Abstract
Objectives
Gait speed is an important marker of health in adults and slows with aging. While knee osteoarthritis (OA) can result in difficulty walking, it is not known if radiographic knee OA (ROA) and/or knee pain are associated with a fast decline trajectory of gait speed over time.
Methods
Gait speed trajectories were constructed using a multinomial modeling strategy from repeated 20-meter walk tests measured annually over four years among participants from the Osteoarthritis Initiative (OAI), a prospective cohort study of adults with or at high risk of knee OA aged 45 to 79 at baseline. We grouped participants into four knee OA categories (having neither ROA nor knee pain, ROA only, knee pain only, or symptomatic knee OA (ROA and pain)) and examined their association with trajectories of gait speed using a multivariable polytomous regression model adjusting for age and other potential confounders.
Results
Of the 4179 participants (mean age (sd) = 61.1 (9.1), women =57.6%, mean BMI =28.5 (4.8) kg/m2), 5% (n=205) were in a fast decline trajectory slowing 2.75%/year. People with symptomatic knee OA had almost a 9-fold risk (OR = 8.9, 95% CI [3.1, 25.5]) of being in a fast decline trajectory compared with those with neither pain nor ROA. Participants with knee pain had 4.5 times the odds of fast decline (95% CI [1.4, 14.6]) and those with ROA only had a slight but non-statistically significant increased risk.
Conclusions
People with symptomatic knee OA have the highest risk of fast decline trajectory of gait speed compared with people with ROA or pain alone.
Keywords: Gait speed, Knee Osteoarthritis, Trajectory
INTRODUCTION
Gait speed is a simple yet important indicator of current health and well-being in older adults, and a powerful predictor of mortality(1-4). As such, gait speed has been proposed as a ‘vital sign’. Similar to other vital signs, gait speed changes with aging. Previous studies have reported that gait speed is relatively stable up to age 65, declines 1%/year from age 65 to 69, and by age 80 declines 4%/year(5, 6). Nevertheless, there is substantial variation in the rate of decline across individuals and the presence of certain comorbidities may be a risk factor for premature decline in gait speed(7, 8).
Knee osteoarthritis (OA) is the most common cause of difficulty walking in older adults (9, 10) and subsequently is associated with slow walking(11, 12). Previous cross-sectional studies report that people with both radiographic knee OA and knee pain, also known as symptomatic knee OA, have slower walking speed than healthy age matched controls(12-15). Nevertheless, little is known about trajectories of gait speed in this population over time. In particular, it is unclear if most people with or at high risk of knee OA decline at more or less the same rate or if distinct trajectories of gait speed are present with some declining faster than others. Moreover, while knee pain is the most common symptom of knee OA, substantial discordance has been reported between radiographic findings of disease and knee pain in adults(16). Thus, it is unclear if structural lesions on radiographs, knee pain, or their combination have unique effects on the trajectory of gait speed. Understanding trajectories are important to clarify the natural history of decline expected in this patient population. Furthermore, recognizing disease-related risk for gait speed trajectories helps set realistic expectations for the extent intervention targeting disease and pain may mitigate future decline.
Therefore, the purpose of this study was to examine the association of radiographic disease and/or knee pain with trajectories of gait speed in people with or at high risk of knee OA. Furthermore, we examined these associations across age strata to determine if knee OA was associated with a premature decline in gait speed.
Methods
Sample
The Osteoarthritis Initiative (OAI) is an ongoing longitudinal cohort study of the risk factors and natural history of OA. Adults between 45 to 79 years of age at enrollment who had or were at high risk of knee OA were recruited from four clinical sites: Baltimore MD, Pittsburgh PA, Pawtucket RI, and Columbus OH. Increased risk was identified from age-specific criteria from established risk factors including knee symptoms in the past 12 months(17), being overweight from gender specific cut-points(17-19), knee injury causing difficulty walking for at least one week(17, 20, 21), any knee surgery history(18, 22), family history of a total knee replacement(23, 24), Heberden’s nodes(17, 25), or repetitive knee bending at work or outside of work(26, 27). People were excluded who had rheumatoid or inflammatory arthritis, end-stage disease defined as severe joint space narrowing in both knees at baseline, or bilateral total knee replacements, positive pregnancy test, or used ambulatory aids other then a cane. More detail regarding the rationale and approach for these criteria can be found at http://www.oai.ucsf.edu/datarelease/About.asp. Participants included in the study were assessed annually. We used data from the baseline visit and the first four years of follow-up for analyses. Institutional Review Board approval was obtained from all OAI sites. In addition, this analysis was approved by the Institutional Review Board at Boston University.
