Abstract
Current adjuvant treatment modalities for breast cancer that express the estrogen receptor or progesterone receptor include adjuvant anti-estrogen therapies, and tamoxifen and aromatase inhibitors. Bone, including the jaw, is an endocrine-sensitive organ, as are other oral structures. This review examines the potential links between adjuvant anti-estrogen treatments in postmenopausal women with hormone receptor positive breast cancer and oral health. A search of PubMed, EMBASE, CENTRAL, and the Web of Knowledge was conducted using combinations of key terms “breast,” “cancer,” “neoplasm,” “Tamoxifen,” “Aromatase Inhibitor,” “chemotherapy,” “hormone therapy,” “alveolar bone loss,” “postmenopausal bone loss,” “estrogen,” “SERM,” “hormone replacement therapy,” and “quality of life.” We selected articles published in peer-reviewed journals in the English. The authors found no studies reporting on periodontal diseases, alveolar bone loss, oral health, or oral health-related quality of life in association with anti-estrogen breast cancer treatments in postmenopausal women. Periodontal diseases, alveolar bone density, tooth loss, and conditions of the soft tissues of the mouth have all been associated with menopausal status supporting the hypothesis that the soft tissues and bone of the oral cavity could be negatively affected by anti-estrogen therapy. As a conclusion, the impact of adjuvant endocrine breast cancer therapy on the oral health of postmenopausal women is undefined. The structures of the oral cavity are influenced by estrogen; therefore, anti-estrogen therapies may carry the risk of oral toxicities. Oral health care for breast cancer patients is an important but understudied aspect of cancer survivorship.
Keywords: Aromatase inhibitors, Breast cancer, Oral health
Introduction
Oral health in those affected by cancer
The Surgeon General's report on Oral Health in America estimates that more than 30–35 % of patients undergoing cancer treatment (400,000 annually) will develop oral complications [1]. As a result, leading health organizations have developed guidelines for the oral care of cancer patients. The National Institutes of Health (NIH) Development Consensus Conference on the Oral Complications of Cancer Therapies strongly recommended oral assessment and oral/dental care prior to cancer therapy, which should be continued during and following therapy [2]. More recently, the American Dental Association and the National Institute of Dental and Cranio-facial Research (NIDCR) have developed brochures and handouts [3, 4] for patients and providers on oral health care guidelines for patients with cancer. While the NIH guidelines have been in place for over 20 years, there is little data as to whether these guidelines are followed by either the medical or dental communities [5, 6].
Cancer treatments and oral health-related quality of life
In the United States, the life time risk of developing cancer is approximately 1 in 2 for men and 1 in 3 for women [7] with breast cancer affecting 1 in 8 American women [8]. Depending on the type of cancer and the treatment, a wide range of oral complications may occur during or after cancer care. Cancer survivors have reported serious oral health-related side effects of radiotherapy and chemotherapy, including xerostomia and mucositis. Oral pain and xerostomia can significantly reduce the quality of life of patients and can seriously affect functional capabilities and thus their nutritional status. Individuals may avoid foods difficult to chew, such as raw vegetables and fruits, as well as other items needed for good nutritional status and overall quality of health [8]. Such factors resulting from oral complications of therapies can compromise patients’ compliance with treatment [9, 10].
The impact of cancer therapy on the oral health of patients with cancer is poorly defined outside of mucositis, head and neck cancers, and in the setting of stem cell transplantation. Thus, oral conditions represent a critical, yet underappreciated, factor in care of thousands of patients with cancer.
Soft tissues and bones of the oral cavity may be considered endocrine-sensitive tissues [11–13]. Breast cancer treatments, such as chemotherapy and anti-estrogen therapies, may promote a low estrogen states, and may affect a woman's oral health [14]. The majority of women diagnosed with breast cancer can expect an excellent outcome, with a 5 year survival rate above 80 % [15]. Currently, over 2.5 million women in the US have carried the diagnosis of breast cancer [15]. Hence, long-term survivorship issues, such as oral health, are important components of breast cancer care and follow-up and reflect an evolving public health concern. Current oral health and breast cancer guidelines do not specifically address anti-estrogen treatments such as tamoxifen or aromatase inhibitors and their potential effects on oral health. In order to assess the data supporting the hypothesis that anti-estrogen therapies used in the management of breast cancer may have an impact on oral health in postmenopausal women, a literature review was performed.
Review of the literature
Four databases, PubMed (January 1966–November 2011), The Cochrane Central Register of Controlled Trials (1941–2011), EMBASE (1974–2011), and the “Web of Knowledge” were searched to identify relevant systematic reviews, meta-analyses, clinical trials, and epidemiologic studies of adjuvant endocrine treatments associated with oral health. The Medical Subject Headings used in single search terms or Boolean combinations included the following: “breast,” “cancer,” “neoplasm,” “tamoxifen,” “aromatase inhibitor,” “chemotherapy,” “hormone therapy,” “alveolar bone loss,” “bone loss,” postmenopausal,” “estrogen,” “selective estrogen receptor modulators,” “hormone replacement therapy,” “quality of life,” and “English language.” The search was supplemented by examining bibliographies of selected articles. The inclusion/exclusion review criteria specified that those papers examining bisphosphonates and osteonecrosis of the jaws, as well as those in languages other than English, were excluded.
Results
The search yielded 45 articles, and 37 of those 45 did not meet the inclusion criteria. Of the remaining eight, seven were reviews which discussed bisphosphonates and osteonecrosis of the jaw; and one included strategies for prevention and treatment of periodontal disease related to bisphosphonates and other agents. Thus, there appears to be a paucity of published works focused specifically on adjuvant endocrine therapy and oral health in postmenopausal women.
