Abstract
Dehydroepiandrosterone (DHEA) levels were reported to associate with increased breast cancer risk in postmenopausal women, but some carcinogen-induced rat mammary tumor studies question this claim. The purpose of this study was to determine how DHEA and its metabolites affect estrogen receptors α or β (ERα or ERβ) -regulated gene transcription and cell proliferation. In transiently transfected HEK-293 cells, androstenediol, DHEA, and DHEA-S activated ERα. In ERβ transfected HepG2 cells, androstenedione, DHEA, androstenediol, and 7-oxo DHEA stimulated reporter activity. ER antagonists ICI 182,780 (fulvestrant) and 4-hydroxytamoxifen, general P450 inhibitor miconazole, and aromatase inhibitor exemestane inhibited activation by DHEA or metabolites in transfected cells. ERβ-selective antagonist R,R-THC (R,R-cis-diethyl tetrahydrochrysene) inhibited DHEA and DHEA metabolite transcriptional activity in ERβ-transfected cells. Expression of endogenous estrogen-regulated genes: pS2, progesterone receptor, cathepsin D1, and nuclear respiratory factor-1 was increased by DHEA and its metabolites in an ER-subtype, gene, and cell-specific manner. DHEA metabolites, but not DHEA, competed with 17β-estradiol for ERα and ERβ binding and stimulated MCF-7 cell proliferation, demonstrating that DHEA metabolites interact directly with ERα and ERβ in vitro, modulating estrogen target genes in vivo.
Keywords: estrogen receptors, DHEA, androstendione, androstendiol, transcription
1. Introduction
In postmenopausal women, androgens are converted to estrogens by aromatase (CYP19A1) [1] and aromatase inhibitors (AI) have recently replaced the selective estrogen receptor modulator (SERM) tamoxifen (TAM) as the most efficacious endocrine therapy for treating postmenopausal patients whose initial breast tumors were estrogen receptor α (ERα) positive [2]. Dehydroepiandrosterone (DHEA) and its sulfate metabolite, DHEA-S, are androgen and estrogen precursors whose role, if any, as source for ER or androgen receptor (AR) ligands in breast cancer progression has yet to be clearly defined.
DHEA is present in normal breast as well as breast tumors DHEA has been suggested to have a protective effect in premenopausal women; however, a positive correlation was reported between DHEA plasma levels and breast cancer risk in postmenopausal women [3]. Analysis of DHEA, DHEA-S, and testosterone levels in 646 postmenopausal women in the Nurses’ Health Study found no significant associations between the breast cancer risk score and androgen levels [4]. However, a weak association was found between higher free testosterone and breast cancer risk [4]. The association with DHEA and breast cancer was less clear [4] More recently, circulating DHEA-S correlated with alcohol consumption, and was strongly associated with several breast cancer risk factors [5]. Circulating DHEA-S levels >90 µg/dL (> 2.4 µM) were suggested as a potential risk factor for breast cancer progression in patients treated with TAM or fulvestrant [6]. Because enzymes involved in DHEA metabolism are expressed in breast tissue [7], local production of estrogenic DHEA metabolites are of keen interest.
Plasma DHEA-S levels are higher than other sterols secreted by the adrenals [8], peak in the second decade of life, and decline thereafter [9, 10]. Circulating DHEA levels are 20–25 nM, with higher levels in peripheral tissues, e.g., prostate [11]. DHEA supplementation has been suggested to have many beneficial effects during aging as endogenous DHEA declines [10]. Although oral DHEA had anti-carcinogenic effects in the mammary gland of rats after chemical, i.e., NMU [12] and DMBA [13] induction of tumors, the role for DHEA as a chemopreventive agent remains uncertain. Recently, 100 µM DHEA, a supraphysiological level [9], was reported to inhibit the proliferation, cell cycle progression, and migration of ERα+ MCF-7 and triple-negative MDA-MB-231 and Hs578T breast cancer cells [14].
However, DHEA (0.5 µM) and ADIOL (2 nM) stimulated MCF-7 cell growth [15]. DHEA activated transfected ERα reporter genes in GT1-7 immortalized mouse hypothalamic neuronal cells [16] and MCF-7 cells [17]. DHEA also stimulated proliferation of MCF-7SH cells, an estrogen-independent MCF-7 variant [17]. The efficacy of DHEA as an ERα ligand was enhanced by aromatase expression, indicating conversion DHEA to estrogenic metabolites in MCF-7 cells [18]. DHEA binds endogenous androgen receptor (AR) in MDA-MB-435 breast or LNCaP prostate cancer cells with a Ki of ~ 1.2 µM and binds ERα or ERβ expressed in COS-1 cells with Ki of ~ 1.1 µM and 500 nM, respectively [11]. However, the role of ER and AR in mediating DHEA responses is uncertain, because neither fulvestrant (ICI 182,780, an ER antagonist) nor flutamide (an AR antagonist) blocked the proliferative activity of 10 nM DHEA in MCF-7 cells [19]. The finding of a lack of inhibition by ICI 182,780 may be because it is a GPR30/GPER agonist [20], and a recent report that one DHEA metabolite 7α-hydroxy-epiandrosterone (7α-OH-EpiA, 1–100 nM) inhibited MCF-7 proliferation through inhibition of GPR30/GPER and ERα and by activation of ERβ [21].
In addition to 7α-OH-EpiA, another metabolite of DHEA, 5-α-androstane-3β,17β-adiol (3β-Adiol) serves as a ligand for ERβ [22–27] and for ERα [28]. ADIOL (100 nM) showed activity similar to 3β-Adiol (100 nM) in stimulating ERα- and ERβ- mediated ERE-luciferase activity in transfected HEK-293 cells [29]. Notably, ADIOL showed greater efficacy compared to DHEA, since 10 nM ADIOL had greater ERβ-stimulatory activity compared to 10 µM DHEA. ADIOL induced transcription of HEM45, an estrogen-responsive gene in MCF-7 cells, presumably through ER-activation, although this was not proven [29].
Evaluating the role of DHEA and its metabolites in activating ER is critical for understanding the biological events of estrogen-mediated gene regulation in normal and diseased tissues. In this study, DHEA and several metabolites were tested for their ability to serve as ligand activators of human ERα and ERβ using in vitro assays.
2. Experimental
2.1. Chemicals
Androstenediol (ADIOL), androstenedione (ADIONE), 5-α-androstane-3β,17β-adiol (3β-Adiol), etiocholanolone (ETIO), DHEA, DHEA-sulfate (DHEA-S), 7α-hydroxy-DHEA (7α-OH-DHEA), 7β-hydroxy-DHEA (7β-OH-DHEA), 7-oxo-DHEA, 11β-hydroxy-DHEA (11β-OH-DHEA), 16α-hydroxy-DHEA (16α-OH-DHEA), and 17β-estradiol (E2) were purchased from Steraloids, Inc. (Wilton, NH). ICI 182,780 was purchased from Tocris, Inc. (Ellisville, MO). Miconazole and 4-hydroxytamoxifen (4-OHT) were purchased from Sigma-Aldrich (St. Louis, MO). Exemestane was a generous gift from Pharmacia Upjohn Corp., Kalamazoo, MI. cis-Diethyl tetrahydrochrysene (R,R-THC, a selective ERβ antagonist/ERα agonist) was a generous gift from Dr. John A Katzenellenbogen of the University of Illinois at Urbana/Champaign [30].
2.2. Plasmids
The pCMV expression plasmid containing the cDNA for human ERα (Reese and Katzenellenbogen, 1991) was a gift from Dr. Benita Katzenellenbogen (University of Illinois at Urbana/Champaign). The pSG5 expression plasmid containing the cDNA for the long form of human ERβ (hERβ1, 530 aa) was a gift from Dr. Eva Enmark, Karolinska Hospital, Stockholm, Sweden [22]. The ERELUC reporter contains three tandem copies of a consensus estrogen response element (ERE) [31]. pCMVβgal control reporter was purchased from CLONTECH (Palo Alto, CA).
2.3. Transient transfection assays
HEK-293, HepG2, CHO-K1, MCF-7, and MDA-MB-231 cells were purchased from ATCC (Manassas, VA). Cells were plated at 1.5 × 105 cells/well in 12-well plates containing 1 ml of MEM supplemented with 5% charcoal-stripped fetal bovine serum (DCC-FBS) Twenty-four hours after plating, cells were transfected in serum free medium with hERα or hERβ expression plasmids (150 ng/ml), ERELUC reporter plasmid (250 ng/ml), and either pCMVβgal (250 ng/ml) or pRL-tk (5 ng/ml, Renilla luciferase control vector, Promega, Madison, WI), for normalization, using 4 µg/ml LipofectAMINE (Invitrogen, Carlsbad, CA). After overnight incubation, the transfection mixture was removed and the cells were treated with phenol red-free MEM supplemented with 5% DCC-FBS. Transfected cells were treated with DHEA and metabolites dissolved in ethanol, and harvested 24 h later with 100 µl of cell lysis buffer (Promega). β-Galactosidase and luciferase activities were determined as described [32]. Since we could not find a single cell line that displays high E2-dependent expression, we therefore used HEK-293-transfectioned assays with ERα-dependent expression and HepG2 cells-transfection assayed with ERβ-dependent cells expression vectors were used demonstrate the independent effects of DHEA and its metabolites on ERα and ERβ transcriptional activity.
For the HEK-293 transient transfection experiment with coactivators SRC-2 or SRC-3, cells were plated in 24 well plates and cotransfected with 250 ng 2EREc38-LUC, 5 ng pCMV-hERα, 5 ng pRL-tk (Promega), and 250 ng of expression plasmids for SRC-2/TIF2/ GRIP1/NCOA2 and SRC-3/AIB1/ACTR/NCOA3, generously provided by Dr. Michael Stallcup [33], as described [34]. Firefly luciferase and Renilla luciferase activities were assayed using Promega’s dual luciferase reporter assay [35]. The data are expressed as luciferase activity relative to β-galactosidase or Renilla luciferase activity to correct for transfection efficiency. All transient transfection experiments were performed in triplicate or quadruplicate, and experiments were repeated at least twice to confirm results.
2.4. Transfection, RNA Isolation, and Quantitative Real-Time-PCR (QRT-PCR)
HEK-293 or HepG2 cells were plated in 6 well plates at a density of 4 × 105 cells/well. HepG2 were plated in phenol red-free DMEM supplemented with 5% DCC-FBS and 1% penicillin/streptomycin. HEK293 were plated in MEM supplemented with 10% DCC-FBS, 1% penicillin/streptomycin, 2% Sodium bicarbonate, and 1% Sodium pyruvate. HEK-293 and HepG2 were transfected with expression plasmids for human ERα or ERβ1, respectively, as described previously [36] using FuGENE HD (Promega, Madison, WI). Twenty-four h post-transfection, the cells were treated with DMSO (vehicle control) or 10 nM E2, 5 µM DHEA, or 5 µM DHEA metabolites for 24 h. RNA was isolated from the cells using QIA Shredder and the RNeasy mini kit (QIAGEN). The High Capacity cDNA Reverse Transcription kit (PE Applied Biosystems, Foster City, CA) was used to reverse transcribe total RNA from random hexamer primers. Taqman primers and probes for TFF1 (pS2), CTSD (cathepsin D), PGR (progesterone receptor (PR)), ESR1 (ERα), ESR2 (ERβ), and 18S rRNA were purchased as Assays-on-Demand™ from PE Applied Biosystems. Sybr green was used for QRT-PCR to measure the expression level of NRF1 (NRF-1) and GAPDH for normalization [35]. Sequences of the forward and reverse primers for NRF-1 were (forward) 5’-5’ GCGGTG GCA TCG TTG GCA GA-3’; ((reverse): 5’-GCT GCT GCG GTT TCC CCA GA-3’. The expression of each target gene was determined in triplicate and normalized using 18S or GAPDH. QRT-PCR was performed in the ABI PRISM 7900 SDS 2.1 (PE Applied Biosystems) using relative quantification. Analysis and fold differences were determined using the comparative CT method. Fold change was calculated from the ΔΔCT values with the formula 2−ΔΔCT and data are presented as relative to expression in DMSO-treated cells, i.e., vehicle control.
