Abstract
We previously demonstrated that chronic hyperglycemia has a detrimental influence on neurovascular coupling in the brain—an effect linked to an alteration in the protein kinase C (PKC)-mediated phosphorylation pattern. Moreover, the activity of PKC was increased, in diabetic rat brain, in a tissue fraction composed primarily of the superficial glia limitans and pial vessels, but trended toward a decrease in cerebral cortical gray matter. However, that study did not examine the expression patterns of PKC isoforms in the rat brain. Thus, , in a rat model of streptozotocin (STZ)-induced chronic type 1 diabetes mellitus (T1DM), and in non-diabetic (ND) controls, two hypotheses were addressed. First, chronic T1DM is accompanied by changes in the expression of PKC-α, βII, γ, δ, and ε. Second, those changes differ when comparing cerebral cortex and glio-pial tissue. In addition, we analyzed the expression of a form of PKC-γ, phosphorylated on threonine 514 (pT514- PKC-γ), as well as the receptor for activated C kinase 1 (RACK1). The expression pattern of different PKC isoforms was altered in a complex and tissue-specific manner during chronic hyperglycemia. Notably, in the gray matter, PKC-α expression significantly decreased, while pT514-PKC-γ expression increased. However, PKC-βII, -γ, -δ, -ε, and RACK1 expressions did not change. Conversely, in glio-pial tissue, PKC-α and RACK1 were upregulated, whereas PKC-γ, pT514-PKCγ, and PKC-ε were downregulated. PKC-βII, and PKC-δ, were unchanged. These findings suggest that the PKC activity increase previously seen in the glio-pial tissue of diabetic rats may be due to the selective upregulation of PKC-α, and ultimately lead to the impairment of neurovascular coupling.
Keywords: Neurovascular coupling, Diabetes, cerebrovascular disease, pial vessels, PKC isoform, Western blot
1. Introduction 1
The protein kinase C (PKC) family of serine/threonine kinases plays a central role in regulating a wide variety of cellular processes in the brain, from cell growth to learning and memory. There are three families of PKC isoforms in vertebrates: classical PKCs (α, βI, βII and γ), novel PKCs (ε, δ, η, and θ), and atypical PKCs (ζ and ι). The classical PKCs are activated by Ca2+ and diacylglycerol (DAG), while the novel PKCs are activated by DAG, but are Ca2+-independent. The atypical PKCs are activated by neither Ca2+ nor DAG.
A major perturbation associated with type 1 (insulin-dependent) diabetes mellitus is chronic hyperglycemia, which, in turn, has been linked to microvascular complications, such as retinopathy, neuropathy and nephropathy (1993). Hyperglycemia may also increase the risk of Alzheimer’s disease (Profenno et al., 2010), stroke, mild cognitive impairment and dementia (Alvarez et al., 2009). Hyperglycemia-associated mechanisms may induce vascular dysfunctions. These include increased polyol pathway flux, altered cellular redox state, increased formation of diacylglycerol (DAG) and the subsequent activation of some PKC isoforms, and accelerated non-enzymatic formation of advanced glycated end products (for review see ref. (Das Evcimen and King, 2007). It is possible that each of these mechanisms may contribute to the different features of diabetic complications. In particular, it has been shown that activation of the DAG-PKC pathway is associated with many vascular abnormalities in the retinal, renal, neural, and cardio- and cerebro-vascular tissues in diabetes mellitus (Geraldes et al., 2009; Koya and King, 1998; Mayhan et al., 2004; Pelligrino and Albrecht, 1991; Pelligrino et al., 1994; Ramana et al., 2005; Shiba et al., 1993).