Discussion
Although there is insufficient data to specifically address the effects of adjuvant endocrine therapy on the oral health of women with breast cancer, there is a body of literature supporting the hypothesis that the soft tissues and bone of the oral cavity could be negatively affected by anti-estrogen therapy. Periodontal disease, conditions of the soft tissues of the mouth, alveolar bone density, and tooth loss have all been associated with menopausal status, as reviewed below.
Estrogen deficiency
For the purpose of this review, hormone therapy refers to supplemental estrogen and/or progesterone treatment of postmenopausal women. Although use of hormone therapy is controversial, the effect of hormone therapy on postmenopausal bone is well established [16] and there is evolving data on the effect of hormone therapy on the tissues of the oral cavity. Studies of the relationship between estrogen and periodontal health are summarized in Table 1. Overall, estrogen appears to improve the periodontal/dental endpoints of bleeding on probing, pocket depth, clinical attachment loss, as well as the number of teeth retained, and the alveolar bone height and density.
Table 1.
Studies of the relationship between estrogen and oral health
| Study | Design | Population demographics | Dental outcome measure | Exposure measure | Covariates adjusted for in analysis | Results |
|---|---|---|---|---|---|---|
| Periodontal diseases | ||||||
| Pizzo et al. [20] | CS | 91 PM women Caucasian (68.3 ± 1.8 years) |
BOP PD CAL |
HT+ HT– |
Decreased BOP HT+ 34.8 % HT– 65.2 % (P = 0.0007) No significant differences with HT+/HT– use and PD, CAL |
|
| Haas et al. [23] | CS | 328 PM women Caucasian (40–69 years) |
CAL | HT+ HT– |
Age, smoking, SES, dental care | Prevalence of individuals with ≥30 % 5 mm CAL: HT+ 46.3 % HT– 64.0 % Odds of having ≥30 % 5 mm CAL among HT– women OR 2.1; 95 % CI: 1.1–4.0 |
| Evio et al. [25] | LS– 2 years | 60 PM women Finnish (65–80 years) |
BOP PD CPITN |
(1)HT (2) Bis (3) HT+ Bis |
No significant differences with HT use and BOP, PD, CPITN | |
| Lopez-Marcos et al. [24] | LS– 1 year | 210 PM women Caucasian Brazilian (40–58 years) |
PD, Mobility, Recession | HT+ HT– |
Changes from baseline to 12 months HT+ decreased PD HT– increased PD (P = 0.004) No significant differences with HT+/HT– use gingival recession |
|
| Ronderos et al. [22] | CS | 11,665 women Representative sample (40–70 years) |
CAL | HT+ HT– |
Age, SES, race/ethnicity, smoking, dental care calculus | Mean CAL associated with HT+ use (SD) Never 1.74 mm (0.029) 1 year or less 1.70 mm (0.059) 2–5 years 1.55 mm (0.073) >5 years 1.56 mm (0.062) (P < 0.05) |
| Reinhardt et al. [19] | LS– 2 years | 59 PM women Caucasian (45–60 years) |
CAL Gingival inflammation |
E+ sufficient E– deficient |
Smoking | Gingival inflammation: E+ women 24.4 % E– women 43.8 % (P < 0.04) No significant differences between E+/E– status and CAL |
| Grossi [21] | CS | 236 PM women Caucasian (50–75 years) |
CAL ABH |
HT+ HT– |
Age | Mean CAL: HT+ 2.1 mm HT– 2.4 mm ABH Loss >3 mm: HT+ 34.0 % HT– 20.0 % |
| Alveolar bone |
||||||
| Payne et al. [26] | LS– 1 year | 24 PM women Caucasian (40–65 years) |
ABD | E+ sufficient E– deficient |
Change in alveolar bone density E+ women 0.30 ± 0.07 E– women 0.44 ± 0.07 (P = 0.0001) |
|
| Civitelli et al. [27] | LS– 3 years | 135 PM women Primarily Caucasian (41–70 years) |
ABD ABH |
HT+ Placebo |
Age, BMI, smoking, years since menopause, parity | Change in alveolar bone density HT+ 1.84 % Placebo +0.95 % (P = 0.04) No significant differences between HT/placebo groups for ABD |
| Tooth loss | ||||||
| Taguchi et al. [34] | CS | 330 PM women Japanese (58.8, ±7.9 years) |
Tooth loss ABH |
HT+ HT– |
Age, years since menopause, BMI, history of hysterectomy or oophorectomy | Mean # of teeth HT+ 23.2 ± 0.9 HT– 21.9 ± 0.4 (P = 0.19) No significant differences with HT/HT– use and ABH |
| Krall [30] | LS– 7 years | 488 PM women Caucasian (75–95 years) |
Tooth loss | HT+ HT– |
Age, smoking, education | Mean # of teeth HT+ 12.5 ± 0.8 HT– 10.7 ± 0.8 (P = 0.04) HT use >8 years: 3.6 more teeth |
| Grodstein et al. [28] | CS | 42,171 women Unspecified race/ethnicity (46–55 years) |
Tooth loss | HT+ HT– |
Age, SES, smoking, dental care | HT duration associated with decreased risk of loss of ≥1 teeth 1 year RR: 0.78; 95 % CI; 0.67–0.94 15+ years RR: 0.73; 95 % CI; 0.65–0.83 |
| Paganini-Hill [31] | LS– 11 years | 3921 women Primarily Caucasian (52–109 years) |
Tooth count | HT+ HT– |
Age, smoking, alcohol, education | Decreased risk of edentia with HT+ use RR 0.64; 95 % CI; 0.51–0.79 |
Estrogen-sufficient E2+, >40 pg 17 β estradiol/ml serum
Estrogen-deficient E2–, <30 pg 17 β estradiol/ml serum
