Abstract
Most vaccines and basic studies of T cell epitopes in M. tuberculosis emphasize water soluble proteins that are secreted into the extracellular space and presented in the context of MHC Class II. Much less is known about the role of antigens retained within the cell wall. We used polyclonal T cells from infected humans to probe for responses to immunodominant antigens in the M. tuberculosis cell wall. We found that the magnitude of response to secreted or cell wall intrinsic compounds was similar among healthy controls, patients with latent tuberculosis, and patients with active tuberculosis. Individual responses to secreted antigens and cell wall extract were strongly correlated (r2=0.495, p=0.001), suggesting that T cells responding to cell wall and secreted antigens are present at similar frequency. Surprisingly, T cell stimulatory factors intrinsic to the cell wall partition into organic solvents; however, these responses are not explained by CD1-mediated presentation of lipids. Instead, we find that molecules soluble in organic solvents are dependent upon MHC Class II and recognized by IFN-γ secreting CD4+ T cells. We reasoned that MHC Class II dependent antigens extracting into lipid mixtures might be found among triacylated lipoproteins present in mycobacteria. We used M. tuberculosis lacking prolipoprotein signal peptidase A (lspA), an enzyme required for lipoprotein synthesis, to demonstrate loss of polyclonal T cell responses. Our results demonstrate the use of bacterial genetics to identify lipoproteins as an unexpected and immunodominant class of cell wall-associated antigens targeted by the polyclonal human T cell response to M. tuberculosis.
Introduction
Mycobacterium tuberculosis remains a leading cause of death worldwide, and CD4-restricted T cell responses have been shown to be critical to controlling infection in both humans and animal models (1, 2). Human tuberculosis occurs along a spectrum ranging from latent infection, in which asymptomatic patients are infected with the bacillus, to active disease, in which patients can transmit the infection to others. Defining the immunodominant targets of responding T cells during natural infection has resulted in major advances in immunodiagnostics as well as new vaccines. For example, the 6 kilodalton early secreted antigenic target (ESAT-6) and 10 kilodalton culture filtrate antigen (CFP-10) are core components of QuantiFERON-TB and T-SPOT.TB, two tests approved by the Food and Drug Administration for the diagnosis of latent tuberculosis infection (3). Members of the antigen 85 complex are immunodominant antigens for T cells included in at least two subunit vaccines currently in Phase II clinical trials (4). ESAT-6, CFP-10, and antigen 85 are among many highly abundant secreted proteins present in culture supernatants that have been the focus of important and productive research for more than twenty years (5–7). However, secreted protein antigens are only a subset of the antigenic pool available to human T cells. Many mycobacterial proteins are embedded in the cell wall, such as proteins that have undergone post-translational glycosylation and acylation (8, 9). Further, recent studies have also shown that the lipid rich cell wall of M. tuberculosis contains mycobacterial lipid antigens presented to T cells in the context of CD1a, CD1b, and CD1c (10–15). Nearly all prior screens for immunodominant antigens emphasize proteins as a source of antigens for T cells, but the discovery of lipid antigen presentation provides a rationale to consider lipidic stimuli of T cells as well. It is currently not known which M. tuberculosis cell wall-associated antigens are most commonly targeted by T cells from infected humans.
To address this question, we used an ex vivo assay to study polyclonal human T cell responses to M. tuberculosis cell wall-associated antigens. As contrasted to antigen screens carried out in small animal models after experimental infection, this approach emphasizes immune responses generated in natural infections and uses human antigen presenting cells (APCs) and T cells that reflect restriction by genetically diverse MHC proteins, nonpolymorphic Group 1 CD1 proteins, and other species-specific aspects of the human APC-T cell interaction. We avoided T cell cloning strategies because long term in vitro culture can induce bias and functional drift in ways that reflect the ability of individual clones to survive rather than their effects occurring directly ex vivo. To capture the clinical complexity of human tuberculosis, we studied healthy controls, patients with latent tuberculosis infection, and patients with active disease.
Contrary to the general view that most or all T cell stimulating factors are secreted, we found strong T cell responses to extracts of molecules embedded within the cell wall. Human polyclonal T cell responses to these factors were strongly correlated with two secreted antigens, ESAT-6 and CFP-10, indicating T cells responding specifically to cell wall intrinsic antigens are present at high frequency. Isolation of T cell stimulatory factors from the cell wall showed that stimulatory substances were enriched with solvents designed to capture lipids. After initial studies ruled out a role for CD1, we demonstrated polyclonal CD4+ T cell responses were dependent on MHC Class II, blocked by chloroquine treatment, and absolutely dependent upon lipoprotein production by M. tuberculosis. Our data reveal that cell wall lipoproteins are major targets of the human T cell response to M. tuberculosis and invoke new models regarding the role of lipoproteins as both adjuvant and T cell immunogen.
Materials and Methods
Bacteria and antigens
M. bovis BCG and M. tuberculosis H37Rv were cultivated in 7H9 medium (Difco) supplemented with 0.05% Tween-80 and 1% glucose. Cell wall extracts were generated by exposing PBS washed cell pellets to chloroform:methanol (2:1, v/v) followed by chloroform:methanol (1:2, v/v) at 20°C for 2 hours (Figure 1A). Subfractions of cell wall extracts were generated by first loading 20 mg onto an open 2 × 20 silica column (Supelco), and then serially eluting with 40 ml chloroform, 40 ml acetone, and 40 ml methanol. For analytical TLC, 150 µg lipids were loaded on a 20 by 20 cm2 Silica 60 TLC plate (Merck) and developed in 60:16:2 v/v/v chloroform:methanol:water. Plates were sprayed with 3% w/v cupric acetate in 8% v/v phosphoric acid, dried and charred for one hour at 140°C in an oven. M. tuberculosis strain H37Rv with the lipoprotein signal peptidase gene (lspA) deleted and complemented have been described previously (16). Prior to enzymatic treatment or cellular assays, antigens were dried onto a glass surface under a stream of sterile nitrogen gas. Antigens were then sonicated for 2 minutes in a water bath sonicator (Branson) in the desired aqueous buffer or tissue culture medium. Overlapping peptide pools of ESAT-6 and CFP-10 were provided by Ajit Lalvani.
