Abstract
Background
The relative impact of human rhino/enteroviruses (HRV/EV) compared to influenza viruses on hospitalized children is unknown.
Objectives
This retrospective study compared the epidemiology and clinical characteristics of hospitalized patients with HRV/EV to patients hospitalized with influenza virus.
Study design
Respiratory specimens from hospitalized children submitted between January 1, 2009 and December 31, 2009 to Children's Hospital Colorado Virology Laboratory in Aurora, CO were tested by a commercial multiplex PCR for 16 respiratory viruses and subtypes. Patients with specimens positive for HRV/EV or influenza virus without bacterial or viral co-infection were selected for retrospective chart review.
Results
Of the 2299 patients with specimens tested during the study period, 427 (18.6%) were singly positive for HRV/EV and 202 (8.8%) for influenza virus (p < 0.01). Children with HRV/EV were more likely to present with increased work of breathing (67.9% vs. 52.5%, p < 0.01) with crackles (36.3% vs. 23.3%, p < 0.01) and wheezing (41.7% vs. 22.8%, p < 0.01) noted on exam. Children hospitalized with HRV/EV had a shorter median length of stay (2 days vs. 3 days, p < 0.01), duration of fever (1 days vs. 3 days, p < 0.01), and duration of hypoxemia (2 days vs. 3 days, p < 0.01) than children with influenza virus. Similar percentages of children with HRV/EV and influenza virus were admitted to the PICU and required positive pressure ventilation. There were no deaths in children hospitalized with HRV/EV, whereas 6 children with influenza virus expired.
Conclusions
HRV/EVs are common pathogens in hospitalized children associated with serious lower respiratory tract disease and significant morbidity, similar to influenza viruses.
Abbreviations: HRV/EV, human rhino/enteroviruses; CHC, Children's Hospital Colorado; PCR, polymerase chain reaction; RVP, Respiratory Virus PCR Panel; CHC, Children's Hospital Colorado; PICU, pediatric intensive care unit
Keywords: Rhinovirus, Enterovirus, Influenza virus, Respiratory virus
1. Background
Human rhinoviruses are the most common cause of acute upper respiratory tract infections, such as the common cold, in children.1, 2 However, they have not traditionally been associated with lower respiratory tract disease or significant morbidity. In contrast, influenza viruses are a known cause of serious lower respiratory tract infection and hospitalization in children.
With the development of molecular assays to detect respiratory viruses, rhinoviruses are being increasingly identified in hospitalized children with serious lower respiratory tract disease. There is now a growing body of literature to support rhinoviruses as a cause of asthma exacerbation, bronchiolitis, and viral pneumonia, which are common reasons for hospitalization in children.3, 4, 5, 6 However, the relative impact of human rhino/enteroviruses (HRV/EV) compared to influenza viruses on hospitalized children is unknown.
2. Objectives
This retrospective study sought to describe the epidemiological and clinical features of human rhino/enterovirus (HRV/EV) associated illnesses among hospitalized children in comparison to influenza.
3. Study design
3.1. Study design
In January 2009, the Virology Laboratory at Children's Hospital Colorado (CHC) began using a multiplex PCR (xTag® Respiratory Virus Panel, RVP, Luminex Molecular Diagnostics, Austin, TX) to identify viral pathogens in respiratory specimens from children with respiratory symptoms. With the start of the 2009 influenza pandemic, RVP testing was recommended for all hospitalized patients at CHC with respiratory symptoms or influenza-like symptoms for epidemiologic purposes and to guide antiviral therapy.
CHC is an academic, tertiary-care, 314-bed hospital serving Colorado and the surrounding states. CHC's primary catchment population is the Denver metro area, which has a population of approximately 2.5 million people. The hospital has approximately 13,000 inpatient admissions and 117,000 emergency room/urgent care visits a year.
