (IgM) (Fig. 1, Supplemental Video 2). In contrast, for cells without mAb or with equal concentrations of the nonprotective mAb 13F1 (IgM) (Fig. 1, Supplemental Videos 3, 4) daughter cells were readily released from the parental cell after budding. These results suggested that the ability to contain bud release was a characteristic of the protective mAbs tested in this study and implied significant alterations in the capsule’s structural and physical properties.
FIGURE 1.
Effect of mAb binding on C. neoformans replication. Micrograph representations of C. neoformans cells at time 0 (left) and after ≥190 min of growth (right) following treatment with 10 μg ml−1 of mAb 18B7 (IgG1), 2D10 (IgM), and 13F1 (IgM). Ab-untreated cells were used as control. Mother cells present at time 0 are labeled M. New budded cells remained trapped in a saclike structure made from the capsule of the mother cell (white arrow). Scale bar, 5 μm.
mAb binding alters capsular elastic properties
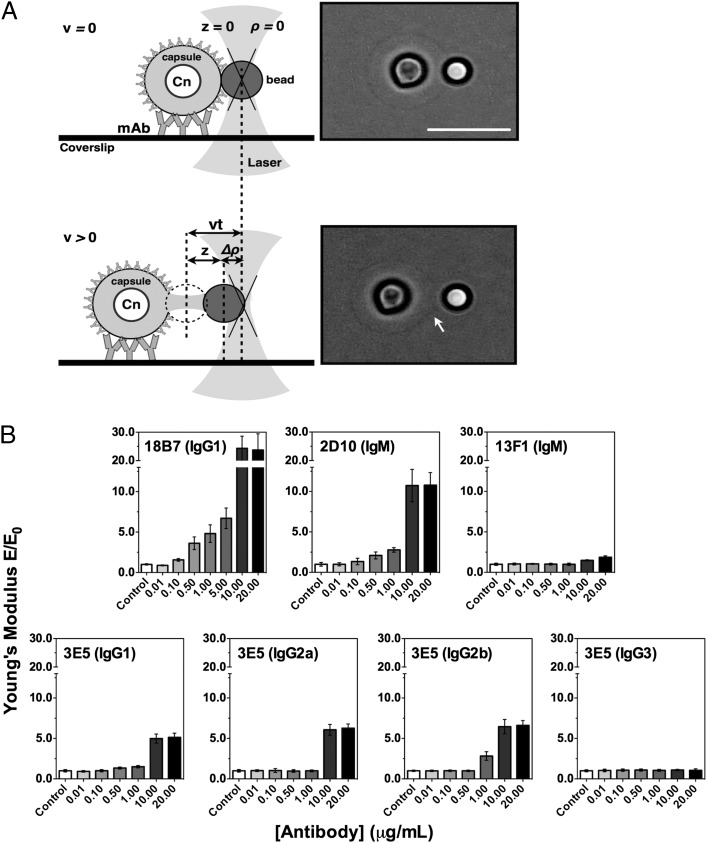
We hypothesized that the ability of protective mAbs to interfere with bud release resulted from alterations in the mechanical properties of the capsule. To explore this possibility, we used optical tweezers microscopy to measure the Young modulus (E) of the PS capsule after mAb binding. E is a measure of the stiffness of an elastic material and can be derived from a form–deformation curve by stretching the PS capsule via micromanipulation of a polystyrene bead (Fig. 2A) (9, 32, 34). A panel of seven mAbs to GXM was studied: 18B7 (IgG1), 2D10 (IgM), 13F1 (IgM), and the 3E5 family (IgG1, IgG2a, IgG2b, IgG3) of variable region–identical isotype switch variants (19). With the exception of the mAb 13F1 (IgM), all other mAbs have been shown to prolong survival of murine models of cryptococcosis (14, 19, 37). The 3E5 IgG3 is not protective in most models of cryptococcosis, although protection has been observed in certain murine genetic backgrounds (38).
FIGURE 2.
Effect on the capsule’s Young's modulus (E) as a function of mAb concentration. (A) Schema showing the C. neoformans capsule’s Young’s modulus determination using optical tweezers (left panel). In the initial situation, the bead is in its equilibrium position in the trap, ρ = 0, and the capsule is not deformed, z = 0. As the microscope stage moves under controlled velocity (V) during a certain time (t), the bead changes its equilibrium position in the trap, Δρ > 0, resulting in capsule deformation, z > 0. Light microscopy micrographs of a top view of a C. neoformans yeast cell treated with 10 μg ml−1 mAb 18B7 (IgG1) (right panel). It shows the actual stretching (arrow) of the PS capsule as the cell is moved opposite the laser-trapped polystyrene bead. Scale bar, 10 μm. Diagram in panel A was adapted with permission from reference (32). (B) Concentration-dependent effect of anti-GXM mAbs on the elastic properties of the PS capsule. Bars represent mean ± SE of at ≥20 different C. neoformans PS capsule Young’s modulus values (E) for each mAb concentration normalized by the Young modulus results from untreated cells (E0).
