Summary
The Plasmodium proteasome has been suggested to be a potential anti-malarial drug target, however toxicity of inhibitors has prevented validation of this enzyme in vivo. We report here a screen of a library of 670 analogs of the recently FDA approved inhibitor, carfilzomib, to identify compounds that selectively kill parasites. We identified one compound, PR3, that has significant parasite killing activity in vitro but dramatically reduced toxicity in host cells. We found that this parasite-specific toxicity is not due to selective targeting of the Plasmodium proteasome over the host proteasome, but instead is due to a lack of activity against one of the human proteasome subunits. Subsequently, we used PR3 to significantly reduce parasite load in P. berghei infected mice without host toxicity, thus validating the proteasome as a viable anti-malarial drug target.
Introduction
Malaria is a devastating disease that affects approximately 250 million people per year, resulting in more than 800,000 deaths annually (World Health Organization, 2011). The majority of human malaria is caused by the pathogen Plasmodium falciparum. Parasite resistance to inexpensive antimalarial drugs, such as chloroquine and sulfadoxine-pyrimethamine, is widespread, and resistance to newer artimisinin-based combination therapies (ACTs), the current gold-standard in malaria treatment, is emerging (Dondorp et al., 2009). Despite a clear need for new antimalarial therapies, the high cost of new drug development and relatively low profit associated with drugs that will be used predominantly in the developing world present many challenges. One approach to lowering the cost of antimalarial drug development is to evaluate compounds that are in clinical development and associated synthetic analog libraries for potential antimalarial activity.
The proteasome is a large multi-subunit protein complex that consists of a barrel shaped proteolytic core (20S) and regulatory factors that flank each end of the barrel to regulate entry of proteins targeted for degradation (Bedford et al., 2010). The microenvironment within the 20S proteasome harbors the sites of protein degradation with its barrel-shaped structure made up of two stacked rings of seven β subunits sandwiched between two rings of α subunits. In the catalytic core of the yeast and mammalian proteasome, only β1, β2 and β5 subunits have catalytic activity. β1 has caspase-like activity, preferring to cleave after acidic residues; β2 has trypsin-like activity, preferring to cleave after basic residues; β5 has chymotrypsin-like activity, preferring to cleave after non-polar residues (Verdoes et al., 2009). Studies using site-directed mutagenesis of the catalytic residues in each of the active sites showed that inactivation of the β5 subunit caused the most drastic phenotypic changes in yeast (Heinemeyer et al., 1997). The proteasome regulates numerous cellular functions, including normal turnover of proteins, degradation of misfolded proteins, and regulation of biological pathways by selective degradation of regulatory proteins and transcription factors (Voges et al., 1999; Voorhees et al., 2003). In rapidly proliferating tumor cells, exposure to proteasome inhibitors leads to cell cycle arrest and subsequent apoptosis (Orlowski and Kuhn, 2008). This increased sensitivity to proteasome inhibition over quiescent cells led to the development of proteasome inhibitors now used for treatment of multiple myeloma.
Proteasome inhibitors have also been found to effectively kill various parasitic organisms such as Mycobacterium tuberculosis (Lin et al., 2009), Trypanosome brucei (Mutomba et al., 1997; Steverding et al., 2011) and Plasmodium spp. Over the last decade, several groups have reported that multiple classes of proteasome inhibitors block P. falciparum asexual replication in red blood cells (Tschan et al., 2011; Li et al., 2012). Some of these compounds have been shown to be effective not only towards asexual stages (Lindenthal et al., 2005; Kreidenweiss et al., 2008) of the P. falciparum life cycle, but also during the sexual (Czesny et al., 2009; Aminake et al., 2011) and insect-vector stages (Gantt et al., 1998). This makes the Plasmodium proteasome a highly attractive drug target as it has important functions in all stages of the parasite life cycle. However, despite promising results in vitro, translation into animal studies has been limited due to toxicity resulting from cross-reactivity with the host proteasome (Gantt et al., 1998). Furthermore, none of the current studies have been able to directly link effects of compounds in parasite replication to proteasome inhibition and a clear understanding of the key parameters required for efficient parasite killing by proteasome inhibitors in vivo is lacking.
Carfilzomib (PR-171; Kyprolis® Trade Name) is a proteasome inhibitor developed by Onyx Pharmaceuticals (South San Francisco) that has just recently been approved by the FDA for the treatment of multiple myeloma. Carfilzomib is a potent, highly selective, and irreversible inhibitor of the proteasome (Demo et al., 2007; Kuhn et al., 2007). Here we describe our efforts to screen a library of 670 analogs of carfilzomib to identify inhibitors that selectively target P. falciparum relative to the human proteasome. We identified one molecule, PR3, that inhibits P. falciparum replication, proteasome activity, and gametocyte survival and has overall low toxicity to host cells. Interestingly, we found that PR3 does not selectively target the Plasmodium proteasome but rather derives its lack of toxicity in the host from its inability to target the human β2 subunit. As a result, PR3 is able to significantly decrease parasitemia in mice with no host toxicity during treatment. The findings from this study validate the Plasmodium proteasome as a target for anti-malaria drugs and provide important insight into the key parameters required of proteasome inhibitors for effective clearance of parasites in vivo.
Results
Anti-parasitic effects of carfilzomib in vitro and in vivo
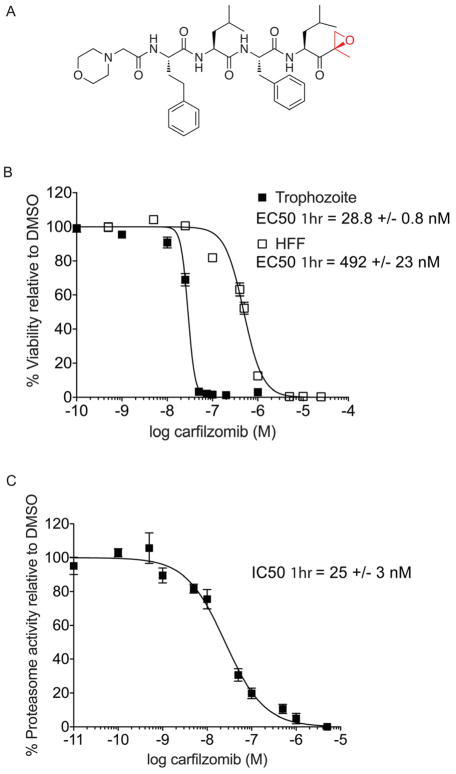
Carfilzomib (Figure 1A) is a synthetic proteasome inhibitor that is an analog of the natural product epoxomicin. While there have not been any reports on the effects of carfilzomib on P. falciparum, the related compound epoxomicin has been shown to block replication of chloroquine-susceptible, chloroquine-resistant, and clinical isolates of P. falciparum in vitro (Kreidenweiss et al., 2008; Czesny et al., 2009). We chose carfilzomib as a starting point for our study because it was recently approved for use for the treatment of multiple myeloma and a significant amount of preclinical toxicity, pharmacokinetic (PK) and adsorption, distribution, metabolism and excretion (ADME) data is available (Yang et al., 2011). To evaluate the potency of carfilzomib against P. falciparum, we monitored its effects on parasite replication in vitro using flow cytometry as previously described (Arastu-Kapur et al., 2008). As carfilzomib has a short half-life in vivo, we chose to perform 1 hr treatment to more closely reflect in vivo conditions. We treated P. falciparum cultures in trophozoite stage with carfilzomib or vehicle for 1 hr at 37°C, washed extensively with fresh media, and then allowed the parasite to replicate. Like epoxomicin, carfilzomib potently blocked parasite replication with effective concentration (EC50) of 28.8 +/− 0.8 nM even with a short exposure time of 1 hr (Figure 1B). We noted that with prolonged treatment (60 hr), there is a 5-fold increase in potency of carfilzomib (Figure S2). We also evaluated general host cell toxicity of carfilzomib using a non-transformed human foreskin fibroblast (HFF) cell line. In order to match the replicative nature of the parasite, we used non-confluent HFF cells grown at low density. As expected, we found that 1 hr treatment with carfilzomib is also able to effectively kill host cells with an EC50 of 492 +/− 23 nM (Figure 1B). This data suggests that there is only a small therapeutic window for use of this drug without causing potentially significant side effects.
