Abstract
Leprosy is caused by the well known Mycobacterium leprae and the newly discovered Mycobacterium lepromatosis. We describe here 2 cases of leprosy with unusual clinical presentation caused by M. lepromatosis. The patients, a 32-year-old man and a 50-year-old woman, both of Mexican origin, manifested high fever, lymphadenopathy, and florid skin lesions in the form of erythema nodosum and Lucio’s phenomenon as the first clinical presentation. Heavy infiltration of acid-fast bacilli was identified in the tissues that led to the diagnosis of lepromatous leprosy or diffuse leprosy. The patients were treated with multi-drug regimen and responded appropriately. From the lymph node tissue, we showed the bacillus to be M. lepromatosis, not M. leprae as presumed previously, by using differential polymerase chain reactions and analysis of gene sequences. These cases add to the growing studies on this organism, expand its endemic regions in Mexico, and provide more clinical insight.
Keywords: Leprosy, diffuse lepromatosis leprosy, Lucio’s phenomenon, erythema nodosum, Mycobacterium lepromatosis
Introduction
Leprosy is one of the oldest human infections that can be traced back to at least 100,000 years along historic human migration tracks across the world.1,2 The etiologic agent of leprosy has long been ascribed solely to M. leprae until 2008, when a new species, named M. lepromatosis, was found to be the cause of diffuse lepromatous leprosy (DLL) in 2 patients of Mexican origin who died of the disease.3 Further analysis of 22,814 nucleotides revealed a 9.1% difference between the two organisms to substantiate a species-level divergence that occurred approximately 10 million years ago.4 The 9.1% sequence difference contracts sharply with the 0.005% difference among worldwide M. leprae strains uncovered by genome sequencing and multi-locus typing.2 The divergence age of ~10 million years between the leprosy bacilli is much longer (~88 times) than the well-known divergence between the tuberculosis bacilli M. tuberculosis and M. bovis (113,000 years ago). Thus, M. lepromatosis is also an ancient organism despite recent recognition.
Two recent papers independently corroborated this new cause of leprosy. Vera-Cabrera et al reported a case of DLL in an 86-year-old woman caused by M. lepromatosis from Mexico.5 From skin biopsy tissue, these authors confirmed the new species by polymerase chain reactions (PCR) and sequencing analyses of 4 genes and also excluded the presence of M. leprae. Jessamine and colleagues reported a case of lepromatous leprosy caused by M. lepromatosis in a 72-year-old native Canadian man from Ontario, Canada.6 This patient had suffered from progressive polyneuropathy of all extremities for 2 years before the onset of a maculopapular skin rash that led to the diagnosis through skin biopsy and detection of acid-fast bacilli (AFB). The authors also excluded M. leprae and confirmed M. lepromatosis by analysis of 2 genes.
In a systematic analysis of tissue specimens from 120 patients with various clinical forms of leprosy from Mexico, we confirmed and differentiated the etiologic mycobacteria in 87 cases.7 Of these, M. lepromatosis alone caused 55 cases, M. leprae alone caused 18 cases, and both species together caused 14 cases. M. lepromatosis caused not only all DLL cases specifically but also more cases of lepromatous leprosy and other clinical forms of leprosy. This study suggests that M. lepromatosis is the dominant cause of leprosy in Mexico and it co-exists with M. leprae in endemic areas as the once elusive second cause of leprosy. Both organisms may cause dual infections in a patient.
In addition to the M. lepromatosis cases from Mexico and Canada, we recently also showed the presence of M. lepromatosis across the Pacific in Singapore.8 Therefore, M. lepromatosis is not so obscure as initially thought. Because DLL and other leprosy forms caused by M. lepromatosis may be misdiagnosed leading to delay in effective treatment, early clinical recognition or suspicion of this infection is important. In this report, we demonstrate that M. lepromatosis was the etiologic agent in two previously featured cases with severe leprosy reactions as the first clinical presentation.
Case Mn-1
This case occurred to a 32-year-old man in 2005 and was reported to illustrate the initial diagnostic challenge.9 The patient was originally from Matamoros, Mexico, but he had lived in Minnesota for 10 years without return visits. He first presented to his primary physician complaining of intermittent high fevers, night sweats, severe joint pain, and skin rash, for which he was prescribed a course of levofloxacin. Persistence of the illness prompted a skin biopsy a month later, which showed subcutaneous panniculitis to suggest erythema nodosum. The patient was referred to the University of Minnesota Medical Center for further workup.