Single-cell analysis of C. neoformans cells demonstrated that binding of mAbs to the capsule results in a concentration-dependent increase in the capsule’s E values (Fig. 2B, Supplemental Videos 5–10). In general, binding of protective mAb produced an allosteric dose–response behavior with an apparent plateau in E after 10 μg ml−1. The mAb 18B7 (IgG1) produced the strongest effect, with >20-fold increase in capsule E values (Table I). Among IgMs, the mAb 2D10 (IgM) produced the strongest effect, with an ∼10-fold increase in E. A moderate increase (<2-fold) by the nonprotective mAb 13F1 (IgM) was observed at the highest concentration only (20 μg ml−1). The ability of these mAbs to affect the mechanical properties of the capsule correlated with their protective efficacy (14, 30, 37) and affinity to GXM (9, 39). Importantly, no increase in capsule E was observed after incubating the cells in 20% mouse serum, demonstrating that simple protein deposition is not sufficient to cause a change in capsular mechanical properties.
Table I. Parameters derived from a log (mAb concentration) versus response (Young's modulus, E) equation.
| Ab | E∞/E0a | C50b | GXM Affinityc |
|---|---|---|---|
| 18B7 (IgG1) | 24.8 | 6.3 | 0.1 |
| 2D10 (IgM) | 10.8 | 1.7 | 0.4 |
| 13F1 (IgM) | 2.3 | 11.4 | 9.5 |
| 3E5 (IgG1) | 5.1 | 1.8 | + |
| 3E5 (IgG2a) | 6.3 | 8.6 | ++ |
| 3E5 (IgG2b) | 6.5 | 1.1 | +++ |
| 3E5 (IgG3) | n.d. | n.d. | + |
E values of the plateau region in relation to the initial (control) E values.
Concentration of mAb that results in E values halfway between the basal (control) and the maximal (plateau) values.
Dissociation constant (Kd) values for 18B7, 2D10, and 13F1 mAbs, using cryptococcal GXM from a 2-d culture of C. neoformans H99 cultures, were taken from Ref. 9. The + symbols represent the intensities of the relative affinities of the 3E5 switch variants, based on binding curves published in Ref. 39.
n.d., Not detectable.
Differences in the ability of the variable region–identical 3E5 switch variant mAbs to increase the capsular stiffness were smaller than for the other mAbs studied (Table I). Whereas 3E5 (IgG2b) showed the strongest effect (≥6-fold increase), followed by IgG2a (∼6-fold increase) and IgG1 (∼5-fold increase), the IgG3 variant had no effect on capsule mechanical properties (Fig. 2B, Supplemental Video 11). The differences observed among the 3E5 switch variants correlated with their opsonic activity (19) and are consistent with previous reports demonstrating the importance of isotype in Ab-binding properties and correlated with their opsonic activity (39). We also determined the C50 (mAb concentration needed for half-maximal E value) for each mAb, using the E values obtained as a function of mAb concentration (Table I). The mAb 3E5 (IgG2b) showed the lowest value, followed by 2D10 (IgM), 3E5 (IgG1), 18B7 (IgG1), 3E5 (IgG2a), and 13F1 (IgM). These results reflect clear differences in both Ag and Ab valence, as well as capsule epitope density. The effect of protective mAbs on the capsule’s elastic properties provides a physical and underlying mechanism for the interference with bud release during C. neoformans replication.