Figure 1.
Anti-parasitic activity and therapeutic window of carfilzomib in vitro. A) Structure of carfilzomib. The epoxyketone reactive functional group is shown in red. B) Dose response of carfilzomib on P. falciparum culture and human foreskin fibroblasts (HFF). Synchronous P. falciparum trophozoites or non-confluent HFF cells were treated for 1 hr followed by washout of the inhibitor and incubation for 60 hr (trophozoite) or 72 hr (HFF). Each concentration was tested at least 3 times; error bars represent standard error of the mean (S.E.M) for each drug concentration from triplicates. EC50 value is shown as mean +/− standard deviation (SD). The dose response curve for 60 hr treatment is shown in Figure S2. C) Proteasome activity in intact trophozoites treated with carfilzomib. Proteasome activity was assessed by monitoring inhibition of the putative β5 subunit (assignment of labeled subunit is shown in Figure S1). Synchronous mid-trophozoite stage parasites were treated with the indicated concentrations of carfilzomib for 1 hr, followed by inhibitor washout and preparation of parasite lysates. Residual proteasome activity of parasite lysate under each drug concentration was determined by labeling with 2 μM MV151. The IC50 curve was obtained from 3 independent experiments; error bars represent S.E.M for each drug concentration from triplicates. A representative probe competition gel is shown in Figure S5. IC50 value is shown as mean +/− standard deviation (SD). (See also Figure S2).
We next wanted to confirm that growth inhibition in the replication assay correlates with inhibition of Plasmodium proteasome activity. Therefore, we exposed intact trophozoites for 1 hr with a range of concentrations of carfilzomib and monitored proteasome activity in the treated parasites using a proteasome selective activity-based probe (MV151; Figure S1; (Verdoes et al., 2006)). Using MV151, we are able to monitor the activity of two (putative β2 and β5; Figure S1) out of three predicted active sites in Plasmodium proteasome. We found that the IC50 for the putative parasite β5 subunit after 1 hr treatment of trophozoites with carfilzomib (25 +/− 3 nM) closely matches the EC50 of the compound for parasite growth (Figure 1C). This data, coupled with the high degree of selectivity of the epoxyketone functional group for the proteasome, suggests that carfilzomib blocks parasite growth as a direct result of specific inhibition of the parasite proteasome.
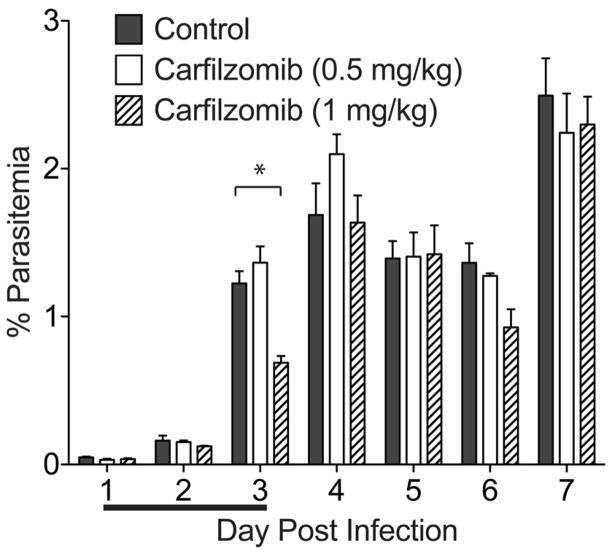
Although we were aware that carfilzomib could only be used at low doses in vivo, we wanted to determine if the drug could reduce parasite burden at its maximum tolerated dose. We used the Plasmodium berghei mouse model of malaria since P. falciparum can only infect humans and New World monkeys (Aotus and Saimiri sp.). Mice were infected with P. berghei via intravenous (I.V.) injection of parasites, treated for 3 consecutive days with daily I.V doses of carfilzomib, and monitored for parasite burden (Figure 2). We tested a range of doses from 0.5 mg/kg to 4 mg/kg, and found that the mice started to develop signs of compound toxicity (scruffy coat, significant weight loss during treatment) at 1 mg/kg of carfilzomib and therefore we had to stop treatment at the higher doses. We noted that the maximum tolerable dose for the P. berghei infected mice was lower than the previously reported 2 mg/kg for 5 consecutive days of treatment (Demo et al., 2007), and we think this is likely because these mice are more susceptible to the toxic effects of the inhibitor as a result of parasite burden. Our results for 3 days of treatment at the tolerated dose of carfilzomib showed only a very minor effect on parasite burden at day 3, and parasitemia returned to control levels by day 4 (Figure 2).
Figure 2.
Carfilzomib fails to effectively block parasite growth in vivo. Female Balb/c mice infected with 1×106 P. berghei parasites via tail vein injection were dosed intravenously with vehicle (n=4), 0.5 mg/kg carfilzomib (n=3) or 1 mg/kg carfilzomib (n=3) on 3 consecutive days (as indicated by line below the x-axis). Parasitemia was quantified by microscopy counting of Giemsa-stained thin blood smears. The asterisk represents p < 0.05.