2.5. [3H] E2 ligand binding assay
Purified recombinant human ERα or ERβ were purchased from Panvera (Madison, WI) and were incubated in a final volume of 54 µL in TDPK111 buffer (40 nM Tris-HCl (pH 7.5), 1 mM DTT, 0.5 mM PMSF, 111 mM KCl) containing 30 nM [3H] E2 (2,3,4,7,-[3H] (N)17β-estradiol, 74 Ci/mmol, NET-317, NEN) for 1 hour at 37°C prior addition of a 10% hydroxyapatite (HAP) solution in TDPK111 for the HAP ligand binding assay [37]. Eight reactions were performed for each concentration of unlabeled competitors: E2, DHEA, DHEA-S, ADIOL, ADIONE, and 7-oxo-DHEA. An additional HAP assay was performed using miconazole (8 concentrations from 1 nM-100 µM) as a competitor for [3H] E2-ERα binding. The percent of specific competitor binding to ER was calculated by first subtracting the nonspecific binding from total [3H] E2 binding [37].
2.6. Cell proliferation BrdU assay
MCF-7 human breast cancer cells were purchased from ATCC, (Manassas, VA.) and maintained in IMEM without phenol red from BioSource International (Camarillo, CA.) supplemented with 10% FBS (Atlanta Biologicals, Norcross, GA.). For the bromodeoxyuridine (BrdU) ELISA assay (Roche Diagnostics, Indianapolis, IN), cells were plated in 96 well plates in normal growth media and allowed to attach to the plates overnight. Media was replaced with phenol red-free IMEM supplemented with 3% DCC-FBS for 24 h. Cells were treated with EtOH (vehicle control) or the indicated compound for 48 h prior to performing the BrdU ELISA assay according to the manufacturer’s instructions. Within each experiment, treatments were performed in quadruplicate and values were averaged. At least 3 separate experiments were performed. Data were analyzed by one-way ANOVA followed by Tukey and Scheffe’s post-hoc testing using GraphPad Prism software(San Diego, CA)..
2.7. Statistical analysis
Experiments were conducted in triplicate or quadruplicate and means ± standard deviations were determined. Statistical comparisons among treatment groups were determined using Student’s t-test or ANOVA in GraphPad Prism, with p < 0.05 as the criterion for significance.
3 Results
3.1. DHEA and metabolites activate ERα and ERβ in transfected cells
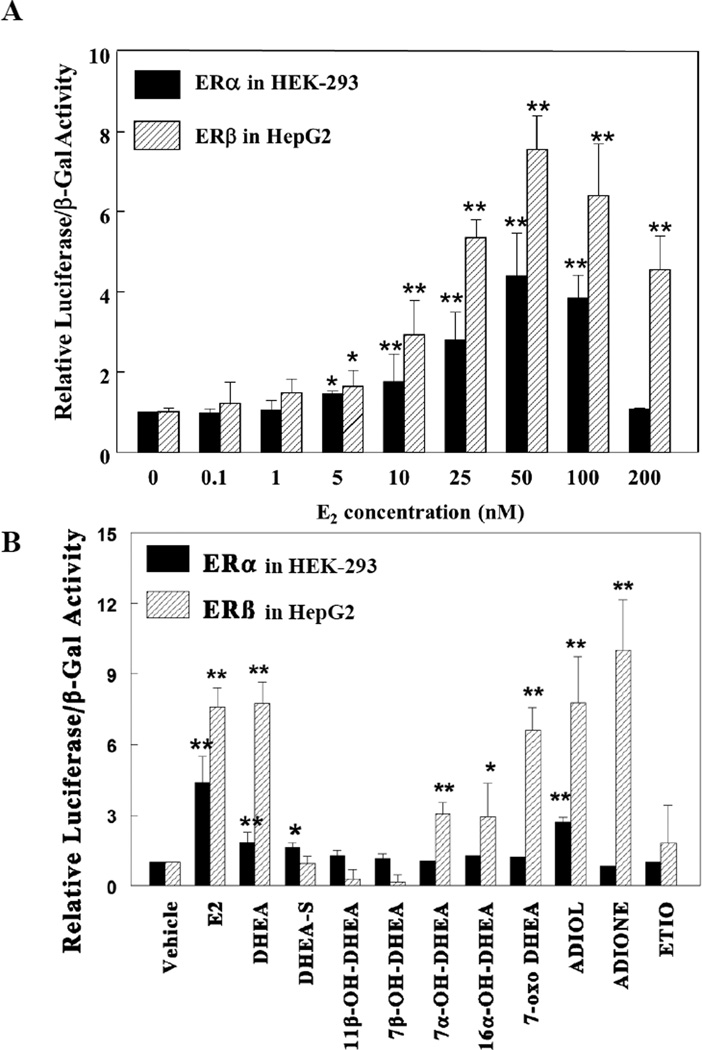
To determine the effect of DHEA on ERα and ERβ individually, a number of ER-null cell lines were tested to achieve the best response to DHEA for each receptor. These cell lines included HEK-293, HepG2 (human hepatoma), CHO-K1 (Chinese hamster ovary), and MDA-MB-231 (triple negative breast cancer) that were transfected with a luciferase reporter plasmid containing three tandem copies of a consensus ERE [31] (ERELUC) and expression vectors for either ERα or ERβ and treated with E2 prior to treatment with DHEA or its metabolites. ERα displayed a high basal (ligand-independent) activity in HepG2 cells, but had a limited, ligand-dependent response to E2 (data not shown). The highest E2-dependent activation of ERα was detected at 50 nM in HEK-293 cells (Fig. 1A). ERβ showed E2-concentration-dependent-activity in HepG2 cells that was similar to or greater than E2-ERα activity in HEK-293 cells (Fig. 1A). Significantly lower levels of E2-ERβ activity was observed in HEK-293 cells. These results for ERα and ERβ are nearly identical to those reported by Martin et al. [38] and therefore, we used HEK-293-transfection assays with ERα expression and HepG2 cells-transfection assays with ERβ expression vectors for subsequent experiments to demonstrate the independent effects of DHEA and its metabolites on ERα and ERβ transcriptional activity.
Figure 1. E2 activates ERE-luciferase activity in transiently transfected cells.
HEK-293 cells were transfected with ERα and HepG2 were transfected with ERβ expression plasmids Both cell lines were cotransfected with ERELUC (estrogen-response element luciferase) and pCMV-β-gal reporters and were treated for 24 h with EtOH (vehicle) or A) the indicated concentrations of E2 or B) 10 nM E2, 5 µM DHEA or 5 µM of the indicated DHEA metabolites. The cells were harvested and the lysates were assayed for β-galactosidase and luciferase activities. Data represent the mean ± S.D. of three separate experiments. Statistical significance is indicated versus vehicle (EtOH control), * p<0.05, ** p<0.01.
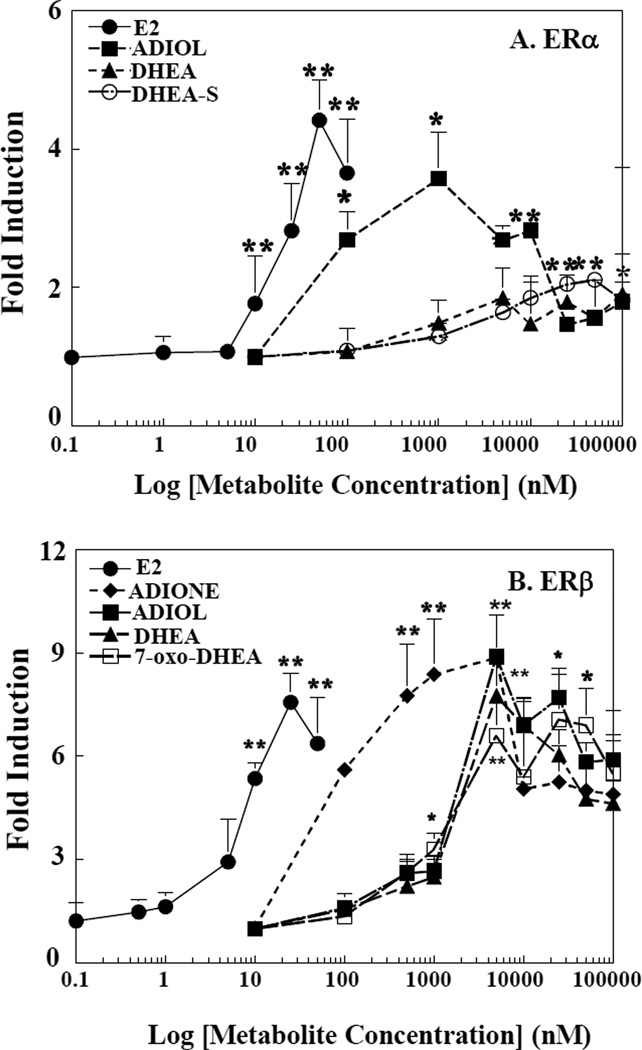
DHEA (5 µM) and many of its known metabolites [39] were tested for their ability to induce ERELUC expression with ERα expressed in HEK-293 cells or ERβ expressed in HepG2 cells (Fig. 1B). As a control, an empty expression vector was co-transfected with ERELUC and after treatment with DHEA or its metabolites. No additional induction of ERELUC over vehicle in either cell line, indicating that both cell types provide a viable null cell-based assay to test ERα and ERβ activation, respectively (data not shown). In HEK-293 cells, DHEA, DHEA-S, and ADIOL significantly induced ERELUC activity around 9-fold via ERα. While 11β-OH-DHEA, and 7β-OH-DHEA suppressed ERELUC activity in ERβ-expressing HepG2 cells, 7α-OH-DHEA, and 16α-OH-DHEA, increased ERELUC activity. Interestingly, DHEA and its cytosolic DHEA metabolites ADIONE and ADIOL, and 7-oxo-DHEA significantly induced ERELUC activity via ERβ in HepG2 cells (Fig. 1B). Concentration-response studies were conducted to evaluate the potency of ERα- and ERβ- mediated induction of ERELUC by DHEA and metabolites (Fig. 2A and 2B). ADIOL was the DHEA metabolite with the greatest ERα agonist activity while DHEA and DHEA-S at higher concentrations induced ERELUC expression ~2-fold with ERα. With ERβ, ADIONE was the most potent activator, inducing expression of ERELUC by ~ 10-to 12-fold. Both androst-5-ene-3,17-dione and androst-4-ene-3,17-dione (data not shown) were effective in activating ERELUC by ERβ in HepG2 cells. The conversion of the 5-ene derivative to the 4-ene form of androstenedione is believed to be an enzyme-mediated process, involving glutathione S-transferases [40]. 7-oxo-DHEA, DHEA, and ADIOL at higher concentrations also induced expression of ERELUC by ~ 6- to 8-fold with ERβ.