In a recent study, we demonstrated that chronic hyperglycemia had a detrimental effect on neurovascular coupling. Moreover, that change was associated with an alteration in the PKC-mediated phosphorylation pattern (Vetri et al., 2012). Curiously, we found that PKC activity was increased, in diabetic rats, in tissue samples largely composed of superficial glia limitans and pial vessels (glio-pial tissue), but not in the bulk cerebral cortex. Moreover, all the features of the diabetes-associated cerebrovascular dysfunction studied in that work were recapitulated by topical application of a PKC activator (Vetri et al., 2012). However, there were some key limitations in that study. First, multiple PKC isoforms known to be present in the rat brain were not considered. Second, the PKC blocker used was not isoform-specific.
Therefore, in a rat model of streptozotocin-induced chronic type 1 diabetes mellitus, and in non-diabetic controls, we monitored the expression of selected PKC isoforms in both cerebral cortex gray matter and glio-pial tissue. We focused on those PKC isoforms which exhibit Ca2+- and/or DAG-dependency, i.e. classical and novel, but not the atypical. The PKC isoforms studied were α, βII, γ, δ, and ε. We also monitored a phosphorylated, and fully active, form of PKC-γ (pT514-PKC-γ), and the receptor for the activated C kinase 1 (RACK1). The latter is important in the maintenance of the activated state of PKC-βII (Ron et al., 1999).
Two hypotheses were addressed. First, chronic type 1 diabetes mellitus affects the expression patterns of PKC isoforms in the rat brain. Second, the expression changes in PKC isoforms during diabetes are different in the cerebral cortex compared to glio-pial tissue.
2. Results
2.1. Expression of PKC isoforms in the rat brain
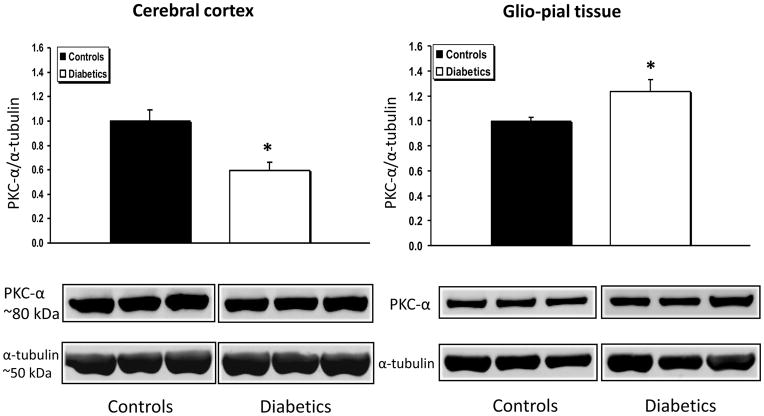
In Fig.1 are shown the expression changes of PKC-α, normalized to α-tubulin, in the cerebral cortex and the glio-pial tissue of diabetic vs non-diabetic rats. In the cerebral cortex, PKC-α expression was decreased by 40% in samples from diabetic rats (n=7), when compared with normoglycemic rats (n=7) (P=0.002). Interestingly, in the glio-pial tissue, the expression change was in the opposite direction, with an increase of 23% of PKC-α in diabetics (n=7 in both groups, P=0.04).
Fig 1.
PKC-α expression profile in diabetic vs non diabetic rat brains in cerebral cortex and glio-pial tissue. Western immunoblot protein analysis of PKC-α in samples obtained from the cerebral cortex (left) or by stripping off pial tissue from the surface of the brain (right) in control and 4 month diabetic rats. The latter samples not only included pial vessels, but also adherent elements of the underlying glia limitans, which is mainly constituted by astrocytes. Also shown are representative blots and quantitative protein expression data for PKC-α and α-tubulin in control and diabetic rats. (*, P < 0.05; n = 7 in each group). Each graph shows the average of 3 independent blots. α-tubulin was used as a loading control. Ratios were normalized to the respective control's group average. The bands within a frame are contiguous, and splicing is shown by a white space between frames.