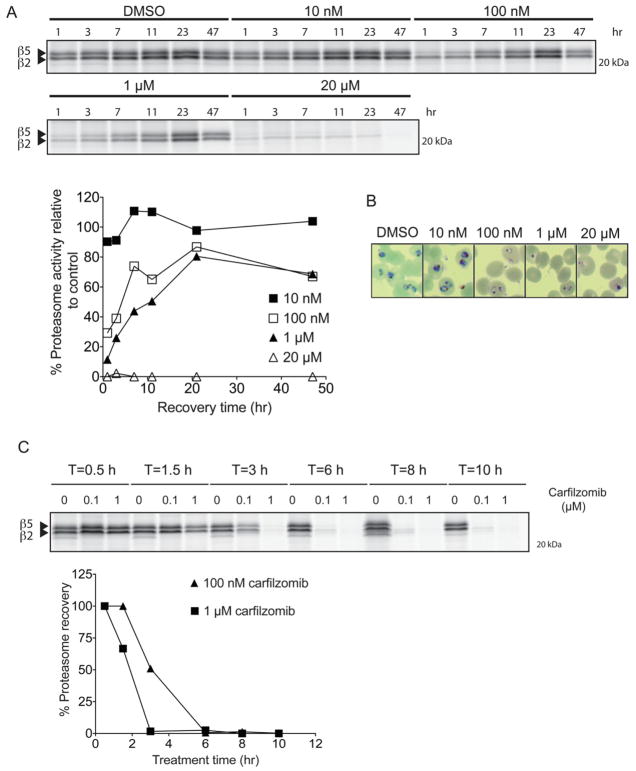
To investigate why carfilzomib only had a moderate effect on lowering parasitemia in vivo, we treated a culture of P. falciparum trophozoites at high parasitemia with carfilzomib for a short time period (1 hr), washed out the inhibitor extensively, and then monitored parasite proteasome activity (Figure 3A). We found that once the inhibitor was removed, proteasome activity recovered within 24 hours or less depending on the dose. Since carfilzomib is a covalent inhibitor and treatment permanently inhibits proteasomes, this recovery of activity is most likely due to new synthesis of the enzyme complex (Meiners et al., 2003). We found that the amount of proteasome recovery is also dependent on the inhibitor dose. A dose that far exceeds the IC50 (1 hr) for carfilzomib (20 μM) was required to achieve complete inhibition of proteasome activity with no further recovery. Surprisingly, even at a relatively high dose of carfilzomib treatment (1 μM), there was substantial recovery of proteasome activity after 47 hrs of compound washout. This data, combined with the data shown in Figure 1, suggests that even partial inhibition of the proteasome is sufficient to significantly block Plasmodium growth. In agreement with previous reports on proteasome inhibitor treatment of P. falciparum (Gantt et al., 1998; Lindenthal et al., 2005), we found that low doses of the inhibitor slowed parasite growth but resulted in parasite death only at high concentrations of the compound (Figure 3B).
Figure 3.
P. falciparum recovers proteasome activity after a short period of inhibition and prolonged proteasome inhibition prevents recovery. A) Recovery of P. falciparum proteasome activity after 1 hr treatment with carfilzomib. A synchronous culture of trophozoites was treated at the indicated concentrations of carfilzomib for 1 hr followed by extensive washout of the inhibitor. Parasites were kept in fresh media for a further 47 h, and proteasome activity in lysates obtained at different time points was determined by labeling with MV151 (see Figure S1). To adjust for variation of proteasome content due to parasite growth, proteasome activity was normalized to DMSO treated parasites obtained at each respective time point. Proteasome activity at each time point was assessed by intensity of probe labeling. Quantification of the putative β5 subunit labeling is shown in the graph below the gel image. B) Giemsa-stained thin blood smears of P. falciparum culture treated with carfilzomib for 1 hr followed by 47 hr washout. C) Synchronous trophozoites were treated with 100 nM or 1 μM carfilzomib for the indicated times followed by inhibitor wash out. Parasites were placed in fresh media, and proteasome activities of all samples were determined by MV151 labeling at 10 hr after inhibitor washout. Quantification of the putative β5 subunit labeling is shown in the graph below the gel image. (See also Figure S1)
We also wanted to use our proteasome probes to determine if prolonged exposure of parasites to the inhibitor leads to sustained proteasome inhibition and therefore parasite death, even at low concentration of the drug. Therefore, we treated P. falciparum trophozoites with two concentrations of carfilzomib for different time periods followed by washout and monitoring of proteasome recovery at the end of the washout period (Figure 3C). These results suggest that increasing treatment time indeed reduced the ability of the parasite to recover proteasome activity. However, at the 100 nM dose (close to EC50 of carfilzomib against parasites in culture), at least 6 hr of inhibitor exposure was necessary to prevent recovery of proteasome activity. This result, combined with our observation of rapid proteasome recovery, likely explains why carfilzomib, which has a short half-life in the bloodstream (Yang et al., 2011), is unable to clear parasites without host toxicity even when used at a dose far above its EC50 for parasite killing in vitro. This data also indicates that any inhibitors designed in the future would likely need to have long half-life in blood to efficiently effect parasite clearance.
Screen to identify P. falciparum proteasome selective inhibitors
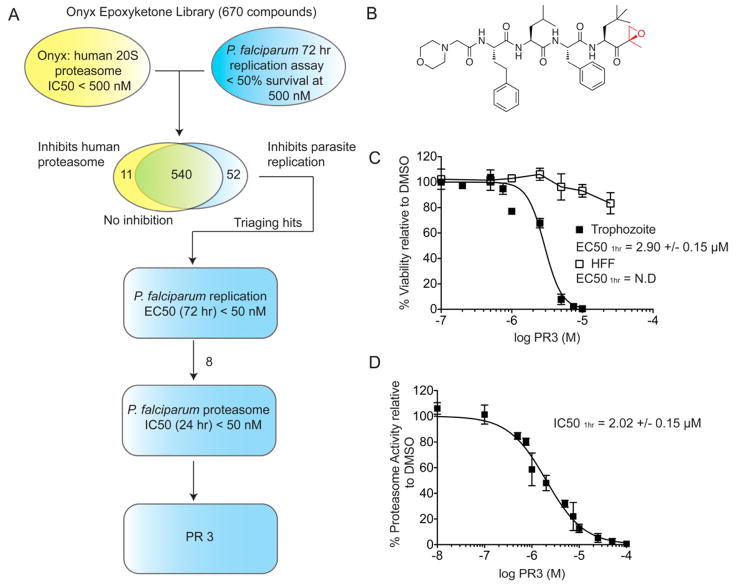
We reasoned that since carfilzomib has high overall toxicity but is an effective inhibitor of parasite growth, it should be possible to find analogs that retain the killing effects against the parasite but that are overall poor inhibitors of the human enzyme. We therefore screened a library of 670 analogs of carfilzomib (generated at Proteolix, acquired by Onyx) for inhibition of ring stage P. falciparum replication in culture after a 72 hr treatment (Figure 4A). From this initial screen, we identified a total of 592 compounds that inhibited parasite replication by greater than 50% at 500 nM. We then cross-referenced these ‘hits’ with their measured potency against the human proteasome. This resulted in an initial 52 preliminary hits that had less than 50% inhibition of human proteasome at 500 nM concentration. We then determined the EC50 values for these hits and selected the top eight compounds with EC50 values for parasite killing at or below 50 nM for 72 hr inhibitor treatment. These eight compounds were then tested for their ability to inhibit partially purified P. falciparum proteasomes that were isolated from parasite extracts using a number of standard protein purification steps (refer to Figure S1 and Experimental Procedure for detailed description on P. falciparum 20S proteasome purification). By assessing the inhibition of parasite proteasome substrate cleavage of Suc-Leu-Leu-Val-Tyr-aminomethylcoumarin (Suc-LLVY-AMC) upon treatment with these compounds, we selected a lead compound that was most potent against the parasite proteasome and also in replication assays but that had the weakest activity against the human enzyme. Interestingly, this compound, given the name PR3, is highly similar in structure to carfilzomib, differing only by the presence of an additional methyl group on the P1 sidechain (Figure 4B).
