Abstract
Oxidized LDLs (ox.LDLs) uptake by macrophages inside the arterial wall is a crucial step in atherosclerotic disease, and some studies suggest that high ox.LDLs plasma levels might be associated with cardiovascular disease (CVD). However, whether high ox.LDLs continue to be a CVD risk factors in older persons is unknown. We investigated the clinical correlates of plasma ox.LDLs, and their role in predicting long-term CVD/cardiac mortality in 1025 older community-dwelling individuals (mean age:75.5±7.4yrs; females:55%) from the InCHIANTI study. Kaplan-Meier curves were fitted to explore the relationship between tertiles of ox.LDLs (ox.LDL/LDL-C ratio) and time to CVD/cardiac death. Hazard Ratios (HR) were estimated by Cox regression analysis.
At multivariate analysis, ox.LDLs were independently associated with LDL-C, triglycerides, and HDL-C (adjusted r2:0.42; P=0.001). The ox.LDL/LDL-C ratio (the extent of LDLs oxidation) was independently correlated with HDL-C, triglycerides, and beta-carotene (adjusted r2:0.15, P=0.001). Among 1025 individuals, 392 died after 9 years, 166 from CVD. The HR for CVD/cardiac mortality was not significantly different across tertiles of ox.LDLs or ox.LDL/LDL-C ratio, both in the whole sample and in individuals with prevalent CVD.
We conclude that in an elderly population LDL-C, triglycerides, and HDL-C are the most important determinants of ox.LDLs levels, indirectly suggesting an association between small dense LDLs and LDLs oxidation. No association emerged between higher ox.LDLs levels and 9-years CVD/cardiac mortality, suggesting that in advanced age the prognostic information added by ox.LDLs on CVD/cardiac mortality might be negligible.
Keywords: Oxidized LDL, Mortality, Cardiovascular Disease, Aging
Introduction
Atherosclerosis is the first cause of death in Western Countries. Current theories on the pathogenesis of atherosclerosis call for an interplay of inflammation and oxidative stress that progressively damage arterial walls. Oxidation of low-density lipoproteins (LDLs) and their subsequent uptake by macrophages inside the arterial wall are considered crucial steps in this process (1). Indeed, macrophages do not take up LDL particles unless they undergo in vivo modifications such as oxidation. Accordingly, cross-sectional studies suggest that the rate of LDLs oxidation might be associated with coronary heart disease (CHD) and carotid atherosclerosis (2, 3). There is some evidence that LDLs are oxidized in the extravascular space of the arterial wall (1, 4) but small part of oxidized LDLs (ox.LDLs) escape the uptake by macrophages or might leak from atherosclerotic plaques and re-enter the blood stream. Of consequence, the measurement of ox.LDLs might contribute to the estimation of cardiovascular risk (5).
Conditions associated with high ox.LDLs plasma levels include a specific lipoprotein profile, LDL particles size (6), diabetes mellitus (7), markers of systemic inflammation (8), and central obesity (9). Interestingly, LDL-cholesterol (LDL-C) and apo B levels have been consistently associated with ox-LDLs plasma levels (10). For this reason, it has been suggested that the ox.LDL/LDL-C ratio (i.e. the relative amount of ox.LDLs) is the best indicator of the risk associated with ox.LDLs levels (8). Although atherosclerosis is common in older people, and ageing itself has been associated with increasing levels of oxidative stress, data about circulating ox.LDLs in older populations are scarce (11). Indeed, only a few studies investigated the impact of increased ox.LDLs levels on the incidence of new cardiovascular events (5), mainly in selected samples of patients with CHD (12-14) or congestive heart failure (15, 16). Furthermore, the clinical value of circulating oxLDL assessment in older person is virtually unknown.
Using data for the InCHIANTI dataset, a population-based study of older people living in the community with a 9-year follow-up, we investigated the clinical correlates of plasma ox.LDLs and tested the hypothesis that baseline circulating ox.LDL levels might predict long-term cardiovascular mortality.
Materials and Methods
This study is part of the “Invecchiare in Chianti” (Aging in the Chianti area, InCHIANTI) study, a prospective population-based study of older persons, designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council of Aging (INRCA, Florence, Italy). The study included participants randomly selected from the residents in two towns of the Chianti area (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy). A detailed description of the sampling procedure and data collection method has been previously published (17). Briefly, in August 1998, 1270 persons ≥65 years old were randomly selected from the population registries. Additional subjects (n. 29) were randomly selected among those who were ≥90 years old, to obtain a sample of 30 men and 30 women in this age group. Of the initial 1299 subjects, 39 were not eligible (they had died or left the area). Clinical visits and assessments were performed in the studyclinic and were preceded by an interview conducted at the participants' homes. Trained interviewers administered structured questionnaires on dietary intakes, household composition, social networks, economical status, education, and general information on health and functional status. The Italian National Research Council on Aging (INRCA) Ethical Committee ratified the entire study protocol.
The analyses here presented are based on data from 1025 individuals aged over 65 in which metabolic parameters including oxidized LDLs, and inflammatory mediators had been measured at the time of baseline visit.
Clinical chemistry parameters
All parameters were measured on fresh serum or plasma drawn after 12 hours overnight fasting, after the patient has been sedentary in sitting or supine position for 15 minutes. Commercial enzymatic tests (Roche Diagnostics) were used for determining serum total cholesterol, triglycerides, and HDL-C concentrations. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald's formula as follows: LDL-C: TC – (TG/5) – HDL-C. As an indirect marker of LDL particle size we used the TG/HDL-C ratio, a validated parameter previously used for assessing the presence of small LDL particles (18, 19). According to data by Muruyama et al., we considered subjects with a TG/HDL-C ratio cut-off > 2.0 as having small LDL particles (18). Fasting insulin was determined using a commercial double-antibody, solid-phase radioimmunoassay (Sorin Biomedica, Milan, Italy). Fasting blood glucose was determined by an enzymatic colorimetric assay using a modified glucose oxidase-peroxidase method (Roche Diagnostics, GmbH, Mannheim, Germany) and a Roche-Hitachi 917 analyzer.