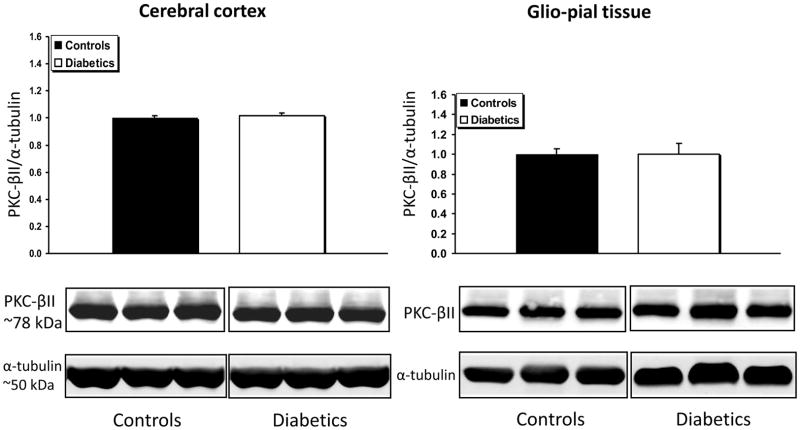
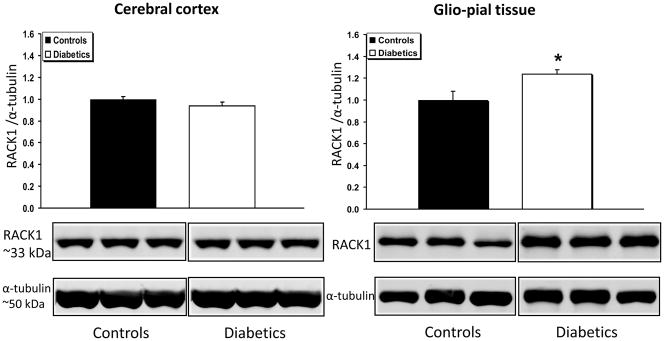
PKC-βII expression, was not affected by the diabetic condition in either bulk cortical gray matter or in glio-pial samples (Fig. 2). However, the expression of the scaffolding protein RACK1, which has been recognized as a stabilizer of the activated PKC-βII (Adams et al., 2011), was found to be increased only in the glio-pial tissue of diabetic rats (Fig. 3) (+23%, n=7 and n=6 in non-diabetic and diabetic rats, respectively; P=0.03). By virtue of the RACK1 increased expression, a gain of activity of PKC-βII is conceivable even in the absence of an increase in PKC-βII expression.
Fig 2.
PKC-βII expression profile in diabetic vs non diabetic rat brains in cerebral cortex gray matter and glio-pial tissue. Western immunoblot protein analysis of PKC-βII in samples obtained from the cerebral cortex (left) or glio-pial tissue (right) in control and 4 month diabetic rats. Representative blots and quantitative protein expression data for PKC-βII and α-tubulin in control and diabetic rats are shown (n = 6–7 in each group). Each graph shows the average of 3 independent blots (see Materials and Methods for technical details).
Fig 3.
RACK1 expression profile in diabetic vs non diabetic rat brains in cerebral cortex and glio-pial tissue. Western immunoblot protein analysis of the receptor for activated C kinase 1 (RACK1) in samples obtained from the cerebral cortex (left) or glio-pial tissue (right) in control and 4 month diabetic rats. Also shown are representative blots and quantitative protein expression data for RACK1 and α-tubulin in control and diabetic rats. (*, P < 0.05; n = 6–7 in each group). See Materials and Methods for technical details.