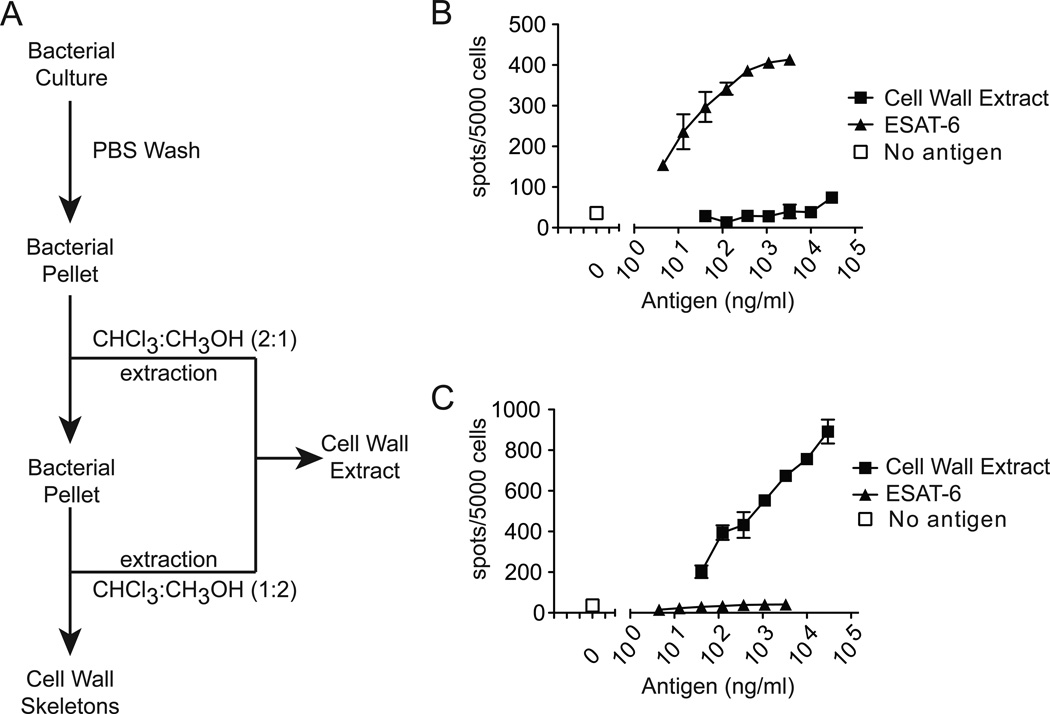
Figure 1.
Generating and assessing cell wall extracts. (A) Scheme for generating cell wall extract by treating bacterial pellets with organic solvents chloroform (CHCl3) and methanol (CH3OH). T-cell clones (B) F5 or (C) LDN5 were co-incubated with monocyte-derived dendritic cells and titrating concentrations of either cell wall extract or whole ESAT-6 protein. Data are representative of three independent experiments.
Digestion of Cell Wall Extracts
Pronase (Roche) and proteinase K (Sigma) were used to digest M. tuberculosis methanol fraction in protease buffer (10mM CaCl, 10mM HEPES buffer, 25mM ammoniumbicarbonate) for 4 h at 40°C, followed by 10 min of inactivation at 85°C as described previously (17). Mock treatment was performed in the same buffer and at the same temperatures, but without addition of the proteases. Lipase treatment was performed using dry lipoprotein lipase (Sigma). The digest was performed in 100 µl PBS containing 0.25 mg/ml cell wall extract and 0.5 mg/ml lipoprotein lipase. After overnight incubation at 37°C, the enzyme was heat inactivated for 10 min at 70°C.
Cellular assays
Peripheral blood mononuclear cells were separated by Ficoll density gradient centrifugation. Monocytes were isolated by adherence to plastic and treatment for 72 hours with granulocyte-macrophage colony-stimulating factor (300 IU/ml) and IL-4 (200 IU/ml) (Peprotech) to generate autologous monocyte-derived dendritic cells (DCs) expressing CD1. Non-adherent cells (called PBMC) and DCs were frozen separately until use. Antigen-specificity and restriction were tested using DCs (5 × 104 cells per well) or CD1a, CD1b, CD1c, or CD1d-transfected K562 cells (2 × 104 cells per well) as antigen presenting cells in RPMI medium supplemented with 10% (v/v) FBS (Hyclone), essential and nonessential amino acids (Gibco), penicillin-streptomycin (Gibco) and β-mercaptoethanol. For IFN-γ ELISPOT assays, co-cultures of APCs and T cells were incubated for 20 hours in Multiscreen-IP filter plate (96 wells; Millipore) coated with anti-IFN-γ according to the manufacturer's instructions (Mabtech), in the presence or absence of the monoclonal antibodies that block MHC II (L243), MHC I and HLA-E (W6/32), CD1a (OKT6), CD1b (BCD1b.3), or CD1d (CD1d.42), or an isotype control (P3), at a final concentration of 10 µg/ml. Overlapping peptide pools of ESAT-6 and CFP-10 were dissolved in DMSO and tested in combination at a concentration of 10 µg/ml. Total cell wall extracts and sub-fractions were tested at a concentration of 5 or 10 µg/ml. For TLR2 blocking experiments, DCs from a donor with latent tuberculosis were pre-incubated in the presence of 15 µg/ml anti-TLR2 blocking antibody or isotype control (clones T2.5 and T2.13, respectively, gifts of Carsten Kirshning (18)) for one hour at 37°C. Subsequently, PBMC and methanol eluates (10µg/ml) were added for overnight incubation prior to IFN-γ ELISPOT. For antigen processing experiments, 25 µM chloroquine (Sigma) was added to DCs for 15 minutes prior to adding antigen and T cells. Chloroquine was continuously present for the duration of the ELISPOT assay.
Flow Cytometry
For isolation of T cell subsets, peripheral blood mononuclear cells were sorted using a FACSAria flow cytometer using CD3 APC-Alexa Fluor 750 (Invitrogen), αβ TCR FITC (BD Bioscience), CD4 APC (BD Bioscience), and CD8 PerCP-Cy5.5 (BD Bioscience). Total T cells were isolated by sorting for CD3 only.