Physical examination revealed a fever of 39.7°C, alopecia areata on the scalp, and supercilliary madarosis. Numerous crops of painful, tender, and erythematous macules and papules spread over the body, except anterior shin, a common location for erythema nodosum. By history the skin lesions would come and go in a few days with residual pigmentation and lichenification. His hands showed motion restriction with tender and swollen joints. The knees and right elbow showed synovial thickening and swelling. No neurologic deficit or nerve thickening was noted; however, the patient complained of paraesthesias and dysesthesias in both forearms. Tender lymph nodes in the left axilla and the right groin were palpated. The spleen was felt firm, enlarged, and tender. The rest of physical examination was unremarkable. Significant laboratory findings included a white blood cell count of 15,100/µl with 89% neutrophils, microcytic and hypochromic anemia, elevated erythrocyte sedimentation rate (85 mm/hr), and elevated C-reactive protein (17.6 mg/dl, normal 0–0.8 mg/dl). The blood angiotensin-converting enzyme level was normal.
The patient was tentatively diagnosed with sarcoidosis and treated with steroids. However, cessation of the steroids brought back the symptoms with severe erythema nodosum and palpable axillary lymph nodes. Biopsy of a node revealed abundant vacuolated histiocytes within parafollicular areas and sinuses on histologic examination; a Fite stain demonstrated innumerable AFB. A review of the initial skin biopsy also showed AFB-laden histiocytes. Based on these findings the diagnosis of lepromatous leprosy with a type 2 reaction (erythema nodosum leprosum) was rendered. The patient received anti-leprosy therapy consisting of daily 100 mg of dapsone, 50 mg of clofazimine, and 200 mg of thalidomide, along with once a month 600 mg of rifampin and 300 mg of clofazimine. He responded adequately to the treatment.
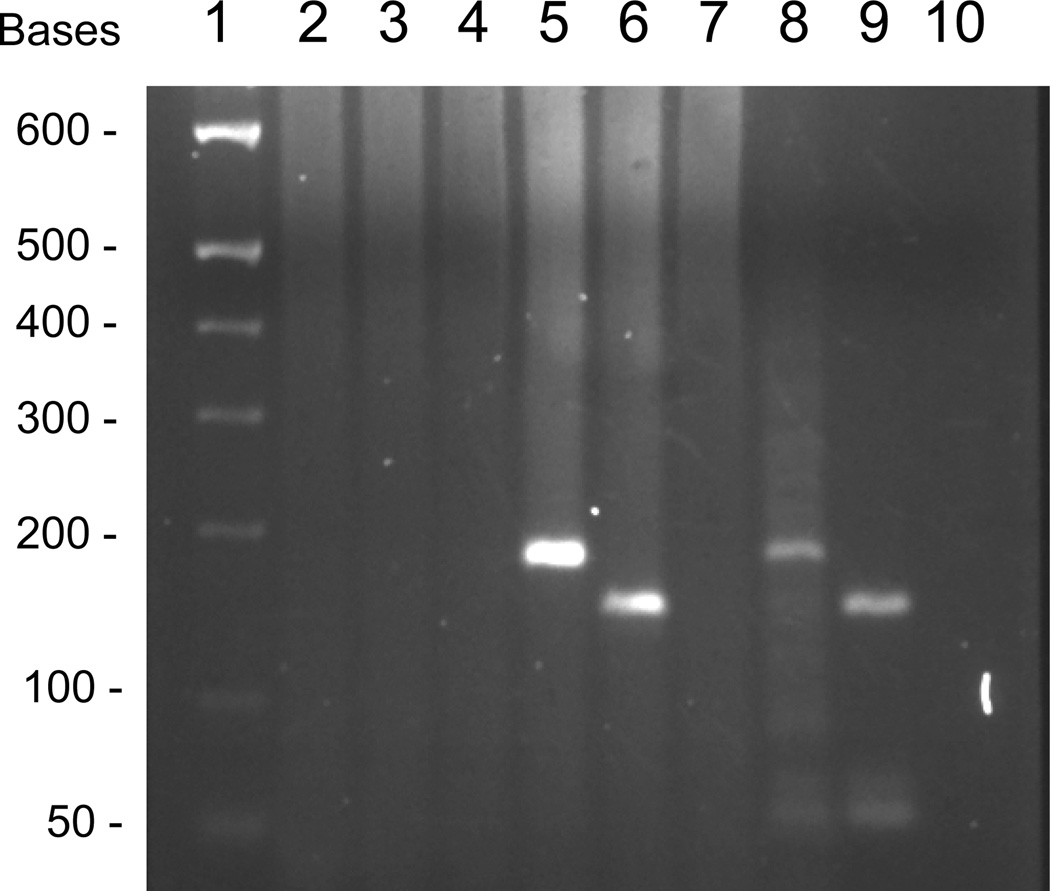
The patient’s unusual presentation and Mexican origin led us to wonder the species identity of this AFB. We extracted DNA from the formalin-fixed and paraffin-embedded (FFPE) lymph node tissue and performed the differential PCRs as described previously.7, 8 The designs of primers and PCRs are shown in Figure 1, and they aim at the 16S rRNA gene. We first targeted the 171-bp to 135-bp amplicons; these reactions revealed the 171-bp common mycobacterial amplicon and the 143-bp M. lepromatosis-specific amplicon, but not the 135-bp M. leprae-specific target (Figure 2, lanes 5–7). Thus this organism was M. lepromatosis, not M. leprae as presumed previously.
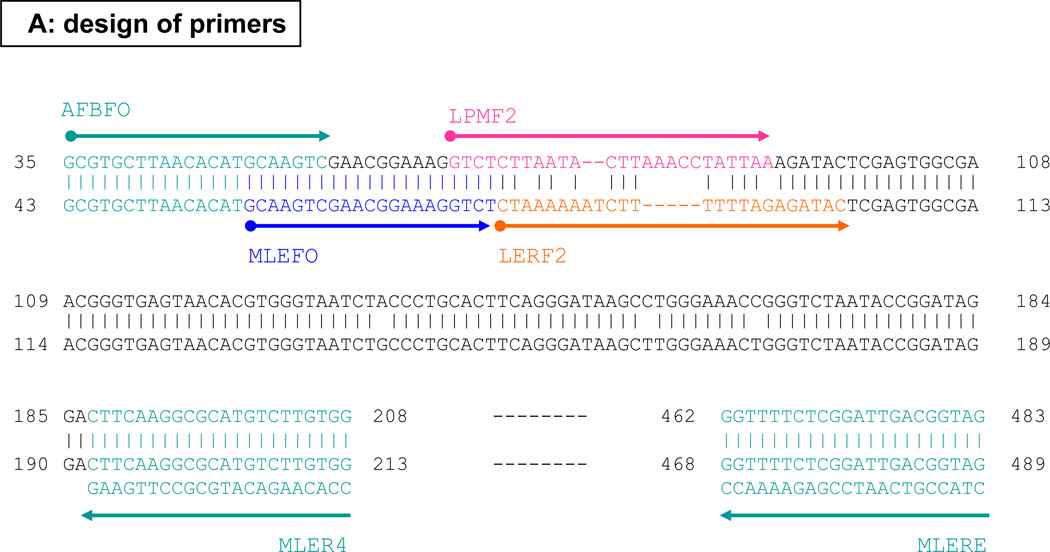
Figure 1.
Designs of leprosy primers (A) and differential polymerase chain reactions (B) for the 16S rRNA gene.