Patients with RVP positive for HRV/EV or influenza virus between January 1, 2009 and December 31, 2009 who were admitted to the pediatric ward or to the intensive care unit of CHC were included. Respiratory specimens submitted for RVP included nasopharyngeal washes, tracheal aspirates, or bronchoalveolar lavages. In order to provide a clear description of symptoms attributable to sole infection with influenza virus or HRV/EV, patients with bacterial or viral co-infections were excluded. Viral co-infection was defined as a RVP result that was positive for more than one virus. Bacterial co-infection was defined as a positive bacterial culture from a sterile site during hospitalization. Nosocomial infections, defined as RVP-positive specimens collected more than 72 h after admission, were also excluded in order to avoid attributing hospitalization for other reasons to a viral infection coincidentally acquired while in the hospital.
Nucleic acids were extracted from specimens submitted for RVP testing using Virus Minikits v.2.0 on BioRobot EZ1 extractors (Qiagen, Valencia, CA) and tested by the classic version of RVP. This test can detect 16 respiratory viruses and subtypes including influenza A (subtypes H1 and H3) and influenza B viruses, parainfluenza viruses 1–4, adenovirus, respiratory syncytial viruses A and B, human metapneumovirus, human coronaviruses 229E, OC43, HKU1, and NL63, and HRV/EV. Human rhinoviruses and enteroviruses are closely related picornaviruses that cannot be distinguished by this assay, therefore throughout this paper these viruses are referred to together as HRV/EVs.
Electronic medical records of all cases were retrospectively reviewed by physicians in the Department of Infectious Diseases or Critical Care. Data collection elements included patient demographics, clinical findings, management, hospital course, laboratory values, and radiographic findings. Hypoxemia was defined as oxygen saturation of less than 90% requiring supplemental oxygen. Fever was defined as subjective history of fever or documented temperature greater than 38.3 °C. Chest radiograph findings were categorized as ‘abnormal’ if focal findings were documented in the radiologist's interpretation, and ‘normal or airways disease’ if no focal process was documented.
The protocol and standardized data collection form were approved by the Colorado Multiple Institutional Review Board. Waiver of informed consent was approved for retrospective chart review of study participants. Study data were collected and managed using REDCap electronic data capture tools hosted at The University of Colorado.7
3.2. Statistical analysis
A 2-sided 0.05 α level was applied for statistical significance. Categorical data was compared using Fischer's exact test or χ 2 test. Continuous variables were compared using Wilcoxon rank sum test. Analyses for this study were performed with SAS software, version 9.2.
4. Results
4.1. Epidemiology
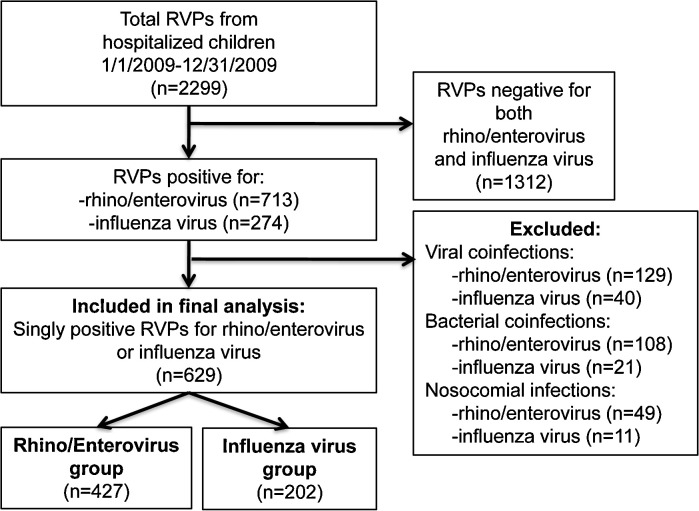
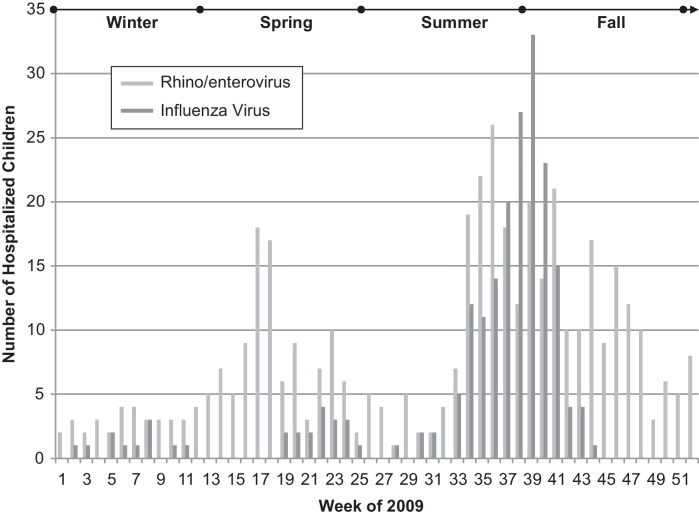