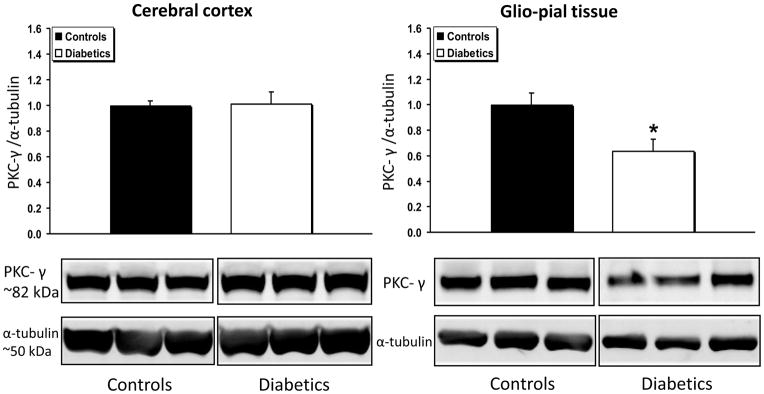
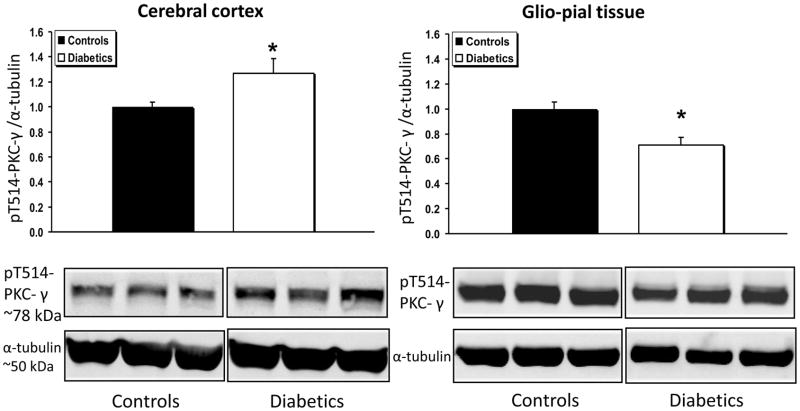
PKC-γ expression (Fig. 4) was unchanged in diabetic vs non-diabetic cerebral cortices, but decreased by 36% in glio-pial tissue of diabetic rats (n=6) relative to non-diabetic rats (n=7, P=0.02). In accordance with this data, the activated form of PKC-γ, namely, the form phosphorylated on threonine 514 (pT514-PKC-γ), was found to be decreased by ~30% in the glio-pial tissue of diabetic vs non-diabetic rats (Fig. 5) (n=7 in both groups, P=0.005). In contrast, in the cerebral cortex of diabetic vs non-diabetic rats, pT514-PKC-γ was increased by 30% (n=7 in both groups, P=0.04).
Fig 4.
PKC-γ expression profile in diabetic vs non diabetic rat brains in cerebral cortex and glio-pial tissue. Western immunoblot protein analysis of PKC-γ in samples obtained from the cerebral cortex (left) or glio-pial tissue (right) in control and 4 month diabetic rats. Also shown are representative blots and quantitative protein expression data for PKC-γ and α-tubulin in control and diabetic rats. (*, P < 0.05; n = 6–7 in each group). See Materials and Methods for technical details.
Fig 5.
pT514-PKC-γ expression profile in diabetic vs non diabetic rat brains in cerebral cortex and glio-pial tissue. Western immunoblot protein analysis of the phosphorylated form of PKC-γ in Threonine 514 (pT514-PKC-γ) in samples obtained from the cerebral cortex (left) or by stripping off pial tissue from the surface of the brain (right) in control and 4 month diabetic rats. Also shown are representative blots and quantitative protein expression data for pT514-PKC-γ and α-tubulin in control and diabetic rats. (*, P < 0.05; n = 7 in each group). See Materials and Methods for technical details.