Gait speed
Gait speed was measured over a 20-meter course in an unobstructed corridor and was reported in meters per second (m/s). Participants were instructed to walk at a usual pace from a starting point to an orange cone indicating the end of the course. Timing started with the first step after the starting line and ended after the first step over the finishing line using a stopwatch. Participants were allowed to use walking aids during the test, such as a cane. High test-retest reliability (intraclass correlation coefficients being greater than 0.9) has been reported for gait speed measured over an 8 meter walkway in older adults with radiographic knee OA(28). Study participants performed two 20-meter walks and gait speed was defined as the mean of both walk speeds, i.e. ([20m/time 1 + 20m/time 2]/2).
Knee OA and pain
Radiographic knee osteoarthritis (ROA) was assessed from weight-bearing posteroanterior and lateral fixed flexion radiographic evaluations of both knees(29). Radiographs were independently graded twice among three expert readers (Two rheumatologists and a musculoskeletal radiologist) for joint space narrowing and osteophytes in the tibiofemoral joint according to Kellgren and Lawrence K/L criteria (grades 0-4)(30). Any disagreements were adjudicated among all three expert readers to reach consensus. There was high agreement between readers with kappa statics ranging from 0.70 to 0.80 for K/L grades. We defined the presence of ROA as a K/L grade ≥ 2. Knee pain (absent/present) was evaluated by asking participants if they had pain, aching, or stiffness in or around each knee on most days for at least one month within the past year. This definition of knee pain been employed previously using data from OAI(31) and in combination with ROA to define symptomatic knee OA(32).
Participants were categorized into four groups: neither ROA nor knee pain, the presence of ROA but no knee pain (ROA only), no ROA but with the presence of knee pain (Pain only), or the presence of both ROA and knee pain (symptomatic knee OA). We assigned a participant’s knee OA category based on the status of his/her worst knee. For instance, a person with symptomatic knee OA in one knee and pain only in the contralateral knee was classified as having symptomatic knee OA, whereas a person with ROA only in one knee and pain only in the contralateral knee was classified as having pain only.
Knee pain was also examined using a severity scale.(33, 34) Participants were asked to rate the worst pain in each knee from the last 7 days. Pain was rated on an ordinal scale ranging from “0” to “10” with “0” representing “No pain” and “10” being “Pain as bad as you can imagine”. We reported values from the knee with higher pain severity.
Potential confounders
We evaluated the following factors as potential confounders given previous literature linking them as risk factors for knee OA or knee pain and slow gait speed. These included age(10, 35), race (Non-White vs White)(10, 36), sex(10, 35), and education level (<some college vs. ≥college)(36, 37) measured from self-report. Body Mass Index (BMI)(10, 38) was computed from standardized weight and height assessments and classified into World Health Organization categories(39). Comorbidities (≥1 vs none)(40, 41) were measured from the modified Charlson comorbidity index(42) and the presence of depressive symptoms(43, 44) was classified using a score ≥ 16 on the Center for Epidemiologic Studies Depression Scale (CES-D)(45). Isometric knee extensor strength(10, 46) was measured using the “Good Strength Chair” (Metitur Oy, Jycaskyla, Finland) with participants positioned seated with their legs hanging over the edge of the chair. After two warm-up repetitions with 50% effort, three maximum isometric knee extensor repetitions at an angle of 60 degrees were performed and averaged together. We then categorized strength into sex-specific and weight adjusted tertiles since no established strength categories are available. Lastly, physical activity(47-49) was measured from the Physical Activity Scale for the Elderly (PASE)(50) and categorized into tertiles since no established physical activity categories are available from PASE.