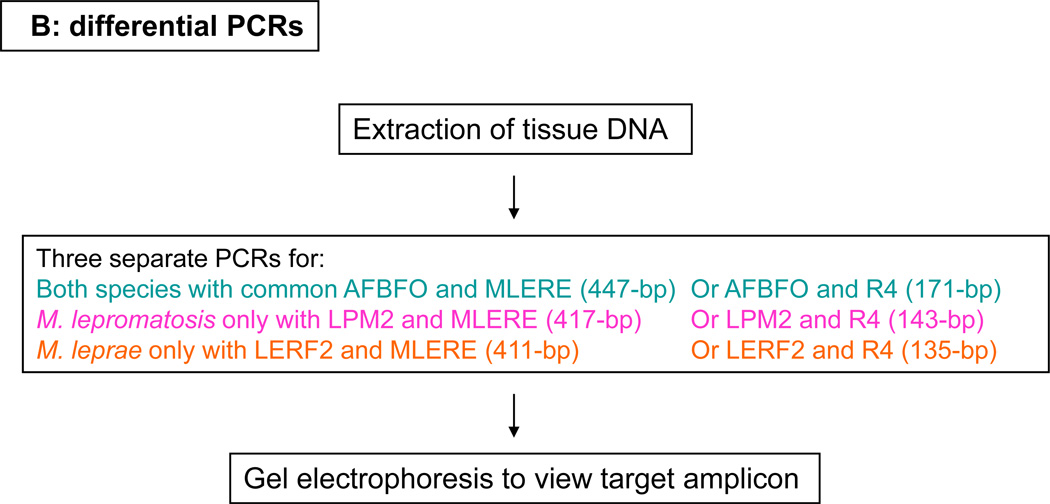
Figure 2.
Detection of Mycobacterium lepromatosis, but not Mycobacterium leprae, by differential polymerase chain reactions for the 16S rRNA gene. Lane 1: DNA size marker; lanes 2, 3, and 4: case Mn-1 with no amplification of the 447-bp, 417-bp, or 411-bp that target both and each species respectively; lanes 5 and 8: common amplicon of 171-bp for cases Mn-1 and S63 respectively; lanes 6 and 9: amplicon of 143-bp of M. lepromatosis for cases Mn-1 and S63 respectively; lanes 7 and 10: lack of the 135-bp amplicon of M. leprae in each case.
We also attempted the larger amplicons of 447-bp, 417-bp, and 411-bp that aimed similarly at both and each specific species respectively. As shown in Figure 2, lanes 2–4, no amplification was detected. This result can be explained by DNA fragmentation below 400-bp in this FFPE tissue with 6 years of archive. It is well known that FFPE processing causes DNA fragmentation to 200–500 bases and archive worsens such DNA degradation over time.
By using an additional common forward primer (MLEFO) to semi-nest the 447-bp common target for a second round PCR with 35 thermocycles (for a total of 70 thermocycles), as used before,8 we amplified successfully the intended 432-bp common target. Sequencing analysis of this amplicon resolved 413 bases that showed 100% match with M. lepromatosis, 95.4% (397 of 416-bp with gap sites) with M. leprae, and even less with other mycobacteria. Because these sequences spanned the M. lepromatosis-specific primer LPMF2 (Figure 1A), they also corroborated the PCR specificity.
Case S63
This featured case occurred in 1963.10 The patient was a 50-year-old Mexican woman who had lived in Ozona, Texas for 7 years. She presented to M. D. Anderson Cancer Center in late January 1963 for treatment of a soft tissue sarcoma in the right lower leg. Aside from the tumor, she had no other complaints. The patient was admitted in February 1963 for anti-cancer chemotherapy, and later in the month, 2 lymph nodes, 1 from the right groin and another “femoral node”, were removed for tumor staging, but histopathology found no tumor.
In the following months, the patient also underwent radiation treatment. But a recurrence of the tumor required amputation of the leg in August 1963. Two weeks after the uneventful operation, the patient suddenly developed fever to 39.6°C, hypotension (80/70 mmHg), and leukocytosis up to 17,100/µl with 88% neurtophils. She received promptly antimicrobial therapy (kanamycin), vasopressors, and blood transfusions. Yet no evidence of blood loss or gross infection of the stump wound was found, and a blood culture was negative. She responded to the treatment with clinical improvement. During the next 2 days, however, skin lesions appeared on her face, arms, buttocks, and left leg. The lesions were described as “flat infiltrative, confluent, deeply livid, sharply outlined, and in variable sizes and shapes.” It was noted at this time that her eyebrows were absent, the left earlobe was indurated, the forehead subcutaneous tissue was swollen, and the sclerae and conjunctivae were injected. These findings led to an examination of a nasal smear, which identified AFB.