Human Subjects
Patients were recruited from the Lemuel Shattuck Hospital (Jamaica Plain, MA) and from employee health services at the Brigham and Women’s Hospital (Boston, MA). This work was approved by the Lemuel Shattuck Hospital and the Partners Healthcare Institutional Review Boards. Our study population consisted of three subgroups (Table 1). Healthy controls (N=11) were uninfected with tuberculosis as demonstrated by negative purified protein derivative (PPD) skin test and no history of immunocompromise or immunosuppressive medications. Subjects with a positive PPD > 10mm (N=33) but with normal chest radiographs and without signs of active disease (cough, fever, weight loss) were considered to have latent tuberculosis infection. Typically, these patients belonged to high-risk groups, such as recent immigrants or injection drug users, and would be offered isoniazid preventive therapy. Finally, patients with active tuberculosis (N=22) were defined by the presence of acid-fast bacilli in expectorated sputum and response to anti-tuberculosis therapy as documented by sputum culture conversion. After obtaining informed consent, 50 mls of blood or a standard blood bank donation were collected.
Table 1.
Patient characteristics of study cohort. Statistical significant was calculated using Fisher’s exact test (Sex and Foreign Born) or Kruskal-Wallis test (Age and Treatment).
| Healthy Controls | Latent TB | Active TB | p-value | |
|---|---|---|---|---|
| N | 11 | 33 | 22 | -- |
| Age (median and IQR) | 47 (30–51) | 37 (28–48) | 47 (38–57) | 0.07 |
| Sex (%F) | 45 | 48 | 17 | 0.04 |
| Foreign Born (%) | 9 | 45 | 70 | 0.003 |
| HIV-infected | 0 | 0 | 0 | -- |
| Treatment (median days and IQR) | -- | 17 (0–243) | 27 (7–195) | 0.24 |
Statistical Methods
T-cell responses among the three groups of subjects were analyzed in Stata IC 11.0 (StataCorp) using a non-parametric test for trend with the assumption that degree of infection is lowest in healthy controls, intermediate during latent infection, and highest during active disease. Continuous variables stratified by subgroups were analyzed by non-parametric Wilcoxon rank sums or Kruskal-Wallis tests. Categorical variables were analyzed using the Fisher’s exact test. Linear regression between two continuous variables was performed in Prism 5.0 (GraphPad Software, Inc.).
Results
To screen for cell wall-associated substances that stimulate human T cells, we first washed intact M. tuberculosis bacilli with phosphate buffered saline (PBS) to remove secreted proteins and then sterilized wet bacterial pellets by extraction with organic solvents. Organic soluble fractions were pooled to produce ‘cell wall extract’ while the organic insoluble fraction, which was enriched for proteins, was designated ‘cell wall skeletons’ (Figure 1A). We tested the ability of M. tuberculosis cell wall extract to stimulate a T-cell clone specific for ESAT-6, but observed no response, confirming the exclusion of secreted protein antigens by washing cells and extracting substances with organic solvent (Figure 1B). By contrast, the glycolipid-specific T-cell clone LDN5 was easily stimulated by cell wall extract but not by ESAT-6 or M. tuberculosis culture supernatants (Figure 1C and data not shown). Thus, M. tuberculosis cell wall lipid antigens targeted by human T-cells can be effectively separated from secreted protein antigens, and neither preparation has broadly active mitogenic effects for T cells.
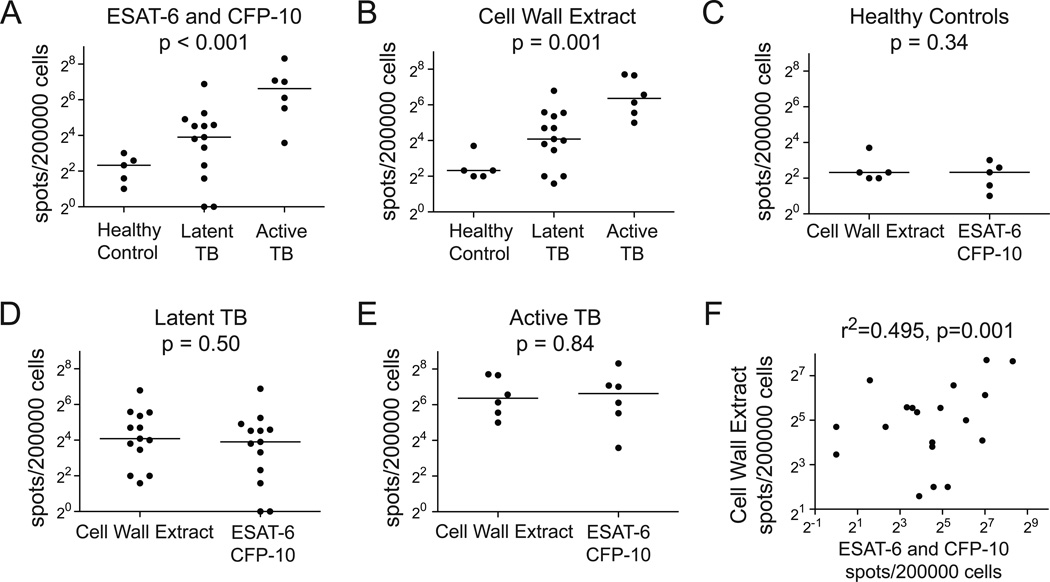
We then compared T cell responses to cell wall extract and secreted antigens ESAT-6 and CFP-10 in a cohort of healthy subjects and subjects infected with M. tuberculosis. We considered the degree of infection to be greatest in those with active disease, intermediate in those with latent disease, and negative in healthy controls. As expected, healthy controls had the fewest IFN-γ spot forming units (SFU) in response to ESAT-6 and CFP-10. Greater responses among subjects with latent tuberculosis confirmed their clinical assignment on the basis of PPD test result. Notably, patients with active tuberculosis had the greatest responses, and we noted a statistically significant increase in IFN-γ SFU with degree of infection (Figure 2A; p<0.001). Similarly, we found that healthy controls had the fewest IFN-γ SFU in response to cell wall extract, and these also increased with degree of infection (Figure 2B; p=0.001). When we compared responses to cell wall extract with ESAT-6 and CFP-10, we found no difference in magnitude of response among any of the three groups of study subjects (Figure 2C, 2D, and 2E). Even though we had shown that cell wall extracts were not likely contaminated with ESAT-6 (Figure 1B), we considered the possibility that the two sets of responses involved antigens that partitioned into both fractions. Importantly, we noted that many individuals responded at high levels to only one or the other antigen preparation. As a group, there was a strong correlation between responses to cell wall extract and ESAT-6 and CFP-10 (Figure 2F; r2=0.495, p=0.001). Thus, cell wall antigens provide a strong stimulus to the human immune system that is distinct from the major secreted antigens used for immunodiagnosis and subunit vaccines.