Figure 2. Concentration-dependent activation of ERα by DHEA, DHEA-S, and ADIOL in ER transfected cells.
HEK-293 and HepG2 cells were transfected with ERELUC reporter plasmid and expression vector for either human ERα (A) or ERβ (B), respectively. Cells were treated for 24 h with varying concentrations of E2, DHEA, DHEA-S, 7-oxo-DHEA, ADIONE and ADIOL. Cells were harvested and lysates were assayed for β-galactosidase and luciferase activities. Data represent the mean ± S.D. of three separate experiments. *, significantly different from vehicle-treated cells, p < 0.05, **,p<0.01. E2, ●; DHEA, ▲; DHEA-S, ○; ADIOL, ■; ADIONE, ♦; and 7-oxo-DHEA, □.
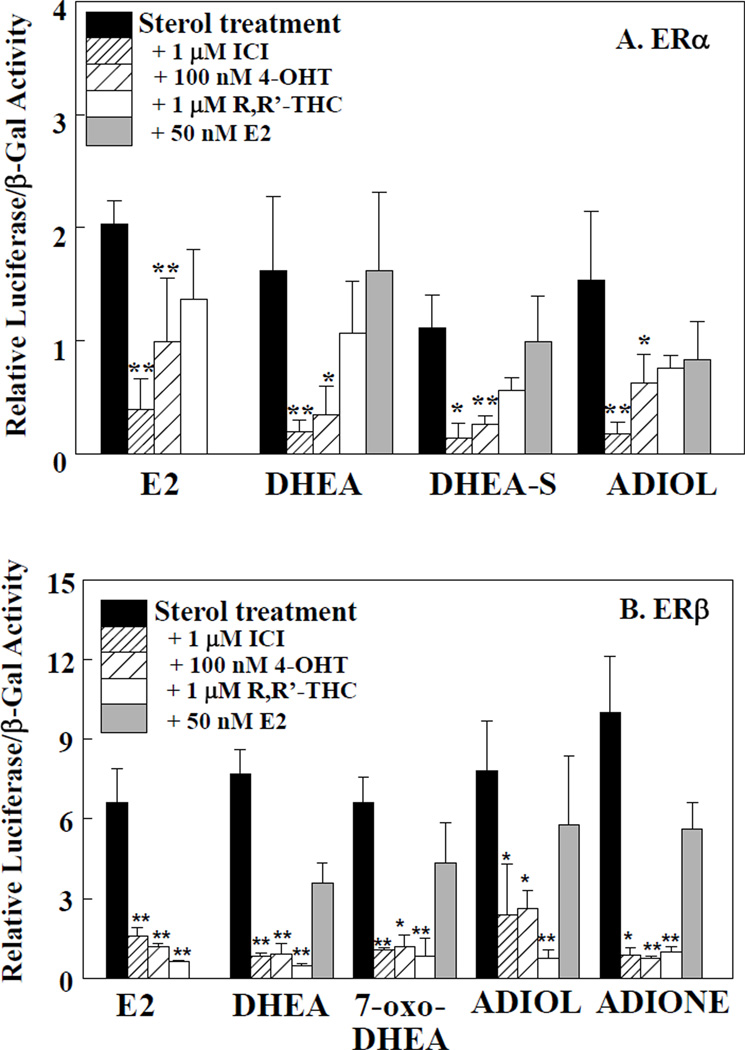
To establish whether the transcriptional response of DHEA and its metabolites seen with ERα and ERβ was mediated by interaction of these sterols with the receptors, cells were co-treated with the ER antagonists 4-hydroxytamoxifen (4-OHT) and ICI 182,780 (ICI) and either E2, DHEA, DHEAS, and ADIOL (Fig. 3A and 3B) ICI and 4-OHT inhibited the ERα-mediated induction of ERELUC by E2, DHEA, DHEA-S, and ADIOL and also the ERβ-mediated induction of ERELUC E2, DHEA, 7-oxo-DHEA, ADIOL, and ADIONE (Fig. 3A and 3B). The ERα agonist/ERβ antagonist R,R-THC [30] significantly inhibited the ERβ-mediated induction of ERELUC activity by E2, DHEA, 7-oxo-DHEA, ADIOL, and ADIONE, but did not inhibit ERELUC activation mediated by ERα. These data indicate that these sterols and their metabolites formed within these cells interact with the ligand binding domain of ERα and ERβ. In addition, E2 did not act synergistically with DHEA metabolites in ERα- or ERβ-mediated induction of ERELUC activity (Fig. 3A and 3B), perhaps because it displaced a lower affinity ligand formed by metabolism. Another possibility is that these data indicate a saturation of the reporter response as often seen due to limiting amounts of coregulators or other transcriptional components.
Figure 3. Inhibition of ERELUC reporter activity in the presence of cotransfected ERα in ER transfected cells.
HEK-293 and HepG2 cells were transfected with an ERELUC reporter plasmid and an expression vector for either human ERα or ERβ, respectively. Cells were treated for 24 h with 5 µM DHEA metabolite in the absence or presence of either 1 µM 182,780 ICI, 100 nM 4-hydroxytamoxifen (4-OHT), 1 µM R,R-THC, or 50 nM 17β-estradiol (E2). Cells were then harvested and lysates assayed for β-galactosidase and luciferase activities. Data represent the mean ± S.D. of three wells. Experiments were repeated three times with similar results. A. ERα-mediated transactivation; B. ERβ-mediated transactivation. Statistical significance was determined using analysis of variance followed by Student's t tests. *, significantly different from treated cells, p < 0.05 *, or **p, 0.01.
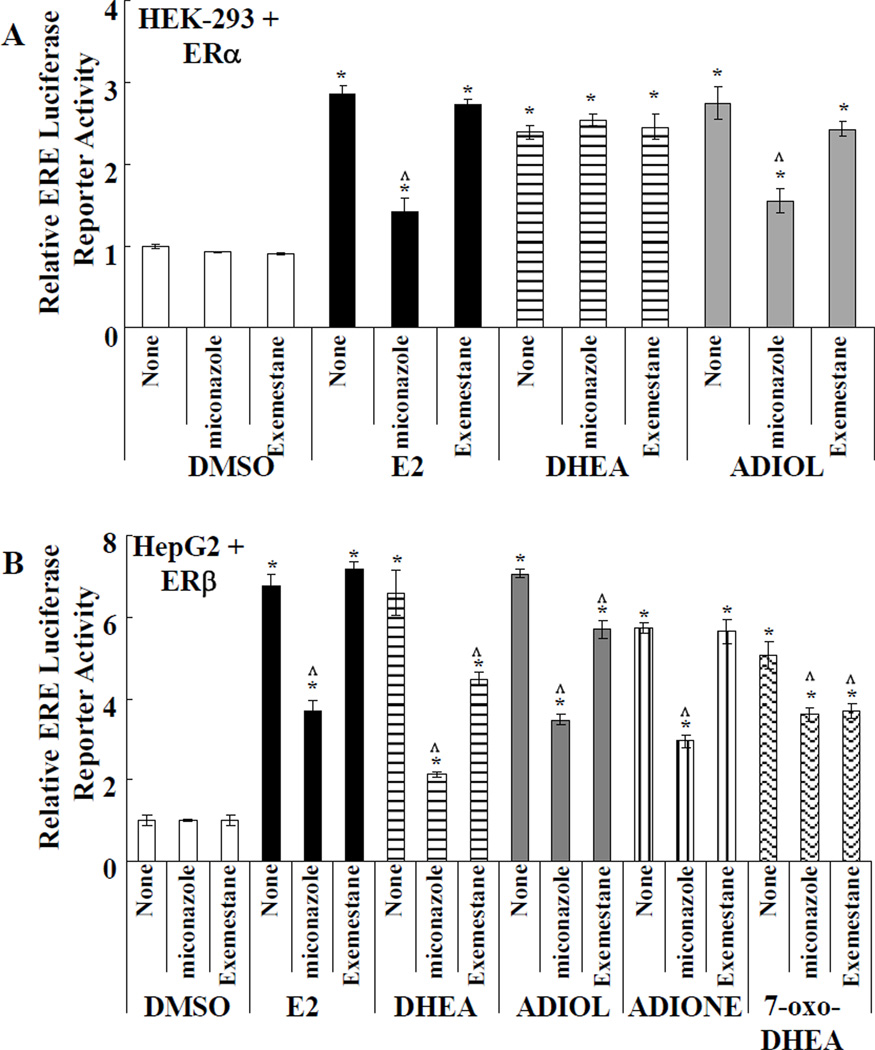
Aromatase inhibitors (AI), by preventing the conversion of androgen precursors into active estrogens, have significantly greater activity against breast cancer in postmenopausal women with estrogen-sensitive tumors compared to TAM [41]. Since HepG2 cells have been reported to have high aromatase activity [42], the effect of DHEA and its metabolites seen in the ERELUC assay may be due to their metabolism to estrogenic compounds. To examine whether the activation of ERELUC in ERα and ERβ transfected cells is mediated by conversion of DHEA metabolites to estrogens, ERα-transfected HEK-293 and ERβ-transfected HepG2 cells were pretreated with 5 µM miconazole, a general P450 inhibitor, or 100 nM exemestane, an AI. Cells treated with miconazole showed reduced ERα- and ERβ- activation of ERELUC activity in response to E2 and ADIOL (Fig. 4A and 4B) and to DHEA, ADIONE, and 7-oxo-DHEA for ERβ (Fig. 4B). Miconazole did not inhibit DHEA-stimulated ERα transcriptional activity (Fig. 4B). Exemestane did not significantly inhibit E2, DHEA, or ADIOL induced ERELUC activity in ERα-transfected cells (Fig. 4A). In ERβ-transfected cells, exemestane significantly inhibited DHEA-, ADIOL-, and 7-oxo-DHEA- induced ERELUC reporter activity ~ 20–30% (Fig 4B). In contrast, exemestane had no effect on ADIONE activation of ERβ (Fig 4B). The inhibition of the transcriptional activity of the tested ligands, except DHEA for ERα, by miconazole may be related to the ability miconazole to inhibit the interaction of coactivators with ERα and ERβ, as was reported for pregnane X receptor (PXR) [43]. To test this idea, HEK-293 cells were transfected with an ERE-LUC reporter plasmid and pCMV-hERα in combination with either an empty expression plasmid or expression plasmids for the SRC-2/TIF2/ GRIP1/NCOA2 or SRC3/AIB1/NCOA3 coactivators and treated with 10 nM E2 in the presence or absence of pretreatment with miconazole. Miconazole increased basal ERE-luciferase reporter activity ~ 0.8-fold, but did not affect Renilla luciferase activity (Supplemental Fig.1 and data not shown). Although the mechanism for this increase is unknown, there is only one report on a direct effect of miconazole on gene expression: miconazole increased the expression of rat hepatic microsomal epoxide hydrolase gene transcription in vivo starting 12 h after oral administration [44]. Miconazole inhibited E2-induced ERα- ERE-LUC activity (Supplemental Fig. 1). Coactivators SRC-2 and SRC-3 relieved the repression by miconazole in (Supplemental Fig. 1). These data are consistent with the idea that miconazole may inhibit ERα-coactivator interaction as reported for PXR [43], but complete confirmation requires additional experiments.