Insulin resistance was calculated according to the Homeostasis Model Assessment (HOMA) as follows: fasting insulin × fasting glucose/22.5. Metabolic syndrome was defined by the criteria of the NCEP-ATP III - AHA/NHLBI statement published in 2005(20). The glomerular filtration rate (GFR) was calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (21).
Determination of plasma oxidized LDLs
Sampled blood was transferred into the tube avoiding haemolysis. Aliquoted, plasma specimens were frozen and stored at −80°C until used for testing. Oxidized LDLs were measured by ELISA (Kit Mercodia AB, Uppsala, Sweden). In this solid-phase two-site enzyme immunoassay two monoclonal antibodies are directed against separate antigenic determinants on the oxidized apolipoprotein B molecule. The detection limit is <1 mU/L. The intrassay and interassay coefficients of variation were 6.3% and 8%, respectively.
Plasma ox.LDL values were strongly correlated with LDL-C plasma concentrations (r: 0.58; P= 0.001); thus, LDL-C was the strongest determinant of ox.LDL absolute levels in our population. To overcome this, we adjusted the plasma values of ox.LDLs (U/L) by the plasma levels of LDL-C (mmol/L) by calculating their ratio (Units of ox.LDLs per mmol of LDL-C). The ratio was used to perform statistical analysis and express the results.
Inflammatory markers
Interleukin-6 (IL-6), interleukin-1 Beta (IL-1β), interleukin-18 (IL-18), Tumor Necrosis Factor-alpha (TNF-α), interleukin-1 Receptor Antagonist (IL-1RA), and high sensitivity C reactive protein (hs.CRP) were measured as previously described (22).
Plasma antioxidants
Plasma Alpha-tocopherol concentrations were measured by reversed-phase high-performance liquid chromatography (HPLC) as previously described (23). Tocopherol concentrations were expressed in μmol/L. Intra- and interbatch CVs were 3% and 4.2%, respectively. Beta-carotene has been measured by HPLC (24). Within-run and between-run coefficients of variation, respectively, were 4.5% and 5.4%. Plasma Selenium was measured by graphite furnace atomic absorption spectrometry in a Perkin Elmer Analyst 600 with Zeeman background correction (25). Within-run and between-run coefficients of variation, respectively, were 3.1% and 7.1%.
Anthropometry
Weight and height were measured by using standard techniques. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Waist circumference was measured to the nearest 0.5 cm by using a non-elastic plastic tape, at the level of the smallest area of the waist between the lower rib margin and the iliac crest.
Dietary variables
Data on dietary daily intake were collected by the questionnaire created for the European Prospective Investigation into Cancer and Nutrition (E.P.I.C.) study (26).
Mortality Follow-up
Participants were evaluated for the 3-year (2001 to 2003), 6-year (2004 to 2006) and 9-year follow-up visits (2007 to 2009). Mortality data of the original InChianti cohort were collected using data from the Mortality General Registry maintained by the Tuscany Region, and the death certificates that are deposited immediately after death at the Registry office of the municipality of residence. Cardiovascular mortality, based on underlying cause of death, was defined as any cardiovascular mortality and coded using the International Classification of Diseases, 9th Revision (ICD-9 codes: 390-459).
Other Measures
The prevalence of specific medical conditions was establishedusing standardized criteria that combined information from self-reported history, medical records, and a clinical medical examination. The following diseases were considered in the present study: coronary heart disease (CHD - acute myocardial infarction, chronic heart failure, cardiac arrest, acute and chronic ischemic heart disease), peripheral arterial disease (PAD), stroke, hypertension, and diabetes. Prevalent cardiovascular disease (CVD) was defined as the as presence of one of the following: coronary heart disease (as previously specified), peripheral arterial disease, and stroke. The ankle-brachial index (ABI) was measured in all subjects by using a Doppler stethoscope (Parks model 41-A; Parks Medical Electronics, Inc; Aloha, Oregon). Besides clinical information, in the presence of ABI value <0.9, the diagnosis of PAD was made.
Statistical analysis
Continuous variables were expressed as mean (SD) or median (interquartile range) when necessary. Means were compared by t test or ANOVA, while medians were compared by Mann-Whitney non-parametric test. Correlations between continuous variables were tested by Pearson's correlation. Due to the skewed distribution of values, cytokines, insulin, TG, and HOMA were log-transformed, in order to approximate a normal distribution, before entering regression analysis. Prevalence was compared by the χ2 test. Multivariate linear regression analysis (method stepwise backward) was performed to test the independent association between ox.LDLs or ox.LDL/LDL-C ratio and other variables of interest.
The parameters included into the Ox.LDL model were: age, gender, LDL-C, HDL-C, TG, uric acid, HOMA, waist circumference, BMI, metabolic syndrome, daily Kcal intake, IL-1β, alpha-tocopherol, beta-carotene, and selenium.
The parameters included into the Ox.LDL/LDL-C ratio model were: age, gender, HDL-C, TG, HOMA, uric acid, WBC count, metabolic syndrome, waist circumference, smoking, CKD-EPI, TNF-α, log IL-18, log IL-1ra, beta-carotene, and selenium.
Kaplan-Meier curves were fitted to explore the relationship between tertiles of plasma ox.LDLs, or ox.LDL/LDL-C ratio, and time to CVD death defined by ICD-9-CM codes 390-459. Death rates per 1.000-person years were also calculated. Hazard Ratios (HR) for each tertile of ox.LDL, or ox.LDL/LDL-C ratio, were separately estimated, in the whole population and in subjects with documented CVD at baseline, by Cox proportional hazard regression analysis. The assumption of proportionality of all variables introduced in the models was assessed through the analysis of Schoenfeld residuals. The lower tertile (I) was considered as the reference category. The Cox models were adjusted for known risk factors for cardiovascular mortality including age, gender, diabetes, hypertension, and smoking; furthermore, CHD and stroke were included into the models for whole population analysis.