During the following few days, the skin lesions became darker and underwent central ulceration. Biopsy specimens of the forearm skin lesion and nasal mucosa confirmed the diagnosis of leprosy. Specifically, the skin biopsy showed foci of necrosis in the dermis and epidermis, vasculitis, and lymphohistiocytic infiltration of the appendages. A Fite stain further revealed AFB in histiocytes in the dermis and subcutaneous tissue, around nerves and arteries, and in the endothelial cells of blood vessels. A bone marrow examination also showed AFB within monocytes. The skin ulcers eventually scarred.
The diagnosis of leprosy led to a review of the histology slides from all previous surgical tissues: chronic lesions of leprosy were detected in the skin, vessels, and nerves surrounding the sarcoma, along with demonstration of AFB. Large nerves and many arteries were surrounded by and infiltrated with histiocytes. In many vessels, endothelial proliferation attenuated the lumens. The constellation of skin lesions and histopathologic findings rendered the diagnosis of diffuse leprosy with Lucio’s phenomenon, or DLL, and the patient was referred to the U. S. Public Health Service Hospital in Carville, Louisiana, for treatment.
We noted this case report through historic literature search and retrieved the medical records and all histology slides and FFPE tissue blocks that have been archived for 50 years. We were impressed by the histopathology of the lymph nodes that was not mentioned in the initial paper. These nodes showed florid foamy histiocytes filled with AFB (Figure 3). From this tissue, we extracted DNA and applied our PCR assay by targeting the 171-bp to 135-bp amplicons. We detected the common mycobacterial target as well as the M. lepromatosis-specific amplicon, but not the M. leprae-specific amplicon (Figure 2, lanes 8–10). The common amplicon was also sequenced, which resolved 159 bases that matched 100% with M. lepromatosis. Therefore, this AFB was clearly M. lepromatosis. Similar to the Mn-1 case examined above, the PCRs aiming at the >400-bp amplicons failed to amplify, even after more semi-nested thermocycles. From a skin tissue with leprosy lesion and AFB, the small amplicon PCRs failed too. These results are also consistent with severe DNA degradation in these FFPE tissues after long archive.
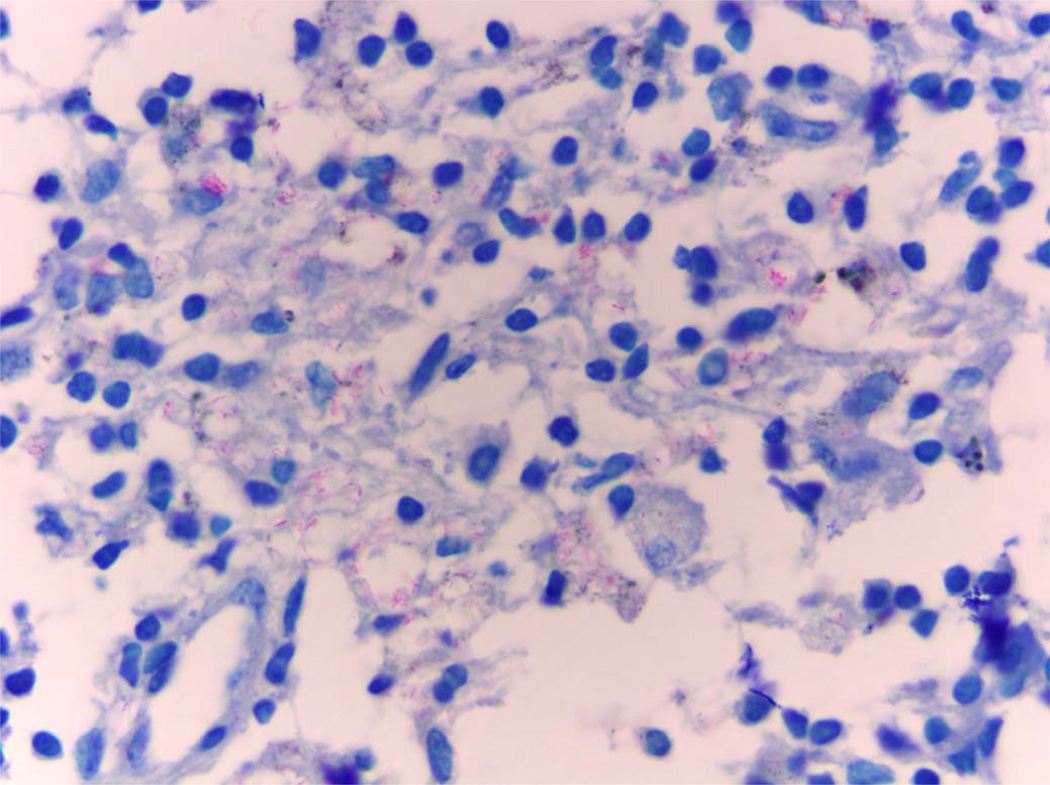
Figure 3.