Figure 2.
Comparing the polyclonal lymphocyte response to cell wall antigens with model secreted antigens. DCs and PBMC were co-incubated overnight in the presence of 10 µg/ml M. tuberculosis cell wall extract or 10 µg/ml ESAT-6 and CFP-10. IFN-γ ELISPOT responses stratified by clinical group for (A) ESAT-6 and CFP-10 or (B) cell wall extract. Nonparametric trend test was used to compare responses among ordered groups. IFN-γ ELISPOT responses stratified by antigen for (C) Healthy Controls, (D) PPD Positive, or (E) Active TB study subjects. Wilcoxon rank sums test was used to test for statistical significance. (F) Linear regression comparing IFN-γ ELISPOT responses between cell wall extract and ESAT-6 and CFP-10 for subjects with latent or active tuberculosis.
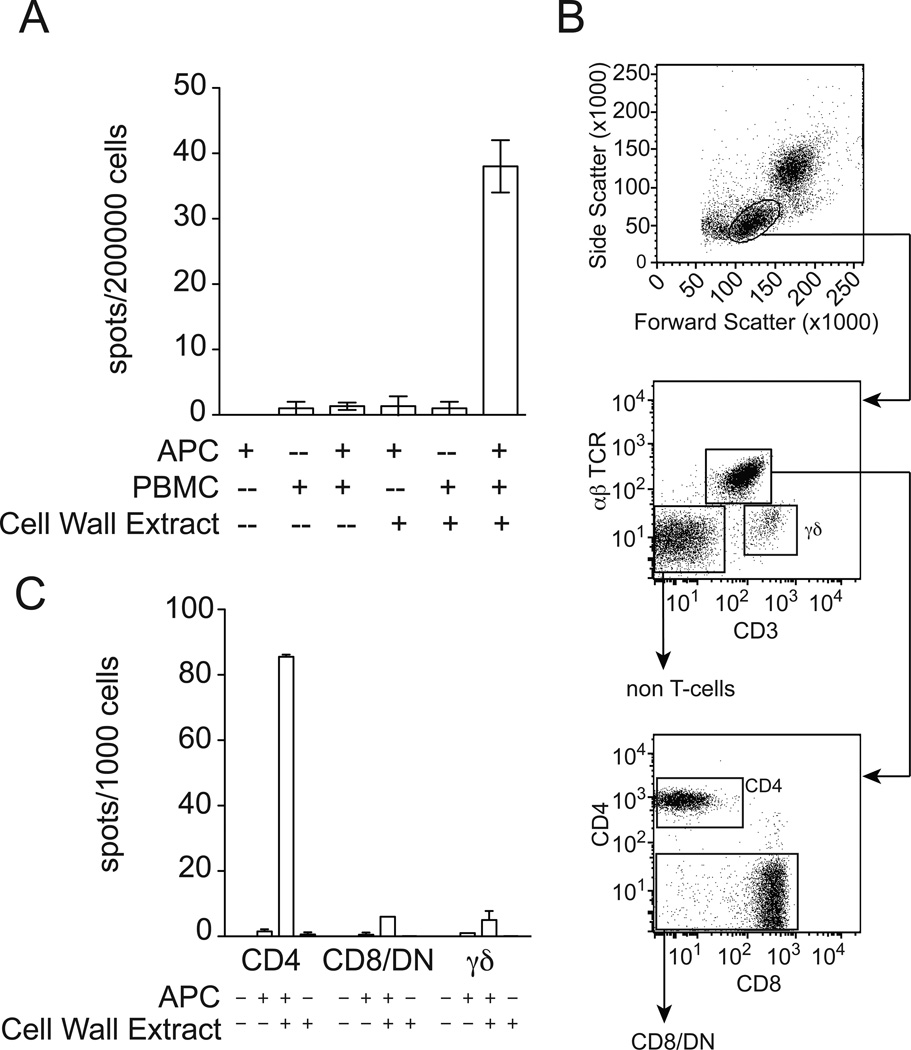
We found that production of IFN-γ by PBMC was absolutely dependent on the presence of added DCs (Figure 3A). Therefore, the stimulatory capacity of cell wall extract was not due to a soluble factor that directly acted on PBMC. Instead, it was more likely dependent on the antigen processing capacity of added DCs. To directly determine if the PBMC response to cell wall extracts enriched for lipids was mediated by T cells, we sorted PBMC into non T-cells, γδ T-cells, and αβ T-cells. αβ T-cells were further sorted into CD4+ or CD8+ and double negative (DN) pools (Figure 3B). Each cell type was tested for the ability to produce IFN-γ in response to M. tuberculosis cell wall extract. Responses were highest in the CD4+ subset of αβ T-cells (Figure 3C and data not shown). Thus, CD4+ T cell activation by lipid-enriched cell wall extract requires cell-to-cell contact or processing by a professional antigen-presenting cell.
Figure 3.
Cellular requirements for the production of IFN-γ by cell wall extracts. (A) PBMC from a subject with latent tuberculosis infection, DCs, and cell wall extract were tested in combination for the ability to induce IFN-γ production by ELISPOT. (B) FACS sorting strategy for PBMC. (C) Lymphocyte sub-populations were co-incubated overnight in the presence of 5 µg/ml cell wall extract and DCs prior to ELISPOT.