Analysis
We compared characteristics of participants across knee OA status categories by performing analysis of variance tests for continuous variables and chi-square tests for categorical variables. Subjects who were included had baseline gait speed and a minimum of two follow-up time points in order to provide an adequate number of data points for trajectory analyses(51, 52). We employed a SAS macro named PROC TRAJ to identify trajectories of gait speed(53). This approach applies a multinomial modeling strategy to identify relatively homogenous clusters of developmental trajectories within a sample population, i.e. the modeling strategy allows for the emergence of more than two trajectories. Trajectory parameters are derived by latent class analysis using maximum likelihood estimation. In particular, the distinctive trajectories of gait speed were derived by modeling gait speed as a function of time, i.e. the number of years in the study. The number of trajectories were determined by the patterns of change in gait speed, and not forced to fit a particular model. We assumed each trajectory of gait speed had a linear pattern of decline. We tested this by also including a quadratic term, which tests for the possibility that change in gait speed has a curved shape (e.g. faster then slower) and evaluated these patterns of decline from p-values for each trajectory group. Linear but not quadratic model terms were statistically significant (p <0.05), therefore we only included a linear term. The optimal number of groups was assessed using model fit from Bayesian Information Criteria (BIC) and trajectory slopes(2, 3). We considered adjacent trajectory groups with slopes differing by more than 10% to be unique. We used the posterior probabilities of group membership from each individual to assess the fit of the model, which was provided by the PROC TRAJ macro. High probability of membership into a single group represents a good model fit.
Next, we examined the association knee OA categories with trajectories of gait speed using a multivariable polytomous regression model adjusting for potential confounders. We repeated these analyses for each of the following age-strata: ≥ 45 to 59 years, ≥ 60 to < 69 years, and ≥ 70 years.
Results
Of the 4796 subjects at baseline, 87% had at least two years of follow-up and were included in analyses and 20 did not have measures of ROA or pain. Of the remaining participants, 25% had ROA only, 19% pain only, and 30% symptomatic knee OA (ROA and pain). Participants with symptomatic knee OA were more likely to be non-white, have less education, more depressive symptoms, a higher BMI, lower knee strength, and higher knee pain severity compared with those in the other knee OA categories (Table 1). A majority of participants (77%) had gait speed measures at all five time points, while 15% had gait speed measures at four time points, and 8% at three time points.
Table 1.
Subject characteristics for all study participants (n=4179) and across knee OA categories.
| Overall (n=4179) | Neither ROA nor no knee pain (n=1079) | ROA only (n=1047) | Pain only (n=797) | Symptomatic OA (n=1236) | p-value | |
|---|---|---|---|---|---|---|
| Age [Mean (sd)] | 61.1 (9.1) | 60.1 (9.1) | 63.8 (8.8) | 58.4 (8.9) | 61.4 (9.0) | <0.0001 |
| Women [%] | 57.6 | 58.4 | 59.2 | 57.5 | 55.7 | 0.35 |
| Non-White [%] | 19.0 | 10.5 | 14.2 | 20.9 | 28.9 | <0.0001 |
| ≤ High School [%] | 14.6 | 11.3 | 13.5 | 13.7 | 19.2 | <0.0001 |
| Number of Comorbidities [Mean (sd)] | 0.4 (0.8) | 0.3 (0.7) | 0.4 (0.8) | 0.4 (0.8) | 0.4 (0.9) | <0.0001 |
| CES-D [Mean (sd)] | 6.3 (6.7) | 5.5 (6.0) | 5.3 (5.8) | 7.1 (6.9) | 7.4 (7.5) | <0.0001 |
| BMI [kg/m] [Mean (sd)] | 28.5 (4.8) | 26.8 (4.4) | 29.0 (4.6) | 27.8 (4.5) | 30.1 (4.9) | <0.0001 |
| <25 [%] | 24.3 | 36.7 | 20.7 | 29.0 | 13.5 | |
| 25-29 [%] | 39.7 | 39.3 | 39.3 | 42.8 | 38.3 | |
| ≥30 [%] | 26.2 | 24.0 | 40 | 28.2 | 48.3 | |
| PASE (Physical activity) [Mean (sd)] | 163.2 (82.0) | 169.2 (79.5) | 151.6 (77.4) | 171.6 (86.7) | 162.5 (83.8) | <0.0001 |
| Knee extensor strength (Newton/kg) [Mean (sd)] | 4.2 (1.5) | 4.6 (1.4) | 4.1 (1.4) | 4.3 (1.5) | 3.8 (1.4) | <0.0001 |
| Knee pain intensity (0-10) [Mean (sd)] | 3.2 (2.7) | 1.7 (2.0) | 2.2 (2.2) | 4.0 (2.5) | 5.0 (2.5) | <0.0001 |
ROA, Radiographic knee osteoarthritis; OA, Osteoarthritis; CES-D, Center for Epidemiologic Studies Depression Scale; PASE, Physical Activity Scale for the Elderly
We identified five distinct trajectories of gait speed. About 5% (n=205) of participants were in a fast decline trajectory of gait speed, slowing 0.025 m/s per year (95% CI [-0.020, -0.031]) or 2.70%/year. The remaining participants had either a stable or slightly declining gait speed, slowing less than 1.0%/year (Figure 1). We found the mean posterior probabilities of group assignment to range from 0.90 to 0.94, indicating good fit of the trajectory model.