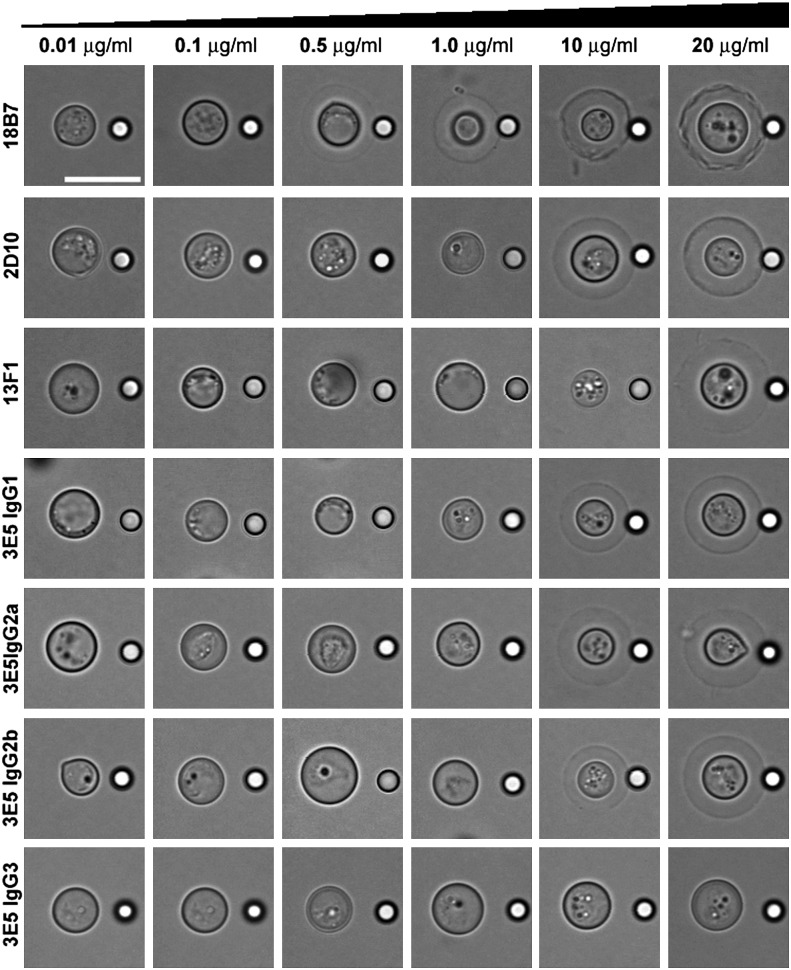
Depending on the mAb and concentration, the capsule became visible by bright-field light microscopy owing to a change in optical path difference, which is the product of refractive index and object thickness (40). This effect is known as capsular quellung or swelling reaction (41), and as confirmed by our data, it increased with mAb concentration (26). With the exception of 3E5 (IgG3), all mAbs produced quellung reactions, at distinct concentrations (Fig. 3). The strongest effect was observed for the mAb 18B7 (IgG1), for which the capsule was discernible beginning at a concentration of 0.5 μg ml−1. Given that mAb incubations were done using 106 yeast cells, addition of ∼0.250 pg of mAb 18B7 (IgG1) per cell appears to be the minimum amount required to mediate this effect. At higher concentrations (>10 μg ml−1), 18B7 binding produced substantial irregularities in the capsule (Fig. 3), consistent with previous reports (23).
FIGURE 3.
Concentration-dependent capsular (quellung) reaction. Light microscopy images of H99 Cryptococcus neoformans cells in the presence of increasing concentration (0.01–20 μg ml−1) of purified mAbs. Scale bar, 10 μm.
MAb-mediated cross-linking of PS molecules in solution
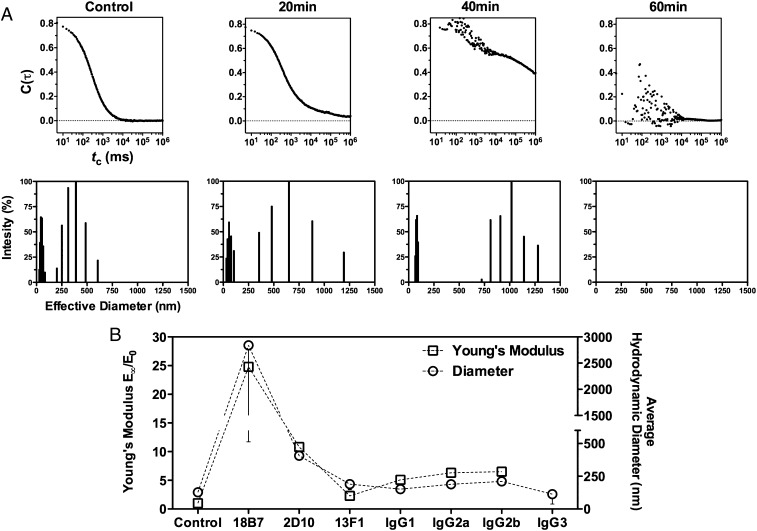
Next, we hypothesized that the ability of mAbs to change the stiffness of the capsule was due to their relative ability to cross-link GXM molecules, which in turn must be determined by the concentration and availability of binding epitopes as well as the binding properties of each mAb. Consequently, we used DLS to monitor changes in average hydrodynamic size of PS solutions after mAb addition. We started by testing mAb 18B7 (IgG1), because it produced the strongest effect on capsule elastic properties. Cross-linking of PS was observed over time after the addition of mAb 18B7 (IgG1), with the appearance of signals corresponding to particles of 15× higher dimensions (∼2 μm) and an increase in autocorrelation function decay time, which relates to how fast particles diffuse in solution (Fig. 4A). Loss of autocorrelation function decay was observed after 40 min of incubation, consistent with a loss of particle Brownian motion as particles exited the scattering volume caused by the formation of large mAb–GXM complexes and/or signal interference by turbidity due to PS aggregation (Fig. 4A, Table II). The presence of a white precipitate after mAb addition and an increase in solution turbidity are consistent with the formation of large mAb–GXM complexes that were no longer soluble.