Figure 4. Effect of non-selective P450 inhibitor miconazole or aromatase inhibitor (AI) exemestane on ER-mediated transcriptional activation by DHEA and metabolites.
A) HEK-293 cells were transfected with ERα (A) and HepG2 cells were transfected with ERβ (B). All cells were co-transfected with ERELUC reporter plasmid and pRL-tk. After 24 h, cells were incubated for 24 h in phenol red-free MEM supplemented with 5% DCC-FBS plus DMSO, 10 nM E2, 5 µM DHEA, 5 µM ADIOL, 5 µM ADIONE, or 5 µM 7-oxo-DHEA, as indicated in each panel. Where indicated, cells were preincubated with 5 µM miconazole or 100 nM Exemestane (AI) for 6 h prior to addition of the hormone treatment. Values are the average of 3 separate determinations +/− SEM * Significantly different from DMSO (vehicle control), p < 0.05. ^ Significantly different from DHEA alone, p < 0.05.
Alternatively, miconazole may destabilize ER-ligand interaction since miconazole inhibits dexamethasone-glucocorticoid receptor (GR)-binding [45]. We examined whether miconazole inhibits E2-ER binding in a ligand binding competition assay and detected only a 15% inhibition of [3H] E2 binding to recombinant human ERα at 100 µM miconazole which is 20 times higher than the 5 µM concentration used in cell treatments (Supplemental Fig. 2). Taken together, these results suggest that DHEA and ADIOL are not converted into E2 or estrone to activate ERα in HEK-293 cells, but DHEA, ADIOL, and 7-oxo-DHEA are at least partially converted to estrogens in HepG2 cells to activate ERβ. In contrast, ADIONE activates ERβ without conversion to an estrogen in HepG2 cells.
3.2. DHEA and metabolites enhance endogenous ER-target gene transcription
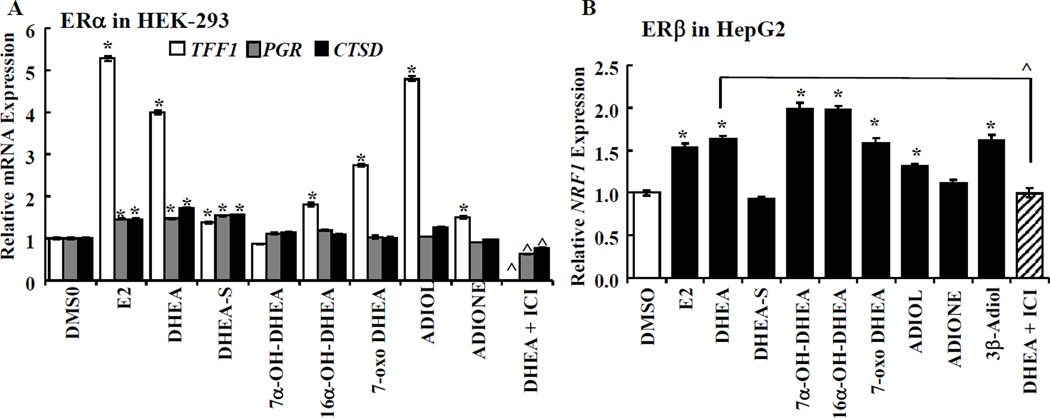
As an initial test to examine how DHEA and its metabolites affect endogenous ER-target gene transcription, HEK-293 cells were transfected with ERα and HepG2 cells were transfected with ERβ. Increased expression of ESR1 and ESR2 were detected in HEK-293 and HepG2, respectively (Supplementary Fig. 3). Twenty-four h after transfection, the cells were treated for 24 h with 10 nM E2 or 5 µM DHEA, DHEA-S, or other DHEA metabolites that had activated ERE-luciferase activity (Fig. 1B). Fig. 5A shows that the positive control E2 increased TFF1 (pS2), PGR (progesterone receptor (PR)), and CTSD (cathepsin D) transcription. The relative induction of pS2 mRNA was higher than that for PR or cathepsin D with 24 h treatment. Similar to the induction of ERE-reporter activity (Fig. 1B), DHEA, DHEA-S, 7-oxo-DHEA, and ADIOL increased TFF1 expression. Likewise, DHEA and DHEA-S increased PGR and CTSD expression. In agreement with the lack of ERα activity with 7α-OH-DHEA in the ERE-luciferase assay (Fig. 1B), no induction of TFF1, PGR, or CTSD was detected (Fig. 5A). While 16α-OH-DHEA and 7-oxo-DHEA showed no activation of PGR and CTSD, TFF1 expression was increased, in contrast to the lack of ERα-induced ERE-luciferase activity with these DHEA metabolites. DIOL increased TFF1, but not PGR or CTSD. We conclude that endogenous ERα target genes show similar, although not identical, responses to DHEA ligands in a gene-specific manner in ERα-transfected HEK-293 cells.
Figure 5. DHEA and metabolites increase endogenous gene transcription.
HEK-293 cells were transfected with ERα (A) and HepG2 were transfected with ERβ (B) expression plasmid for 24 h and then treated with the indicated hormones (10 nM E2 or 5 µM sterols) or 100 nM ICI 182,780 (ER antagonist) for 24 h. QPCR for A) TFF1 (pS2), PGR (PR), CTSD (cathepsin D) mRNA was normalized to 18S. B) NRF1 (NRF-1) was normalized to GAPDH. Values are the avg. ± SEM of triplicate determinations. * Significantly different from DMSO (vehicle control), p < 0.05. ^ Significantly different from DHEA alone, p < 0.05.
For ERβ-transfected HepG2 cells, we did not detect induction of TFF1, PGR, or CTSD (data not shown). ERβ regulates NRF1 (nuclear respiratory factor-1, NRF-1) expression in response to E2 and 4-OHT in breast and lung cancer cells [35, 36]. In agreement with the ERELUC activation data for ERβ (Fig. 1B), a statistically significant increase in NRF-1 mRNA expression was seen with E2, DHEA, α-OH-DHEA, 16α-OH-DHEA, and ADIOL (Fig. 5B). In contrast to the ERELUC for ERβ (Fig. 1B), ADIONE did not increase endogenous NRF-1 expression. On the other hand, 3β-Adiol, identified as an ERβ-selective agonist in prostate [23, 46], increased NRF-1 expression in HepG2 cells. We conclude that some DHEA ligands show ERβ agonist activity on NRF-1 expression, but ADIONE does not.
3.3 DHEA metabolites compete with E2 for binding ERα and ERβ
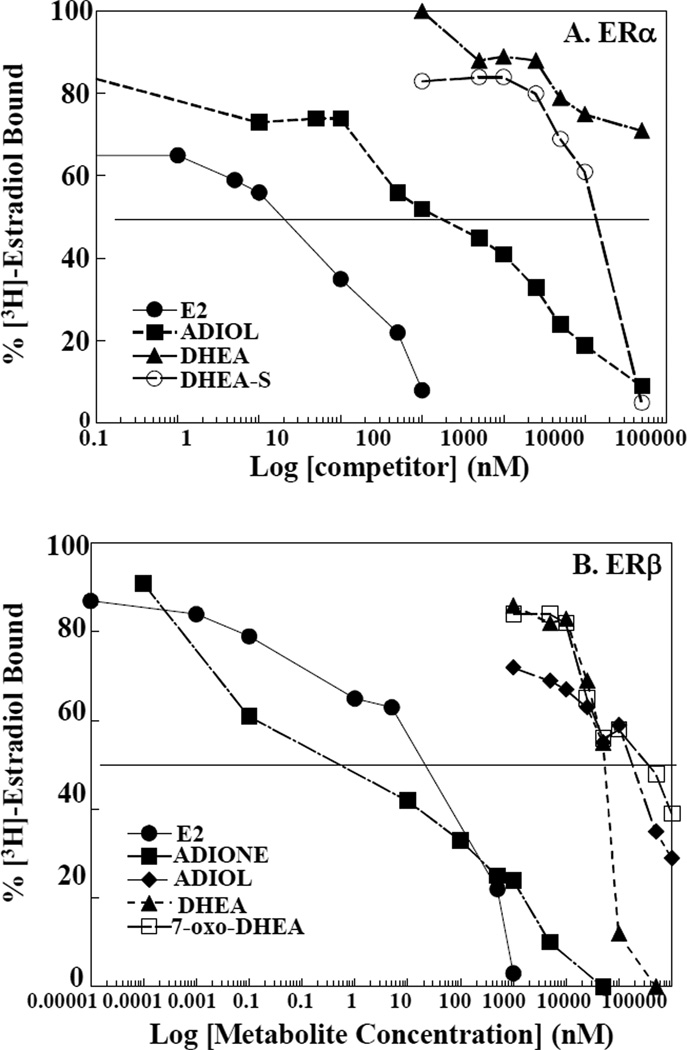
To determine if DHEA and its metabolites serve as ligands for ERα and ERβ, in vitro ligand binding competition assays [37]. Fig. 6A shows that in our hands, the IC50 value of E2 for ERα is ~1–10 nM, similar to the value reported in the literature [22, 47]. ADIOL bound to ERα with an IC50 of ~0.1 µM. DHEA and DHEA-S bound ERα with IC50s of >500 µM and 100–500 µM, respectively. Fig. 6B shows that the IC50 of E2 for ERβ is ~1–5 nM, in agreement with previous studies [22, 47]. Like ERα, ADIOL exhibited significant binding to ERβ with an IC50 of ~50 nM followed by ADIONE with an IC50 of 50 µM. DHEA and 7-oxo-DHEA exhibited IC50 values for ERβ of 500 µM and did not exhibit significant ERα binding. Previous work by Gustafsson’s group demonstrated that 4-androstene-3,17-dione did not compete with E2 for ERα or ERβ [22]. To our knowledge, no one has evaluated the ER binding of 5-androstene-3,17-dione that would be the anticipated product of oxidation of DHEA and, this intermediate may have appreciable rates of formation in mammalian tissues. The DHEA concentrations at which E2 is displaced are not physiologically relevant [9], and thus we conclude that DHEA and DHEA-S do not compete with E2 for binding ERα or ERβ.
Figure 6. Competitive binding of DHEA metabolite to ERα and ERβ.