CS cross-sectional, LS longitudinal study, PM postmenopausal, BOP bleeding on probing, PD pocket depth, CAL clinical attachment loss, CPITN community periodontal index of treatment needs, Mm millimeters, SD standard deviation, ABH alveolar bone height, ABD alveolar bone density, HT+ hormone therapy supplements usually consisting of estrogen or estrogen plus a progestin, HT– no therapy, Bis bisphosphonate, BMI body mass index, SES socio-economic status, NS no significant association
Periodontal disease
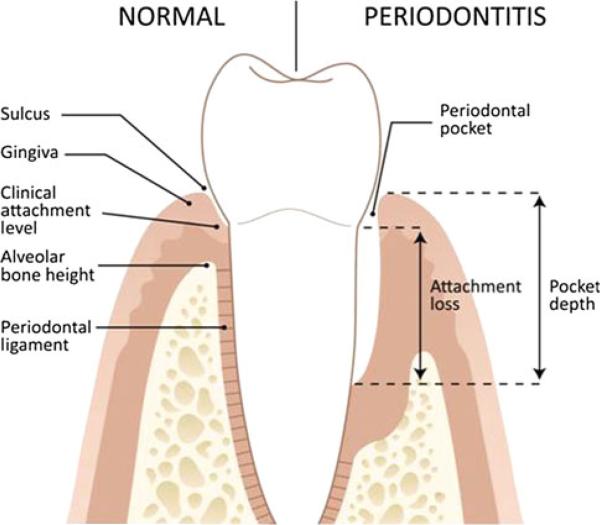
Periodontal disease is a general term referring to inflammatory processes that occurs in the tissues surrounding the teeth in response to bacterial accumulations (dental plaque) on the teeth. Gingivitis, is the inflammation of the gingival tissues, whereas, periodontitis is a destructive inflammatory disease of the deeper supporting tissues of the teeth (bone, ligament), which may result in tooth loss [17] (Fig. 1).
Fig. 1.
Oral structures are illustrated
The primary symptoms of periodontitis are a loss of tooth-supporting tissues (attachment loss) and periodontal pocket formation. Typically the space between the tooth and the gingiva, termed the gingival sulcus, is a blind cul-de-sac where food and bacterial plaque can accumulate. In health, the depth of the sulcus ranges from 1 to 3 mm. If the pocket depth is over 4 mm, it often harbors anaerobic bacteria that can lead to irritation and ulceration of the sulcus lining epithelium which can lead to gingival bleeding. Using periodontal probe, dental providers measure periodontal pockets and clinical attachment levels, typically measured from a fixed point on the tooth (e.g., the cementoenamel junction to the depth of the periodontal pocket), to determine a tooth's stability and the loss of bone support around a tooth. Radiographic examination of the alveolar bone height using dental bitewing X-rays provides another estimate of the bony support around the teeth [17]. Table 2 gives an overview of dental anatomy terms.
Table 2.
Periodontal Definitions
| Definitions | |
|---|---|
| Alveolar bone height (ABH) | The highest crest of the alveolar bone |
| Alveolar process | The bony portion of the maxilla and mandible where the teeth are embedded and tooth roots are supported (via a ligamentous attachment called the periodontal ligament). Also includes a thin layer of compact bone which forms the tooth socket |
| Attachment loss (AL) | Attachment loss (AL) is the extent of periodontal support (bone and tissues) that has been destroyed around a tooth |
| Bleeding on probing (BOP) | Bleeding on gentle probing within the gingival sulcus is a sign of inflammation |
| Clinical attachment level (CAL) | The clinical attachment loss (CAL) is the estimated position of the structures that support the tooth as measured with a periodontal probe. The CAL provides the tooth's stability and the loss of bone support. Typically the CAL is measured from a fixed point on a tooth to the base of the periodontal pocket |
| Gingiva (Gums) | Part of the oral mucosa which covers the alveolar process; the most peripheral component of the periodontium |
| Gingivitis | Inflammation of the gingival tissues without the involvement of the supporting tissues (bone and periodontal ligament) |
| Periodontal pocket | A pathologic deepening of the gingival sulcus produced by the destruction of the supporting tissues with an apical migration of the epithelial attachment |
| Periodontitis | A chronic inflammatory disease affecting the gingival (gums) along with destruction of the supporting structures surrounding the tooth including the periodontal ligament and alveolar bone. Periodontal pockets form as a result |
| Pocket depth (PD) | A periodontal pocket depth is measured using a periodontal probe |
| Sulcus | The space between the gum and the tooth which can be probed and measured and is a site where bacterial plaque can accumulate |
| Soft tissues of the mouth | Mucosal tissue of the lips, cheeks, palate, the tongue, gums (gingival), and floor of the mouth |
Periodontal disease and hormonal therapy
Hormone therapy has been suggested to be beneficial in optimizing healthy periodontal tissues in postmenopausal women, although the mechanism by which hormone therapy alters periodontal disease remains to be elucidated. Clinical studies demonstrate association between hormone therapy use and decreased gingival bleeding [18–20]. A recent, [20] cross-sectional study comparing the periodontal status of 91 postmenopausal women using hormone therapy as compared to non-hormone therapy users showed that no significant differences were noted in other clinical parameters; however, plaque levels and bleeding on probing were significantly lower in the group taking hormone therapy. Earlier studies showed that the percent of gingival sites with bleeding was significantly lower in women who used hormone therapy compared to women who did not use hormone therapy, independent of the levels of plaque accumulation [18, 19].