All analyses were performed by the SPSS for Windows statistical package, version 13.0 (SPSS, Inc, Chicago, IL).
Results
One thousand twenty five community dwelling older individuals were enrolled into the present study; the characteristics of the sample are reported in TABLE 1. Ox.LDLs values were normally distributed, with a mean of 42.14±11.72 U/L. On the whole population, 226 (22%) individuals resulted affected by CVD at baseline. Ox.LDLs levels (43.5 vs 41.8 U/L; P=0.07) as well as ox.LDL/LDL-C ratio (12.8 vs 12.1U/mmol; P=0.10) were slightly higher in subjects with CVD compared with controls.
TABLE 1.
principal characteristics of 1025 community dwelling older individuals enrolled in the InChianti study.
| PARAMETER | |
|---|---|
| Age (years) | 75.5±7.4 |
| Gender (female) | 55% |
| Oxidized LDLs (U/L) | 42.14±11.72 U/L |
| Total cholesterol (mg/dl) | 217±40 |
| LDL-Cholesterol (mg/dl) | 136±34 |
| HDL-Cholesterol (mg/dl) | 56±15 |
| Triglycerides (mg/dl) ° | 110 (83-150) |
| Fasting Glucose (mg/dl) | 96±26 |
| HOMA score ° | 2.25 (1.54-3.28) |
| Uric acid (mg/dl) | 5.2±1.4 |
| Creatinine (mg/dl) | 0.93±0.24 |
| Body Mass Index (kg/m2) | 27.4±4.0 |
| Waist circumference (cm) | 92.7±10.2 |
| WBC count (×1000/mm3) | 6.14±1.60 |
| Ankle Brachial Index (ABI) | 1.03±0.16 |
| IL -1 β (pg/ml) ° | 0.12 (0.08-0.19) |
| IL-1 ra (pg/ml) ° | 132 (97-187) |
| TNF-α (pg/ml) ° | 5.07 (2.85-7.64) |
| IL-6 (pg/ml) ° | 1.47 (0.87-2.29) |
| IL-18 (ug/ml) ° | 386 (303-484) |
| Hs.CRP (mg/L) ° | 2.81 (1.32-5.84) |
| α Tocopherol (umol(L) | 33.6±7.6 |
| B Carotene (umol/L) ° | 0.35 (0.24-0.53) |
| Selenium (ug/L) | 74.4±13 |
| Diet (daily intake) | |
| - Kcalories | 1910±563 |
| - Cholesterol (mg) | 268±96 |
| - Saturated fatty acids (g) | 22±7.5 |
| - Alcohol (g) ° | 6.2 (0-20) |
| Smoking habit (past/current) | 41% |
| Metabolic Syndrome (by NCEP-ATP III) | 31% |
| Diabetes | 11.9% |
| Hypertension (by NCEP-ATP III) | 93% |
| Coronary Heart Disease (CHD) | 7.6% |
| Stroke | 6.6% |
| Peripheral Arterial Disease (PAD) | 12.1% |
HOMA: homeostasis model assessment; WBC: white blood cells; CHD: coronary heart disease
Median (interquartile range)
Metabolic syndrome (MS) was more frequent in subjects with high ox.LDLs (>42.12 U/L) while history of CHD, stroke, PAD, diabetes, and smoking was similar across ox.LDL tertiles. By multivariate regression analysis we found that LDL-C was strongly related to ox.LDLs (β: 0.21; SE: 0.010; P=0.001), explaining more than one third of ox.LDLs total variability (adjusted r2:0.36; P=0.001; age and gender covariates). When other variables associated with ox.LDLs at univariate analysis were forced into the model, also TG (β: 0.042; SE: 0.005; P=0.001), and HDL-C, (β: −0.11; SE: 0.026; P=0.001) resulted independently associated with ox.LDLs (adjusted r2: 0.42; P=0.001). In TABLE 2 are described the characteristics of the sample according to low or high ox.LDL/LDL-C ratio (below/above mean value). Participants with high ox.LDL/LDL-C ratio had higher values of TG, fasting glucose, HOMA score, uric acid, creatinine, waist circumference, white blood cells (WBC) count, IL-1 receptor antagonist (IL-1ra) and IL-18, and by lower levels of HDL-C and beta-carotene. Participants with high ox.LDL/LDL.C ratio were also more likely to be smokers, have a MS diagnosis, and to report a history of diabetes or stroke (P=0.07)
TABLE 2.
principal characteristics of 1025 community dwelling older individuals enrolled in the InChianti study according to the presence of low (<12.28 U/mmol) or high (≥12.28 U/mmol) values of ox.LDL/LDL-C ratio.