FIGURE 4.
Ab-mediated cross-linking of soluble PS molecules correlates with the capsule’s elastic properties. (A) The ability of Ab-mediated cross-linking of PS molecules was studied by monitoring the changes in normalized scattered intensity autocorrelation function decay of a PS solution (top) and the corresponding multimodal hydrodynamic size distribution (bottom), before (control) and 20, 40, and 60 min after addition of mAb (IgG1 18B7, 10 μg ml−1). Data represent average values from 10 repeated measurements. (B) Correlation between fold increase of E and average hydrodynamic size of PS solutions after mAb addition (10 μg ml−1) following 30-min incubation at 37°C. PS solution in the absence of mAb was used as control. Data are averages from 10 repeated measurements.
Table II. Ab-mediated changes in average hydrodynamic size and polydispersity of PS solution.
| Time (min) | Effective Diameter (nm) | Polydispersity |
|---|---|---|
| 0 | 126 ± 1 | 0.361 ± 0.004 |
| 10 | 179 ± 7 | 0.371 ± 0.005 |
| 20 | 200 ± 23 | 0.371 ± 0.004 |
| 30 | 194 ± 8 | 0.580 ± 0.036 |
| 40 | (194 ± 95) × 10 | 0.083 ± 0.054 |
| 50 | n.d. | n.d. |
n.d., Not detectable.
We then compared the cross-linking capacity between our panel of anti-GXM mAbs following a 30-min incubation period. Depending on the mAb, an increase in the average hydrodynamic size of PS solutions was observed relative to the control (PS alone) (Fig. 4B). Differences in the ability of protective mAbs to increase average hydrodynamic sizes of PS molecules correlated with the capacity to increase the capsule’s E values (Fig. 4B). To our knowledge, these results provide, for the first time, both quantitative and qualitative evidence for mAb-mediated cross-linking of GXM molecules and suggest a molecular explanation for the mAb-mediated increase in capsule stiffness.
Discussion
Abs to microbial surfaces can contribute to host defense through multiple mechanisms (1). In addition to functioning as a connection between the microbe and cells of the immune system through Fc–FcR interactions, the binding of some Abs to microbial Ags can directly modify the microbe’s normal physiology, also contributing to protection of the infected host (1). For encapsulated pathogens such as C. neoformans, the Ab–capsule interaction is important for protection because it can result in increased drug susceptibility, altered gene expression, and prevention of PS release (2, 23). The present study examines this Ab–capsule interaction and the effect of protective and nonprotective mAbs on C. neoformans replication and the capsule’s physical properties. Our results indicate that protective mAbs can directly alter cell division by trapping and preventing the full release of newly budded cells. This effect is caused by an Ab-mediated increase in capsule stiffness, involving cross-linking of capsular PS molecules. The ability of mAbs to impair C. neoformans budding through changes in the capsule’s mechanical properties indicates a new, nonclassical mechanism of Ab function and presents important implications for understanding Ab-mediated immunity.
The increase in capsule stiffness by protective mAbs, however, was not sufficient to affect the yeast budding rate, consistent with previous reports that Ab binding to the capsule does not prevent cell growth (42–44). This finding suggests that cellular division in C. neoformans is not physically influenced by the capsule and/or that intracellular-derived turgor forces (45) can overcome the mechanical resistance that could potentially be exerted by an Ab-coated capsule.