From January 1, 2009 to December 31, 2009, respiratory specimens were submitted from 2299 hospitalized children for RVP to the Virology Laboratory at CHC. Before exclusion criteria were applied, 713 (31%) patients were positive for HRV/EV and 274 (11.9%) were positive for influenza virus (Fig. 1 ). After exclusion of viral and bacterial co-infections and nosocomial infections, 629 patients were included in the final analysis: 427 (18.6%) singly positive for HRV/EV and 202 (8.8%) singly positive for influenza virus (p < 0.01). In the final influenza group, there were 191 (95%) patients with 2009 influenza A (H1N1) virus, 10 (5%) with seasonal influenza A virus, and 1 (0.5%) patient with influenza B virus. The epidemiology curve for patients positive for HRV/EV or influenza virus by week is displayed in Fig. 2 .
Fig. 1.
Study inclusion algorithm.
Fig. 2.
Epidemiology curve of hospitalized children with HRV/EV or influenza virus.
4.2. Study group demographics
Demographics and past medical history of children with HRV/EV and influenza virus are displayed in Table 1 . The mean age of patients with HRV/EV was 2.4 years compared to 6.6 years with influenza virus (p < 0.01). Sixty-one percent of patients in the study group had an underlying medical condition, which was less common in patients with HRV/EV than with influenza virus (56% vs. 70.3%, p < 0.01). However, premature birth was more common in patients with HRV/EV (14.3% vs. 4.5%, p < 0.01). There was no difference in the underlying prevalence of asthma, cardiac disease, neurologic disease, or the proportion of patients who were immunocompromised.
Table 1.
Demographics and underlying medical conditions of hospitalized children with HRV/EV compared to influenza virus.
| Rhino/enterovirus n = 427 | Influenza virus n = 202 | p value | |
|---|---|---|---|
| Demographics | |||
| Age (mean) | 2.4 | 6.6 | <0.01 |
| Male | 257 (60.2%) | 117 (57.9%) | 0.6 |
| Underlying medical condition | 239 (56.0%) | 142 (70.3%) | <0.01 |
| Asthma | 88 (20.6%) | 50 (24.8%) | 0.26 |
| Cardiac | 31 (7.3%) | 14 (6.9%) | 1 |
| Immunocompromised | 27 (6.3%) | 17 (8.4%) | 0.4 |
| Neurologic | 72 (16.9%) | 40 (19.8%) | 0.37 |
| Prematurity (<36 weeks) | 61 (14.3%) | 9 (4.5%) | <0.01 |
4.3. Clinical features
The presenting symptoms and major findings on physical exam of children with HRV/EV or influenza virus are presented in Table 2 . Children with HRV/EV were more likely to present with nasal congestion (79.2% vs. 56.4%, p < 0.01) and increased work of breathing (67.9% vs. 52.5%, p < 0.01) with crackles (36.3% vs. 23.3%, p < 0.01) and wheezing (41.7% vs. 22.8%, p < 0.01) noted on physical exam. Children with HRV/EV were less likely than those with influenza to present with fever (67% vs. 83.2%, p < 0.01), sore throat (8.7% vs. 14.4%, p = 0.03) and cough (74% vs. 82.7%, p < 0.01). The most common reason for admission in both patients with HRV/EV and influenza was respiratory difficulty (62.8% vs. 58.4%, p = 0.5), which included hypoxemia, respiratory distress, and asthma.
Table 2.