| ox.LDL/LDL-C ratio | |||
|---|---|---|---|
| PARAMETER | LOW | HIGH | P |
| Age (years) | 75.1±7 | 75.9±7.6 | 0.09 |
| Gender (female) | 58.5% | 53% | 0.07 |
| Total cholesterol (mg/dl) | 223±39 | 208±39 | 0.001 |
| LDL-C (mg/dl) | 142.5±33 | 126±34 | 0.001 |
| HDL-C (mg/dl) | 58±15 | 53±15 | 0.001 |
| Triglycerides (mg/dl) ° | 101 (76-137) | 124 (87-172) | 0.001 |
| Fasting Glucose (mg/dl) | 94±25 | 98±27 | 0.005 |
| HOMA score ° | 2.05 (1.36-3.03) | 2.21 (1.543.32) | 0.001 |
| Uric acid (mg/dl) | 5±1.4 | 5.4±15 | 0.001 |
| Creatinine (mg/dl) | 0.92±0.22 | 0.95±0.23 | 0.05 |
| BMI (kg/m2) | 27.3±3.9 | 27.7±4.1 | 0.20 |
| Waist circumference (cm) | 92±10 | 93.1±10 | 0.02 |
| ABI | 1.02±.015 | 1.03±0.18 | 0.69 |
| WBC count (×1000/mm3) | 6.05±1.53 | 6.27±1.68 | 0.04 |
| IL -1 β (pg/ml) ° | 0.12 (0.07-0.19) | 0.12 (0.08-1.20) | 0.91 |
| IL-1 ra (pg/ml) ° | 127 (92-178.4) | 137 (98.4-189) | 0.01 |
| TNF-α (pg/ml) ° | 5.02 (2.83-7.62) | 5.32 (2.89-7.91) | 0.23 |
| IL-6 (pg/ml) ° | 1.22 (0.70-1.98) | 1.29 (0.80-2.09) | 0.14 |
| IL-18 (ug/ml) ° | 358 277-461) | 382 (302-484) | 0.002 |
| Hs.CRP (mg/L) ° | 2.32 (1.07-5.26) | 2.50 (1.23-5.32) | 0.17 |
| α Tocopherol (umol(L) | 33.8 | 33.2 | 0.21 |
| B Carotene (umol(L) ° | 0.37 (0.27-0.55) | 0.34 (0.21-0.50) | 0.005 |
| Selenium (ug/L) | 74.8 | 73.8 | 0.17 |
| Diet (daily intake) | |||
| - Kcal | 1892±544 | 1939±586 | 0.18 |
| - Cholesterol (mg) | 267±95 | 267±89 | 0.92 |
| - Saturated fatty acids (g) | 22.1±7.5 | 21.6±7.3 | 0.74 |
| - Alcohol users | 65% | 63% | 0.30 |
| Smoking habit (smokers) | 37% | 45% | 0.01 |
| Metabolic Syndrome | 27% | 38% | 0.001 |
| Diabetes | 10.2% | 14% | 0.07 |
| CHD | 7.2% | 8.4% | 0.30 |
| Stroke | 5.4% | 7.8% | 0.07 |
| PAD | 11.2% | 13.4% | 0.17 |
HOMA: homeostasis model assessment; BMI: body mass index; ABI: ankle brachial index; WBC: white blood cells; CHD: coronary heart disease;
Median (interquartile range)
The ox.LDL/LDL-C ratio correlated positively with TG, fasting glucose, HOMA score, uric acid, creatinine, CKD-EPI values, waist circumference, WBC count, IL-1ra, TNF-alpha, and IL-18, and negatively with HDL-C, beta-carotene and selenium (Table 3).
TABLE 3.
correlation coefficients between ox.LDL/LDL-C ratio and other parameters in 1025 community dwelling older individuals.
| PARAMETER | r | P |
|---|---|---|
| Age | 0.032 | 0.30 |
| Metabolic Syndrome | 0.167 | 0.001 |
| HDL-C (mg/dl) | −0.238 | 0.001 |
| Triglycerides (mg/dl) | 0.249 | 0.001 |
| Fasting Glucose (mg/dl) | 0.096 | 0.002 |
| HOMA score | 0.087 | 0.006 |
| Uric acid (mg/dl) | 0.137 | 0.001 |
| AST (U/L) | 0.013 | 0.98 |
| ALT (U/L) | 0.037 | 0.23 |
| Creatinine (mg/dl) | 0.108 | 0.001 |
| GFR by CKD-EPI (ml/m/1.73 m2) | −0.132 | 0.001 |
| BMI (kg/m2) | 0.053 | 0.10 |
| Waist circumference (cm) | 0.101 | 0.002 |
| WBC count (/mm3) | 0.079 | 0.01 |
| Kcal/day | 0.058 | 0.06 |
| IL -1 β (pg/ml) | 0.013 | 0.67 |
| IL-1 ra (pg/ml) | 0.066 | 0.03 |
| TNF-α (pg/ml) | 0.060 | 0.05 |
| IL-6 (pg/ml) | 0.037 | 0.23 |
| IL-18 (ug/ml) | 0.075 | 0.01 |
| hs.CRP (mg/L) | 0.011 | 0.73 |
| α Tocopherol (umol(L) | 0.20 | 0.52 |
| B Carotene (umol(L) | −0.12 | 0.001 |
| Selenium (ug/L) | −0.07 | 0.05 |
HOMA: homeostasis model assessment; GFR: glomerular filtration rate; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; BMI: body mass index; WBC: white blood cells
At multivariate regression analysis the ox.LDL/LDL-C ratio correlated with HDL-C (β: −0.032; SE: 0.008; P= 0.001), TG (β: 0.014; SE: 0.002; P= 0.001), and beta-carotene (β: −1.14; SE: 0.43; P= 0.008) (adjusted r2: 0.15, P=0.001), independent of potential confounders.
We successively investigated the possible association between ox.LDLs levels (or ox.LDL/LDL-C ratio) and nine-years CVD mortality. Among the 1025 individuals enrolled into the present study, 392 had died after 9 years. The principal causes of death was the following: 42 % (166) were from CVD (among this 71 from cardiac causes), 20 % from neoplasm, 5 % from diseases of the respiratory system, 5 % from fracture and accidents due to natural and environmental factors, 4 % from diseases of nervous system, 4 % from diseases of gastro-intestinal tract, 3 % from endocrine/nutritional/metabolic diseases, and immunity disorders. In FIGURE 1 are reported the Kaplan-Meier survival curves for CVD mortality in the whole population, according to ox.LDLs (A) or ox.LDL/LDL-C ratio tertiles (B). The adjusted HR for CVD mortality according to ox.LDLs tertiles was 1.09 (95%CI: 0.73-1.61) for tertile II, and 1.17 (95%CI: 0.79-1.73) for tertile III. As regards ox.LDL/LDL-C ratio, the HR was 0.84 (95%CI: 0.51-1.12) for tertile II, and 0.89 (95%CI: 0.55-1.18) for tertile III.