Figure 4.
Identification of PR3, an inhibitor of parasite growth with low host cell toxicity through screening of a focused library of carfilzomib analogs. A) Synchronous ring stage parasites were treated for 72 hr with 500 nM of each 670 carfilzomib analogs. Inhibition of parasite replication was quantified by flow cytometry and compared to the Onyx dataset for human 20S proteasome inhibition. The 52 preliminary hits were further tested in a dilution series in parasite replication assay. Eight compounds with EC50 < 50 nM were selected for a 24 hr proteasome inhibition assay with enriched P. falciparum 20S proteasome. PR3 was identified as the most potent anti-parasitic compound. B) Structure of PR3. C) Dose response of PR3 on P. falciparum culture and non-confluent HFF. Conditions were as described in Figure 1B. EC50 values are shown as mean +/− standard deviation (SD). Dose response for 60 hr treatment is shown in Figure S2, and effect of PR3 on P. falciparum gametocytes is shown in Figure S3. (D) Proteasome activity in intact trophozoites after PR3 treatment. Proteasome activity was determined by monitoring inhibition of the putative β5 subunit using MV151 (2 μM). The IC50 curve was obtained from 3 independent experiments (error bars are shown as S.E.M); IC50 value is shown as mean +/− SD. A representative probe competition gel is shown in Figure 5a (lower panel). (See also Figure S2 and S3)
We next assessed the potency of PR3 in P. falciparum trophozoites and compared these results to the activity of the compound against non-confluent human foreskin fibroblast (HFF) cells. Although PR3 was only 100 times less potent than carfilzomib at killing parasites (EC50 = 2.90 +/− 0.15 μM), it showed dramatically reduced toxicity in the HFF cells (Figure 4C) in 1 hr inhibitor treatment. Even at the solubility limit of the compound (~ 50 μM), it only reduced viability of the HFF cells by 20%. Since potency of a covalent inhibitor is dependent the length of treatment, a short (1 hr) treatment of trophozoites resulted in an effective concentration that was higher than that identified in our preliminary screen, where ring stage P. falciparum was treated for an extended period (72 hr). Similar to carfilzomib, the IC50 of the putative β5 proteasome subunit inhibition in trophozoites after 1 hr treatment (2.05 +/− 0.22 μM) closely matched the EC50 value for parasite killing in the replication assay (Figure 4D). Thus, PR3 has weak but effective activity against parasites and reduced toxicity to host cells.
PR3 is a weak inhibitor of both the human and parasite proteasome
The fact that PR3 remained effective at killing parasites yet is a poor inhibitor of the human proteasome suggested that it might be acting through a mechanism of selective parasite proteasome inhibition. We initially thought that the addition of the methyl group on the P1 sidechain could enhance binding in the P. falciparum 20S proteasome active site or reduce binding to the human active site. To investigate the selectivity of PR3, we performed a series of detailed kinetic analysis of both carfilzomib and PR3 in purified human and parasite 20S proteasomes using the fluorogenic substrate Suc-LLVY-AMC that reports activity of the chymotrypsin-like activity of the proteasome (associated with the β5 subunit). (Table 1, see also Figure S4).
TABLE 1.
Inhibition constants of carfilzomib and PR3 in purified proteasome. (See also Figure S4)
| Human 20S | Plasmodium 20S | |||
|---|---|---|---|---|
|
| ||||
| PR171 | PR3 | PR171 | PR3 | |
| Kinact (s−1) | 0.00119 ± 0.00005 | 0.0012 ± 0.0001 | 0.00128 ± 0.00005 | 0.0028 ± 0.0009 |
| Ki (nM) | 13.3 ± 1.2 | 3900 ± 900 | 126 ± 6 | 30000 ± 11000 |
| * kinact/Ki (M−1/s−1) | 90100 ± 5300 | 310 ± 50 | 10200 ± 100 | 94 ± 4 |
The second order inhibition constant ki is equals to kinact/Ki
We first determined the observed rate constant (kobs) for human and P. falciparum 20S proteasome inhibition over a range of inhibitor concentrations for both carfilzomib and PR3. We then fit the kobs values to the inhibition model shown in Table 1 that accounts for reversible binding of the inhibitor (Ki) followed by covalent modification by the reactive functional group (kinact; Figure S5). These data suggest that the differences in potency between these two inhibitors are the result of differences in binding to the proteasome active site (Ki) rather than differences in reactivity of the epoxyketone towards the active site threonine (kinact).
Because epoxyketones are covalent inhibitors, the best measure of inhibitor potency is the second order inhibition constant (ki = kinact/Ki ). Ki represents the dissociation constant, and kinact, the rate of covalent inactivation of the proteasome by the warhead after inhibitor binding. Calculation of the inhibition constants (ki) for carfilzomib and PR3 against the human proteasome confirmed that PR3 is nearly 300 times less potent than carfilzomib, with a ki of 90,100 and 310 M−1s−1 for carfilzomib and PR3, respectively (Table 1). PR3 is also 100 fold less potent than carfilzomib for the P. falciparum proteasome, with a ki for carfilzomib and PR3 of 10,200 and 94 M−1s−1, respectively. Interestingly, PR3 is slightly more potent against the human β5 proteasome suggesting that the increased therapeutic window is not due to selective targeting of the parasite proteasome.
PR3 is a partial inhibitor of the host proteasome
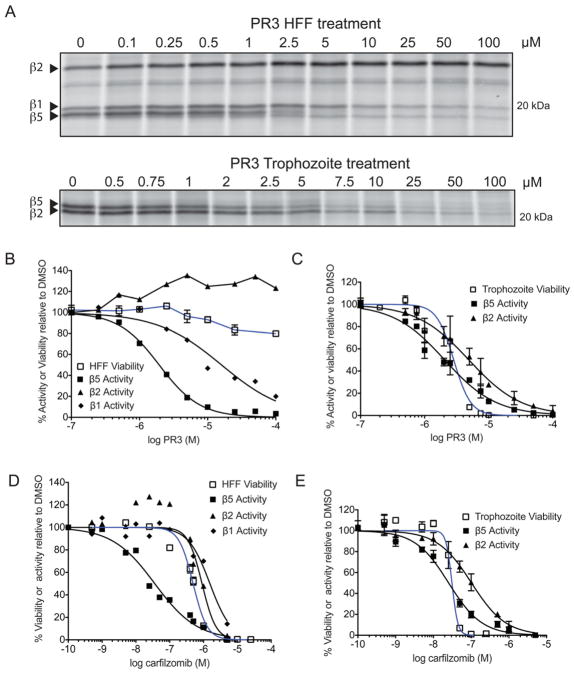
Because our results indicated that PR3 is not a selective inhibitor of the parasite proteasome, we wanted to determine how this inhibitor was able to kill parasites but not the host HFF cells. To compare the effect of a short treatment of PR3 on proteasome inhibition, we treated intact HFF cells as well as intact P. falciparum infected red blood cells for 1 hr with the inhibitor at various concentrations and monitored the levels of residual activity of the primary proteasome catalytic subunits by labeling with MV151 (Figure 5A). We found that PR3 has similar potency against the human and parasite chymotrypsin-like β5 subunit activity but importantly, it does not inhibit the β2 subunit in the host proteasome, even at the highest dose tested. Interestingly, while close to full inhibition of the β5 subunit in the host cell only has a small effect on viability (Figure 5B), inhibition of this same activity of the parasite proteasome directly correlates with a decrease in P. falciparum growth (Figure 5C, E). Furthermore, in agreement with recent data suggesting that inhibition of multiple proteasome activity sites is required to induce cell killing of mammalian cells (Britton et al., 2009; Mirabella et al., 2011), we find that a decrease in HFF viability correlates with inhibition of all three active subunits in the host cell (Figure 5D, see also Figure S5). Taken together, these data suggest that parasites, unlike the host cell, are not able to survive when even one of the three active sites of the proteasome is blocked. Therefore, the window of therapy observed for PR3 is likely due to fact that PR3 is unable to inhibit all of the host proteasome subunits.