Clinical characteristics of hospitalized children with HRV/EV compared to influenza virus.
| Rhino/enterovirus n = 427 | Influenza virus n = 202 | p value | |
|---|---|---|---|
| Symptoms | |||
| Fever | 286 (67%) | 168 (83.2%) | <0.01 |
| Conjunctivitis | 25 (5.9%) | 9 (4.5%) | 0.57 |
| Nasal congestion | 338 (79.2%) | 114 (56.4%) | <0.01 |
| Sore throat | 37 (8.7%) | 29 (14.4%) | 0.03 |
| Cough | 316 (74%) | 167 (82.7%) | <0.01 |
| Increased work of breathing | 290 (67.9%) | 106 (52.5%) | <0.01 |
| Vomiting | 135 (31.6%) | 72 (35.6%) | 0.32 |
| Diarrhea | 40 (9.4%) | 26 (12.9%) | 0.21 |
| Rash | 27 (6.3%) | 7 (3.5%) | 0.19 |
| Mental status changes | 20 (4.7%) | 13 (6.4%) | 0.44 |
| Physical exam findings | |||
| Crackles | 155 (36.3%) | 47 (23.3%) | <0.01 |
| Wheeze | 178 (41.7%) | 46 (22.8%) | <0.01 |
| Decreased aeration | 135 (31.6%) | 64 (31.7%) | 0.93 |
| Hypoxia | 246 (57.6%) | 125 (61.9%) | 0.34 |
| Chest X-ray | n = 287 (67.2%) | n = 155 (76.7%) | 0.02 |
| Normal or airways disease | 137 (47.7%) | 47 (30.3%) | <0.01 |
| Abnormal X-ray | 150 (52.3%) | 108 (69.7%) | <0.01 |
Of the 442 patients with a chest radiograph, patients with HRV/EV were less likely than those with influenza to have abnormal focal findings (52.3% vs. 69.7%, p < 0.01). There were no significant differences in frequency of abnormal laboratory values on initial white blood cell count, maximum C-reactive protein or erythrocyte sedimentation rate.
4.4. Hospital course
Differences in duration of symptoms, management, and outcomes are displayed in Table 3 . Patients with HRV/EV had a shorter median duration of fever (1 day vs. 3 days, p < 0.01) and hypoxemia (2 days vs. 3 days, p < 0.01), as well as a shorter median length of hospitalization (2 days vs. 3 days, p < 0.01). Similar proportions of patients with HRV/EV and influenza virus received albuterol (49.2% vs. 42.1%, p = 0.1), but patients with HRV/EV were more likely to receive steroids (44% vs. 33.7%, p = 0.02). Sixty percent of all patients received antibiotics, 50.1% of those with HRV/EV and 64.4% of those with influenza virus (p < 0.01). Fifty-five patients with HRV/EV (12.9%) received oseltamivir for suspected influenza and 160 (83.7%) patients with confirmed influenza virus received oseltamivir (p < 0.01).
Table 3.
Hospital course, management, and outcomes of hospitalized children with HRV/EV compared to influenza virus.
| Rhino/enterovirus n = 427 | Influenza virus n = 202 | p value | |
|---|---|---|---|
| Duration of symptoms (days) | |||
| Median length of fever | 1 | 3 | <0.01 |
| Median length of hypoxia | 2 | 3 | <0.01 |
| Duration of hospitalization (days) | |||
| Median length of stay | 2 | 3 | <0.01 |
| Interventions | |||
| Albuterol | 210 (49.2%) | 85 (42.1%) | 0.1 |
| Steroids | 188 (44%) | 68 (33.7%) | 0.02 |
| Antibiotics | 214 (50.1%) | 130 (64.4%) | <0.01 |
| Oseltamivir | 55 (12.9%) | 169 (83.7%) | <0.01 |
| Pediatric intensive care unit admission | n = 88 (20.6%) | n = 45 (22.3%) | 0.68 |
| Median length of PICU stay (days) | 3 | 3 | 0.09 |
| Noninvasive positive pressure ventilation | 23 (26.1%) | 14 (31.1%) | 0.68 |
| Intubation | 26 (29.5%) | 20 (44.4%) | 0.12 |
| Mortality | |||
| Deceased | 0.0% | 6 (3%) | <0.01 |
Similar percentages of children with HRV/EV and influenza virus required admission to the PICU (20.6% vs. 22.3%, p = 0.68) with similar median length of stay (3 days vs. 3 days, p = 0.09). Similar percentages of children required positive pressure ventilation and intubation in both groups. There were no deaths in children with HRV/EV and six deaths in children with influenza virus (3.0%, p < 0.01).