FIGURE 1.
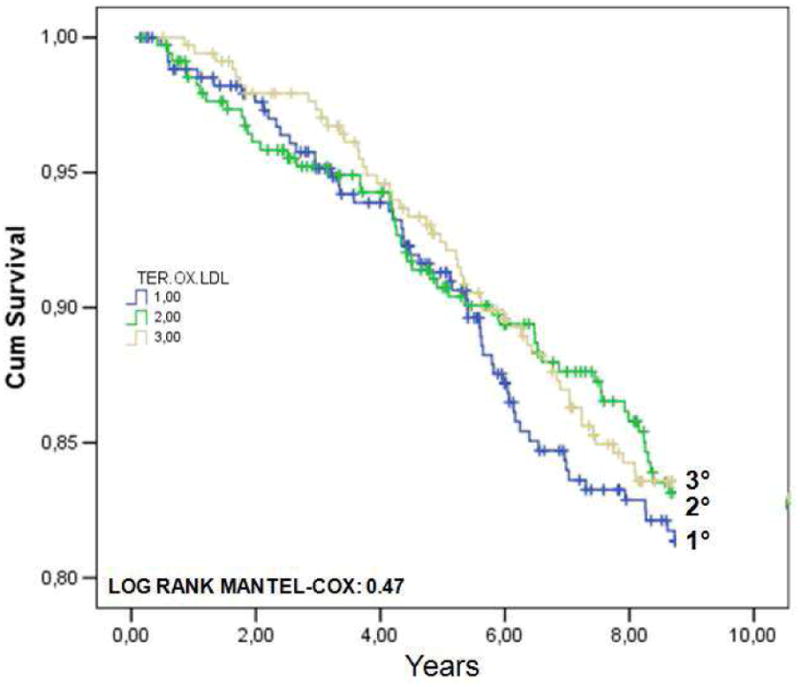
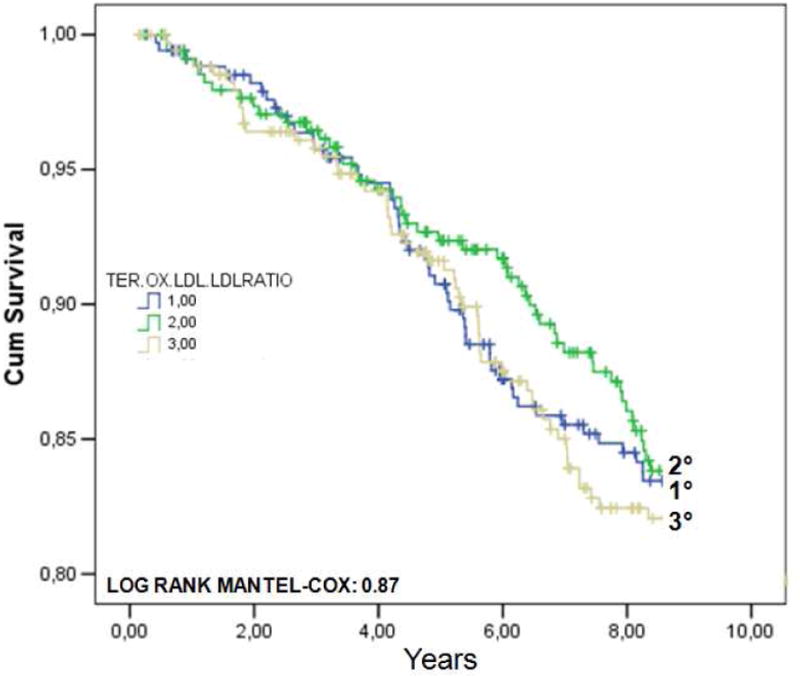
Kaplan-Meier survival curves for cardiovascular (CVD) mortality in 1025 older individuals from the InChianti study, according to ox.LDLs (A) or ox.LDL/LDL-C ratio tertiles (B).
In FIGURE 2 are shown the Kaplan-Meier survival curves for CVD mortality in subjects with prevalent CVD across tertiles of plasma ox.LDLs (A) or ox.LDL/LDL-C ratio (B). As expected, mortality rates were much higher compared to the whole population. Furthermore, participants with CVD were older, more often males, and had greater prevalence of diabetes, hypertension, and other risk factors. According to ox.LDLs tertiles, the adjusted HR for CVD mortality in subjects with prevalent baseline CVD was 1.09 (95%CI: 0.65-2.12) for tertile II, and 1.12 (95%CI: 0.69-2.14)for tertile III. As regards ox.LDL/LDL-C ratio tertiles, the adjusted HR was 0.95 (95%CI: 0.54-1.77) for tertile II, and 1.07 (95%CI: 0.58-1.94) for tertile III.
FIGURE 2.