The molecular basis for the increase in capsule stiffness could be explained by mAb-mediated cross-linking of GXM molecules upon mAb binding to the capsule. This hypothesis is supported by the correlation of the E data with the average hydrodynamic size increase of PS solutions. These results also provide insight into another classical Ab function: Ab-mediated precipitation. Mixing of mAb and PS could result in the formation of complexes that increased in hydrodynamic size with time, after which they left the solution state to form precipitates. Although the size at which precipitation occurred may differ with the type of complex, our results indicate that the transition from the solution to the precipitate state takes time and occurs rapidly once a threshold mass is reached.
The importance of Ab isotype and binding properties in the ability of anti-GXM mAbs to affect the physical properties of the capsule was evident from the different dose–response curves. The contributions of the isotype and epitope specificities may be interrelated because C region type can affect fine specificity (39). For instance, the inability of the mAb 3E5 (IgG3) to increase the E was striking when compared with the mAb 3E5 (IgG1) because these Abs have same variable gene regions. We can imagine two explanations for this result that are not exclusive to one another. First, the 3E5 (IgG1) and 3E5 (IgG3) differ in epitope specificity for GXM, despite identical V regions (46), and it is conceivable that the latter binds to an epitope that precludes formation of larger Ag–Ab aggregates. Second, IgG subclasses differ in the angle and flexibility of the two binding regions and the particular configuration of IgG3 (47). This property, in combination with the configuration of its epitopes in GXM, may not favor formation of larger aggregates. It is also important to mention that structural features of Abs can also determine the formation of Ab complexes or aggregates, thus affecting their overall functionality (48–50). Although Ab aggregate formation should not be favored under the diluted concentrations used in this study, Ab–Ab interaction capacity could potentially influence their ability to mediate cross-linking of PS molecules and increase the stiffness of the capsule.
Our study also provides insights into the mechanism of the capsular (quellung) reaction. The capsular reaction was first described by Neufeld in 1902 upon the addition of type-specific immune sera to Streptococcus pneumoniae (41) and continues to be clinically useful in the typing of pneumococcus (51). Despite its fundamental importance in the development of immunological concepts of specificity, the physical basis of the capsular reaction has never been rigorously studied, possibly because of the relatively small dimensions of encapsulated bacterial cells. One advantage of the C. neoformans system is that the fungal cell volume is enormous relative to bacterial cells (> 200× larger), and this allows the type of mechanoelastic measurements done in this study. In C. neoformans, capsular reactions have been correlated with protection (26). When viewed by differential interference contrast, protective and nonprotective mAbs produce distinct capsular reactions—rim and puffy, respectively (26). Importantly, similar to the increase in capsule stiffness described in this study, it was suggested that the capsular reaction resulted from Ab-mediated cross-linking and/or localized precipitation of PS molecules at the capsule surface (26, 52). The poor reactivity (inferred from capsule stiffness, visibility, and average hydrodynamic size of PS solutions) observed for 13F1 and 3E5 IgG3, relative to the rest of the mAbs, also supports this view and suggests that both mechanical and optical changes on Ab-coated capsules arise from the cross-linking capacity of capsular mAbs. Thus the capsular reaction most likely results from alterations in capsular matrix organization or density, thus affecting the absorptive, reflective, and/or refractive properties of the capsule. In addition, the formation of larger Ab–PS complexes may result in higher molar refractivity based on the Lorentz–Lorenz relation, which positively correlates refractive index to the number of molecules per unit of volume (53).
Our results provide information that leads to a better understanding of the direct effects of mAbs on the anti–C. neoformans immunity. Recently, mAb binding to the capsule was shown to induce changes in gene expression and fungal metabolic activity (2). This phenomenon has also been observed with S. pneumoniae, another encapsulated pathogen (3). The ability of mAb 18B7 (IgG1) and 13F1 (IgM) to induce changes in gene expression also correlated with binding locations in the capsule (2). The mechanism by which fungal cells respond to mAb–capsule interactions is not understood, especially when binding occurs at a distance of several microns from the cell wall and given the limited permeability of the capsule to large molecules (9, 54). In this regard, mAb-mediated increase in capsule stiffness could provide an explanation to such general alterations in C. neoformans physiology based on a mechanical sensing-transduction phenomenon. In fact, the magnitude of alterations in microbial gene expression by mAbs 18B7 and 13F1 (2) correlated with their ability to increase capsule E. We propose that mAb-mediated cross-linking results in an increase in capsule stiffness, which generates a mechanical stimulus that could affect cell wall integrity sensors (55) and consequently triggers changes in gene expression, as well as potentially other physiological parameters such as PS release (23) and biofilm formation (24).