The data addressing the effects of hormone therapy on clinical attachment level and pocket depths predominantly demonstrate the beneficial effects of hormone therapy on health and maintenance of oral structures. In 1998, a prospective clinical study [21] examined the impact of hormone therapy use and attachment loss and alveolar bone height among pre and postmenopausal women. When adjusted for age, postmenopausal women not receiving estrogen supplements were twice as likely as those who received estrogen supplements, and three times more likely than premenopausal women, to demonstrate severe attachment loss and alveolar bone height. Likewise, population-based studies examining the association between hormone therapy and periodontal disease demonstrated that postmenopausal non-users of hormone therapy had an increased prevalence of attachment loss than women taking hormone therapy, after adjusting for covariates [22, 23]. In a longitudinal study, investigators followed 59 osteoporotic postmenopausal women for 1 year [19] and found that estradiol supplementation was associated with less clinical attachment loss, suggesting that hormone therapy plays a protective role in preventing periodontitis in postmenopausal women. A prospective cohort study [24] investigated the differences in pocket depth between hormone therapy and non-hormone therapy groups at the beginning of the therapy with a reevaluation at 6 and 12 months after the beginning of the hormone therapy. The hormone therapy users demonstrated a reduction in periodontal pocket depths.
However, two small clinical studies [20, 25] examining hormone therapy use found no significant differences in clinical attachment loss or periodontal pocket measurements among postmenopausal women who use hormone therapy as compared to nonusers. The present data suggests that a low estrogen environment may influence the progression of periodontal disease via decreased bone mass of the jaw. Further investigation is warranted to further define these correlations.
Alveolar bone and hormonal therapy
Clinical studies have demonstrated that hormone therapy improves alveolar bone density and tooth retention [26–31]. Alveolar bone density determined by radiographic absorptiometry or computer-assisted densitometric image analysis (CADIA), reflects the bone density of the jaw and is clinically important for the support of the teeth for mastication.
In a prospective study, the association between estrogen status and alveolar bone density was examined in postmenopausal women with a history of periodontitis [26]. Women within 7 years of menopause were classified as either estrogen-sufficient or estrogen-deficient (E2- < 30 pg 17 β-estradiol/ml serum). The estrogen-sufficient women demonstrated a net gain in alveolar bone density; and the estrogen-deficient women displayed a mean net loss in alveolar bone density over the 1 year course of the study, suggesting that estrogen influences alveolar bone density, as would be expected. Similarly, a randomized double-blind placebo-controlled trial over a 3 year period with 135 postmenopausal women reported that estrogen therapy significantly increased alveolar bone density compared with placebo [27]. Furthermore, bone mineral density of the proximal femur significantly increased in the hormone therapy group, as did alveolar bone, demonstrating a systemic effect of hormone therapy use.
Tooth loss and hormonal therapy
Tooth loss is considered the ultimate endpoint in periodontal disease and is a clinically important marker of the disease. Trauma, extensive dental caries, smoking, and patterns of tooth extraction, which vary by geographic region, also influence tooth retention [32, 33]. Epidemiologic studies of the relationship between estrogen and tooth loss have shown that hormone therapy protects against tooth loss and reduces the risk of edentulism [28, 30, 31]. In the Framingham study, [30] estrogen use independently predicted the number of teeth remaining among 488 postmenopausal women. Furthermore, individuals who used estrogen >8 years averaged 3.6 more teeth than women who never used estrogen. Similarly, a positive relationship between hormone therapy use, tooth loss and the need for dentures in older women was demonstrated in an analysis of women from the Leisure World Cohort [31]. Rates of edentia were significantly lower in hormone therapy users as compared to nonusers even after adjusting for age. Furthermore, the need for dentures was less common among estrogen users than among nonusers. These results demonstrate that estrogen use may promote tooth retention by strengthening the periodontal attachment and/or alveolar bone density. In contrast to these studies, a clinical study using a Japanese population [34] found no difference in tooth retention or oral bone porosity between hormone therapy users and non-hormone therapy users.
Osteoporosis and oral health
Both osteoporosis and periodontal disease are conditions associated with excessive bone resorption. These diagnoses are host-dependent, multi-factorial diseases whose conditions are regulated by local and systemic cytokines (e.g., IL-1 and IL-6), [35] and hormones. Low skeletal bone mineral density has been shown to be associated with reduced alveolar bone height and residual alveolar bone resorption [36–38]. Moreover, epidemiologic studies report a significant relationship between systemic bone loss and tooth loss [29, 39, 40]. While the literature certainly supports a link between systemic bone health and oral bone structures [37, 41–43], there remains much to be further defined on this topic.
Soft tissues of the mouth and hormonal therapy
Hormone therapy has been shown to impact the mouth beyond the periodontal tissues and bone [12, 44, 45]. Estrogen deficiency among postmenopausal women has been associated with decreased salivary flow unrelated to medications [46]. Decreased saliva flow can result in an increased number of dental caries and may be responsible for an increased prevalence of oral dysesthesia and alterations in taste [47–51]. Other oral symptoms associated with decreased estrogen levels include red and shiny gingival tissues (menopausal gingivostomatitis) [45]. Reduced levels of sex hormones as a result of menopause have been implicated in the etiopathogenesis of burning mouth syndrome [45, 52, 53], but this association is not fully established. Burning mouth syndrome, a chronic condition, is characterized by burning of the oral mucosa associated with a sensation of dry mouth and/or taste alterations in the absence of visible pathologic lesions [54]. Burning mouth syndrome typically occurs at more than one oral site; the tongue is the most commonly affected site, followed by the lips, palate, and cheeks [55]. Breast cancer treatments that reduce estrogen levels may increase the risk for soft tissue complications in the mouth.