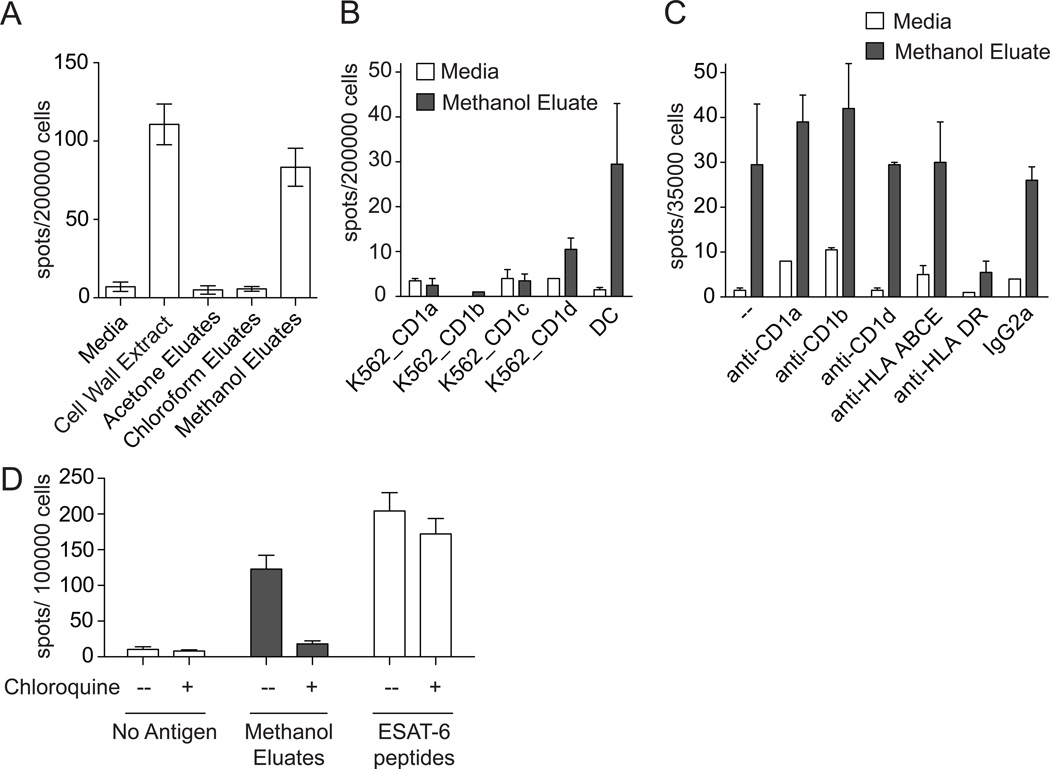
To more directly characterize its biochemical properties, cell wall extract was passed over an open silica column and serially extracted with chloroform, acetone, and methanol to separate stimulatory factors with low, intermediate, or high polarity, respectively. Fractions were dried, sonicated into media, and tested for stimulation of IFN-γ release from PBMC. Only the methanol eluate retained stimulatory capacity, indicating that the stimulatory factor, although extractable in chloroform and methanol, was relatively polar (Figure 4A). Our prior work revealed that methanol extracts are normally enriched in lipopeptides, so we attempted to reduce the biologic activity of methanol eluates by protease and lipase digestion (17). However, these experiments were inconclusive because antigenicity was reduced with heat treatment alone, and this step is required for inactivation of enzymes after digestion (data not shown). We also attempted direct identification of antigens using thin layer chromatography and mass spectrometry, but biochemical separation by normal phase chromatography failed to resolve the biological activity into any single fraction (data not shown). Methanol eluates containing partially purified polar lipids were then used in subsequent experiments.
Figure 4.
Restricting element for presentation of antigens in cell wall extract. (A) Cell wall extract from M. tuberculosis and sub-fractions (10 µg/ml) were co-incubated overnight with PBMC and DCs from a subject with latent tuberculosis infection. (B) Sorted T-cells were co-incubated overnight with methanol eluates and either DCs or K562 cells transfected to express CD1a, CD1b, CD1c, or CD1d. (C) Sorted T-cells were co-incubated overnight with DCs, methanol eluates, and 10 µg/ml blocking antibody against CD1a (OKT6), CD1b (BCD1b.3), CD1d (CD1d42), MHC Class I and HLA-E (W6/32), HLA-DR (L243), or isotype control (IgG2a). (D) DCs were pre-incubated with 25 µM chloroquine for 15 minutes. These were co-incubated overnight with sorted T-cells in the continuous presence of 25 µM chloroquine and either 10 µg/ml methanol eluates or 10 µg/ml ESAT-6 peptides. In all cases, IFN-γ production was assessed by ELISPOT.
Because methanol eluates of cell wall extracts contain lipids, including known CD1 antigens like dideoxymycobactins and phosphomycoketides, we hypothesized that the dominant antigens in this fraction would be CD1-restricted. Human DCs express five CD1 proteins of which four (CD1a, CD1b, CD1c, and CD1d) present lipid antigens at the cell surface. To identify which CD1 protein might mediate T cell activation, we co-incubated polyclonal T cells with methanol eluates in the presence of K562 cells stably transfected with CD1a, CD1b, CD1c, or CD1d. Surprisingly, we were unable to detect T cell activation in response to cells expressing any of the four CD1 proteins (Figure 4B). Failure to stimulate T cells was not likely due to an intrinsic defect in K562 cells because each of these transfectants has been shown to efficiently present exogenous lipid antigens and efficiently activate T cells (19). Nevertheless, we could directly test CD1 proteins in a different system in which the APC was proven to be sufficient for activation and then attempt to block the response using antibodies that recognize and inhibit the function of CD1a, CD1b, and CD1d. This experiment showed that anti-CD1 did not block activation, confirming results using transfected cells. Surprisingly, anti-MHC II, but not an isotype matched control antibody, blocked polyclonal T cell activation nearly to baseline (Figure 4C). Taken together, these data show that organic soluble cell wall factors stimulate polyclonal T cells in an MHC Class II dependent rather than CD1 dependent manner. MHC Class II antigen loading canonically occurs via the endosomal processing pathway, but can also occur at the cell surface. To distinguish between these two possibilities, we performed a T cell stimulation assay in the continuous presence of chloroquine, which inhibits endosomal acidification and has been shown to reduce MHC Class II antigen processing and presentation (20). As expected, we found no effect on the capacity of DCs to present ESAT-6 peptides, which do not require cellular processing and are likely loaded at the cell surface (Figure 4D). This result also revealed that the continuous presence of chloroquine did not globally impair DC function or lead to non-specific T cell activation. On the other hand, the stimulatory capacity of methanol eluates was markedly reduced by chloroquine treatment of DCs indicating that stimulatory factors in methanol eluates require endosomal processing.