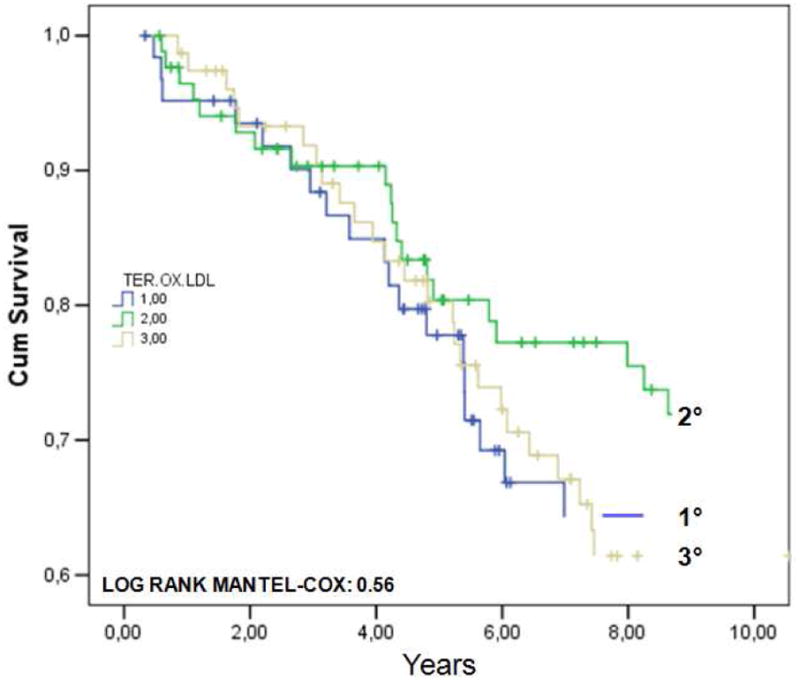
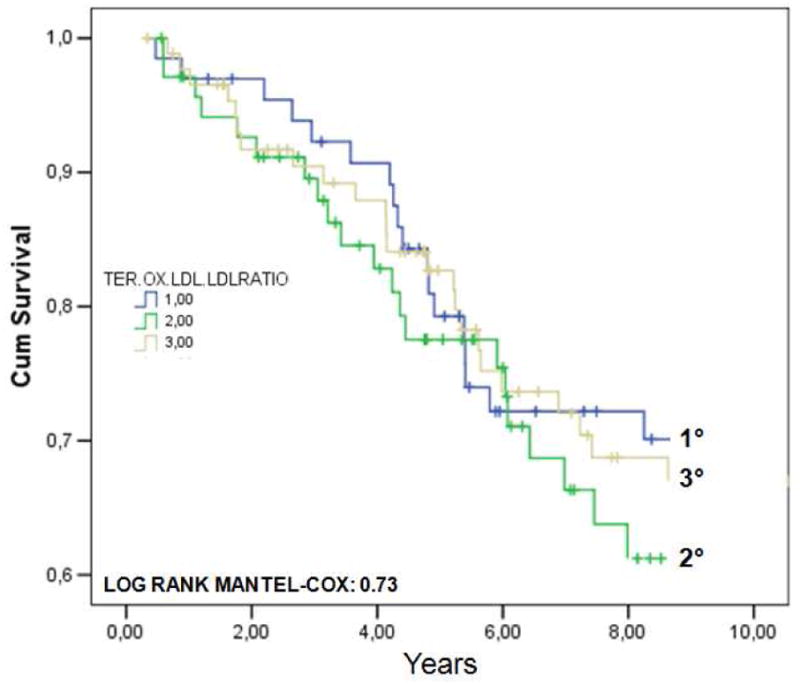
Kaplan-Meier survival curves for cardiovascular (CVD) mortality in 226 older individuals with prevalent CVD at baseline, from the InChianti study, across tertiles of plasma ox.LDLs (A) or ox.LDL/LDL-C ratio (B).
Finally, Cox multivariate analysis was specifically focused on cardiac mortality (71 subjects; ICD-9 codes 410, 411, 414, 427.5, and 428). Adjusted HR for cardiac mortality according to ox.LDLs tertiles was 1.27 (95%CI: 0.54-2.32) for tertile II, and 1.33 (95%CI: 0.64-2.74) for tertile III, while for the ox.LDL/LDL-C ratio the HR was 0.59 (95%CI: 0.39-1.20) for tertile II, and 0.67 (95%CI: 0.43-1.37) for tertile III.
Discussion
We evaluated plasma ox.LDLs levels in a large sample of community dwelling older individuals from the InChianti study. The levels we measured in our sample were in line with those previously reported by using the same ELISA method. In healthy individuals, Nakhjavani reported a mean value of 42 U/L (7), while Nordin reported a mean value of 48 U/L (27).
The major determinants of ox.LDLs in this population were the levels of LDL-C, TG, and HDL-C. In agreement with other reports, LDL-C was by far the strongest determinant, explaining 36% of total variability. This result further supports the concept that the absolute rate of LDLs oxidation depends not only on the levels of oxidative stress, but also on the amount of substrate (8). Interestingly, a negative association between ox.LDLs levels and age was found in our population (r:−0.101; P=0.001), perfectly mirroring the relationship between LDL-C and age (r:−0.110; P=0.001). This finding suggests that after 65 years of age, although the oxidative stress might increase with ageing, circulating ox.LDLs tend to decrease as a consequence of the progressive reduction of the substrate.
TG levels were also positively associated with ox.LDLs levels. It is known that high TG values are strongly associated with the presence of small, dense LDL particles, the “pattern B” LDLs (28). Interestingly, Kondo et al. demonstrated that decreased LDLs size together with increased TG leads to increased ox.LDLs levels (29). Of consequence, we advance that the relationship between TG and ox.LDLs levels might be mediated by the small size of LDL particles. Supporting this hypothesis, we found that the TG/HDL-C ratio, a validated indicator of small LDLs size (18, 19), was associated with ox.LDLs levels. Indeed, the prevalence of high ox.LDLs in subjects with TG/HDL-C ratio below or above 2.0 was 39.5% and 66%, respectively (P=0.001); moreover, the risk of high ox.LDLs was associated with a TG/HDL-C ratio >2.0 (OR: 3.06; 95%I.C.:2.36-3.97).
HDL-C was also negatively associated with ox.LDLs, independent of TG values. HDLs might contribute in reducing LDL oxidation (30); indeed, HDLs contain enzymes (e.g. paraoxonase 1-3) able to destroy lipid hydro peroxides that oxidize LDLs, while Apo A-I removes lipid hydro peroxides from LDLs.