Breast cancer adjuvant aromatase inhibition
Osteoporosis may serve as a risk factor for periodontitis [35] and tooth loss [40], which suggests that cancer therapies that promote osteoporosis may have a negative impact on oral health. Certain breast cancer therapies may accelerate bone loss. For example, chemotherapy is associated with premature ovarian failure and subsequent loss of bone mineral density [14, 56, 57]. In addition, supportive medications used with chemotherapy often include glucocorticoids which in themselves can induce osteoporosis [58]. Glucocorticoid exposure has been shown to decrease bone mineral density in the radius and jawbone to similar extents [59]. The aromatase inhibitors are associated with bone loss and fracture [60–62].
The American Society of Clinical Oncology (ASCO) clinical practice guidelines on adjuvant endocrine therapy for postmenopausal women with hormone receptive positive cancer (Estrogen Receptor or Progesterone Receptor) breast cancer recommend that aromatase inhibition be included in adjuvant treatment of postmenopausal women [63]. As noted previously, aromatase inhibitors reduce bone mass. Therefore, tens of thousands of women exposed to an aromatase inhibitor may be at increased risk of bone loss. At present, there is insufficient data addressing oral health during the course of adjuvant endocrine therapy with an aromatase inhibitor.
The bone preserving effect of tamoxifen in postmenopausal bone is in contrast to the loss of bone mineral density seen with the aromatase inhibitors [64]. Tamoxifen has been shown to maintain or slightly increase bone mineral density in postmenopausal women [65, 66]. At present, no clinical data are available regarding the impact of tamoxifen and oral health on postmenopausal breast cancer patients. However, human gingival fibroblasts are a target tissue for sex hormones and in vitro studies have demonstrated that tamoxifen may decrease the stimulatory effect of estrogen on human gingival fibroblast proliferation [67, 68].
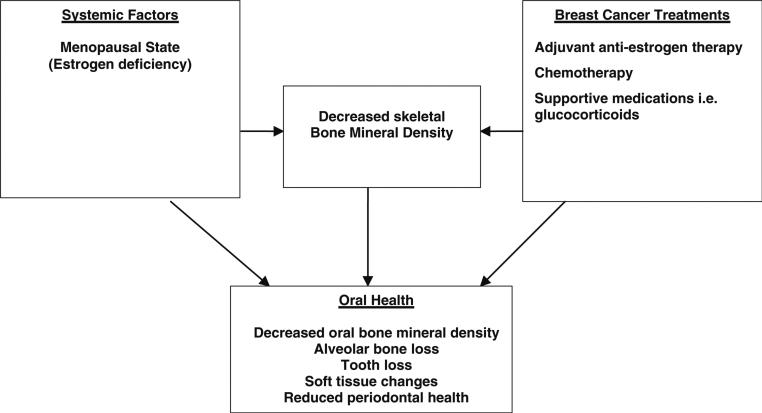
The possible oral effects of adjuvant endocrine therapies commonly used in the management of early stage breast cancer remain undefined, yet a plausible rationale exists for negative oral conditions among postmenopausal women on an aromatase inhibitor as proposed in Fig. 2. Maintaining oral health, particularly in the aging population is a complex medical concern which is complicated further by access to dental insurance coverage. It is possible that changes to the oral cavity associated with aromatase inhibitors may lead to public health concern, the magnitude of which has not yet been defined.
Fig. 2.
Potential relationships between adjuvant endocrine breast cancer treatments, systemic osteopenia/osteoporosis, and oral bone health
The clinical relevance of oral health in populations affected by cancer
Research on oral complications of cancer therapy is very much needed, especially with the growing knowledge that oral and systemic health is linked. Current research to define these risks and toxicities is scant. Ongoing areas of study include clinical trials investigating therapies to prevent or treat mucositis/stomatitis (NCT00385515). Again, the head and neck tumors lead the way in this arena (NCT01283906, NCT00131638, NCT01403064). In addition, association of osteonecrosis of the jaw with potent osteoclast inhibitors such as bisphosphonates (NCT01325142) or denosumab frequently used in metastatic breast cancer treatment, is being investigated both clinically and pre-clinically [69].
A recent literature review showed that the majority of clinical trials testing novel anticancer treatment regimens with tyrosine kinase inhibitors are not formally and specifically investigating oral toxicities or including oral exams as part of the drug toxicity evaluation [70]. In fact, many of the most promising targeted cancer therapies, including agents targeting epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF). Targeting these pathways may have severe oral complications, thus warranting investigation. Drugs that affect the production and/or binding of estrogen to its receptor are also targeted therapies and these anti-estrogen drugs may also affect bone and/or soft tissue of the oral cavity. The endocrine treatment regimens may have a profound impact on public health due to the frequency with which endocrine therapies are used in the management of breast cancer. In order to investigate the questions raised here, the authors are investigating the impact that adjuvant aromatase inhibition therapy has on women with early stage breast cancer through clinical trial NCT01272579 and 1K23DEO21779 and additional studies are warranted. Detailed dental data and oral health-related quality of life information are needed to better understand the significance of this potential treatment related outcome.
Conclusions
Breast cancer affects approximately 200,000 American women annually. The majority of cases will be diagnosed in postmenopausal women and approximately 70 % of these cancers express the estrogen receptor and/or the progesterone receptor for which adjuvant endocrine therapy with an aromatase inhibitor has become a standard intervention. Advancing age places survivors at an increased risk for declining oral health, estrogen deficiency, and osteoporosis. Research is warranted to understand if patients receiving adjuvant aromatase inhibition are at increased risk for oral complications due to therapy. As we gain a greater understanding of the linkage between oral and systemic health, the need for closer collaboration between dental and medical professionals is important for the care of patients with cancer.