Figure 5.
PR3 is non-toxic to host cells due to partial inhibition of the human proteasome. A) MV151 labeling of intact HFF (top) and P. falciparum trophozoites (bottom) cells treated with PR3. Quantification of each labeled subunit (indicated by arrow) is shown below. B–E) Comparison of viability (blue line) versus inhibition of the catalytic proteasome subunit activities (black lines) after 1 hr incubation of HFF (B, D) or trophozoites (C, E) with PR3 (B–C) or carfilzomib (D–E). Proteasome activity was determined by competition of MV151 labeling from the gels shown in (A) and supplementary Figure S5. The effect of carfilzomib and PR3 in live trophozoites are obtained from 3 independent experiments; error bars represent S.E.M for each drug concentration from triplicates. (See also Figure S5).
PR3 reduces parasite growth in vivo without any host toxicity
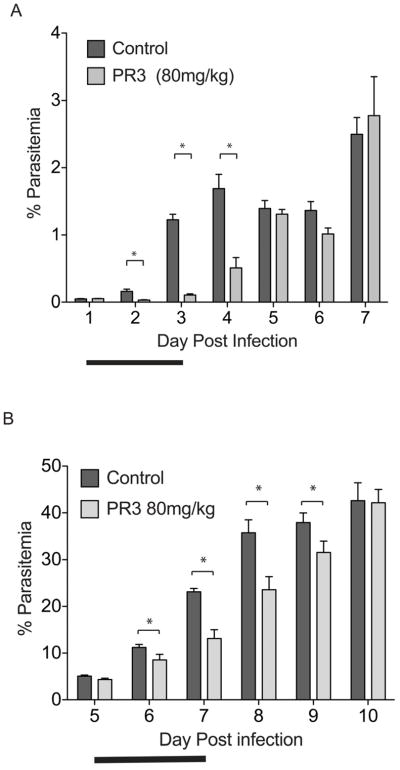
Encouraged by the selective parasite killing of PR3 in our culture model, we proceeded to test PR3 in the in vivo Plasmodium berghei model. In our studies with the Balb/c female P. berghei infection model, we consistently observed that parasitemia develops fairly slowly around the first 5 days of infection, and thereafter reaches an aggressive and exponential stage of high parasitemia. Mice were infected with P. berghei and treated with PR3 through 3 consecutive days of intravenous injections (Figure 6). We tested a series of doses of PR3 up to 80 mg/kg (data not shown), and found no noticeable toxic effect in the mice even at the highest drug dose. To test the effect of PR3 treatment at low parasitemia, we treated mice with 80 mg/kg dose of PR3 (n=3) for 3 consecutive days starting 1 day after infection, when the initial parasitemia is low (~ 0.05%; Figure 6A). We observed a highly significant decrease in parasitemia compared to vehicle treated mice (n=4) on days 2, 3 and 4. Parasitemia decreased by nearly 90% compared to vehicle treated mice after 2 days of drug treatment. To investigate if the effect of PR3 is significant when treatment was administered at higher parasitemia, where infection reaches an exponential stage, we treated mice with 80 mg/kg dose of PR3 (n=5) for 3 consecutive days 5 days after infection when initial parasitemia is ~ 4.7 % (Figure 6B). Again, we found that PR3 has a significant effect at lowering parasitemia up to four days after treatment, though the effect is less drastic than treatment 1 day after infection. In both treatment regimes, we were unable to clear parasites from the host at the end of the drug treatment, suggesting that the in vivo concentration of PR3 is insufficient to sustain parasite proteasome inhibition and hence only had a partial effect on parasite growth. Since PR3 is highly similar in structure to carfilzomib, we postulate that it is also likely to have a short half-life in vivo and is rapidly degraded after dosing.
Figure 6.
PR3 significantly reduces parasite growth in an in vivo model of malaria. A) Treatment of mice with PR3 when parasitemia is low (one day after infection, initial parasitemia ~ 0.05% for all groups). Mice were inoculated (via i.v.) with 1×106 of P. berghei parasites and treated with vehicle (n=4) or 80 mg/kg PR3 (n=3) for 3 consecutive days (indicated by bold line) starting one day after infection. None of the PR3 treated mice showed signs of compound toxicity. Parasitemia was monitored daily from Giemsa-stained thin blood smears. B) Treatment of mice with PR3 when parasite burden is already high (five days after infection, initial parasitemia ~4.7%). Mice were inoculated (via i.p.) with 1×106 of Plasmodium berghei parasites and treated with vehicle (n = 5) or 80 mg/kg PR3 (n = 5) via intravenous injections for 3 consecutive days (indicated by bold line) starting on day 5 after infection. Both treatment groups had similar parasitemia (4–5%) before treatment was administered. None of the PR3 treated mice showed signs of compound toxicity. * = p <0.05. Error bars represent S.E.M.
Discussion
The proteasome is an essential multi-subunit protease complex in all eukaryotic cells. Because it is involved in many critical cellular processes, many have considered it as a therapeutic target in disease models such as cancer, tuberculosis and African sleeping sickness. Lactacystin was the first reported proteasome inhibitor that showed anti-malarial effects. Although lactacystin has potent anti-parasitic effects in vitro, it causes significant host toxicity when administered in a rat P. berghei model (Gantt et al., 1998). Subsequently, even though many groups have reported potent effects of a variety of proteasome inhibitors against asexual, sporozoite (insect-vector) and gametocyte stages of P. falciparum, the proteasome has yet to be validated as a true anti-malarial target due to toxicity of these inhibitors to the host organism.
We report here the first studies on the anti-malarial effect of a clinical drug carfilzomib. We found that carfilzomib is a nano-molar inhibitor of P. falciparum in the intra-erythrocytic life cycle, and is also effective towards gametocytes (Figure S3). However, in an in vivo P. berghei model, we found that a modest decrease in host infection is accompanied by drug-induced toxicity during treatment. We postulate that this is due to the short half-life of carfilzomib (t1/2 ranges from 5 to 20 min; Yang et al., 2011). In our work, we find that a short exposure to low concentrations of carfilzomib only results in growth retardation of the parasite and this is most likely due to the fact that the parasites are able to rapidly replenish their proteasomes. As carfilzomib is rapidly degraded in the bloodstream, the blood concentration of the drug is insufficient to result in parasite clearance without significant compromise to the health of the host.