Figure 1.
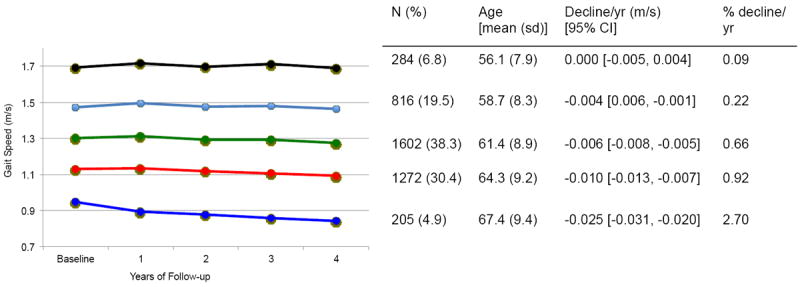
Gait speed trajectory groups and unadjusted absolute and relative decline in gait speed.
In general, trajectory groups with faster declines in gait speed had a higher proportion of participants with symptomatic knee OA and a lower proportion of participants with neither ROA nor pain (Figure 2). Participants with symptomatic knee OA had the highest risk of a fast decline in gait speed followed by those with knee pain only and those with ROA only (Table 2). Compared with those with neither pain nor ROA, participants with symptomatic knee OA had almost 9 times the odds (OR = 8.9, 95% CI [3.1, 25.5]) of being in the fastest decline trajectory after adjustment for potential confounders. Participants with knee pain only had an increased risk of being in the fastest decline trajectory, although the magnitude of association was less than those with symptomatic knee OA (OR = 4.5 [1.4, 14.6]). People with ROA only had a slightly increased risk, albeit this was not statistically significant compared with those with neither pain nor ROA (OR = 1.7 [0.6, 4.7]).
Figure 2.
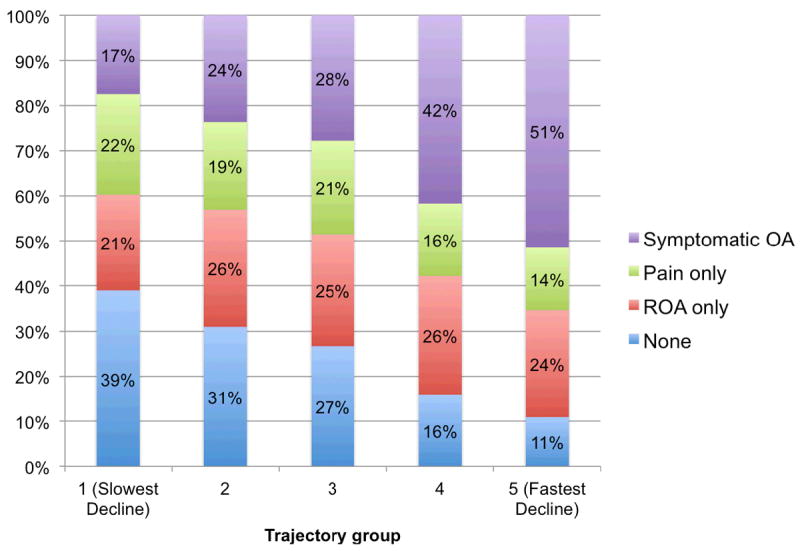
Percentage of participants within each knee OA status category across gait speed trajectory groups.
Table 2.
Adjusted* association knee OA status with membership within each trajectory group
| Knee OA Status | 1 (Slowest Decline) (n=284) OR |
2 (n=1272) OR (95% CI) |
3 (n=1602) OR (95% CI) |
4 (n=816) OR (95% CI) |
5 (Fastest Decline) (n=205) OR (95% CI) |
|---|---|---|---|---|---|
| ROA only vs neither ROA nor pain | 1.0 | 0.9 (0.5, 1.6) | 0.9 (0.5, 1.6) | 1.3 (0.7, 2.4) | 1.7 (0.6, 4.7) |
| Pain Only vs neither ROA nor pain | 1.0 | 1.2 (0.6, 2.6) | 1.9 (0.9, 3.9) | 2.0 (0.9, 4.7) | 4.5 (1.4, 14.6) |
| Symptomatic OA vs neither ROA nor pain | 1.0 | 1.7 (0.8, 3.5) | 2.5 (1.3, 5.2) | 4.1 (1.9, 8.8) | 8.9 (3.1, 25.5) |
OR, Odds Ratio; 95% CI, 95% Confidence Interval
Adjusted for age, sex, race, education, comorbidities, depressive symptoms, BMI, Knee extensor strength, and physical activity.