Acknowledgments
This review was supported with funding from the Michigan Institute for Clinical Health Research/CTSA pilot grant UL1RR024986 and the National Institute on Dental and Craniofacial Research (NIDCR) grant 5K23DE020197 and 1K23DEO21779. The authors appreciate the assistance of Mark Mac Eachern of the University of Michigan, Taubman Health Sciences Library, for his assistance with the search of the scientific literature and Dr. Rudy Schmerl for his editorial assistance.
Footnotes
Conflict of interest The authors have no conflict of interest to declare in association with this paper
Contributor Information
L. Susan Taichman, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, 1011 North University Ave., Ann Arbor, MI 48109-1078, USA.
Aaron M. Havens, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, 1011 North University Ave., Ann Arbor, MI 48109-1078, USA hipolite@umich.edu Department of Orthodontics and Pediatric Dentistry, University of Michigan School of Dentistry, 1011 North University Ave., Ann Arbor, MI 48109-1078, USA.
Catherine H. Van Poznak, Division of Hematology and Oncology, Department of Internal Medicine, University of Michigan, 1500 E. Medical Center Drive, Ann Arbor, MI 48109, USA cvanpoz@umich.edu; cvanpoz@med.umich.edu
References
- 1.US Department of Health and Human Services . Oral health in America: a report of the surgeon general. U.S. Department of Health and Human Services; National Institute of Dental and Craniofacial Research; National Institute of Health; Rockville: 2000. [Google Scholar]
- 2.NIH [1 April 2012];Oral complications of cancer therapies: diagnosis, prevention and treatment consensus statement. 1989 7:1–11. http://consensus.nih.gov/1989/1989OralComplicationsCancerTherapy073html.htm. [PubMed] [Google Scholar]
- 3.National Institute of Dental and Craniofacial Research [1 April 2012];Cancer treatment and you: three good reasons to see a dentist before cancer treatment. 2011 http://www.nidcr.nih.gov/NR/rdonlyres/04330D33-58E8-4265-A918-1001DC630980/0DentalTeam.pdf.
- 4.National Institute of Dental and Craniofacial Research [1 April 2012];Oral complications of cancer treatment: What the dental team can do. 2008 http://www.nidcr.nih.gov/NR/rdonlyres/4FB39055-1788-4A9E-ADE6-FFBF2C1B6FE9/0/ThreeGoodReasons.pdf.
- 5.McGuire DB. Barriers and strategies in implementation of oral care standards for cancer patients. Support Care Cancer. 2003;11:435–441. doi: 10.1007/s00520-003-0466-4. [DOI] [PubMed] [Google Scholar]
- 6.Epstein JB, Parker IR, Epstein MS, et al. A survey of National Cancer Institute-designated comprehensive cancer centers’ oral health supportive care practices and resources in the USA. Support Care Cancer. 2007;15:357–362. doi: 10.1007/s00520-006-0160-4. [DOI] [PubMed] [Google Scholar]
- 7.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. SEER cancer statistics review, 1975–2008. National Cancer Institute; Bethesda, MD: [30 April 2012]. http://seer.cancer.gov/csr/1975_2008. [Google Scholar]
- 8.Sheiham A, Steele JG, Marcenes W, et al. The impact of oral health on stated ability to eat certain foods; findings from the National Diet and Nutrition Survey of older people in Great Britain. Gerodontology. 1999;16:11–20. doi: 10.1111/j.1741-2358.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 9.Sonis ST, Fey EG. Oral complications of cancer therapy. Oncology (Williston Park) 2002;16:680–686. discussion 686, 691-692, 695. [PubMed] [Google Scholar]
- 10.Epstein JB, Parker IR, Epstein MS, et al. Cancer-related oral health care services and resources: a survey of oral and dental care in Canadian cancer centres. J Can Dent Assoc. 2004;70:302–304. [PubMed] [Google Scholar]
- 11.Mariotti A. Sex steroid hormones and cell dynamics in the periodontium. Crit Rev Oral Biol Med. 1994;5:27–53. doi: 10.1177/10454411940050010201. [DOI] [PubMed] [Google Scholar]
- 12.Leimola-Virtanen R, Salo T, Toikkanen S, et al. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas. 2000;36:131–137. doi: 10.1016/s0378-5122(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 13.Karthik SJ, Arun KV, Sudarsan S, et al. Evaluation of estrogen receptor and circulating estradiol levels in pre- and postmenopausal women with periodontal disease. J Int Acad Periodontol. 2009;11:202–205. [PubMed] [Google Scholar]
- 14.Khan MN, Khan AA. Cancer treatment-related bone loss: a review and synthesis of the literature. Curr Oncol. 2008;15:S30–S40. doi: 10.3747/co.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute [1 April 2012];Breast cancer statistics. 2011 http://seer.cancer.gov/statfacts/html/breast.html#incidence-mortality.