In an effort to identify a proteasome inhibitor that has anti-parasitic effects with low host cell toxicity, we performed a screen of a focused library of epoxyketone analogs of carfilzomib. In this screen, we found that most inhibitors that block P. falciparum replication are also potent human proteasome inhibitors, suggesting there is a strong homology in the active sites of both the host and parasite proteasome. However, we did identify one compound, PR3, that is only 100-fold less potent than carfilzomib in asexual P. falciparum culture, but has virtually no toxicity in short treatment of HFF cells and produces no toxic effects when dosed in mice. Like carfilzomib, PR3 also targets the sexual stage of P. falciparum (gametocytes) at sub-micromolar concentrations with 24 hr treatment (Figure S3). Although we initially thought that this was due to selectivity towards P. falciparum proteasome, our kinetics studies revealed that ironically, PR3 is a slightly better inhibitor for the chymotrypsin-like activity of the host proteasome than the parasite proteasome. Using the proteasome activity-based probe MV151, we were able to assess inhibition of proteasome activity in intact host and parasite cells to compare effects of proteasome inhibition with cell viability. We found that inhibition of the proteasome activity even for a short exposure had a detrimental effect on P. falciparum viability, and furthermore parasite survival (EC50) closely correlated with the extent of the putative β5 subunit inhibition as measured using the active site probe. In host HFF cells however, nearly full inhibition of the chymotrypsin-like proteasome activity for 1 hr had no significant effect on cell viability. Interestingly, Czesny et al. also observed a similar effect with epoxomicin, a precursor of carfilzomib (Czesny et al., 2009). In their study, 1 μM of epoxomicin, a nano-molar inhibitor of human proteasome only resulted in a 20% decrease in viability in A549 and NIH3T3 cells while completely blocking P. falciparum replication. This, together with the work presented in this study, shows that partial inhibition of the parasite proteasome can bring about a significant block to Plasmodium growth.
It is tempting to postulate that just as proliferating cancer cells are especially sensitive to proteasome inhibition, the parasite’s highly replicative nature makes it similarly sensitive to proteasome inhibition. Specifically, proteasome inhibition leads to cell cycle arrest and activation of apoptotic pathway in cancer cells. It is likely that proteasome inhibition similarly blocks cell cycle progression in the parasite, consistent with our findings that low doses of the compounds slow parasite development (Figure 3B). Furthermore, our finding that PR3 only inhibits β1 and β5 subunits of the human proteasome while leaving β2 subunit fully active, even at the maximum compound solubility, gives us further insight into design of proteasome inhibitors to target P. falciparum. As host cells are vulnerable to co-inhibition of all the subunits as demonstrated by carfilzomib (Figure 5 D–E), we postulate that proteasome inhibitors that do not block all host proteasome subunit activities will likely be able to serve as more selective anti-parasitic agents.
Finally, we show that PR3 is able to significantly block Plasmodium growth in a P. berghei mouse model with no host toxicity. However, at the dose given, we were unable to clear parasitemia and we think this is likely due to poor pharmacokinetic properties of the compound. PR3 only differs from carfilzomib by the presence of a single methyl group suggesting that both inhibitors are likely to have similar PK properties. As such, even at a high dose of 80 mg/kg, the inhibitor is likely to be rapidly degraded and the resulting concentration of PR3 in blood is insufficient to lead to complete parasite clearance.
Significance
This study provides evidence that the P. falciparum proteasome can be safely targeted by small molecule inhibitors. We find that it is possible to selectively kill Plasmodium using our lead compound PR3 due to the inability of PR3 to fully inhibit all of the host proteasome subunits. Furthermore, partial inhibition of the proteasome is sufficient to significantly block Plasmodium growth and this suggests that inhibitors with selectivity for the Plasmodium proteasome over the human proteasome are likely to have an even greater therapeutic window in vivo. Furthermore, as sustained inhibition of the parasite proteasome will lead to parasite death, compounds with better stability in vivo will be necessary for any anti-malarial agents based on proteasome inhibitors to be effective. As such, the P. falciparum proteasome represents a promising therapeutic target that can complement other existing anti-malarial drugs in novel combination therapies. Studies that focus on compounds with enhanced selectivity for the P. falciparum proteasome are ongoing.
Experimental Procedure
Parasite culture, harvesting of life cycle stages, and lysate preparation
Clones 3D7 and D10 of P. falciparum were cultured and synchronized in media containing the serum substitute Albumax (Invitrogen) using standard procedures (Trager and Jensen, 1976). Synchronous parasite cultures for life stage lysates and replication assays were obtained using treatments with 5% sorbitol to maintain parasites that have an ~6 hr window of synchrony.
To release parasites from infected red blood cells, infected red cells were selectively lysed with 0.15% saponin (Calbiochem) and the parasite pellet was prepared in 1X phosphate buffered saline (PBS). Pelleted parasites were stored at −80°C for further analyses. Lysates from asynchronous and synchronous parasite pellets were prepared by the addition of an equal volume of hypotonic lysis buffer (50 mM Tris pH 7.4, 5 mM MgCl2, 1 mM DTT) to the parasite pellet on ice for 1 hr with occasional vortexing. Lysates were spun at 6000 × g for 10 min and the resulting supernatant was quantified by Bradford assay (Pierce) and used for activity assays.
Tightly synchronized parasites for stage specificity experiments were obtained by enriching for mature schizonts on a 70% percoll gradient as previously described (Blackman, 1994). Enriched schizonts were subsequently allowed to infect fresh red blood cells, and schizonts that did not rupture in the first hour were lysed by sorbitol treatment to create an ~1 hr window of synchrony.
P. falciparum replication assay and proteasome inhibitor screen
To evaluate carfilzomib and to screen the Onyx (formerly Proteolix) compound library, synchronous ring-stage parasites (9 hr post invasion; 1% parasitemia; 0.5% hematocrit) were treated with the library of inhibitors at a final concentration of 500 nM for 72 hr at 37 °C in a 96-well-plate format, fixed with 0.05% glutaraldehyde (Sigma) in 1X phosphate-buffered saline (PBS) overnight at 4 °C, permeabilized with 0.25% Triton X-100 in 1X PBS for 5 min at room temperature (25 °C), resuspended in 200 uL 1X PBS, and stained with a 1:100 dilution of a 5 mg/ml working solution of propidium iodide (Sigma) in deionized water. Parasite replication was monitored by observation of a propidium iodide positive population (infected) and propidium iodide negative population (uninfected) using a BD FACScan flow cytometer (Becton, Dickinson and Co.) located in the Stanford Shared FACS Facility at Stanford University. The infected and uninfected red blood cell populations were quantified using Flowjo 9.1.11. Parasitemia was calculated as the number of infected cells divided by the number of total cells and was normalized to the average of the DMSO treated controls (average of 3–6 DMSO controls per compound plate). Screening was performed in duplicate. Any compound giving a value below 0.5 relative parasitemia was considered a preliminary hit. Parasite replication data was compared to the existing Onyx human data set using software available on the Collaborative Drug Discovery database website (http://www.collaborativedrug.com/).