For participants 45 to 59 years at baseline, we identified 3 distinct gait speed trajectories. Those in the fastest decline trajectory slowed 0.006 m/s per year (0.72%/year from baseline to the last follow-up visit). Symptomatic knee OA was associated with the highest risk of decline in gait speed (OR=2.3 [1.4, 3.7]) (See Supplementary Figure A, available on the Arthritis Care & Research website). Three distinct gait speed trajectories were also found for participants 60 to 69 years at baseline. Those in the fastest decline group slowed 0.003 m/s per year (0.68%/year from baseline to the last follow-up visit). Symptomatic knee OA and knee pain only had a similar risk of decline in gait speed (OR =3.2, 95% CI [1.7, 6.02], and 3.0 [1.4, 6.1], respectively) (See Supplementary Figure B, available on the Arthritis Care & Research website). For participants 70 years of age or older, we identified five trajectories of gait speed. The fastest decline group slowed 0.038 m/s per year (5.55%/year from baseline to the last follow-up). Participants with symptomatic knee OA again had the highest risk of decline in gait speed (OR=6.0 [1.2, 31.1]) (See Supplementary Figure C, available on the Arthritis Care & Research website).
Discussion
Among people with or at high risk of knee OA, approximately 5% had an underlying trajectory of fast decline in gait speed. People with symptomatic knee OA had the highest risk of being on this trajectory of fast decline compared with those with neither ROA nor knee pain. The current study findings add that people with symptomatic knee OA have a predilection for premature slowing of gait speed. Given the strong link between slow gait speed and mortality, people with a slowing gait speed may also be at risk of morbidity and mortality(4). Moreover, those in the fastest decline trajectory slow at a rate equivalent to what is expected in healthy adults 5 to 12 years older on average. For instance, those in the fastest decline trajectory were 67.4 years old on average and based on normative data around a 1%/year decline gait speed would be expected(6). However, their actual rate of decline was 2.75%/year, which is typically observed in healthy adults aged 75 to 80 years old, which we believe is a difference of clinical relevance.
We find it noteworthy that people with symptomatic knee OA were generally at a higher risk of a fast decline in gait speed than those with pain alone. While previous literature suggests a discordance between radiographic findings and symptoms(16), pain is a subjective factor whose response is unique to each person. A recent study that accounted for between person factors reported a strong association between radiographic severity and pain(54). Therefore, one explanation why people with symptomatic knee OA were at higher risk of a fast decline in gait speed is that they had more severe pain than those with knee pain only. In the present study, the mean pain severity on the 0 to 10 scale for people with symptomatic knee OA was 5, while for those with pain only was 4. While a one unit change in pain may not represent a clinically significant difference for an individual, this mean difference between groups still supports the notion that the effect of knee OA on trajectories of gait speed is perhaps mediated by pain severity, one of the most common symptoms of knee OA.
The study findings show that people with the slowest gait speed at baseline were likely to be in the fast decline gait speed trajectory. Similar phenomenon has also been observed in the other numerical quantities of physiological measures, such as blood pressure in adults and knee cartilage loss among OA participants(55, 56). Nevertheless, the strong correlation between an absolute value and the rate of change is akin to a “horse-racing” effect, i.e. one would expect the fast horses in a race to be out in front at any given time point(57). Thus, it is not that a slow gait speed at baseline causes a trajectory of decline in gait speed, but rather the trajectory of gait speed determines the current gait speed. Symptomatic knee OA may have altered the trajectory of gait speed well before the start of the study, thereby resulting in slower walking at any subsequent point in time.
Despite the fact that increased age is strongly associated with a slower gait speed(35), we consistently found participants with symptomatic knee OA to have the highest risk of a fast decline in gait speed across age stratum. However, it is noteworthy that the rate of decline among those with symptomatic knee OA was by far the fastest within the highest age stratum of participants > 70 years of age (5.55%/year), compared with those 60 to 69 years (0.68%/year) and those 45 to 59 years of age (0.72%/year). Likewise across the overall sample, participants in the fast decline trajectory had the highest mean age (67.4 years) compared with the other trajectory groups (Figure 1). Hence, the presence of symptomatic knee OA may be most detrimental for adults with more advanced age when declines in gait speed are common.