When comparing the relative opsonization capacity between the mAbs tested in this study, we also noticed an association between their capacity to increase in capsule stiffness and promote phagocytosis (19, 56). We propose that a more rigid microbial surface could favor cell–cell interactions and particle engulfment by macrophages. In this regard, the physical properties of a targeted particle, such as stiffness and shape, are known to influence phagocytosis by macrophages, indicating the existence of a physical sensing mechanism by these key effector cells (57, 58).
In conclusion, our data illustrate a new direct Ab-mediated effect on microbial physiology involving alterations in C. neoformans replication and bud mobility associated with a change in the mechanical properties of the PS capsule. The relative contribution of this mechanism to Ab-mediated protection against encapsulated microbes is still unknown However, this biophysical effect has important implications for understanding the mechanisms of mAb action, because trapping of cells will 1) confine the fungal mass to a specific locale and 2) enable a more localized and targeted antimicrobial response by cytotoxic and phagocytic cells. This mechanism could also help to explain the finding that Ab-mediated phagocytosis is followed by nonlytic exocytosis in the form of microcolonies, whereas complement-mediated phagocytosis produces exocytosis in the form of planktonic cells (59). The ability of the different mAbs to increase the capsule stiffness correlates with their in vivo and in vitro biological activity (19, 37, 56), suggesting that this physical effect is, in fact, an important determinant for mAb protective efficacy against C. neoformans. Given that protective Ab responses are known to produce quellung reactions in bacterial pathogens (41, 60), it is conceivable that capsule-binding Abs produce similar effects on encapsulated bacteria and that the effects reported can be generalized to other systems.
Supplementary Material
This work was supported by National Institutes of Health Grants AI033774, HL059842, and AI033142 to A.C. and R.J.B.C. R.J.B.C. was also supported in part by the Training Program in Cellular and Molecular Biology and Genetics Grant T32 GM007491. The Casadevall laboratory is part of and participates in the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center, funded by the National Institutes of Health (AI51519). B.P. and N.B.V. received grants from Conselho Nacional de Desenvolvimento Tecnológico, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Instituto Nacional de Ciência e Tecnologia de Fluidos Complexos. S.F. received grants from Conselho Nacional de Desenvolvimento Tecnológico. M.L.R. and L.N. received grants from Conselho Nacional de Desenvolvimento Tecnológico, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Fundação de Amparo à Pesquisa do Estado de São Paulo, and Financiadora de Estudos e Projetos.
The online version of this article contains supplemental material.
- DLS
- dynamic light scattering
- GXM
- glucuronoxylomannan
- PS
- polysaccharide.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Casadevall A., Pirofski L. A. 2012. A new synthesis for antibody-mediated immunity. Nat. Immunol. 13: 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClelland E. E., Nicola A. M., Prados-Rosales R., Casadevall A. 2010. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J. Clin. Invest. 120: 1355–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yano M., Gohil S., Coleman J. R., Manix C., Pirofski L. A. 2011. Antibodies to Streptococcus pneumoniae capsular polysaccharide enhance pneumococcal quorum sensing. MBio. 2: pii . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClelland E. E., Bernhardt P., Casadevall A. 2005. Coping with multiple virulence factors: which is most important? PLoS Pathog. 1: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y. C., Kwon-Chung K. J. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14: 4912–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozel T. R., Pfrommer G. S., Guerlain A. S., Highison B. A., Highison G. J. 1988. Role of the capsule in phagocytosis of Cryptococcus neoformans. Rev. Infect. Dis. 10(Suppl 2): S436–S439 [DOI] [PubMed] [Google Scholar]
- 7.Kozel T. R., Gotschlich E. C. 1982. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 129: 1675–1680 [PubMed] [Google Scholar]