- 16.Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 17.Newman MG, Takei HH, Klokkevold PR, Carranza FA. Carranza's clinical periodontology. Saunders Elsevier; St. Louis: 2011. [Google Scholar]
- 18.Norderyd OM, Grossi SG, Machtei EE, et al. Periodontal status of women taking postmenopausal estrogen supplementation. J Periodontol. 1993;64:957–962. doi: 10.1902/jop.1993.64.10.957. [DOI] [PubMed] [Google Scholar]
- 19.Reinhardt RA, Payne JB, Maze CA, et al. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. J Periodontol. 1999;70:823–828. doi: 10.1902/jop.1999.70.8.823. [DOI] [PubMed] [Google Scholar]
- 20.Pizzo G, Guiglia R, Licata ME, et al. Effect of hormone replacement therapy (HRT) on periodontal status of postmenopausal women. Med Sci Monit. 2011;17:PH23–PH27. doi: 10.12659/MSM.881700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossi SG. Effect of estrogen supplementation on periodontal disease. Compend Contin Educ Dent Suppl. 1998:S30–S36. [PubMed] [Google Scholar]
- 22.Ronderos M, Jacobs DR, Himes JH, et al. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from NHANES III. J Clin Periodontol. 2000;27:778–786. doi: 10.1034/j.1600-051x.2000.027010778.x. [DOI] [PubMed] [Google Scholar]
- 23.Haas AN, Rosing CK, Oppermann RV, et al. Association among menopause, hormone replacement therapy, and periodontal attachment loss in southern Brazilian women. J Periodontol. 2009;80:1380–1387. doi: 10.1902/jop.2009.090082. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Marcos JF, Garcia-Valle S, Garcia-Iglesias AA. Periodontal aspects in menopausal women undergoing hormone replacement therapy. Med Oral Patol Oral Cir Bucal. 2005;10:132–141. [PubMed] [Google Scholar]
- 25.Evio S, Tarkkila L, Sorsa T, et al. Effects of alendronate and hormone replacement therapy, alone and in combination, on saliva, periodontal conditions and gingival crevicular fluid matrix metalloproteinase-8 levels in women with osteoporosis. Oral Dis. 2006;12:187–193. doi: 10.1111/j.1601-0825.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 26.Payne JB, Zachs NR, Reinhardt RA, et al. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol. 1997;68:24–31. doi: 10.1902/jop.1997.68.1.24. [DOI] [PubMed] [Google Scholar]
- 27.Civitelli R, Pilgram TK, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement therapy: a randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2002;162:1409–1415. doi: 10.1001/archinte.162.12.1409. [DOI] [PubMed] [Google Scholar]
- 28.Grodstein F, Colditz GA, Stampfer MJ. Post-menopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc. 1996;127:370–377. doi: 10.14219/jada.archive.1996.0208. quiz 392. [DOI] [PubMed] [Google Scholar]
- 29.Krall EA. Osteoporosis and the risk of tooth loss. Clin Calcium. 2006;16:287–290. [PubMed] [Google Scholar]
- 30.Krall EA, Dawson-Hughes B, Hannan MT, et al. Post-menopausal estrogen replacement and tooth retention. Am J Med. 1997;102:536–542. doi: 10.1016/s0002-9343(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 31.Paganini-Hill A. The benefits of estrogen replacement therapy on oral health. The Leisure World cohort. Arch Intern Med. 1995;155:2325–2329. [PubMed] [Google Scholar]
- 32.Albandar JM, Streckfus CF, Adesanya MR, et al. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol. 2000;71:1874–1881. doi: 10.1902/jop.2000.71.12.1874. [DOI] [PubMed] [Google Scholar]
- 33.Krall EA, Dawson-Hughes B, Garvey AJ, et al. Smoking, smoking cessation, and tooth loss. J Dent Res. 1997;76:1653–1659. doi: 10.1177/00220345970760100601. [DOI] [PubMed] [Google Scholar]
- 34.Taguchi A, Sanada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Menopause. 2004;11:556–652. doi: 10.1097/01.gme.0000113845.74462.bf. [DOI] [PubMed] [Google Scholar]
- 35.Megson E, Kapellas K, Bartold PM. Relationship between periodontal disease and osteoporosis. Int J Evid Based Healthc. 2011;8:129–139. doi: 10.1111/j.1744-1609.2010.00171.x. [DOI] [PubMed] [Google Scholar]
- 36.Payne JB, Reinhardt RA, Nummikoski PV, et al. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 37.Klemetti E, Kolmakov S, Kroger H. Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res. 1994;102:68–72. doi: 10.1111/j.1600-0722.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 38.Al Habashneh R, Alchalabi H, Khader YS, et al. Association between periodontal disease and osteoporosis in postmenopausal women in Jordan. J Periodontol. 2011;81:1613–1621. doi: 10.1902/jop.2010.100190. [DOI] [PubMed] [Google Scholar]
- 39.Nicopoulou-Karayianni K, Tzoutzoukos P, Mitsea A, et al. Tooth loss and osteoporosis: the OSTEODENT study. J Clin Periodontol. 2009;36:190–197. doi: 10.1111/j.1600-051X.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- 40.Tezal M, Wactawski-Wende J, Grossi SG, et al. Periodontal disease and the incidence of tooth loss in postmenopausal women. J Periodontol. 2005;76:1123–1128. doi: 10.1902/jop.2005.76.7.1123. [DOI] [PubMed] [Google Scholar]
- 41.Krall EA, Dawson-Hughes B, Papas A, et al. Tooth loss and skeletal bone density in healthy postmenopausal women. Osteoporos Int. 1994;4:104–109. doi: 10.1007/BF01623233. [DOI] [PubMed] [Google Scholar]
- 42.Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent. 1990;63:218–222. doi: 10.1016/0022-3913(90)90108-o. [DOI] [PubMed] [Google Scholar]