Subsequent replication assays were performed as described above, but with synchronous population of early-mid trophozoites with various inhibitor concentrations by treating cultures for 1 hr followed by washout of inhibitor to better mimic in vivo treatment or 60 hr continuous treatment. For the washout assays, cultures were treated with inhibitor for 1 hr at 37°C, washed three times with fresh media, and incubated with fresh media for a further 59 hr. Data was obtained and analyzed using Graphpad Prism 5.0d.
Enrichment of the P. falciparum proteasome from schizont lysate
The P. falciparum proteasome was enriched through a two-step column purification. A synchronous population of late schizonts (40–48 hr) was harvested from culture and lysates were obtained as described above. Lysates were then pooled and concentrated on a 100 kD spin column (Amicon Ultra 100k Ultracel) for 20 minutes at 4°C yielding ~ 500μL containing 20 mg/mL total parasite protein. The entire sample was loaded onto a 5 mL anion exchange HiTrap DEAE-FF column (GE Healthcare) and eluted with a gradient buffer from 20 mM Tris pH 7.4 to 1M NaCl in 20 mM Tris pH7.4. 1.5 mL fractions were collected, and fractions were analyzed for presence of proteasome using fluorogenic assay with 20 μM Succinyl-Leu-Leu-Val-Tyr-aminocoumarin (Suc-LLVY-amc, Boston Biochem)). Fractions with Suc-LLVY-amc activity were pooled and concentrated on a 100kD spin column into gel filtration buffer (25 mM Tris pH 7.4, 100 mM NaCl, 10% glycerol). This is then loaded onto a Superose-6 gel filtration column (GE Healthcare) and 500 μL fractions were collected. Fractions were analyzed for the presence of proteasome by fluorgenic substrate assay (as above) and the proteasome activity based probe MV151 (Verdoes et al., 2006). Active fractions were combined and concentrated on a 100kD spin column. The final proteasome enriched preparation was evaluated for purity by silver stain (Figure S1E; Invitrogen SilverQuest).
Proteasome substrate activity assays
Proteasome chymotrypsin-like activity was determined using succinyl-Leu-Leu-Val-Tyr-AMC (LLVY-AMC; 20 μM) with purified human 20S proteasome (2nM) as previously described (Demo et al., 2007) or enriched parasite proteasome. For the human 20S proteasome, inhibition was assessed by adding inhibitor and substrate simultaneously to the enzyme in proteasome assay buffer (50mM HEPES pH 8.0, 0.5mM EDTA, 0.03% SDS) and measuring AMC fluorophore release at 340/465 nm at 27°C with a plate-based spectophotometer (Spectramax M5). Slopes of AMC product formation between 60 and 75 minutes were determined and normalized relative to DMSO. The 50% inhibitory concentration (IC50) for each inhibitor was determined by non-linear regression using GraphPad Prism software. For the enriched P. falciparum proteasome, inhibition was assessed by adding inhibitor and substrate simultaneously to the enzyme in assay buffer (50 mM Tris pH 7.4, 5 mM MgCl2, 1 mM DTT), and measuring AMC fluorophore release at 340/465 nm at 27°C.
In order to calculate inhibition constants in human and P. falciparum proteasome, the LLVY-AMC cleavage assay described above was repeated 200 μM LLVY-AMC and a range of inhibitor concentrations (0–5 nM for carfilzomib and 0–10 μM for PR-3). Slopes for each inhibitor concentration were calculated at 30 minute intervals over 3 hr and normalized to DMSO to generate inactivation curves. First-order rate constants (kobs) were derived from inactivation curves, and inhibition constants (kI) were calculated from the relationship of kobs with each inhibitor concentration using KaleidaGraph software
Proteasome labeling with MV151
The synthesis of MV151 is described in Verdoes et al.(2006). For all lysates or purified proteasome labeling experiments, MV151 is used at a final concentration of 2 μM followed by incubation at 37°C for 1hr. The samples are then denatured by adding loading dye and boiling, and ran on a 12% SDS PAGE. Gels are then scanned at the TAMRA channel on a Typhoon Scanner (GE Healthcare). Quantification of the intensity of the labeled proteins is done using Image J.
Assessment of P. falciparum proteasome activity in intact inhibitor treated parasites
P. falciparum is cultured at around 15% parasitemia to ensure sufficient parasite is available for proteasome labeling. P. falciparum culture is first treated for the indicated amount of time, and spun down at 3200 rpm to remove inhibitor. Culture is then washed 3 times in fresh media, and parasite pellets are obtained as described above. P. falciparum proteasome is labeled by incubating at least 2 μg of parasite lysate obtained from the pellet (see above) with 2 μM of MV151. To assess proteasome inhibition, the intensity of the proteasome labeling is quantified by Image J and the amount of proteasome inhibition is taken as a percentage of the DMSO treated parasite lysate. IC50 curves are obtained by fitting the data using GraphPad Prism 5.0d.
Infection with P. berghei and inhibitor treatment of mice
All mouse experiments were approved by the Stanford Administration Panel on Laboratory Animal Care and strictly followed their specific guidelines. For each drug assay, Balb/c mice (~20 g) were infected by intravenous (I.V) or intra-peritoneal (I.P) injection with 1×106 P. berghei parasites isolated from an infected mouse on Day 0. Carfilzomib (0.5 and 1 mg/kg), PR-3 (80 mg/kg), or vehicle (10 mM citrate buffer, 20% kleptose) was administered by intravenous injection for 3 consecutive days on days indicated. For each drug dose, 3–5 mice were used. Due to technical challenge of 3 consecutive days of tail vein injections, some doses were administered as retro-orbital injection when required. Treatment with 80 mg/kg PR3 (figure 5) for all mice is administered as tail vein injection (day 5) and retro-orbital injections (day 6 and 7). During drug treatment, health of mice is assessed by observing physical appearances and activities of mice. Weight of mice is also monitored daily after infection. Parasitemia was monitored daily by thin blood smear and quantified by light microscope counting or flow cytometry as described above for P. falciparum. For quantification (for < 20% parasitemia) with thin blood smears, a minimum of 5 fields each containing at least 400 red blood cells were counted for parasite infection. A student’s one-tailed T-test was performed to assess statistical relevance of difference in treatment groups.
P. falciparum gametocyte viability assay
P. falciparum (3D7) parasites were induced to produce gametocytes by standard method (Ifediba and Vanderberg, 1981). Inhibitor efficacy was tested ten days after setting up the gametocyte culture, when it contained stage III and IV gametocytes. One ml of the culture was transferred to each well of a 24-well plate and treated with inhibitor or DMSO as a control for 24 hr at 37°C. Coded Giemsa-stained parasite smears from each well were counted and gametocyte stage determined by morphology by an experienced technician. Small, amorphous, densely-stained inclusions in the RBCs were not counted as live parasites.