Our study has several limitations. First, since only four years of follow-up were available, we were unable to link membership in trajectory groups with subsequent risk of health outcomes, such as total knee replacement, hospitalization, or mortality. Second, it is important to note that there is still variation of changes in gait speed within each of the five gait speed trajectories despite the fact the methods we employed allowed identification of distinctive trajectories. Finally, an individual’s gait speed trajectory is not likely to be discerned in the clinic without collecting standardized measures of gait speed over multiple time points; thus the feasibility of using trajectories in the clinic setting needs to be evaluated in future studies.
Despite these limitations, our study has several strengths. First, we employed a novel methodology whose underlying assumption fits well with gait speed trajectories. Previous longitudinal studies have charted the individual variability of gait speed around a mean population trend(8, 58-60). A limitation to this approach is that meaningful subgroups of change that follow a distinct trajectory cannot be identified(51). As an alternative, a multinomial modeling strategy allows for the emergence of distinct trajectories of gait speed that affords the ability to investigate distinctive patterns within a study population instead of assuming common rate of decline. Furthermore, using this method we found the posterior probability of allocating each study participant into trajectory groups to be over 90%, indicating a good fit of the model of group trajectories to individual trajectories. Second, we used data collected from a large multi-center longitudinal cohort study conducted among people with or at high risk of knee OA. Such data allowed us to describe gait speed trajectories among a large number of people who were likely to experience a decline in gait speed.
Among older adults with or at high risk of knee OA, clinicians should be most concerned about health outcomes in those with symptomatic knee OA given this group is most likely to have a fast decline in gait speed. Currently, clinicians could consider prescribing a walking program to their patients with or at high risk of knee OA in order to promote the preservation or improvement of gait speed. This assertion is based on data from clinical trials that reported improvements in walking performance following the completion of a walking program in people with symptomatic knee OA.(61, 62) In particular, a walking program involving the use of a pedometer to record steps/day paired with a step count goal has been shown to be effective with increasing physical activity and reducing blood pressure in adults.(63) A similar method could be considered to treat people with or at high risk of knee OA. Future research is needed, however, to confirm if such an approach increases gait speed and changes gait speed trajectories in people with or at high risk of knee OA.
In conclusion, 5% of people with or at high risk of knee OA had a fast decline trajectory of gait speed slowing 3% per year in gait speed. This magnitude of slowing is typical in adults 5 to 12 years older on average than our sample. People with symptomatic knee OA had the highest risk of fast decline in gait speed followed by those with pain without ROA. These findings were consistent across age stratum suggesting that symptomatic knee OA is a risk factor for a premature decline in gait speed independent of age. Future studies should examine if intervention of symptomatic disease, such as walking programs, can prevent future declines in gait speed and whether fast decline in gait speed is associated with poor health outcomes.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
Approximately 5% of participants with or at high risk of knee OA had an underlying trajectory of fast decline in gait speed, which is a strong indicator of future poor health outcomes.
Participants with symptomatic knee OA (radiographic knee OA and knee pain) had almost a 9-fold increased risk of being on a trajectory of fast decline compared with those with neither radiographic knee OA nor knee pain.
Participants with knee pain alone or radiographic knee OA alone also had a higher risk of fast decline, however the magnitude of such risk was less than those with symptomatic knee OA.
Acknowledgments
The authors would like to acknowledge and thank the participants in OAI for providing data used in this study
FUNDING
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Funding for the authors was provided by the ACR/REF Rheumatology Investigator Award, Boston Claude D. Pepper Older Americans Independence Center (P30-AG031679), the Foundation for Physical Therapy Geriatric Research Grant, and NIH AR47885.