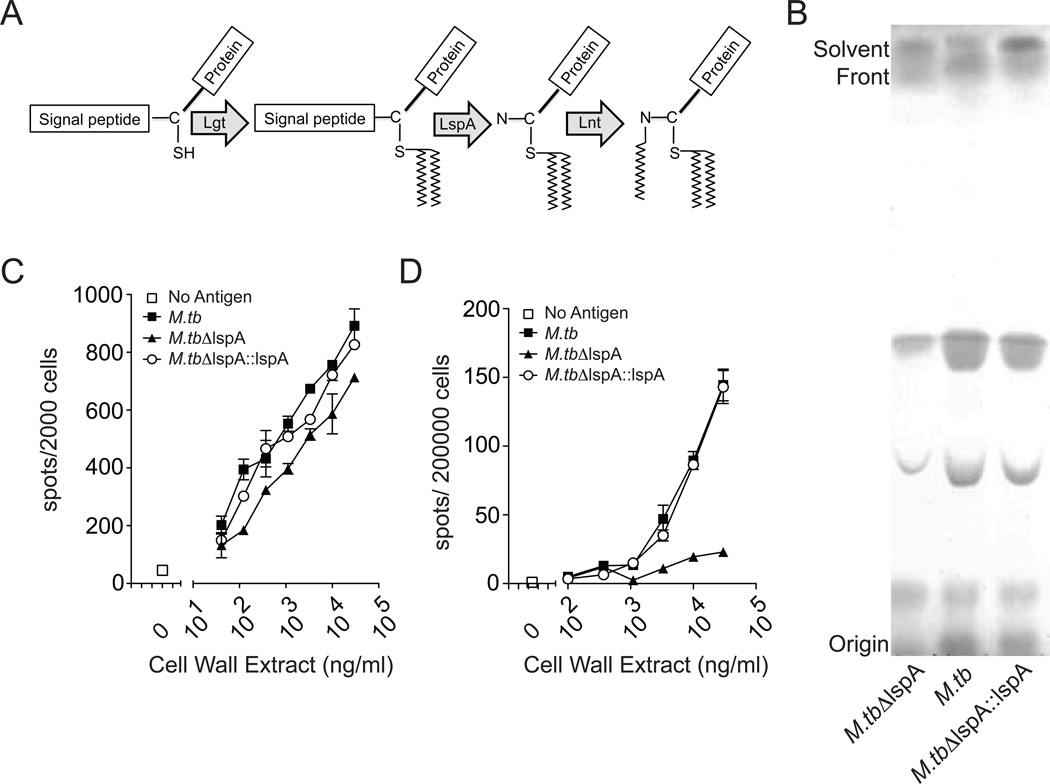
We found that T cells were activated by an antigen-presenting molecule that binds peptides, yet the antigens were extracted by solvents commonly used for lipids. To reconcile these apparently contradictory observations, we hypothesized that MHC Class II restricted hydrophobic antigens included lipoproteins. Supporting this hypothesis, MHC Class II epitopes have been identified from at least four M. tuberculosis genes annotated as lipoproteins (www.immuneepitope.org). Also, a prior study implicated mycobacterial lipopeptides as T cell antigens using biochemical criteria (21). Bacterial lipoprotein synthesis is mediated by the consecutive activity of three enzymes: prolipoprotein diacylglyceryl transferase (Lgt), prolipoprotein signal peptidase II (Lsp), and lipoprotein N-acyl transferase (Lnt), resulting in triacylation of a conserved and essential N-terminal cysteine residue (Figure 5A). Mycobacteria produce a number of terminally acylated lipoproteins, which might have explained our prior inability to isolate stimulatory factors into a single chromatographic fraction. Rather than testing bacteria deficient in any one lipoprotein, we reasoned that deletion of the mycobacterial signal peptidase lspA would represent a reliable method to eliminate this entire class of candidate T cell antigens genetically. We generated cell wall extracts from wild type M. tuberculosis strain H37Rv (M.tb), bacteria that were deficient in lspA (M.tbΔlspA), and bacteria that had lspA complemented (M.tbΔlspA::lspA) (16). Thin layer chromatography analysis of cell wall extract showed no broad differences in the migration of bands corresponding to the most abundant lipids, indicating that there was not a global change in lipid content among the bacterial mutants (Figure 5B). This method also allowed us to normalize the abundance of total lipids derived from the three bacteria. There was minimal effect of lspA deletion on stimulation of the glycolipid-specific and CD1b-restricted T-cell clone LDN5, which recognizes glucose monomycolate, a glycolipid that is not expected to be altered by lspA deletion (Figure 5C). However, the capacity of M.tbΔlspA extracts to stimulate polyclonal T cells was markedly diminished compared to wild-type, and genetic complementation of lspA restored the antigenic activity (Figure 5D). Since lspA is required for the production of mature lipoproteins, these data indicate that lipoproteins are the source of immunodominant MHC Class II mediated stimuli in M. tuberculosis cell wall extracts.
Figure 5.
Analysis of T cell responses to cell wall extracts derived from lspA mutant bacteria. (A) Scheme depicting bacterial lipoprotein synthesis. lspA is responsible for cleaving the signal peptide and exposing a free amine on the modified cysteine residue for the final N-acylation reaction. (B) Thin layer chromatography of cell wall extract from wild-type M. tuberculosis strain H37Rv, ΔlspAand ΔlspA::lspA bacteria. (C) CD1-restricted T-cell clone LDN5 and (D) PBMC were co-incubated overnight with titrating concentrations of cell wall extract and DCs prior to IFN-γ ELISPOT. Data in C and D are representative of two independent experiments.
Lipoproteins present in cell wall extracts stimulate T cells when processed and presented by DCs. However, lipoproteins are also known agonists of Toll-like receptor 2 (TLR2), so we considered the possibility of secondary stimulation of T cells after TLR2 engagement. Cell wall extracts contain a mixture of compounds including CD1-restricted glycolipids and phospholipids as well as Toll-like receptor 2 (TLR2) agonist glycolipids (22). We attempted to reduce the stimulatory effect of cell wall extracts by using an antibody that directly binds to and inhibits the function of TLR2. Pre-incubating dendritic cells with TLR2 blocking antibody did not abrogate the T cell stimulatory effect of methanol eluates (Supplemental Figure 1). Despite the presence of multiple TLR2 agonist ligands in M. tuberculosis cell wall extract, such as phosotidyl-myo-inositol mannosides and triacylated lipoproteins, these data reveal that T cell stimulation occurs independently of TLR2.