The mean value of the ox.LDL/LDL-C ratio was also similar to those previously reported in other studies (31, 32). Again, TG and HDL-C were the major determinants of the ox.LDL/LDL-C ratio explaining, together with beta carotene, 15% of its total variability. A TG/HDL-C ratio >2.0 was associated with high values of ox.LDL/LDL-C ratio (OR:1.69; 95%CI:1.31-2.18). Thus, our data suggest that TG and HDL-C are independent predictors of both absolute levels of ox.LDLs and of the extent of LDLs oxidation. Interestingly, beta carotene was negatively correlated with the ox.LDL/LDL-C ratio, suggesting that it might improve the oxidative status of circulating LDLs in this older population. Different antioxidants have been shown to inhibit in vitro LDL oxidation (33-35), and to reduce the susceptibility of LDLs to oxidation in humans (34, 36). Since plasma levels of beta carotene can be increased by diet or dietary supplementation without side effects, they may show promise in the prevention of atherosclerosis even in older individuals.
We also investigated the relationship between plasma ox.LDLs (or ox.LDL/LDL-C ratio) and nine years CVD/cardiac mortality. Indeed, it has been suggested that higher ox.LDLs levels might have a prognostic value in predicting future CVD or CHD (5, 11, 13, 14). Our results did not demonstrate any significant association between the absolute or relative amount of plasma ox.LDLs and nine years CVD/cardiac mortality; these results were found both in the whole population and in subjects with prevalent CVD at baseline. Actually, prospective data on the clinical significance of ox.LDLs are scarce, and most of the studies have been conducted on relatively small samples of patients with prevalent CHD (5, 12-14).
Only one study, conducted on 3033 subjects aged 70 to 79 years from the Healthy ABC study has been focused on the elderly population (11). Although these Authors could not demonstrate any association between ox.LDLs and CHD incidence (4 years follow up), the risk for MI was significantly increased in participants with higher ox.LDLs.
The reason for our negative results might be different. In one hand it is possible that in older individuals plasma ox.LDLs do not strictly reflect the real amount of ox.LDLs and/or the levels of oxidative stress inside the arterial wall. On the other hand, ox.LDLs might lose their predictive value with advancing age since individuals disposed to develop precocious atherosclerosis have already died, while ageing population might be progressively selected for resistance to risk factors. This phenomenon has been already observed for other cardiovascular risk factors including plasma lipids (37). Furthermore, we evaluated the risk for CVD/cardiac mortality, whereas previous studies investigated the incidence of all CVD events, an outcome that may be more specifically related to lipid abnormalities; this methodological aspect might also contribute to explain our findings. Finally, since the aim of the study was to test the ability of ox.LDL to predict 9-years CV death, we measured this variables only at baseline; nevertheless, information about ox.LDLs concentration over time might give further risk indications.
Some limitations of the study must be acknowledged. First, LDL-C was estimated by the Friedewald formula, and not directly. Second, we used an indirect indicator of LDL particles size, the TG/HDL-C ratio. Although this marker has been validated (18, 19), and is much easier to use in a large population based study, a direct measure such as polyacrylamide linear gradient gel electrophoresis or NMR would be much more precise.
We also would like to underline the strength of the study. To our knowledge, this is at present time the population-based longitudinal study on circulating ox.LDLs with the longest follow-up period.
Conclusion
We confirmed in an elderly population that, besides LDL-C, TG and HDL-C are two independent determinants of ox.LDLs levels, supporting the existence of an association between small, dense LDLs phenotype and LDLs oxidation. Most important, we could not demonstrate any association between higher ox.LDLs plasma levels and nine years CVD/cardiac mortality rates, both in the whole sample and in participants with prevalent CVD. This result suggests that in advanced age the prognostic information added by ox.LDLs on future CVD/cardiac mortality might be negligible.
Highlights.
We studied 1025 older community-dwelling subjects with 9-years follow up
LDL-C, TG, and HDL-C are the most important determinants of ox.LDLs levels
The extent of LDLs oxidation was independently correlated with HDL-C, TG, and b-carotene.
No association emerged between higher ox.LDLs levels and 9-years CVD/cardiac mortality.