- 8.Zaragoza O., Rodrigues M. L., De Jesus M., Frases S., Dadachova E., Casadevall A. 2009. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 68: 133–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordero R. J., Pontes B., Guimarães A. J., Martinez L. R., Rivera J., Fries B. C., Nimrichter L., Rodrigues M. L., Viana N. B., Casadevall A. 2011. Chronological aging is associated with biophysical and chemical changes in the capsule of Cryptococcus neoformans. Infect. Immun. 79: 4990–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordero R. J., Frases S., Guimaräes A. J., Rivera J., Casadevall A. 2011. Evidence for branching in cryptococcal capsular polysaccharides and consequences on its biological activity. Mol. Microbiol. 79: 1101–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frases S., Pontes B., Nimrichter L., Viana N. B., Rodrigues M. L., Casadevall A. 2009. Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc. Natl. Acad. Sci. U S A 106: 1228–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall A., Pirofski L. A. 2012. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe 11: 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanford J. E., Lupan D. M., Schlageter A. M., Kozel T. R. 1990. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect. Immun. 58: 1919–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee J., Scharff M. D., Casadevall A. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60: 4534–4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleuridor R., Zhong Z., Pirofski L. 1998. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J. Infect. Dis. 178: 1213–1216 [DOI] [PubMed] [Google Scholar]
- 16.Dromer F., Charreire J., Contrepois A., Carbon C., Yeni P. 1987. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55: 749–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen R. A., Pappas P. G., Perfect J., Aberg J. A., Casadevall A., Cloud G. A., James R., Filler S., Dismukes W. E. 2005. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 49: 952–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casadevall A., Cleare W., Feldmesser M., Glatman-Freedman A., Goldman D. L., Kozel T. R., Lendvai N., Mukherjee J., Pirofski L. A., Rivera J., et al. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42: 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan R. R., Spira G., Oh J., Paizi M., Casadevall A., Scharff M. D. 1998. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect. Immun. 66: 1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taborda C. P., Rivera J., Zaragoza O., Casadevall A. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170: 3621–3630 [DOI] [PubMed] [Google Scholar]
- 21.Taborda C. P., Casadevall A. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166: 2100–2107 [DOI] [PubMed] [Google Scholar]
- 22.Nussbaum G., Cleare W., Casadevall A., Scharff M. D., Valadon P. 1997. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J. Exp. Med. 185: 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez L. R., Moussai D., Casadevall A. 2004. Antibody to Cryptococcus neoformans glucuronoxylomannan inhibits the release of capsular antigen. Infect. Immun. 72: 3674–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez L. R., Casadevall A. 2005. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 73: 6350–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClelland E. E., Casadevall A. 2012. Strain-related differences in antibody-mediated changes in gene expression are associated with differences in capsule and location of binding. Fungal Genet. Biol. 49: 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacGill T. C., MacGill R. S., Casadevall A., Kozel T. R. 2000. Biological correlates of capsular (quellung) reactions of Cryptococcus neoformans. J. Immunol. 164: 4835–4842 [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee J., Cleare W., Casadevall A. 1995. Monoclonal antibody mediated capsular reactions (Quellung) in Cryptococcus neoformans. J. Immunol. Methods 184: 139–143 [DOI] [PubMed] [Google Scholar]
- 28.Cleare W., Brandt M. E., Casadevall A. 1999. Monoclonal antibody 13F1 produces annular immunofluorescence patterns on Cryptococcus neoformans serotype AD isolates. J. Clin. Microbiol. 37: 3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan R., Casadevall A., Spira G., Scharff M. D. 1995. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J. Immunol. 154: 1810–1816 [PubMed] [Google Scholar]
- 30.Mukherjee J., Casadevall A., Scharff M. D. 1993. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J. Exp. Med. 177: 1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spira G., Paizi M., Mazar S., Nussbaum G., Mukherjee S., Casadevall A. 1996. Generation of biologically active anti-Cryptococcus neoformans IgG, IgE and IgA isotype switch variant antibodies by acridine orange mutagenesis. Clin. Exp. Immunol. 105: 436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frases S., Pontes B., Nimrichter L., Rodrigues M. L., Viana N. B., Casadevall A. 2009. The elastic properties of the Cryptococcus neoformans capsule. Biophys. J. 97: 937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicola A. M., Frases S., Casadevall A. 2009. Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryot. Cell 8: 1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araujo Gde. S., Fonseca F. L., Pontes B., Torres A., Cordero R. J., Zancopé-Oliveira R. M., Casadevall A., Viana N. B., Nimrichter L., Rodrigues M. L., et al. 2012. Capsules from pathogenic and non-pathogenic Cryptococcus spp. manifest significant differences in structure and ability to protect against phagocytic cells. PLoS ONE 7: e29561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teraoka I. 2002. Polymer Solutions: An Introduction to Physical Properties. Wiley, New York [Google Scholar]