Discussion
In summary, our data reveal two key findings regarding human immunity to M. tuberculosis. First, using a system that emphasizes natural infection and human DCs and T cells, we identified cell wall-associated immunostimulatory factors that are distinct from the secreted antigens currently the focus of vaccine and immunodiagnostic development. Second, mechanistic analysis of the response shows that this response is dependent on DCs, involves CD4+ T cells, and is dependent on MHC Class II via endosomal processing pathways as well as bacterial lspA function. Though detailed studies regarding the breadth of ex vivo T cell responses to cell wall-associated antigens are limited, our data reveal that such studies are feasible in a way that captures the immunologic and clinical complexity of human tuberculosis.
Our findings confirm and extend a previous study which used biochemical criteria to identify mycobacterial lipopeptides as antigens for cytotoxic CD4+ lymphocytes (21). Though we show that polyclonal T cell responses to cell wall antigens are strongly correlated with secreted antigens, the antigenic activity of cell wall extracts is likely due to a number of lipoproteins whereas we compared this with overlapping peptide pools of just two proteins, ESAT-6 and CFP-10. Thus, the contribution of any single immunodominant lipoprotein remains to be determined. Little is known about the breadth of human T-cell responses to mycobacterial lipoproteins. Sutcliffe and Harrington identified 99 lipoproteins in M. tuberculosis by bio-informatic analysis, representing 2.5% of the proteome (23). The vast majority of these are pending biochemical validation, and systematic T cell epitope mapping has not been reported.
Our data show that lspA function is required for T cell activation. Since lspA catalyzes the release of the signal peptide from nascent prolipoproteins, polyclonal T cells may be recognizing signal peptide sequences as dominant antigens. Lipoproteins are characterized by the presence of a conserved ‘lipobox’ motif (LVI/ASTVI/GAS/C) on the C-terminal portion of the signal peptide (24). The N-terminus of the signal peptide is less conserved and varies in length from 16–33 amino acids (23). While this is an appropriate range for an MHC Class II restricted peptide antigen, it is less likely that such peptides would partition into organic solvents and survive solid phase extraction in our system. Another possibility is that the failure to cleave the signal peptide results in an inability of Lnt to catalyze the final N-acylation reaction (Figure 4A). Therefore, partially acylated lipoproteins may fail to insert into the cell wall and extract into organic solvents. Supporting this hypothesis, lspA deficient M. tuberculosis bacilli are more susceptible to killing by malachite green and show decreased virulence, possibly as a result of increased cell wall permeability (16, 25–27). Even if partially acylated lipoproteins extract into organic solvents, they may fail to engage the proper antigen-processing pathway. Diacylated lipoproteins bind the TLR2/TLR6 heterodimer while triacylated lipoproteins bind the TLR1/TLR2 heterodimer. Previous studies have shown that the 19-kilodalton mycobacterial lipoprotein inhibits MHC Class II antigen processing via a TLR2 mediated mechanism (28). However, we show that MHC Class II dependent T cell stimulation occurs despite TLR2 blocking (Supplemental Figure 1), suggesting lipoproteins are heterogeneous in their effects on antigen processing. Further, it is unlikely that TLR activation is a primary or specific mechanism of the CD4+ T cell activation occurring here because many TLR2 agonist phospholipids are present in lspA mutants, and we failed to block the response with anti-TLR2 antibody. The fact that lipoproteins are a natural combination of adjuvant and antigen may be one reason for the observed immunodominance in our polyclonal system. Recently, synthetic lipoproteins have been used successfully as vaccine immunogens in the absence of additional adjuvant (29–31). Further, vaccination with a lipid-modified epitope of the 16 kDa antigen from M. tuberculosis was shown to protect mice against aerosol challenge to a greater extent that peptide vaccination alone (32). Our results invite a reconsideration of whether such stimulatory molecules operate through the T cell receptor, TLRs, or both.
A significant body of literature exists to support the importance of M. tuberculosis secreted protein antigens, and some have recently suggested that the strength of T cell responses to these antigens may paradoxically assist the bacteria in its life cycle (33). T cell responses to lipids and lipoproteins are common at the polyclonal level and provide another ‘axis’ by which we can assess the natural history of infection in humans.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the patients and staff at the Lemuel Shattuck Hospital for their participation. We would also like to thank Ajit Lalvani for recombinant ESAT-6 and CFP-10 peptide pools, John Annand for generating bacterial lipid extracts, Annemieke de Jong for providing K562 transfectants, William Jacobs for providing ΔlspA mutant and complemented bacteria, and Gwendolyn Swarbrick for logistical support.
Footnotes
This work was supported by the National Institutes of Health (T32-AI007061 to CS and R01 AI 049313 to DBM), the Irvington Institute Fellowship Program of the Cancer Research Institute (CS), and Nederlands Wetenschappelijk Onderzoek (Meervoud grant 836.08.001 to IVR).
Author Contributions
CS, IVR, DML, and DBM designed the cellular experiments. CS and MTT designed the clinical study and enrolled subjects. CS and IVR conducted the experiments. All authors analyzed the data. CS and IVR wrote the manuscript with contributions from all authors.