Acknowledgments
The InCHIANTI study baseline (1998-2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health, and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336). The Follow-up 1 (2001-2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the Follow-up 2 and 3 studies (2004-2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002). Supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Steinberg D, Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997:951062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 2.Salonen JT, Yla-Herttuala S, Yamamoto R, et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 3.Toshima S, Hasegawa A, Kurabayashi M, et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:2243–2247. doi: 10.1161/01.atv.20.10.2243. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 5.Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–657. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]
- 6.Scheffer PG, Bos G, Volwater HG, Dekker JM, Heine RJ, Teerlink T. Associations of LDL size with in vitro oxidizability and plasma levels of in vivo oxidized LDL in Type 2 diabetic patients. Diabet Med. 2003;20:563–567. doi: 10.1046/j.1464-5491.2003.00975.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakhjavani M, Khalilzadeh O, Khajeali L, et al. Serum oxidized-LDL is associated with diabetes duration independent of maintaining optimized levels of LDL-cholesterol. Lipids. 2010;45:321–327. doi: 10.1007/s11745-010-3401-8. [DOI] [PubMed] [Google Scholar]
- 8.van der Zwan LP, Teerlink T, Dekker JM, et al. Circulating oxidized LDL: determinants and association with brachial flow-mediated dilation. J Lipid Res. 2009;50:342–349. doi: 10.1194/jlr.P800030-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Njajou OT, Kanaya AM, Holvoet P, et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev. 2009;25:733–739. doi: 10.1002/dmrr.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Tits LJ, van Himbergen TM, Lemmers HL, de GJ, Stalenhoef AF. Proportion of oxidized LDL relative to plasma apolipoprotein B does not change during statin therapy in patients with heterozygous familial hypercholesterolemia. Atherosclerosis. 2006;185:307–312. doi: 10.1016/j.atherosclerosis.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Holvoet P, Kritchevsky SB, Tracy RP, et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53:1068–1073. doi: 10.2337/diabetes.53.4.1068. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Mokuno H, Matsunaga E, et al. Predictive value of circulating oxidized LDL for cardiac events in type 2 diabetic patients with coronary artery disease. Diabetes Care. 2004;27:843–844. doi: 10.2337/diacare.27.3.843. [DOI] [PubMed] [Google Scholar]
- 13.Shimada K, Mokuno H, Matsunaga E, et al. Circulating oxidized low-density lipoprotein is an independent predictor for cardiac event in patients with coronary artery disease. Atherosclerosis. 2004;174:343–347. doi: 10.1016/j.atherosclerosis.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Johnston N, Jernberg T, Lagerqvist B, Siegbahn A, Wallentin L. Oxidized low-density lipoprotein as a predictor of outcome in patients with unstable coronary artery disease. Int J Cardiol. 2006;113:167–173. doi: 10.1016/j.ijcard.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui T, Tsutamoto T, Wada A, et al. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;39:957–962. doi: 10.1016/s0735-1097(02)01721-7. [DOI] [PubMed] [Google Scholar]
- 16.Charach G, George J, Afek A, et al. Antibodies to oxidized LDL as predictors of morbidity and mortality in patients with chronic heart failure. J Card Fail. 2009;15:770–774. doi: 10.1016/j.cardfail.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 18.Maruyama C, Imamura K, Teramoto T. Assessment of LDL particle size by triglyceride/HDL-cholesterol ratio in non-diabetic, healthy subjects without prominent hyperlipidemia. J Atheroscler Thromb. 2003;10:186–191. doi: 10.5551/jat.10.186. [DOI] [PubMed] [Google Scholar]
- 19.Boizel R, Benhamou PY, Lardy B, Laporte F, Foulon T, Halimi S. Ratio of triglycerides to HDL cholesterol is an indicator of LDL particle size in patients with type 2 diabetes and normal HDL cholesterol levels. Diabetes Care. 2000;23:1679–1685. doi: 10.2337/diacare.23.11.1679. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuliani G, Galvani M, Maggio M, et al. Plasma soluble gp130 levels are increased in older subjects with metabolic syndrome. The role of insulin resistance. Atherosclerosis. 2010;213:319–324. doi: 10.1016/j.atherosclerosis.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin A, Zulueta J, Hassoun P, Blumberg JB, Meydani M. Effect of vitamin E on hydrogen peroxide production by human vascular endothelial cells after hypoxia/reoxygenation. Free Radic Biol Med. 1996;20:99–105. doi: 10.1016/0891-5849(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 24.van LM, Harries AD, Kumwenda JJ, et al. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis. 2004;4:61. doi: 10.1186/1471-2334-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauretani F, Semba RD, Bandinelli S, et al. Low plasma selenium concentrations and mortality among older community-dwelling adults: the InCHIANTI Study. Aging Clin Exp Res. 2008;20:153–158. doi: 10.1007/bf03324762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 27.Nordin FG, Hedblad B, Berglund G, Nilsson J. Plasma oxidized LDL: a predictor for acute myocardial infarction? J Intern Med. 2003;253:425–429. doi: 10.1046/j.1365-2796.2003.01128.x. [DOI] [PubMed] [Google Scholar]
- 28.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 29.Kondo A, Muranaka Y, Ohta I, et al. Relationship between triglyceride concentrations and LDL size evaluated by malondialdehyde-modified LDL. Clin Chem. 2001;47:893–900. [PubMed] [Google Scholar]
- 30.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 31.Vasankari T, Ahotupa M, Toikka J, et al. Oxidized LDL and thickness of carotid intima-media are associated with coronary atherosclerosis in middle-aged men: lower levels of oxidized LDL with statin therapy. Atherosclerosis. 2001;155:403–412. doi: 10.1016/s0021-9150(00)00573-6. [DOI] [PubMed] [Google Scholar]
- 32.Stephens JW, Gable DR, Hurel SJ, Miller GJ, Cooper JA, Humphries SE. Increased plasma markers of oxidative stress are associated with coronary heart disease in males with diabetes mellitus and with 10-year risk in a prospective sample of males. Clin Chem. 2006;52:446–452. doi: 10.1373/clinchem.2005.060194. [DOI] [PubMed] [Google Scholar]
- 33.Jialal I, Norkus EP, Cristol L, Grundy SM. beta-Carotene inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1991;1086:134–138. doi: 10.1016/0005-2760(91)90164-d. [DOI] [PubMed] [Google Scholar]
- 34.Karppi J, Nurmi T, Kurl S, Rissanen TH, Nyyssonen K. Lycopene, lutein and beta-carotene as determinants of LDL conjugated dienes in serum. Atherosclerosis. 2010;209:565–572. doi: 10.1016/j.atherosclerosis.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Kontush A, Weber W, Beisiegel U. Alpha- and beta-carotenes in low density lipoprotein are the preferred target for nitric oxide-induced oxidation. Atherosclerosis. 2000;148:87–93. doi: 10.1016/s0021-9150(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 36.Jialal I, Grundy SM. Effect of combined supplementation with alpha-tocopherol, ascorbate, and beta carotene on low-density lipoprotein oxidation. Circulation. 1993;88:2780–2786. doi: 10.1161/01.cir.88.6.2780. [DOI] [PubMed] [Google Scholar]
- 37.Gordon DJ, Rifkind BM. Treating high blood cholesterol in the older patient. Am J Cardiol. 1989;63:48H–52H. doi: 10.1016/0002-9149(89)90116-1. [DOI] [PubMed] [Google Scholar]