Demonstration of the acid-fast bacillus Mycobacterium lepromatosis in the lymph node tissue in case S63 (Fite, × 1000).
The patient was cured at Carville; she died in 1998 at the age of 85. On review of the February 1963 admission records, we spotted “The patient appears tense, nose feels dry, complains of poor circulation in hands and feet.” In retrospect, these subtle complaints are typical of the early signs of DLL. The words “appears tense” can be taken as facial swelling and possibly loss or thinning of eyebrows. These manifestations coincided with the enlarged lymph nodes that were removed during that admission or six months before the overt manifestation and diagnosis of leprosy.
Discussion
Several aspects of the present cases may be instructive of the epidemiologic and clinical features of leprosy caused by M. lepromatosis. Both patients were of Mexican origin and presented with severe leprosy reactions with involvement of the lymph nodes and other internal organs. Both had a long incubation period of over 10 years and 7 years respectively and showed an adequate response to therapy.
Erythema nodosum is one of several similar skin manifestations of panniculitis of the subcutaneous soft tissue. It can be seen in infections due to viruses, bacteria, and fungi, and in non-infectious entities, such as sarcoidosis and inflammatory bowel disease. It is considered as an antigen-antibody immune complex disease. Erythema nodosum leprosum, or ENL, denotes such reaction in leprosy due to its common occurrence. ENL most commonly occurs after initiation of specific anti-leprosy treatment and is usually mild with low-grade fever.11
The ENL of case Mn-1 appears unusual and severe in that the patient manifested this reaction as the first clinical presentation with high fever and prolonged duration. To our knowledge, this is the first report among several cases of documented M. lepromatosis infection.3,5,6,8 Earlier clinical studies of DLL did not mention this reaction either.12,13 Our Mexico leprosy study could not address this aspect due to the lack of clinical details.7 The severity of this ENL is likely related to the heavy burden of the mycobacterium as revealed in the tissues of skin and lymph node. It remains to be seen whether M. lepromatosis is specific for this type of ENL.
DLL is a unique severe form of leprosy initially recognized by Lucio and Alvarado in Mexico in 1852,14 and further studied by Latapi and Chevez-Zamora in 1948.12 Thus, DLL is also known as diffuse leprosy of Lucio and Latapi,15,16 leprosy with Lucio’s phenomenon,13,17 or merely Lucio’s leprosy. It has been endemic in Mexico and Costa Rica12,18 and rarely reported elsewhere. DLL is characterized clinically by diffuse, non-nodular cutaneous infiltration, manifesting recurrent crops of large sharply demarcated skin lesions called Lucio’s phenomenon, which may become ulcerated or even generalized as the disease progresses.11,12 On histopathology, DLL shows usual AFB infiltration to the skin and nerves, panniculitis, vasculitis, and in the late stage, unique endothelial proliferation and occlusion of small to intermediate arteries.3,12,13,16 DLL is caused specifically by M. lepromatosis.7 As illustrated in the present cases, recent species-confirmed cases,3 and early clinical studies,12,13,16,19,20 this organism may disseminate to internal organs, such as lymph nodes, bone marrow, spleen, and liver.
DLL differs from lepromatous leprosy only by the lack of lepromata, which manifest as visual skin nodules. In this regard, the lack of conspicuous skin nodules in the Mn-1 case and the Canadian case6 is a compatible feature; hence, the diagnosis of DLL might also fit. But skin nodules usually appear in long-standing leprosy whereas these 2 cases could well be in early clinical stage. Thus, the diagnosis of lepromatous leprosy seems more appropriate at the time. This issue on the diagnosis of DLL versus lepromatous leprosy and other clinical forms first emerged in the Mexico study in which the 55 cases of M. lepromatosis infection represented 34 clinical diagnoses of lepromatous leprosy, 13 diagnoses of DLL, and 8 other forms.7 Considering factors that may influence initial diagnosis, it is likely that non-DLL diagnoses could be in early stage, without the Lucio’s phenomenon (yet), insufficient pathologic features due to tissue sampling bias, individual variations of the patients, or in combination thereof.