- 36.Jain N., Cook E., Xess I., Hasan F., Fries D., Fries B. C. 2009. Isolation and characterization of senescent Cryptococcus neoformans and implications for phenotypic switching and pathogenesis in chronic cryptococcosis. Eukaryot. Cell 8: 858–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee J., Nussbaum G., Scharff M. D., Casadevall A. 1995. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J. Exp. Med. 181: 405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera J., Casadevall A. 2005. Mouse genetic background is a major determinant of isotype-related differences for antibody-mediated protective efficacy against Cryptococcus neoformans. J. Immunol. 174: 8017–8026 [DOI] [PubMed] [Google Scholar]
- 39.Torres M., May R., Scharff M. D., Casadevall A. 2005. Variable-region-identical antibodies differing in isotype demonstrate differences in fine specificity and idiotype. J. Immunol. 174: 2132–2142 [DOI] [PubMed] [Google Scholar]
- 40.Murphy D. B. 2001. Fundamentals of Light Microscopy and Electronic Imaging. Wiley-Liss, New York [Google Scholar]
- 41.Neufeld F. Über die Agglutination der Pneumokokken und über die Theorieen der Agglutination. Z. Hyg. Infektionskr. 1902;40 [Google Scholar]
- 42.Mukherjee J., Feldmesser M., Scharff M. D., Casadevall A. 1995. Monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan enhance fluconazole efficacy. Antimicrob. Agents Chemother. 39: 1398–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dromer F., Perronne C., Barge J., Vilde J. L., Yeni P. 1989. Role of IgG and complement component C5 in the initial course of experimental cryptococcosis. Clin. Exp. Immunol. 78: 412–417 [PMC free article] [PubMed] [Google Scholar]
- 44.Diamond R. D., Root R. K., Bennett J. E. 1972. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J. Infect. Dis. 125: 367–376 [DOI] [PubMed] [Google Scholar]
- 45.Campbell N. A., Reece J. B. 2009. Biology. Pearson Benjamin Cummings, San Francisco [Google Scholar]
- 46.Torres M., Fernández-Fuentes N., Fiser A., Casadevall A. 2007. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J. Biol. Chem. 282: 13917–13927 [DOI] [PubMed] [Google Scholar]
- 47.Roux K. H., Strelets L., Michaelsen T. E. 1997. Flexibility of human IgG subclasses. J. Immunol. 159: 3372–3382 [PubMed] [Google Scholar]
- 48.Abdelmoula M., Spertini F., Shibata T., Gyotoku Y., Luzuy S., Lambert P. H., Izui S. 1989. IgG3 is the major source of cryoglobulins in mice. J. Immunol. 143: 526–532 [PubMed] [Google Scholar]
- 49.Gavin A. L., Barnes N., Dijstelbloem H. M., Hogarth P. M. 1998. Identification of the mouse IgG3 receptor: implications for antibody effector function at the interface between innate and adaptive immunity. J. Immunol. 160: 20–23 [PubMed] [Google Scholar]
- 50.Greenspan N. S., Cooper L. J. 1992. Intermolecular cooperativity: a clue to why mice have IgG3? Immunol. Today 13: 164–168 [DOI] [PubMed] [Google Scholar]
- 51.Siira L., Kaijalainen T., Lambertsen L., Nahm M. H., Toropainen M., Virolainen A. 2012. From Quellung to multiplex PCR, and back when needed, in pneumococcal serotyping. J. Clin. Microbiol. 50: 2727–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans E. E. 1960. Capsular reactions of Cryptococcus neoformans. Ann. N. Y. Acad. Sci. 89: 184–192 [Google Scholar]
- 53.Aspnes D. E. 1982. Local-field effects and effective-medium theory—a microscopic perspective. Am. J. Phys. 50: 704–709 [Google Scholar]
- 54.Gates M. A., Thorkildson P., Kozel T. R. 2004. Molecular architecture of the Cryptococcus neoformans capsule. Mol. Microbiol. 52: 13–24 [DOI] [PubMed] [Google Scholar]
- 55.Levin D. E. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69: 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLean G. R., Torres M., Elguezabal N., Nakouzi A., Casadevall A. 2002. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 169: 1379–1386 [DOI] [PubMed] [Google Scholar]
- 57.Beningo K. A., Wang Y. L. 2002. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 115: 849–856 [DOI] [PubMed] [Google Scholar]
- 58.Underhill D. M., Goodridge H. S. 2012. Information processing during phagocytosis. Nat. Rev. Immunol. 12: 492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez M., Saylor C., Casadevall A. 2008. Antibody action after phagocytosis promotes Cryptococcus neoformans and Cryptococcus gattii macrophage exocytosis with biofilm-like microcolony formation. Cell. Microbiol. 10: 1622–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexander H. E. 1943. Treatment of Haemophilus influenzae infections and of meningococcic and pneumococcic meningitis. Am. J. Dis. Child. 66: 172–187 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.