HFF cell toxicity assays and human proteasome labeling
Supplementary Material
Highlights.
Purification of Plasmodium proteasomes and labeling with an activity-based probe
Identification of an epoxyketone-based inhibitor with parasite-specific toxicity
Demonstration that Plasmodium is sensitive to partial inhibition of its proteasome
Determination of key parameters for selective parasite killing with proteasome inhibitors
Acknowledgments
We would like to thank Juan Valderramos for technical assistance, and Prof. Herman Overkleeft from Leiden University for kindly providing proteasome probes. H.L. is supported by the National Science Scholarship from Agency for Science, Technology and Research (A*STAR) Singapore. This work was made possible by grants from the National Institutes of Health R01 AI078947 and R21 AI088541 to M.B. C.K. is an employee of Onyx Pharmaceutical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aminake MN, Schoof S, Sologub L, Leubner M, Kirschner M, Arndt HD, Pradel G. Thiostrepton derivatives exhibit antimalarial and gametocytocidal activity by dually targeting parasite proteasome and apicoplast. Antimicrob Agents Chemother. 2011 doi: 10.1128/AAC.01096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arastu-Kapur S, Ponder EL, Fonović UP, Yeoh S, Yuan F, Fonović M, Grainger M, Phillips CI, Powers JC, Bogyo M. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol. 2008;4:203–213. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- Bedford L, Paine S, Sheppard PW, Mayer RJ, Roelofs J. Assembly, structure, and function of the 26S proteasome. Trends Cell Biol. 2010;20:391–401. doi: 10.1016/j.tcb.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman MJ. Purification of Plasmodium falciparum merozoites for analysis of the processing of merozoite surface protein-1. Methods Cell Biol. 1994;45:213–220. doi: 10.1016/s0091-679x(08)61853-1. [DOI] [PubMed] [Google Scholar]
- Britton M, Lucas MM, Downey SL, Screen M, Pletnev AA, Verdoes M, Tokhunts RA, Amir O, Goddard AL, Pelphrey PM, et al. Selective inhibitor of proteasome’s caspase-like sites sensitizes cells to specific inhibition of chymotrypsin-like sites. Chem Biol. 2009;16:1278–1289. doi: 10.1016/j.chembiol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesny B, Goshu S, Cook JL, Williamson KC. The Proteasome Inhibitor Epoxomicin Has Potent Plasmodium falciparum Gametocytocidal Activity. Antimicrob Agents Chemother. 2009;53:4080–4085. doi: 10.1128/AAC.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo S, Kirk C, Aujay M, Buchholz T. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Research. 2007 doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- Dondorp A, Nosten F, Yi P, Das D. Artemisinin Resistance in Plasmodium falciparum Malaria — NEJM. … England Journal of …. 2009 doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt SM, Myung JM, Briones MR, Li WD, Corey EJ, Omura S, Nussenzweig V, Sinnis P. Proteasome inhibitors block development of Plasmodium spp. Antimicrob Agents Chemother. 1998;42:2731–2738. doi: 10.1128/aac.42.10.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf DH. The active sites of the eukaryotic 20 S proteasome and their involvement in subunit precursor processing. J Biol Chem. 1997;272:25200–25209. doi: 10.1074/jbc.272.40.25200. [DOI] [PubMed] [Google Scholar]
- Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- Kreidenweiss A, Kremsner PG, Mordmuller B. Comprehensive study of proteasome inhibitors against Plasmodium falciparum laboratory strains and field isolates from Gabon. Malaria Journal. 2008:7. doi: 10.1186/1475-2875-7-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, Demo SD, Bennett MK, van Leeuwen FWB, Chanan-Khan AA, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Child MA, Bogyo M. Proteases as regulators of pathogenesis: Examples from the Apicomplexa. Biochim Biophys Acta. 2012;1824:177–185. doi: 10.1016/j.bbapap.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Li D, de Carvalho LPS, Deng H, Tao H, Vogt G, Wu K, Schneider J, Chidawanyika T, Warren JD, et al. Inhibitors selective for mycobacterial versus human proteasomes. Nature. 2009;461:621–626. doi: 10.1038/nature08357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenthal C, Weich N, Chia YS, Heussler V, Klinkert MQ. The proteasome inhibitor MLN-273 blocks exoerythrocytic and erythrocytic development of Plasmodium parasites. Parasitology. 2005;131:37–44. doi: 10.1017/s003118200500747x. [DOI] [PubMed] [Google Scholar]
- Meiners S, Heyken D, Weller A, Ludwig A, Stangl K, Kloetzel PM, Krüger E. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of Mammalian proteasomes. J Biol Chem. 2003;278:21517–21525. doi: 10.1074/jbc.M301032200. [DOI] [PubMed] [Google Scholar]
- Mirabella AC, Pletnev AA, Downey SL, Florea BI, Shabaneh TB, Britton M, Verdoes M, Filippov DV, Overkleeft HS, Kisselev AF. Specific cell-permeable inhibitor of proteasome trypsin-like sites selectively sensitizes myeloma cells to bortezomib and carfilzomib. Chem Biol. 2011;18:608–618. doi: 10.1016/j.chembiol.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutomba MC, To WY, Hyun WC, Wang CC. Inhibition of proteasome activity blocks cell cycle progression at specific phase boundaries in African trypanosomes. Molecular and Biochemical Parasitology. 1997;90:491–504. doi: 10.1016/s0166-6851(97)00197-7. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- Steverding D, Baldisserotto A, Wang X, Marastoni M. Trypanocidal activity of peptidyl vinyl ester derivatives selective for inhibition of mammalian proteasome trypsin-like activity. Exp Parasitol. 2011;128:444–447. doi: 10.1016/j.exppara.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tschan S, Mordmuller B, Kun JF. Threonine peptidases as drug targets against malaria. Expert Opin Ther Targets. 2011 doi: 10.1517/14728222.2011.555399. [DOI] [PubMed] [Google Scholar]
- Verdoes M, Florea BI, Menendez-Benito V, Maynard CJ, Witte MD, van der Linden WA, van den Nieuwendijk AMCH, Hofmann T, Berkers CR, van Leeuwen FWB. A Fluorescent Broad-Spectrum Proteasome Inhibitor for Labeling Proteasomes In Vitro and In Vivo. Chem Biol. 2006;13:1217–1226. doi: 10.1016/j.chembiol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Verdoes M, Florea BI, van der Marel GA, Overkleeft HS. Chemical Tools To Study the Proteasome. Eur J Org Chem. 2009;2009:3301–3313. [Google Scholar]
- Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- Voorhees PM, Dees EC, O’Neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316–6325. [PubMed] [Google Scholar]
- World Health Organization. World malaria report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- Yang J, Wang Z, Fang Y, Jiang J, Zhao F, Wong H, Bennett MK, Molineaux CJ, Kirk CJ. Pharmacokinetics, pharmacodynamics, metabolism, distribution, and excretion of carfilzomib in rats. Drug Metab Dispos. 2011;39:1873–1882. doi: 10.1124/dmd.111.039164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.