The DLL diagnosis of case S63 was aided by the unique histopathology demonstrated in the large surgically resected tissues. On the contrary, a routine skin biopsy only offers limited pathologic assessment due to the minute size. The usual depth of 4 mm may be too shallow to obtain vessels and nerves that run in the subcutis. This was true for the Canadian case in which no nerve was sampled in the skin biopsy to back the patient’s long and severe polyneuropathy.6 Thus, for biopsy of leprous skin tissue, DLL in particular, Rea and Jerskey have called to use a 6 mm punch.13
Lucio’s phenomenon, or erythema necrotican, is also considered a leprosy reaction, similar to ENL. But the skin eruptions of Lucio’s reaction end with ischemic ulceration of the epidermis whereas ENL does not. Lucio’s reaction usually starts insidiously without associated fever, tender, or leukocytosis, and runs a chronic course. By contrast, the Lucio’s reaction of case S63 was acute and severe with high fever, marked leukocytosis, and hypotension. We speculate that this abruption was precipitated by the surgery and months of chemoradiation therapy for the tumor. Similarly, in a previous fatal case (Az-1),3 the 53-year-old man also suddenly developed high fever, hypotension, and multi-organ failure, shortly after treatment with ampicillin. In the Singapore DLL cases,8,21 both patients also died of shock soon after the leprosy diagnosis and antimicrobial treatment. These deaths were likely due to end-stage or fulminant Lucio’s reaction in view of the longstanding disease of the patients and heavy mycobacterial burden.
Multi-bacillary leprosy requires a treatment regimen with multiple drugs. The 8 cases of M. lepromatosis infection reported thus far, including the present ones, 2 from Arizona,3 2 from Singapore,8 and 1 of each from Mexico5 and Canada,6 were all multi-bacillary. The treatment success of the present cases with a multi-drug regimen and the similar experience of Rea and Jerskey with DLL13 support this empiric approach for M. lepromatosis. Apparently, more organized studies are needed for optimal treatment.
In Mexico, M. lepromatosis infection is endemic along the western and central Pacific coast and around Mexico City.7 Along the Gulf of Mexico States, on the other hand, M. leprae cases dominated, as was also shown in the study by Matsuoka et at.22 In the present study, the man of case Mn-1 came from Matamoros, a city of Tamaulipas that borders the Gulf of Mexico and Brownsville, the southern tip of Texas, USA; the woman of case S63 lived in Ozona, south central Texas that is ~80 miles to Coahuila. The DLL case reported by Vera-Cabrera et al5 came from Nuevo Leon that lies between the States of Tamaulipas and Coahuila and also borders Texas. These cases thus suggest the presence of M. lepromatosis in the northeastern Mexico in the past decades.
Finally, the present study also shows that for archived FFPE tissue small PCR amplicons of <200-bp work better than larger amplicons. The finding supports our earlier choice of the 143-bp amplicon for M. lepromatosis and the 135-bp amplicon for M. leprae in the Mexico study.7 At the time of that study, the amount of tissue DNA obtained from minute skin biopsy was too limited to evaluate the effect of amplicon size.