Footnotes
COMPETING INTERESTS
None
References
- 1.Hall WJ. Update in geriatrics. Ann Intern Med. 2006;145(7):538–43. doi: 10.7326/0003-4819-145-7-200610030-00012. [DOI] [PubMed] [Google Scholar]
- 2.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–80. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 3.Ayis S, Ebrahim S, Williams S, Juni P, Dieppe P. Determinants of reduced walking speed in people with musculoskeletal pain. J Rheumatol. 2007;34(9):1905–12. [PubMed] [Google Scholar]
- 4.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med Sci Sports Exerc. 1988;20(2):161–6. doi: 10.1249/00005768-198820020-00010. [DOI] [PubMed] [Google Scholar]
- 6.Forrest KY, Zmuda JM, Cauley JA. Correlates of decline in lower extremity performance in older women: A 10-year follow-up study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1194–200. doi: 10.1093/gerona/61.11.1194. [DOI] [PubMed] [Google Scholar]
- 7.Rosano C, Longstreth WT, Jr, Boudreau R, Taylor CA, Du Y, Kuller LH, et al. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. J Am Geriatr Soc. 2011;59(3):390–7. doi: 10.1111/j.1532-5415.2010.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onder G, Penninx BW, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, et al. Change in physical performance over time in older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M289–93. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- 9.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 11.Ko SU, Simonsick EM, Husson LM, Ferrucci L. Sex-specific gait patterns of older adults with knee osteoarthritis: results from the Baltimore longitudinal study of aging. Curr Gerontol Geriatr Res. 2011;2011:175763. doi: 10.1155/2011/175763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowers M, Jannausch ML, Gross M, Karvonen-Gutierrez CA, Palmieri RM, Crutchfield M, et al. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoarthritis. Am J Epidemiol. 2006;163(10):950–8. doi: 10.1093/aje/kwj109. [DOI] [PubMed] [Google Scholar]
- 13.Al-Zahrani KS, Bakheit AM. A study of the gait characteristics of patients with chronic osteoarthritis of the knee. Disabil Rehabil. 2002;24(5):275–80. doi: 10.1080/09638280110087098. [DOI] [PubMed] [Google Scholar]
- 14.Mundermann A, Dyrby CO, Hurwitz DE, Sharma L, Andriacchi TP. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: reduced walking speed. Arthritis Rheum. 2004;50(4):1172–8. doi: 10.1002/art.20132. [DOI] [PubMed] [Google Scholar]
- 15.Zeni JA, Jr, Higginson JS. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: a result of altered walking speed? Clin Biomech (Bristol, Avon) 2009;24(4):372–8. doi: 10.1016/j.clinbiomech.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27(6):1513–7. [PubMed] [Google Scholar]
- 17.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43(5):995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41(8):1343–55. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Manninen P, Riihimaki H, Heliovaara M, Makela P. Overweight, gender and knee osteoarthritis. Int J Obes Relat Metab Disord. 1996;20(6):595–7. [PubMed] [Google Scholar]
- 20.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40(4):728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 21.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–7. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 22.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687–93. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312(7036):940–3. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chitnavis J, Sinsheimer JS, Clipsham K, Loughlin J, Sykes B, Burge PD, et al. Genetic influences in end-stage osteoarthritis. Sibling risks of hip and knee replacement for idiopathic osteoarthritis. J Bone Joint Surg Br. 1997;79(4):660–4. doi: 10.1302/0301-620x.79b4.7437. [DOI] [PubMed] [Google Scholar]
- 25.Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999;42(1):17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Felson DT, Hannan MT, Naimark A, Berkeley J, Gordon G, Wilson PW, et al. Occupational physical demands, knee bending, and knee osteoarthritis: results from the Framingham Study. J Rheumatol. 1991;18(10):1587–92. [PubMed] [Google Scholar]
- 27.Cooper C, McAlindon T, Coggon D, Egger P, Dieppe P. Occupational activity and osteoarthritis of the knee. Ann Rheum Dis. 1994;53(2):90–3. doi: 10.1136/ard.53.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fransen M, Crosbie J, Edmonds J. Reliability of gait measurements in people with osteoarthritis of the knee. Phys Ther. 1997;77(9):944–53. doi: 10.1093/ptj/77.9.944. [DOI] [PubMed] [Google Scholar]
- 29.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 30.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattler M, Dannhauer T, Hudelmaier M, Wirth W, Sanger AM, Kwoh CK, et al. Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain - data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2012 doi: 10.1016/j.joca.2012.02.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68(5):674–9. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellamy N. Musculoskeletal Clinical Metrology. London: Kluwer Academic Publishers; 1993. [Google Scholar]