An ligand binding competition assay using [3H] E2 was performed using baculovirus expressed ERα (A) or ERβ (B) with increasing concentrations of nonradiolabeled E2, DHEA or DHEA metabolites (DHEA-S, 7-oxo-DHEA, ADIONE and ADIOL). The values on the Y-axes are expressed as the percentage of [3H] E2 bound and each data point represents the mean of two independent binding assays. The competitor concentration causing 50% reduction in [3H] E2 binding (IC50) is found at the intersection of the binding curves with the 50% binding line (---------). E2, ●; DHEA, ▲; DHEA-S, ♦; ADIOL, ■; ADIONE, ♦; and 7-oxo-DHEA, □.
3.4 DHEA and its metabolites stimulate MCF-7 cell proliferation
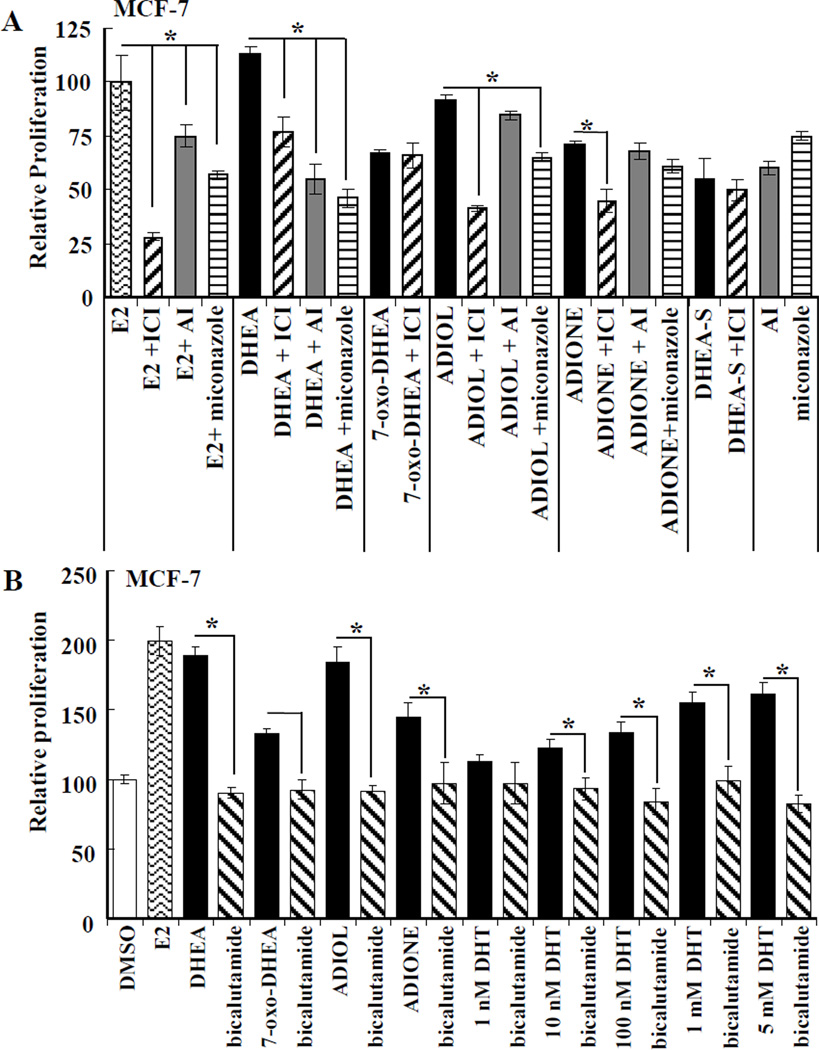
To examine the biological activity of the DHEA metabolites in a cell line expressing endogenous ERα and low levels of ERβ [36], a 48 h BrdU assay was performed in MCF-7 breast cancer cells (Fig. 7). As a positive control, the E2-induced stimulation of MCF-7 cell proliferation was inhibited by the selective estrogen receptor disrupter (SERD) ICI 182,780 (fulvestrant), demonstrating that ER was responsible for E2-induced cell proliferation (Fig. 7A). Similarly, DHEA, DHEA-S, 7-oxo-DHEA, ADIOL and ADIONE, all at 5 µM, increased MCF-7 proliferation (Fig. 7A). or The aromatase inhibitor (AI) exemestane inhibited the proliferative activity of DHEA by ~ 50% while showing no inhibitory activity with ADIOL or ADIONE. The general P450 inhibitor miconazole inhibited MCF-7 cell proliferation induced by E2, DHEA, ADIOL, and ADIONE, again indicating that miconazole appears to have off target effects. To address the potential involvement of endogenous AR in MCF-7 cells in the proliferative effects of DHEA and its metabolites, cells were pretreated with bicalutamide, an AR antagonist, and also treated with dihydrotestosterone (DHT). Bicalutamide inhibited DHEA-, 7-oxo-DHEA-, ADIOL-, and ADIONE- induced proliferation in MCF-7 cells. DHT induced a concentration-dependent increase in MCF-7 cell proliferation that was blocked by bicalutamide. These later results are in agreement with a report showing that testosterone stimulated MCF-7 cell proliferation [17] and AR protein expression increases ~ 2–3 fold with 48 h treatment of MCF-7 cells with 10 nM DHT [48]. Together these data suggest that DHEA is metabolized to compounds that stimulate MCF-7 cell proliferation through both ER- and AR- mediated pathways.
Figure 7. DHEA metabolites stimulate the proliferation of MCF-7 breast cancer cells.
The effect of E2 (10 nM), and 5 µM of either DHEA, DHEA-S, 7-oxo-DHEA, ADIOL or ADIONE alone or in combination with 100 nM ICI 182,780 (fulvestrant, a pure ER antagonist, pretreatment for 6 h), 100 nM Exemestane (AI), or 5 µM miconzaole (A) or in the presence of 10 µM bicalutamide (Casodex, pretreatment for 4 h) on the proliferation of MCF-7 cells was measured by BrdU assay after 48 h treatment as described in Experimental. A) The absorbance values were converted to percent of E2 values for relative proliferation values. B) Values were normalized to DMSO vehicle control. The data are the mean ± S.E.M. of 3–4 independent experiments in which each treatment was performed in quadruplicate. *, Statistically different from that particular indicated ligand treatment alone; p < 0.05 (one-way ANOVA followed by Student, Newman, Keuls post-hoc testing).
4. Discussion
DHEA has been suggested to share some of the same beneficial properties as estrogens in diseases of aging including atherosclerosis, osteoporosis, depression, and Alzheimer's disease, but lack their carcinogenic effects in breast [8, 10, 49]. Here we demonstrate that DHEA metabolite ADIOL activated ERα transcriptional activity in HEK-293 cells; whereas, ADIONE, ADIOL, DHEA, 7-oxo-DHEA, 7α-OH-DHEA, and 16α-OH-DHEA activated ERβ-mediated transcription in HepG2 cells. One caveat of our studies is the use of aromatase null HEK-293 [50–53] and aromatase positive HepG2 [42, 54]. Thus, it is possible that some of the DHEA metabolites were converted to estrogens in HepG2, but not in HEK-293 cells. Figure 8 presents a scheme of the DHEA metabolites examined in the present study and their role in activation of ERα and ERβ. In support of these findings, a recent paper reported that the androgenic DHEA metabolite 7β-Hydroxy-epiandrosterone (7β-OH-EpiA) activated ERβ in transfected MDA-MB-231 cells and GPER/GPR30 in SKBR3 cells [21]. Alone at 1–100 nM concentrations 7β-OH-EpiA did not affect cell proliferation, but inhibited E2-induced proliferation of MCF-7 and ERβ-overexpressing MDA-MB-231 cells with no dose-response detected [21]. The observation that different sterols (ADIOL or ADIONE) stimulated activation of ERα and ERβ to levels similar to those by E2, respectively, is reminiscent of the differences seen between the rodent versus human PXR, for which we reported that ADIOL was the preferred ligand for the murine and ADIONE for human PXR [55]. Human and murine PXR are also regulated differentially by other compounds, such as rifampicin (hPXR) and pregnenolone-16α-carbonitrile [56, 57]. The finding that DHEA and its metabolites showed greater ERβ agonist activity agrees with their higher affinity ERβ interaction, as shown here and reported previously [22], but is the first systematic study of DHEA and its metabolites as activators of ERα and ERβ transcriptional responses.
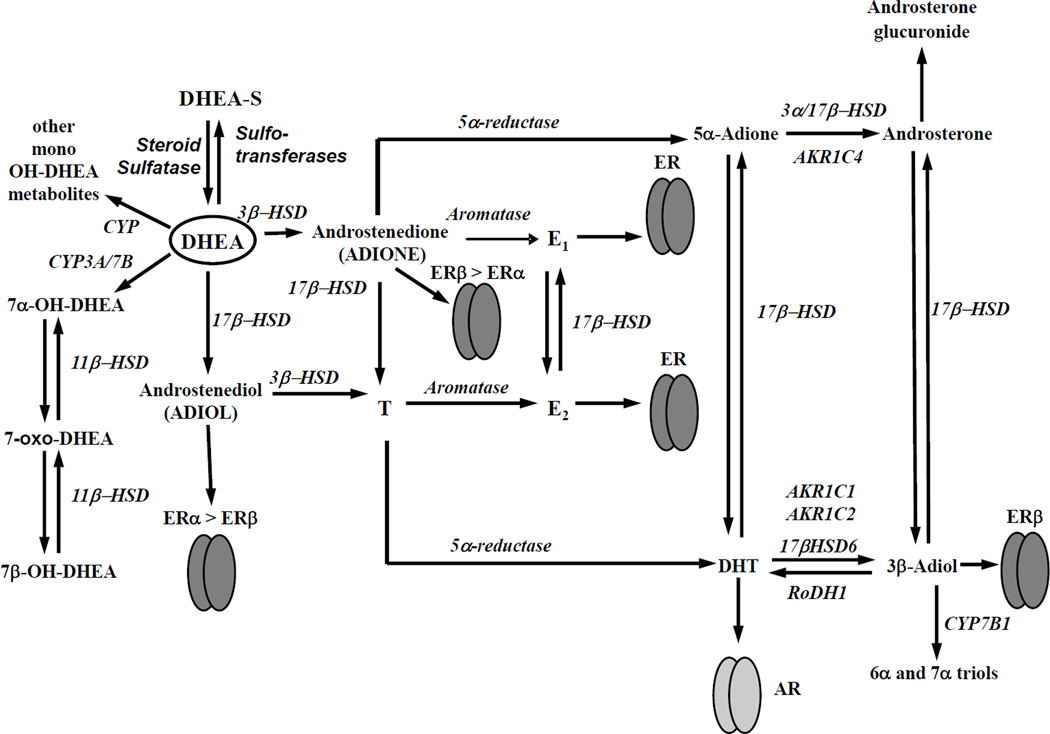
Figure 8. Model of how DHEA metabolites activate ERα and ERβ.
DHEA is converted to ADIOL, ADIONE, and mono-hydroxylated metabolites capable of activating the estrogen receptors. ADIOL and ADIONE are high affinity ligand activators of ER similar to the 3β-diol derived from DHT as demonstrated by Muthusamy et al [46]. Other DHEA metabolites, 7-hydroxy- and 7-keto-DHEA are low affinity ligand activators of ER, relative to ADIOL or ADIONE. Further information on the synthesis and metabolism of DHEA is reviewed in [39, 90–92].