5. Discussion
This study demonstrates that HRV/EVs are common pathogens in hospitalized children associated with serious lower respiratory tract disease and significant morbidity. Amongst children ill enough to be hospitalized at our tertiary care institution, HRV/EVs were the most frequently identified viruses. HRV/EVs were over twice as common as influenza viruses, which are known to be major causes of hospitalization in children.8, 9
While the morbidity of rhinovirus has been associated with the presence of underlying medical conditions,10 nearly half of patients hospitalized with HRV/EV in our tertiary care hospital were previously healthy. When compared to children with influenza virus, children hospitalized with HRV/EV were less likely to have a predisposing underlying medical condition. This suggests that HRV/EVs are associated with severe disease requiring hospitalization even in previously healthy children.
Patients with HRV/EV were equally likely as patients with influenza to present with severe respiratory disease requiring intensive care and positive pressure ventilation. This finding is consistent with a study in patients of all ages hospitalized during the peak of the 2009 influenza season in Rhode Island which found no difference in the likelihood of requiring ICU care between patients with influenza virus and those with rhinovirus.11 Similarly, a multicenter US study of 287 children hospitalized with rhinovirus compared to 588 children hospitalized with other non-rhinovirus viral infections found no significant difference in the rates of ICU admission or mechanical ventilation.12 Despite the severity of disease presentation in our study, HRV/EVs were associated with decreased duration of illness and no mortality, compared to influenza viruses.
Though traditionally associated with mild upper respiratory tract disease, this study demonstrates that HRV/EVs are associated with serious lower respiratory tract disease in hospitalized children. Patients with HRV/EV were more likely than patients with influenza virus to have lower respiratory tract findings, such as increased work of breathing, crackles, and wheezing, and equally likely to be hypoxemic. While rhinoviruses have been identified as the most common trigger for asthma exacerbation in children,13 there was no difference in the rates of underlying asthma between the HRV/EV and influenza virus groups. In spite of this, patients with HRV/EV were still more likely than those with influenza to present with asthma-like symptoms, such as wheezing, and be treated accordingly with steroids.
Over half of the patients in this study received antibiotics even though only a viral pathogen was identified. Patients with influenza virus were more likely to receive antibiotics than those with HRV/EV. This may have been due to the frequency of abnormal X-rays and a reluctance of clinicians to attribute focal X-ray findings to a viral pathogen. It is important to note that bacterial cultures are not often sent from pediatric patients with suspected bacterial pneumonia, so patients with bacterial pneumonia diagnosed clinically or radiographically would not be excluded from this study.
The increased use of RVP testing at our institution during the 2009 influenza pandemic allowed for detection of one of the largest single-center cohorts of children hospitalized with HRV/EV. However, the unique characteristics of the 2009 influenza pandemic are not representative of a normal influenza season and affect our comparison of HRV/EVs with influenza viruses. For instance, children hospitalized with HRV/EV in this study were on average 4.2 years younger than those with influenza virus. This is likely due to the uniquely high reported age of children hospitalized with the 2009 influenza A (H1N1) virus, which at our institution had a reported median age of 6 years compared to 2008 when the median age of those hospitalized with influenza was 1.8 years.14 During a typical influenza season, patients with HRV/EV would be expected to be the same age or slightly older than those with influenza virus.
The epidemiology curve of HRV/EVs was also affected by the presence of the 2009 influenza A (H1N1) virus. The bimodal distribution of the HRV/EV epidemiology curve with seasonal peaks in the spring and fall is consistent with previous descriptions.15 However, the larger peak of HRV/EV activity in the late summer/early fall coinciding with the influenza pandemic is exaggerated by an increase in RVP testing for influenza virus during this period. It is likely that a proportion of patients being tested for influenza-like illnesses were found to actually have HRV/EV by RVP.