References
- 1.Winslow GM, Cooper A, Reiley W, Chatterjee M, Woodland DL. Early T-cell responses in tuberculosis immunity. Immunol Rev. 2008;225:284–299. doi: 10.1111/j.1600-065X.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 2002;4:635–646. doi: 10.1016/s1286-4579(02)01582-4. [DOI] [PubMed] [Google Scholar]
- 3.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011;10:645–658. doi: 10.1586/erv.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins FM, Lamb JR, Young DB. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988;56:1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb JR, Young DB. A novel approach to the identification of T-cell epitopes in Mycobacterium tuberculosis using human T-lymphocyte clones. Immunology. 1987;60:1–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Jackett PS, Bothamley GH, Batra HV, Mistry A, Young DB, Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988;26:2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moody DB. The surprising diversity of lipid antigens for CD1-restricted T cells. Advances in immunology. 2006;89:87–139. doi: 10.1016/S0065-2776(05)89003-0. [DOI] [PubMed] [Google Scholar]
- 9.Bell C, Smith GT, Sweredoski MJ, Hess S. Characterization of the Mycobacterium tuberculosis proteome by liquid chromatography mass spectrometry-based proteomics techniques: a comprehensive resource for tuberculosis research. J Proteome Res. 2012;11:119–130. doi: 10.1021/pr2007939. [DOI] [PubMed] [Google Scholar]
- 10.Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, Porcelli SA. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 11.Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, Besra GS, Porcelli SA. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 12.Van Rhijn I, Zajonc DM, Wilson IA, Moody DB. T-cell activation by lipopeptide antigens. Current opinion in immunology. 2005;17:222–229. doi: 10.1016/j.coi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 14.Layre E, Collmann A, Bastian M, Mariotti S, Czaplicki J, Prandi J, Mori L, Stenger S, De Libero G, Puzo G, Gilleron M. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem Biol. 2009;16:82–92. doi: 10.1016/j.chembiol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Bohmer G, Prandi J, Mori L, Puzo G, De Libero G. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banaiee N, Kincaid EZ, Buchwald U, Jacobs WR, Jr, Ernst JD. Potent inhibition of macrophage responses to IFN-gamma by live virulent Mycobacterium tuberculosis is independent of mature mycobacterial lipoproteins but dependent on TLR2. J Immunol. 2006;176:3019–3027. doi: 10.4049/jimmunol.176.5.3019. [DOI] [PubMed] [Google Scholar]
- 17.Van Rhijn I, Young DC, De Jong A, Vazquez J, Cheng TY, Talekar R, Barral DC, Leon L, Brenner MB, Katz JT, Riese R, Ruprecht RM, O'Connor PB, Costello CE, Porcelli SA, Briken V, Moody DB. CD1c bypasses lysosomes to present a lipopeptide antigen with 12 amino acids. J Exp Med. 2009;206:1409–1422. doi: 10.1084/jem.20082480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest. 2004;113:1473–1481. doi: 10.1172/JCI20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong A, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nature immunology. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lombard-Platlet S, Bertolino P, Deng H, Gerlier D, Rabourdin-Combe C. Inhibition by chloroquine of the class II major histocompatibility complex-restricted presentation of endogenous antigens varies according to the cellular origin of the antigen-presenting cells, the nature of the T-cell epitope, and the responding T cell. Immunology. 1993;80:566–573. [PMC free article] [PubMed] [Google Scholar]
- 21.Bastian M, Braun T, Bruns H, Rollinghoff M, Stenger S. Mycobacterial lipopeptides elicit CD4+ CTLs in Mycobacterium tuberculosis-infected humans. J Immunol. 2008;180:3436–3446. doi: 10.4049/jimmunol.180.5.3436. [DOI] [PubMed] [Google Scholar]
- 22.Layre E, Sweet L, Hong S, Madigan CA, Desjardins D, Young DC, Cheng TY, Annand JW, Kim K, Shamputa IC, McConnell MJ, Debono CA, Behar SM, Minnaard AJ, Murray M, Barry CE, 3rd, Matsunaga I, Moody DB. A comparative lipidomics platform for chemotaxonomic analysis of Mycobacterium tuberculosis. Chem Biol. 2011;18:1537–1549. doi: 10.1016/j.chembiol.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutcliffe IC, Harrington DJ. Lipoproteins of Mycobacterium tuberculosis: an abundant and functionally diverse class of cell envelope components. FEMS Microbiol Rev. 2004;28:645–659. doi: 10.1016/j.femsre.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Babu MM, Priya ML, Selvan AT, Madera M, Gough J, Aravind L, Sankaran K. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J Bacteriol. 2006;188:2761–2773. doi: 10.1128/JB.188.8.2761-2773.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rampini SK, Selchow P, Keller C, Ehlers S, Bottger EC, Sander P. LspA inactivation in Mycobacterium tuberculosis results in attenuation without affecting phagosome maturation arrest. Microbiology. 2008;154:2991–3001. doi: 10.1099/mic.0.2008/018895-0. [DOI] [PubMed] [Google Scholar]
- 26.Sander P, Rezwan M, Walker B, Rampini SK, Kroppenstedt RM, Ehlers S, Keller C, Keeble JR, Hagemeier M, Colston MJ, Springer B, Bottger EC. Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;52:1543–1552. doi: 10.1111/j.1365-2958.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- 27.Banaei N, Kincaid EZ, Lin SY, Desmond E, Jacobs WR, Jr, Ernst JD. Lipoprotein processing is essential for resistance of Mycobacterium tuberculosis to malachite green. Antimicrob Agents Chemother. 2009;53:3799–3802. doi: 10.1128/AAC.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol. 2004;173:2660–2668. doi: 10.4049/jimmunol.173.4.2660. [DOI] [PubMed] [Google Scholar]
- 29.Jackson DC, Lau YF, Le T, Suhrbier A, Deliyannis G, Cheers C, Smith C, Zeng W, Brown LE. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101:15440–15445. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J Immunol. 2002;169:4905–4912. doi: 10.4049/jimmunol.169.9.4905. [DOI] [PubMed] [Google Scholar]
- 31.Andrieu M, Desoutter JF, Loing E, Gaston J, Hanau D, Guillet JG, Hosmalin A. Two human immunodeficiency virus vaccinal lipopeptides follow different cross-presentation pathways in human dendritic cells. J Virol. 2003;77:1564–1570. doi: 10.1128/JVI.77.2.1564-1570.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gowthaman U, Singh V, Zeng W, Jain S, Siddiqui KF, Chodisetti SB, Gurram RK, Parihar P, Gupta P, Gupta UD, Jackson DC, Agrewala JN. Promiscuous peptide of 16 kDa antigen linked to Pam2Cys protects against Mycobacterium tuberculosis by evoking enduring memory T-cell response. J Infect Dis. 2011;204:1328–1338. doi: 10.1093/infdis/jir548. [DOI] [PubMed] [Google Scholar]
- 33.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.