ERs plays an important role in the physiology of many tissues [58, 59] by direct binding to EREs, by interaction with other transcription factors; e.g., AP-1 and Sp1, binding to their responsive elements [60], and by activating membrane ER or GPR30/GPER [61, 62]. Here we focused on DHEA and metabolite action on genomic/transcriptional ER activity. The ER antagonists ICI 182,780 and 4-OHT blocked the agonist activity of DHEA and its activating metabolites with both ERs. Likewise, the P450 inhibitor, miconazole, blocked the transcriptional activity of E2, DHEA, except for ERα, and all DHEA metabolites, thus suggesting an ‘off target’ effect, e.g., inhibition of coactivator-ER interaction as indicated by the ability of transfected SRC-2/GRIP1/NCOA3 and SRC-3/AIB1/NCOA to alleviate the repression of E2-induced luciferase reporter activity by miconazole in ERα-transfected HEK-293 cells (Supplemental Fig. 1) as reported previously for PXR [43]. The reason for lack of inhibition of DHEA-induced ERα transcriptional activity by miconazole in HEK-293 cells is unclear. It is possible that DHEA stimulates ERα transcription indirectly through DHEA activation of its GPR plasma membrane receptor [63], thus possibly activating ERα phosphorylation. Alternatively, DHEA may interact with a second site in ERα other than the LBD, as has been reported for 4-hydroxytamoxifen [64]. Both of these ideas would require further testing beyond the scope of the present study. In contrast to a report that miconazole inhibited DEX-GR binding [45], miconazole did not compete with E2 for ERα binding, indicating that miconazole is not inhibiting transcriptional activity by reducing ligand-ER interaction. The aromatase inhibitor, exemestane inhibited DHEA-, ADIOL-, and 7-oxo-DHEA-activativation of ERβ-induced reporter expression in HepG2 cells, but did not affect ERα transcriptional activity. This suggests conversion of DHEA, ADIOL, and 7-oxo-DHEA to active ERβ ligands in HepG2 cells, but not HEK-293 cells, a result congruent with steroid metabolic activity in HepG2 cells [39, 65]. Since HEK-293 cells are routinely used for transfection studies, knowledge of their endogenous sterol metabolism appears not to have been extensively studied. Indeed, exemestane inhibited DHEA-induced MCF-7 cell proliferation, indicating that metabolism is involved. Others reported that DHEA is metabolized to E2 in MCF-7 cells [66] which agrees with our MCF-7 cell proliferation and ERβ-transcriptional responses in HepG2 cells. In contrast, ADIONE activated ERβ transcriptional activity was not inhibited by exemestane. We noted that ADIONE competed with E2 for binding ERβ with an IC50 of ~ 50 µM (Fig. 6). This observation accounts for the direct transactivation of ERβ by ADIONE. We also note that DHEA and its metabolites stimulate MCF-7 cell proliferation by activating AR, results in agreement with previous reports [17, 67].
Our data are concordant with and extend previous reports on the estrogenic activity of DHEA metabolites. DHEA was reported to bind ERα and ERβ expressed in COS-1 cells with Kd ~ 1.2 and 0.5 µM, respectively [11]. We did not detect DHEA binding as a ligand to baculovirus-expressed rh ERα and ERβ at physiologically relevant DHEA concentrations in our studies. DHEA, its metabolites ADIOL, 3β-Adiol, testosterone, and DHT all stimulated proliferation and ERE-driven luciferase activity in MCF-7 cells, albeit at significantly higher (1–10 µM) concentrations than E2 [17]. DHEA is metabolized by CYP7B to 7α-hydroxy-DHEA in human prostate tissues and CYP3A4 in human liver [38, 39]. 7α-Hydroxy-DHEA (50 µM) stimulated activity of an ERE-TK-luciferase reporter in COS cells transactivated with ERα or ERβ [38]. Similarly, 0.1 µM 3β-Adiol and 5-androstene-3β,17β-diol (ADIOL) activated ERE-luciferase activity in HEK-293 cells transfected with ERα or ERβ [29].
Reports on the activities of DHEA and its metabolites in cancer cells have been contradictory. Early studies with diethylnitrosamine-induced hepatic tumors in rats demonstrated that DHEA (0.6% in the diet) inhibited tumor cell growth, perhaps by the inhibition of glucose-6-phosphate dehydrogenase, a key enzyme in the biosynthesis of both NADPH and ribose phosphate derivatives [68]. Likewise, estrone-stimulated growth of ZR-75-1 breast cancer xenografts in nude mice was suppressed by twice daily s.c. injections of 0.3 mg DHEA [69]. Conversely, studies with MCF-7 breast cancer cells demonstrated that ADIOL, DHEA, and DHEA-S increased cell proliferation, albeit less than E2 [17] [70]. Over-expression of aromatase stimulated DHEA-dependent MCF-7 cell proliferation, implicating conversion to estrogens [18]. Recently, interest in DHEA metabolites has been revived with the observation that DHT and its metabolite 3β-Adiol stimulated MCF-7 and T47D breast cancer cell proliferation through ERα [28, 71]. These and related studies suggested that DHEA and its metabolites may serve as activators of ERα upon metabolism by aromatase to form estrogens.
We detected gene-specific and DHEA metabolite-specific differences in induction of endogenous gene transcription in HEK-293 transfected with ERα and HepG2 transfected with ERβ. There are many reasons that could account for the different responses of ERα detected with 16α-OH-DHEA, 7-oxo-DHEA, or ADIONE treatment in HEK-293 cells between the ERE-luciferase assay and induction of endogenous TFF1 expression. First, the ERE-luciferase reporter is an artificial construct with three tandem perfect, consensus EREs, which binds ERs with high affinity [31, 72, 73], compared to the endogenous TFF1 which is regulated by imperfect ERE and an AP-1 element [74]. Second, endogenous genes have chromatin structure and epigenetic modifications that have profound effects on transcription. In agreement with previous reports of low ERβ activity in transfected HepG2 cells [75], we detected low induction of endogenous NRF-1 expression in response to E2, DHEA, 7α-OH-DHEA, 16α-OH-DHEA, 7-oxo-DHEA, and 3β-Adiol. ADIONE gave the highest activation of ERELUC by ERβ in transiently transfected HepG2, the reason for the lack of ADIONE-induction of NRF-1 expression in HepG2-ERβ cells is unknown. Certainly, the relative deficiency of specific coregulators or differences in the epigenome may explain the lack of effect of ADIONE on NRF-1 expression. To our knowledge, only one report on NRF-1 regulation in HepG2 has been published and that showed that an 8 h combined treatment of proinflammatory LPS and TNFα increased NRF-1 by activating NFκB [76]. It is clear from genome-wide studies identifying ERβ-regulated genes in U2OS [77], HEK293 [78], T47D [79], and MCF-7 [80] cells overexpressing ERβ, that there are cell-type differences in gene regulation. Thus, the selection of another ERβ target gene in HepG2 cells would necessitate a separate study and would be of limited physiological relevance since ERβ is not expressed in human liver [81].
There are significant differences in the transactivation of ERs based on relative AF-1 activation differences between cell lines [82–85]. Since AF-1 is stimulated by phosphorylation of ser 118 in ERα by MAPK [86, 87] and DHEA activates a membrane-initiated G-protein-coupled receptor (GPCR)- MAPK pathway in human endothelial cells [88, 89], we examined if the MEK1/2 inhibitor, PD98059 would block transcriptional activation of ERα and ER by DHEA and its metabolites. However, PD998059 did not block DHEA or metabolite-induced ERELUC activity, suggesting that ERα- and ERβ-dependent activation of transcription by DHEA and its metabolites is independent of MAPK-mediated phosphorylation (data not shown).
In conclusion, these studies demonstrate that DHEA and its metabolites activate ERα and ERβ in an ER-subtype and cell-selective manner. The most potent ligands for ERα and ERβ are ADIOL and ADIONE, respectively, in an ERELUC assay, but potencies differ for endogenous estrogen target genes. DHEA, ADIOL, and 7-oxo-DHEA are converted by aromatase to estrogens to activate ERβ in HepG2 cells, but ADIONE appears to directly activate ERβ, albeit with low affinity (IC50 ~50 µM). Since circulating levels of DHEA and DHEA-S are high and variable in humans and DHEA can be metabolized in peripheral and tumor tissues, our results provide new insight into the mechanism of action of DHEA and its metabolites as possible contributors in the progression of hormone-sensitive cancer in patients expressing high circulating and tissue levels of these sterols and their metabolizing enzymes.
Supplementary Material
Highlights.
DHEA and its metabolites increased ERα and ERβ transcriptional activity
Androstenediol binds ERα with an IC50 ~0.1 µM
Androstenedione binds ERβ with an IC50 ~5 nM
DHEA metabolites stimulate MCF-7 cell proliferation
Miconazole inhibits ERα-coactivator interaction
ACKNOWLEDGEMENTS
The authors are grateful to Mary Pendleton and Nalinie S. Wickramasinghe for expert technical assistance with cell culture, cell proliferation, and transient transfection assays. Supported by USPHS grant DK54774 to RAP, DK053220 to CMK, and Kentucky Affiliate American Heart Association Predoctoral Fellowships to KKMM (0110109) and KAM (315097B).
Abbreviations
- ADIOL
androstenediol (androst-5-ene-3β,17β-diol)
- 3β-Adiol
(5α-androstane-3β,17β-adiol)
- ADIONE
androstenedione (androst-5-ene-3,17-dione)
- CYP
cytochrome P450
- DHEA
dehydroepiandrosterone (3β-hydroxy-androst-5-ene-17-one)
- DHEA-S
DHEA 3β-sulfate
- DMSO
dimethyl sulfoxide
- E2
17β-estradiol
- ER
estrogen receptor
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- ERE
estrogen response element
- ETIO
etiocholanolone (3α-hydroxy-5β-androstan-17-one)
- HAP
hydroxyapatite
- NRF-1
nuclear respiratory factor-1
- SD
standard deviation
- 4-OHT
4-hydroxytamoxifen
- 7α-OH-DHEA
7α-hydroxy-DHEA
- 7β-OH-DHEA
7β-hydroxy-DHEA
- 11β-OH-DHEA
11β-hydroxy-DHEA
- 16α-OH-DHEA
16α-hydroxy-DHEA
- 7-oxo-DHEA
3β-hydroxyandrost-5-ene-7,17-dione
- ICI 182,780
(ICI, Fulvestrant)
- R,R-THC
cis-diethyl tetrahydrochrysene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version.