Several studies have demonstrated that non-influenza viruses, such as rhinoviruses, can clinically mimic influenza.11, 16 In our study, several statistically significant differences in clinical features were found between groups due to the large sample sizes and amount of clinical data analyzed. Nonetheless, small differences in the frequency of clinical signs and symptoms do not help to differentiate HRV/EV from influenza virus in the individual patient. The similarity in clinical presentations and seasonal overlap of these viruses highlights the usefulness of molecular diagnostic tests to differentiate influenza from other respiratory viruses, such as HRV/EVs, and determine appropriate patients to receive influenza-targeted antivirals.
Our study has several limitations. We acknowledge that the presence of viral RNA in a respiratory specimen does not always reflect the cause of current symptoms. It is possible that some patients had a positive RVP result due to prolonged shedding from a prior unrelated infection or asymptomatic carriage of virus with signs and symptoms due to another disease process or infectious agent. By excluding patients with bacterial and viral co-infections from the study, we limited this effect as much as possible. With regards to viral shedding, early studies that did not type individual rhinoviruses suggested that rhinovirus RNA can persist in nasal secretions up to 5–6 weeks, in comparison to a median of 6 days for 2009 influenza A (H1N1) virus in children.17, 18 However more recent studies that included typing have found that the RNA of individual rhinoviruses typically persists for less than 2 weeks, with sequential infections due to the same or different rhinovirus types giving the appearance of prolonged shedding.19, 20 Rhinovirus RNA has been identified in up to 15% of asymptomatic children,21 whereas influenza virus infection is rarely asymptomatic. Given the longer shedding and asymptomatic carriage of rhinoviruses, the effect of incidental detection of viral RNA may have disproportionately increased the prevalence of HRV/EVs compared to influenza virus.
This study only included children hospitalized at our tertiary care facility, selecting the more seriously ill end of the spectrum of HRV/EV and influenza virus infection. In addition, the selective RVP testing by clinicians of patients with respiratory symptoms or influenza-like symptoms is likely to describe more symptomatic patients. Therefore, our data do not necessarily reflect the overall burden of HRV/EV and influenza virus respiratory disease in the general pediatric population.
Picornaviruses were not subtyped in this study. Rhinoviruses and enteroviruses are closely related RNA viruses. However, specific species and types of rhinovirus and enterovirus have been associated with specific clinical features and severe disease.22, 23, 24, 25 It would be of interest to distinguish rhinoviruses from enteroviruses, as well as determine if there were any predominant circulating types which were associated with particular disease presentations or clinical severity.
In conclusion, HRV/EVs are significant pathogens associated with serious lower respiratory tract disease and significant morbidity in hospitalized children. This study adds to the growing body of literature describing the burden of disease due to HRV/EVs in children and finds it comparable to that of influenza. As the impact of HRV/EVs on children becomes better characterized, the magnitude of research efforts into preventative and therapeutic interventions for HRV/EVs should also expand.
Funding
Supported by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154.
Competing of interest
None declared.
Ethical approval
Not required.