Acknowledgments
Funding support: This work was supported in part by a grant from the University Cancer Foundation of the University of Texas M. D. Anderson Cancer Center (MDACC) and by the National Institutes of Health (NIH) grant CA16672 for MDACC core facilities for DNA analysis and histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
References
- 1.Monot M, Honoré N, Garnier T, et al. On the origin of leprosy. Science. 2005;308:1040–1042. doi: 10.1126/science/1109759. [DOI] [PubMed] [Google Scholar]
- 2.Monot M, Honoré N, Garnier T, Zidane N, et al. Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nat Genet. 2009;41:1282–1289. doi: 10.1038/ng.477. [DOI] [PubMed] [Google Scholar]
- 3.Han XY, Seo Y-H, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856–864. doi: 10.1309/AJCPP72FJZZRRVMM. [DOI] [PubMed] [Google Scholar]
- 4.Han XY, Sizer KC, Thompson EJ, et al. Comparative sequence analysis of Mycobacterium leprae and the new leprosy-causing Mycobacterium lepromatosis. J Bacteriol. 2009;191(19):6067–6074. doi: 10.1128/JB.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vera-Cabrera L, Escalante-Fuentes WG, Gomez-Flores M, et al. A Case of Diffuse Lepromatous Leprosy associated with Mycobacterium lepromatosis. J Clin Microbiol. 2011;49:4366–4368. doi: 10.1128/JCM.05634-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jessamine PG, Desjardins M, Gillis T, et al. Leprosy-like illness in a patient with Mycobacterium lepromatosis from Ontario, Canada. J Drugs Dermatol. 2012;11:229–233. [PubMed] [Google Scholar]
- 7.Han XY, Sizer KC, Velarde-Felix JS, et al. The leprosy agents Mycobacterium lepromatosis and Mycobacterium leprae in Mexico. Int J Dermatol. 2012 Aug;(issue) doi: 10.1111/j.1365-4632.2011.05414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han XY, Sizer KC, Tan HH. Identification of the leprosy agent Mycobacterium lepromatosis in Singapore. J Drugs Dermatol. 2012;11:168–172. [PubMed] [Google Scholar]
- 9.Patnaik MM, Hammerschmidt D, van Burik JAH, Jessurun J, Smyth P. Lepromatous Leprosy Masquerading as Acute Sarcoidosis: A Case Report and Literature Review. Minnesota Medicine. 2008 Nov; [PubMed] [Google Scholar]
- 10.Donner RS, Shively JA. The “Lucio phenomenon” in diffuse leprosy. Ann Intern Med. 1967;67:831–836. doi: 10.7326/0003-4819-67-4-831. [DOI] [PubMed] [Google Scholar]
- 11.Gelber RH. Leprosy (Hansen’s Disease) In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005. pp. 966–972. [Google Scholar]
- 12.Latapi F, Chevez-Zamora A. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr. 1948;16:421–437. [Google Scholar]
- 13.Rea TH, Jerskey RS. Clinical and histologic variations among thirty patients with Lucio's phenomenon and pure and primitive diffuse lepromatosis (Latapi's lepromatosis) Int J Lepr Other Mycobact Dis. 2005;73(3):169–188. [PubMed] [Google Scholar]
- 14.Lucio R, Alvarado Y. Opusculo sobre el mal de San Lazaro o elefantiasis de los Griegos. Mexico: M. Murguia y Cia; 1852. p. 53. [Google Scholar]
- 15.Frenken JH. Diffuse leprosy of Lucio and Latapi. Oranjestad, Aruba, Netherlands Antilles: De Wit; 1963. [Google Scholar]
- 16.Vargas-Ocampo F. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78(3):248–260. [PubMed] [Google Scholar]
- 17.Rea TH. Lucio's phenomenon: an overview. Lepr Rev. 1979;50:107–112. doi: 10.5935/0305-7518.19790016. [DOI] [PubMed] [Google Scholar]
- 18.Romero A, Ibarra AB, Fallas M. Clinical study of lepromatous leprosy in Costa Rica. Int J lepr. 1949;17:27–33. [PubMed] [Google Scholar]
- 19.Rea TH, Bevans L, Taylor CR. The histopathology of the spleen from a patient with lepromatous leprosy. Int J Lepr Other Mycobact Dis. 1980;48(3):285–290. [PubMed] [Google Scholar]
- 20.Derbes VJ, Samuels M, Williams OP, Walsh JJ. Diffuse leprosy; case in a Louisiana Negro. Arch Dermatol. 1960 Feb;81:210–224. doi: 10.1001/archderm.1960.03730020046009. [DOI] [PubMed] [Google Scholar]
- 21.Ang P, Tay YK, Ng SK, Seow CS. Fatal Lucio's phenomenon in two patients with previously undiagnosed leprosy. J Am Acad Dermatol. 2003;48:958–961. doi: 10.1067/mjd.2003.326. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka M, Gonzalez AV, Estrada I, et al. Various genotypes of Mycobacterium leprae from Mexico reveal distinct geographic distribution. Lepr Rev. 2009;80:322–326. [PubMed] [Google Scholar]