- 43.Takaishi Y, Okamoto Y, Ikeo T, et al. Correlations between periodontitis and loss of mandibular bone in relation to systemic bone changes in postmenopausal Japanese women. Osteoporos Int. 2005;16:1875–1882. doi: 10.1007/s00198-005-1955-8. [DOI] [PubMed] [Google Scholar]
- 44.Valimaa H, Savolainen S, Soukka T, et al. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol. 2004;180:55–62. doi: 10.1677/joe.0.1800055. [DOI] [PubMed] [Google Scholar]
- 45.Wardrop RW, Hailes J, Burger H, et al. Oral discomfort at menopause. Oral Surg Oral Med Oral Pathol. 1989;67:535–540. doi: 10.1016/0030-4220(89)90269-7. [DOI] [PubMed] [Google Scholar]
- 46.Streckfus CF, Baur U, Brown LJ, et al. Effects of estrogen status and aging on salivary flow rates in healthy Caucasian women. Gerontology. 1998;44:32–39. doi: 10.1159/000021980. [DOI] [PubMed] [Google Scholar]
- 47.Mott AE, Grushka M, Sessle BJ. Diagnosis and management of taste disorders and burning mouth syndrome. Dent Clin North Am. 1993;37:33–71. [PubMed] [Google Scholar]
- 48.Lopez-Jornet P, Camacho-Alonso F, Andujar-Mateos P, et al. Burning mouth syndrome: an update. Med Oral Patol Oral Cir Bucal. 2010;15:e562–e568. doi: 10.4317/medoral.15.e562. [DOI] [PubMed] [Google Scholar]
- 49.Ferris GM. Alteration in female sex hormones: their effect on oral tissues and dental treatment. Compendium. 1993;14:1558–15564. 1566. quiz 1571. [PubMed] [Google Scholar]
- 50.Zakrzewska JM. Women as dental patients: are there any gender differences? Int Dent J. 1996;46:548–557. [PubMed] [Google Scholar]
- 51.Zakrzewska JM, Glenny AM, Forssell H. Interventions for the treatment of burning mouth syndrome. Cochrane Database Syst Rev. 2001;1:CD002779. doi: 10.1002/14651858.CD002779. [DOI] [PubMed] [Google Scholar]
- 52.Gao J, Chen L, Zhou J, et al. A case-control study on etiological factors involved in patients with burning mouth syndrome. J Oral Pathol Med. 2009;38:24–28. doi: 10.1111/j.1600-0714.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- 53.Forabosco A, Criscuolo M, Coukos G, et al. Efficacy of hormone replacement therapy in postmenopausal women with oral discomfort. Oral Surg Oral Med Oral Pathol. 1992;73:570–574. doi: 10.1016/0030-4220(92)90100-5. [DOI] [PubMed] [Google Scholar]
- 54.Scala A, Checchi L, Montevecchi M, et al. Update on burning mouth syndrome: overview and patient management. Crit Rev Oral Biol Med. 2003;14:275–279. doi: 10.1177/154411130301400405. [DOI] [PubMed] [Google Scholar]
- 55.Mock D, Chugh D. Burning mouth syndrome. Int J Oral Sci. 2010;2:1–4. doi: 10.4248/IJOS10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hadji P, Ziller M, Maskow C, et al. The influence of chemotherapy on bone mineral density, quantitative ultrasonometry and bone turnover in pre-menopausal women with breast cancer. Eur J Cancer. 2009;45:3205–3212. doi: 10.1016/j.ejca.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Robinson WR, Luck M, Omar H, et al. A pilot study of bone density loss in menopausal women treated with chemotherapy for cancer. Support Care Cancer. 2005;13:663–667. doi: 10.1007/s00520-005-0798-3. [DOI] [PubMed] [Google Scholar]
- 58.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 59.von Wowern N, Klausen B, Olgaard K. Steroid-induced mandibular bone loss in relation to marginal periodontal changes. J Clin Periodontol. 1992;19:182–186. doi: 10.1111/j.1600-051x.1992.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 60.Van Poznak C, Sauter NP. Clinical management of osteoporosis in women with a history of breast carcinoma. Cancer. 2005;104:443–456. doi: 10.1002/cncr.21201. [DOI] [PubMed] [Google Scholar]
- 61.VanderWalde A, Hurria A. Aging and osteoporosis in breast and prostate cancer. CA Cancer J Clin. 2011;61:139–156. doi: 10.3322/caac.20103. [DOI] [PubMed] [Google Scholar]
- 62.Santen RJ. Clinical review: effect of endocrine therapies on bone in breast cancer patients. J Clin Endocrinol Metab. 2011;96:308–319. doi: 10.1210/jc.2010-1679. [DOI] [PubMed] [Google Scholar]
- 63.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force Report: bone health in cancer care. J Natl Compr Canc Netw 7 Suppl. 2009;3:S1–S32. doi: 10.6004/jnccn.2009.0076. quiz S33-S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 66.Powles TJ, Hickish T, Kanis JA, et al. Effect of tamoxifen on bone mineral density measured by dual-energy X-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 67.Soory M, Tilakaratne A. Modulation of androgen metabolism by phenytoin, oestradiol and tamoxifen in human gingival fibroblasts. J Clin Periodontol. 2003;30:556–561. doi: 10.1034/j.1600-051x.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 68.Tilakaratne A, Soory M. Modulation of androgen metabolism by estradiol-17beta and progesterone, alone and in combination, in human gingival fibroblasts in culture. J Periodontol. 1999;70:1017–1025. doi: 10.1902/jop.1999.70.9.1017. [DOI] [PubMed] [Google Scholar]
- 69.U.S. National Institutes of Health Clinical Trials Registry [1 April 2012]; http://www.clinicaltrials.gov/.
- 70.Watters AL, Epstein JB, Agulnik M. Oral complications of targeted cancer therapies: a narrative literature review. Oral Oncol. 2011;47:441–448. doi: 10.1016/j.oraloncology.2011.03.028. [DOI] [PubMed] [Google Scholar]