References
- 1.Vesa S., Kleemola M., Blomqvist S., Takala A., Kilpi T., Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20(6):574. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Makela M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimaki M. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36(2):539. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louie J.K., Roy-Burman A., Guardia-Labar L., Boston E.J., Kiang D., Padilla T. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J. 2009;28(4):337–339. doi: 10.1097/INF.0b013e31818ffc1b. [DOI] [PubMed] [Google Scholar]
- 4.El-Sahly H.M., Atmar R.L., Glezen W.P., Greenberg S.B. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31(1):96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan W.C. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11(1):21. doi: 10.1097/01.mcp.0000146781.11092.0d. [DOI] [PubMed] [Google Scholar]
- 6.Mak R.K., Tse L.Y., Lam W.Y., Wong G.W., Chan P.K., Leung T.F. Clinical spectrum of human rhinovirus infections in hospitalized Hong Kong children. Pediatr Infect Dis J. 2011;30(9):749–753. doi: 10.1097/INF.0b013e31821b8c71. [DOI] [PubMed] [Google Scholar]
- 7.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izurieta H.S., Thompson W.W., Kramarz P., Shay D.K., Davis R.L., DeStefano F. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342(4):232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 9.Grijalva C.G., Craig A.S., Dupont W.D., Bridges C.B., Schrag S.J., Iwane M.K. Estimating influenza hospitalizations among children. Emerg Infect Dis. 2006;12(1):103–109. doi: 10.3201/eid1201.050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheuk D.K., Tang I.W., Chan K.H., Woo P.C., Peiris M.J., Chiu S.S. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr Infect Dis J. 2007;26(11):995–1000. doi: 10.1097/INF.0b013e3181586b63. [DOI] [PubMed] [Google Scholar]
- 11.Chan P.A., Mermel L.A., Andrea S.B., McCulloh R., Mills J.P., Echenique I. Distinguishing characteristics between pandemic 2009–2010 influenza A (H1N1) and other viruses in patients hospitalized with respiratory illness. PLoS ONE. 2011;6(9):e24734. doi: 10.1371/journal.pone.0024734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwane M.K., Prill M.M., Lu X., Miller E.K., Edwards K.M., Hall C.B. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204(11):1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos N.G., Christodoulou I., Rohde G., Agache I., Almqvist C., Bruno A. Viruses and bacteria in acute asthma exacerbations—a GA2 LEN-DARE systematic review. Allergy. 2011;66(4):458–468. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagdure D., Curtis D.J., Dobyns E., Glodé M.P., Dominguez S.R. Hospitalized children with 2009 pandemic influenza A (H1N1): comparison to seasonal influenza and risk factors for admission to the ICU. PLoS ONE. 2010;5(12):e15173. doi: 10.1371/journal.pone.0015173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monto A.S. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin Ther. 2002;24(12):1987–1997. doi: 10.1016/S0149-2918(02)80093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnepf N., Resche-Rigon M., Chaillon A., Scemla A., Gras G., Semoun O. High burden of non-influenza viruses in influenza-like illness in the early weeks of H1N1v epidemic in France. PLoS ONE. 2011;6(8):e23514. doi: 10.1371/journal.pone.0023514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jartti T., Lehtinen P., Vuorinen T., Koskenvuo M., Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72(4):695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 18.Bhattarai A., Villanueva J., Palekar R.S., Fagan R., Sessions W., Winter J. Viral shedding duration of pandemic influenza A H1N1 virus during an elementary school outbreak—Pennsylvania, May–June 2009. Clin Infect Dis. 2011;52(January (Suppl. 1)):S102–S108. doi: 10.1093/cid/ciq026. [DOI] [PubMed] [Google Scholar]
- 19.van der Zalm M.M., Wilbrink B., van Ewijk B.E., Overduin P., Wolfs T.F., van der Ent C.K. Highly frequent infections with human rhinovirus in healthy young children: a longitudinal cohort study. J Clin Virol. 2011;52(4):317–320. doi: 10.1016/j.jcv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Jartti T., Lee W.M., Pappas T., Evans M., Lemanske R.F., Gern J.E. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32(2):314–320. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jartti T., Jartti L., Peltola V., Waris M., Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J. 2008;27(12):1103–1107. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 22.Imamura T., Fuji N., Suzuki A., Tamaki R., Saito M., Aniceto R. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis. 2011;17(8):1430–1435. doi: 10.3201/eid1708.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo C., Casas I., García-García M.L., Pozo F., Reyes N., Cruz N. Role of rhinovirus C respiratory infections in sick and healthy children in Spain. Pediatr Infect Dis J. 2010;29(8):717–720. doi: 10.1097/INF.0b013e3181d7a708. [DOI] [PubMed] [Google Scholar]
- 24.Lee W.M., Kiesner C., Pappas T., Lee I., Grindle K., Jartti T. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khetsuriani N. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis. 2008;14(11):1793–1796. doi: 10.3201/eid1411.080386. [DOI] [PMC free article] [PubMed] [Google Scholar]