REFERENCES
- 1.Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis-some new perspectives. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 2.Janni W, Hepp P. Adjuvant aromatase inhibitor therapy: Outcomes and safety. Cancer Treat Rev. 2010;36:249–261. doi: 10.1016/j.ctrv.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PHM, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 4.Danforth KN, Eliassen AH, Tworoger SS, Missmer SA, Barbieri RL, Rosner BA, et al. The association of plasma androgen levels with breast, ovarian and endometrial cancer risk factors among postmenopausal women. Int J Cancer. 2010;126:199–207. doi: 10.1002/ijc.24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endogenous H Breast Cancer Collaborative G. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011 doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calhoun KE, Pommier RF, Muller P, Fletcher WS, Toth-Fejel S. Dehydroepiandrosterone sulfate causes proliferation of estrogen receptor-positive breast cancer cells despite treatment with fulvestrant. Arch Surg. 2003;138:879–883. doi: 10.1001/archsurg.138.8.879. [DOI] [PubMed] [Google Scholar]
- 7.Aguiar M, Masse R, Gibbs BF. Regulation of cytochrome P450 by posttranslational modification. Drug Metab Rev. 2005;37:379–404. doi: 10.1081/dmr-46136. [DOI] [PubMed] [Google Scholar]
- 8.Labrie F. DHEA, Important Source of Sex Steroids in Men and Even More in Women. In: Luciano M, editor. Prog Brain Res. Elsevier; 2010. pp. 97–148. [DOI] [PubMed] [Google Scholar]
- 9.Labrie F, Bélanger A, Cusan L, Gomez J-L, Candas B. Marked Decline in Serum Concentrations of Adrenal C19 Sex Steroid Precursors and Conjugated Androgen Metabolites During Aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 10.Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)—A Precursor Steroid or an Active Hormone in Human Physiology (CME) The Journal of Sexual Medicine. 2011;8:2960–2982. doi: 10.1111/j.1743-6109.2011.02523.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, et al. Direct Agonist/Antagonist Functions of Dehydroepiandrosterone. Endocrinology. 2005;146:4568–4576. doi: 10.1210/en.2005-0368. [DOI] [PubMed] [Google Scholar]
- 12.Shilkaitis A, Green A, Punj V, Steele V, Lubet R, Christov K. Dehydroepiandrosterone inhibits the progression phase of mammary carcinogenesis by inducing cellular senescence via a p16-dependent but p53-independent mechanism. Breast Cancer Research. 2005;7:R1132–R1140. doi: 10.1186/bcr1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakkak R, Shaaf S, Jo CH, MacLeod S, Korourian S. Dehydroepiandrosterone intake protects against 7,12-dimethylbenz(a)anthracene-induced mammary tumor development in the obese Zucker rat model. Oncol Rep. 2010;24:357–362. doi: 10.3892/or_00000867. [DOI] [PubMed] [Google Scholar]
- 14.López-Marure R, Contreras PG, Dillon JS. Effects of dehydroepiandrosterone on proliferation, migration, and death of breast cancer cells. Eur J Pharmacol. 2011;660:268–274. doi: 10.1016/j.ejphar.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Boccuzzi G, Brignardello E, di Monaco M, Forte C, Leonardi L, Pizzini A. Influence of dehydroepiandrosterone and 5-en-androstene-3 beta, 17 beta-diol on the growth of MCF-7 human breast cancer cells induced by 17 beta-estradiol. Anticancer Res. 1992;12:799–803. [PubMed] [Google Scholar]
- 16.Bruder JM, Sobek L, Oettel M. Dehydroepiandrosterone stimulates the estrogen response element. The Journal of Steroid Biochemistry and Molecular Biology. 1997;62:461–466. doi: 10.1016/s0960-0760(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 17.Maggiolini M, Donze O, Jeannin E, Ando S, Picard D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alpha. Cancer Res. 1999;59:4864–4869. [PubMed] [Google Scholar]
- 18.Maggiolini M, Bonofiglio D, Pezzi V, Carpino A, Marsico S, Rago V, et al. Aromatase overexpression enhances the stimulatory effects of adrenal androgens on MCF7 breast cancer cells. Mol Cell Endocrinol. 2002;193:13–18. doi: 10.1016/s0303-7207(02)00091-6. [DOI] [PubMed] [Google Scholar]
- 19.Gayosso V, Montano LF, Lopez-Marure R. DHEA-induced antiproliferative effect in MCF- 7 cells is androgen- and estrogen receptor-independent. Cancer J. 2006;12:160–165. [PubMed] [Google Scholar]
- 20.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 21.Niro S, Pereira E, Pélissier M-A, Morfin R, Hennebert O. The DHEA metabolite 7β-hydroxy-epiandrosterone exerts anti-estrogenic effects on breast cancer cell lines. Steroids. 2012;77:542–551. doi: 10.1016/j.steroids.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Kuiper GG, Carlsson B, Grandien J, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 23.Weihua Z, Lathe R, Warner M, Gustafsson J-Å. An endocrine pathway in the prostate, ERβ, AR, 5α-androstane-3β,17β-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci U S A. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omoto Y, Lathe R, Warner M, Gustafsson J-A. Early onset of puberty and early ovarian failure in CYP7B1 knockout mice. PNAS. 2005;102:2814–2819. doi: 10.1073/pnas.0500198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettersson H, Holmberg L, Axelson M, Norlin M. CYP7B1-mediated metabolism of dehydroepiandrosterone and 5α-androstane-3β,17β-diol – potential role(s) for estrogen signaling. FEBS J. 2008;275:1778–1789. doi: 10.1111/j.1742-4658.2008.06336.x. [DOI] [PubMed] [Google Scholar]
- 26.Dondi D, Piccolella M, Biserni A, Della Torre S, Ramachandran B, Locatelli A, et al. Estrogen receptor beta and the progression of prostate cancer: role of 5alpha-androstane-3beta,17beta-diol. Endocr Relat Cancer. 2010;17:731–742. doi: 10.1677/ERC-10-0032. [DOI] [PubMed] [Google Scholar]
- 27.Norata GD, Cattaneo P, Poletti A, Catapano AL. The androgen derivative 5[alpha]-androstane-3[beta],17[beta]-diol inhibits tumor necrosis factor [alpha] and lipopolysaccharide induced inflammatory response in human endothelial cells and in mice aorta. Atherosclerosis. 2010;212:100–106. doi: 10.1016/j.atherosclerosis.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5alpha-androstane-3beta,17beta-diol (3betaAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat. 2009;115:289–296. doi: 10.1007/s10549-008-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson H, Lundqvist J, Norlin M. Effects of CYP7B1-mediated catalysis on estrogen receptor activation. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2010;1801:1090–1097. doi: 10.1016/j.bbalip.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology. 1999;140:800–804. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- 31.Klinge CM. Estrogen receptor binding to estrogen response elements slows ligand dissociation and synergistically activates reporter gene expression. Mol Cell Endocrinol. 1999;150:99–111. doi: 10.1016/s0303-7207(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 32.Falkner KC, Rushmore TH, Linder MW, Prough RA. Negative Regulation of the Rat GlutathioneS-Transferase A2 Gene by Glucocorticoids Involves a Canonical Glucocorticoid Consensus Sequence. Mol Pharmacol. 1998;53:1016–1026. [PubMed] [Google Scholar]
- 33.Koh SS, Chen D, Lee YH, Stallcup MR. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem. 2001;276:1089–1098. doi: 10.1074/jbc.M004228200. [DOI] [PubMed] [Google Scholar]
- 34.Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J Mol Endocrinol. 2004;33:387–410. doi: 10.1677/jme.1.01541. [DOI] [PubMed] [Google Scholar]
- 35.Ivanova MM, Luken KH, Zimmer AS, Lenzo FL, Smith RJ, Arteel MW, et al. Tamoxifen increases nuclear respiratory factor 1 transcription by activating estrogen receptor β and AP-1 recruitment to adjacent promoter binding sites. The FASEB Journal. 2011;25:1402–1416. doi: 10.1096/fj.10-169029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of Nuclear Respiratory Factor-1 and increases mitochondrial biogenesis. Mol Endocrinol. 2008;22:609–622. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavlik EJ, Coulson PB. Hydroxylapatite "batch" assay for estrogen receptor: Increased sensitivity over present receptor assays. J Steroid Biochem. 1976;7:357–368. doi: 10.1016/0022-4731(76)90095-9. [DOI] [PubMed] [Google Scholar]
- 38.Martin C, Ross M, Chapman KE, Andrew R, Bollina P, Seckl JR, et al. CYP7B Generates a Selective Estrogen Receptor β Agonist in Human Prostate. J Clin Endocrinol Metab. 2004;89:2928–2935. doi: 10.1210/jc.2003-031847. [DOI] [PubMed] [Google Scholar]
- 39.Miller KK, Cai J, Ripp SL, Pierce WM, Jr, Rushmore TH, Prough RA. Stereo- and regioselectivity account for the diversity of dehydroepiandrosterone (DHEA) metabolites produced by liver microsomal cytochromes P450. Drug Metab Dispos. 2004;32:305–313. doi: 10.1124/dmd.32.3.305. [DOI] [PubMed] [Google Scholar]
- 40.Benson AM, Talalay P, Keen JH, Jakoby WB. Relationship between the soluble glutathione-dependent delta 5-3-ketosteroid isomerase and the glutathione S-transferases of the liver. Proc Natl Acad Sci U S A. 1977;74:158–162. doi: 10.1073/pnas.74.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gluck S. Exemestane as first-line therapy in postmenopausal women with recurrent or metastatic breast cancer. Am J Clin Oncol. 2010;33:314–319. doi: 10.1097/COC.0b013e31819fdf9b. [DOI] [PubMed] [Google Scholar]
- 42.Castagnetta LA, Agostara B, Montalto G, Polito L, Campisi I, Saetta A, et al. Local estrogen formation by nontumoral, cirrhotic, and malignant human liver tissues and cells. Cancer Res. 2003;63:5041–5045. [PubMed] [Google Scholar]
- 43.Dvorak Z. Drug–drug interactions by azole antifungals: Beyond a dogma of CYP3A4 enzyme activity inhibition. Toxicol Lett. 2011;202:129–132. doi: 10.1016/j.toxlet.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Kim SG. Transcriptional regulation of rat microsomal epoxide hydrolase gene by imidazole antimycotic agents. Mol Pharmacol. 1992;42:273–279. [PubMed] [Google Scholar]
- 45.Duret C, Daujat-Chavanieu M, Pascussi J-M, Pichard-Garcia L, Balaguer P, Fabre J-M, et al. Ketoconazole and Miconazole Are Antagonists of the Human Glucocorticoid Receptor: Consequences on the Expression and Function of the Constitutive Androstane Receptor and the Pregnane X Receptor. Mol Pharmacol. 2006;70:329–339. doi: 10.1124/mol.105.022046. [DOI] [PubMed] [Google Scholar]
- 46.Muthusamy S, Andersson S, Kim H-J, Butler R, Waage L, Bergerheim U, et al. Estrogen receptor β and 17β-hydroxysteroid dehydrogenase type 6, a growth regulatory pathway that is lost in prostate cancer. Proceedings of the National Academy of Sciences. 2011;108:20090–20094. doi: 10.1073/pnas.1117772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Branham WS, Dial SL, Moland CL, Hass BS, Blair RM, Fang H, et al. Phytoestrogens and Mycoestrogens Bind to the Rat Uterine Estrogen Receptor. The Journal of Nutrition. 2002;132:658–664. doi: 10.1093/jn/132.4.658. [DOI] [PubMed] [Google Scholar]