- 34.Turk D, Melzack R. Handbook of pain assessment. 2. New York: Guilford Press; 2001. [Google Scholar]
- 35.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182–9. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Haas SA, Krueger PM, Rohlfsen L. Race/Ethnic and nativity disparities in later life physical performance: the role of health and socioeconomic status over the life course. J Gerontol B Psychol Sci Soc Sci. 2012;67(2):238–48. doi: 10.1093/geronb/gbr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannan MT, Anderson JJ, Pincus T, Felson DT. Educational attainment and osteoarthritis: differential associations with radiographic changes and symptom reporting. J Clin Epidemiol. 1992;45(2):139–47. doi: 10.1016/0895-4356(92)90006-9. [DOI] [PubMed] [Google Scholar]
- 38.Samson MM, Crowe A, de Vreede PL, Dessens JA, Duursma SA, Verhaar HJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging (Milano) 2001;13(1):16–21. doi: 10.1007/BF03351489. [DOI] [PubMed] [Google Scholar]
- 39.WHO. WHO Technical Report Series 894. Geneva: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. [PubMed] [Google Scholar]
- 40.van Dijk GM, Veenhof C, Schellevis F, Hulsmans H, Bakker JP, Arwert H, et al. Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord. 2008;9:95. doi: 10.1186/1471-2474-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rantanen T, Guralnik JM, Ferrucci L, Penninx BW, Leveille S, Sipila S, et al. Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc. 2001;49(1):21–7. doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]
- 42.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Salaffi F, Cavalieri F, Nolli M, Ferraccioli G. Analysis of disability in knee osteoarthritis. Relationship with age and psychological variables but not with radiographic score. J Rheumatol. 1991;18(10):1581–6. [PubMed] [Google Scholar]
- 44.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279(21):1720–6. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 45.Radloff L. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 46.Sayers SP, Guralnik JM, Thombs LA, Fielding RA. Effect of leg muscle contraction velocity on functional performance in older men and women. J Am Geriatr Soc. 2005;53(3):467–71. doi: 10.1111/j.1532-5415.2005.53166.x. [DOI] [PubMed] [Google Scholar]
- 47.Fries JF, Bruce B, Shoor S. Osteoarthritis, exercise, and knee replacement. J Rheumatol. 2012;39(4):669–71. doi: 10.3899/jrheum.111087. [DOI] [PubMed] [Google Scholar]
- 48.Gelber AC. Exercise and Osteoarthritis: Not Necessarily an All-or-Nothing Proposition! J Rheumatol. 2012;39(4):672–4. doi: 10.3899/jrheum.111340. [DOI] [PubMed] [Google Scholar]
- 49.Yorston LC, Kolt GS, Rosenkranz RR. Physical activity and physical function in older adults: the 45 and up study. J Am Geriatr Soc. 2012;60(4):719–25. doi: 10.1111/j.1532-5415.2012.03906.x. [DOI] [PubMed] [Google Scholar]
- 50.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 51.Nagin D. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 52.Hejazi S, Dahinten VS, Marshall SK, Ratner PA. Developmental pathways leading to obesity in childhood. Health Rep. 2009;20(3):63–9. [PubMed] [Google Scholar]
- 53.Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Social Methods Res. 2001;29:374–93. [Google Scholar]
- 54.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartlett SJ, Ling SM, Mayo N, Scott S, Bingham CO., 3rd Identifying common trajectories of joint space narrowing over two years in knee Osteoarthritis. Arthritis Care Res (Hoboken) 2011 doi: 10.1002/acr.20614. [DOI] [PubMed] [Google Scholar]
- 56.Why Does Blood-pressure Rise with Age? The Lancet. 1981;318(8241):289–90. [PubMed] [Google Scholar]
- 57.Peto R. The horse-racing effect. Lancet. 1981;2(8244):467–8. doi: 10.1016/s0140-6736(81)90791-1. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, van Belle G, Kukull WB, Larson EB. Predictors of functional change: a longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc. 2002;50(9):1525–34. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- 59.Beckett LA, Brock DB, Lemke JH, Mendes de, Leon CF, Guralnik JM, Fillenbaum GG, et al. Analysis of change in self-reported physical function among older persons in four population studies. Am J Epidemiol. 1996;143(8):766–78. doi: 10.1093/oxfordjournals.aje.a008814. [DOI] [PubMed] [Google Scholar]
- 60.Buchner DM, Cress ME, Esselman PC, Margherita AJ, de Lateur BJ, Campbell AJ, et al. Factors associated with changes in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 1996;51(6):M297–302. doi: 10.1093/gerona/51a.6.m297. [DOI] [PubMed] [Google Scholar]
- 61.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 62.Ettinger WH, Jr, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST) Jama. 1997;277(1):25–31. [PubMed] [Google Scholar]
- 63.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