- 48.Greeve M, Allan R, Harvey J, Bentel J. Inhibition of MCF7 breast cancer cell proliferation by 5alpha-dihydrotestosterone; a role for p21(Cip1/Waf1) J Mol Endocrinol. 2004;32:793–810. doi: 10.1677/jme.0.0320793. [DOI] [PubMed] [Google Scholar]
- 49.Labrie F, Luu-The V, Martel C, Chernomoretz A, Calvo E, Morissette J, et al. Dehydroepiandrosterone (DHEA) is an anabolic steroid like dihydrotestosterone (DHT), the most potent natural androgen, and tetrahydrogestrinone (THG) The Journal of Steroid Biochemistry and Molecular Biology. 2006;100:52–58. doi: 10.1016/j.jsbmb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Saarinen N, Joshi SC, Ahotupa M, Li X, Ammala J, Makela S, et al. No evidence for the in vivo activity of aromatase-inhibiting flavonoids. J Steroid Biochem Mol Biol. 2001;78:231–239. doi: 10.1016/s0960-0760(01)00098-x. [DOI] [PubMed] [Google Scholar]
- 51.Bobes RJ, Miranda C, Pérez-Martinez M, Luu-The V, Romano MC. Isolation and characterization of goat ovarian aromatase cDNA: assessment of the activity using an intact cell system and placental expression. Theriogenology. 2004;62:532–543. doi: 10.1016/j.theriogenology.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Wang D-S, Kobayashi T, Zhou L-Y, Paul-Prasanth B, Ijiri S, Sakai F, et al. Foxl2 Up-Regulates Aromatase Gene Transcription in a Female-Specific Manner by Binding to the Promoter as Well as Interacting with Ad4 Binding Protein/Steroidogenic Factor 1. Mol Endocrinol. 2007;21:712–725. doi: 10.1210/me.2006-0248. [DOI] [PubMed] [Google Scholar]
- 53.Hu Y, Ghosh S, Amleh A, Yue W, Lu Y, Katz A, et al. Modulation of aromatase expression by BRCA1: a possible link to tissue-specific tumor suppression. Oncogene. 2005;24:8343–8348. doi: 10.1038/sj.onc.1208985. [DOI] [PubMed] [Google Scholar]
- 54.Granata OM, Cocciadifero L, Campisi I, Miceli V, Montalto G, Polito LM, et al. Androgen metabolism and biotransformation in nontumoral and malignant human liver tissues and cells. The Journal of Steroid Biochemistry and Molecular Biology. 2009;113:290–295. doi: 10.1016/j.jsbmb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Ripp SL, Fitzpatrick JL, Peters JM, Prough RA. Induction of CYP3A Expression by Dehydroepiandrosterone: Involvement of the Pregnane X Receptor. Drug Metab Disposition. 2002;30:570–575. doi: 10.1124/dmd.30.5.570. [DOI] [PubMed] [Google Scholar]
- 56.Kojima H, Sata F, Takeuchi S, Sueyoshi T, Nagai T. Comparative study of human and mouse pregnane X receptor agonistic activity in 200 pesticides using in vitro reporter gene assays. Toxicology. 2011;280:77–87. doi: 10.1016/j.tox.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, et al. The Pregnane X Receptor: A Promiscuous Xenobiotic Receptor That Has Diverged during Evolution. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 58.Sugiyama N, Barros RPA, Warner M, Gustafsson J-Å. ER[beta]: recent understanding of estrogen signaling. Trends in Endocrinology & Metabolism. 2010;21:545–552. doi: 10.1016/j.tem.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 60.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levin ER. Minireview: Extranuclear Steroid Receptors: Roles in Modulation of Cell Functions. Mol Endocrinol. 2011;25:377–384. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filardo EJ, Thomas P. Minireview: G Protein-Coupled Estrogen Receptor-1, GPER-1: Its Mechanism of Action and Role in Female Reproductive Cancer, Renal and Vascular Physiology. Endocrinology. 2012;153 doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olivo HF, Perez-Hernandez N, Liu D, Iruthayanathan M, O'Leary B, Homan LL, et al. Synthesis and application of a photoaffinity analog of dehydroepiandrosterone (DHEA) Bioorg Med Chem Lett. 2010;20:1153–1155. doi: 10.1016/j.bmcl.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kojetin DJ, Burris TP, Jensen EV, Khan SA. Implications of the binding of tamoxifen to the coactivator recognition site of the estrogen receptor. Endocr Relat Cancer. 2008;15:851–870. doi: 10.1677/ERC-07-0281. [DOI] [PubMed] [Google Scholar]
- 65.Carruba G. Aromatase in Nontumoral and Malignant Human Liver Tissues and Cells. Ann N Y Acad Sci. 2009;1155:187–193. doi: 10.1111/j.1749-6632.2009.03706.x. [DOI] [PubMed] [Google Scholar]
- 66.Schmitt M, Klinga K, Schnarr B, Morfin R, Mayer D. Dehydroepiandrosterone stimulates proliferation and gene expression in MCF-7 cells after conversion to estradiol. Mol Cell Endocrinol. 2001;173:1–13. doi: 10.1016/s0303-7207(00)00442-1. [DOI] [PubMed] [Google Scholar]
- 67.Maggiolini M, Carpino A, Bonofiglio D, Pezzi V, Rago V, Marsico S, et al. The direct proliferative stimulus of dehydroepiandrosterone on MCF7 breast cancer cells is potentiated by overexpression of aromatase. Mol Cell Endocrinol. 2001;184:163–171. doi: 10.1016/s0303-7207(01)00563-9. [DOI] [PubMed] [Google Scholar]
- 68.Feo F, Daino L, Seddaiu MA, Simile MM, Pascale R, McKeating JA, et al. Differential effects of dehydroepiandrosterone and deoxyribonucleosides on DNA synthesis and de novo cholesterogenesis in hepatocarcinogenesis in rats. Carcinogenesis. 1991;12:1581–1586. doi: 10.1093/carcin/12.9.1581. [DOI] [PubMed] [Google Scholar]
- 69.Couillard S, Labrie C, Belanger A, Candas B, Pouliot F, Labrie F. Effect of dehydroepiandrosterone and the antiestrogen EM-800 on growth of human ZR-75-1 breast cancer xenografts. J Natl Cancer Inst. 1998;90:772–778. doi: 10.1093/jnci/90.10.772. [DOI] [PubMed] [Google Scholar]
- 70.Billich A, Nussbaumer P, Lehr P. Stimulation of MCF-7 breast cancer cell proliferation by estrone sulfate and dehydroepiandrosterone sulfate: inhibition by novel non-steroidal steroid sulfatase inhibitors. The Journal of Steroid Biochemistry and Molecular Biology. 2000;73:225–235. doi: 10.1016/s0960-0760(00)00077-7. [DOI] [PubMed] [Google Scholar]
- 71.Sikora M, Strumba V, Lippman M, Johnson M, Rae J. Mechanisms of estrogen-independent breast cancer growth driven by low estrogen concentrations are unique versus complete estrogen deprivation. Breast Cancer Res Treat. 2012:1–13. doi: 10.1007/s10549-012-2032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klinge CM, Jernigan SC, Smith SL, Tyulmenkov VV, Kulakosky PC. Estrogen response element sequence impacts the conformation and transcriptional activity of estrogen receptor α. Mol Cell Endocrinol. 2001;174:151–166. doi: 10.1016/s0303-7207(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 73.Tyulmenkov VV, Klinge CM. A mathematical approach to predict the affinity of estrogen receptors alpha and beta binding to DNA. Mol Cell Endocrinol. 2001;182:109–119. doi: 10.1016/s0303-7207(01)00508-1. [DOI] [PubMed] [Google Scholar]
- 74.Barkhem T, Haldosén L-A, Gustafsson J-Å, Nilsson S. pS2 Gene Expression in HepG2 cells: Complex Regulation through Crosstalk between the Estrogen Receptor α, an Estrogen-Responsive Element, and the Activator Protein 1 Response Element. Mol Pharmacol. 2002;61:1273–1283. doi: 10.1124/mol.61.6.1273. [DOI] [PubMed] [Google Scholar]
- 75.Delaunay F, Pettersson K, Tujague M, Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol. 2000;58:584–590. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- 76.Suliman HB, Sweeney TE, Withers CM, Piantadosi CA. Co-regulation of nuclear respiratory factor-1 by NF{kappa}B and CREB links LPS-induced inflammation to mitochondrial biogenesis. J Cell Sci. 2010;123:2565–2575. doi: 10.1242/jcs.064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vivar OI, Zhao X, Saunier EF, Griffin C, Mayba OS, Tagliaferri M, et al. Estrogen receptor [beta] binds to and regulates three distinct classes of target genes. J Biol Chem. 2010;285:22059–22066. doi: 10.1074/jbc.M110.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao C, Gao H, Liu Y, Papoutsi Z, Jaffrey S, Gustafsson J-Å, et al. Genome-Wide Mapping of Estrogen Receptor-β–Binding Regions Reveals Extensive Cross-Talk with Transcription Factor Activator Protein-1. Cancer Res. 2010;70:5174–5183. doi: 10.1158/0008-5472.CAN-09-4407. [DOI] [PubMed] [Google Scholar]
- 79.Williams C, Edvardsson K, Lewandowski SA, Strom A, Gustafsson JA. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019–1032. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- 80.Grober O, Mutarelli M, Giurato G, Ravo M, Cicatiello L, De Filippo M, et al. Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genomics. 2011;12:36. doi: 10.1186/1471-2164-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 82.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, et al. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 84.Metivier R, Stark A, Flouriot G, Hubner MR, Brand H, Penot G, et al. A dynamic structural model for estrogen receptor-alpha activation by ligands, emphasizing the role of interactions between distant A and E domains. Mol Cell. 2002;10:1019–1032. doi: 10.1016/s1097-2765(02)00746-3. [DOI] [PubMed] [Google Scholar]
- 85.Penot G, Le Peron C, Merot Y, Grimaud-Fanouillere E, Ferriere F, Boujrad N, et al. The Human Estrogen Receptor-{alpha} Isoform hER{alpha}46 Antagonizes the Proliferative Influence of hER{alpha}66 in MCF7 Breast Cancer Cells. Endocrinology. 2005;146:5474–5484. doi: 10.1210/en.2005-0866. [DOI] [PubMed] [Google Scholar]
- 86.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 87.Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 88.Liu D, Dillon JS. Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: evidence for a cell surface receptor. Steroids. 2004;69:279–289. doi: 10.1016/j.steroids.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Liu D, Iruthayanathan M, Homan LL, Wang Y, Yang L, Wang Y, et al. Dehydroepiandrosterone Stimulates Endothelial Proliferation and Angiogenesis through Extracellular Signal-Regulated Kinase 1/2-Mediated Mechanisms. Endocrinology. 2008;149:889–898. doi: 10.1210/en.2007-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. The Journal of Steroid Biochemistry and Molecular Biology. 2008;108:281–286. doi: 10.1016/j.jsbmb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kihel LE. Oxidative metabolism of dehydroepiandrosterone (DHEA) and biologically active oxygenated metabolites of DHEA and epiandrosterone (EpiA) – Recent reports. Steroids. 2012;77:10–26. doi: 10.1016/j.steroids.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 92.Labrie F, Luu-The V, Bélanger A, Lin S-X, Simard J, Pelletier G, et al. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.