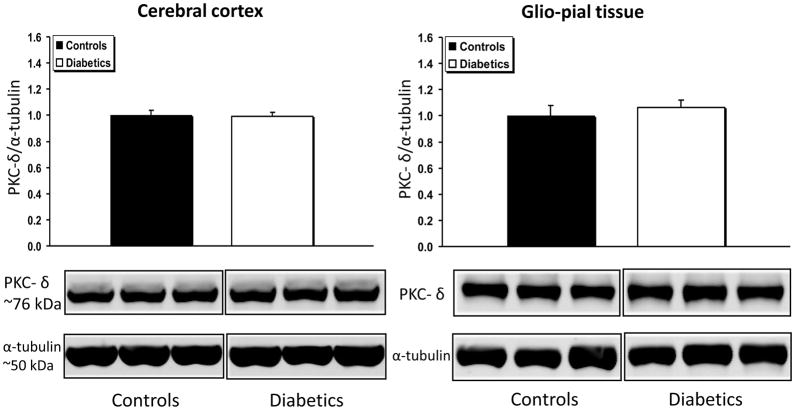
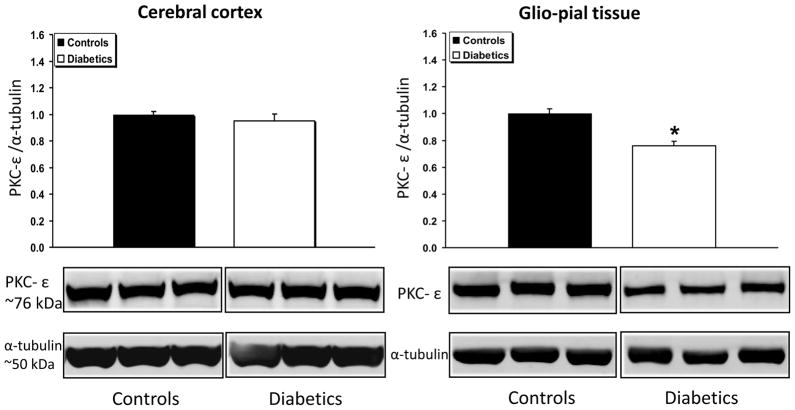
The expression of the novel isoform PKC-δ (Fig. 6) was unchanged in both cerebral cortex and glio-pial tissue of diabetics (n=7) vs non-diabetics (n=7) (P>0.05 for both groups). Compared to non-diabetic rats, PKC-ε expression was significantly decreased (by 30%) in the glio-pial tissue of diabetic rats (n=5 in both groups, P=0.004), but not in the cerebral cortex. A summary of the complex patterns of PKC isoform expression modifications associated with type 1 diabetes mellitus in the two tissues examined is provided in Table 1.
Fig 6.
PKC-δ expression profile in diabetic vs non diabetic rat brains in cerebral cortex and glio-pial tissue. Western immunoblot protein analysis of PKC-δ in samples obtained from the cerebral cortex (left) or by stripping off pial tissue from the surface of the brain (right) in control and 4 month diabetic rats. Representative blots and quantitative protein expression data for PKC-δ and α-tubulin in control and diabetic rats are shown (n = 7 in each group). See Materials and Methods for technical details.
Table 1.
Differences in expression of PKC isoforms in the brain of diabetic rats, compared to normoglycemic controls, in cerebral cortex and glio-pial tissue. Summary of the differential expression pattern of PKC isoforms in the brain of diabetic rats compared to normoglycemic controls in specimens derived from cerebral cortex or glio-pial tissue (see text for details).
| PKC isoform | Cerebral cortex | Glio pial tissue |
|---|---|---|
| PKC-α | ⬇ | ⬆ |
| PKC-βII | ⬌ | ⬌ |
| PKC-γ | ⬌ | ⬇ |
| pT514-PKC-γ | ⬆ | ⬇ |
| PKC-δ | ⬌ | ⬌ |
| PKC-ε | ⬌ | ⬇ |
3. Discussion
This study shows that in chronically hyperglycemic rats with type 1 diabetes mellitus, the expression patterns of PKC isoforms in the rat brain are affected in a complex manner. Moreover, the levels and expression patterns of several PKC isoforms differ in the two types of tissues studied, namely cerebral cortex gray matter, and glio-pial tissue. The latter is enriched in astrocytic and vascular elements.
The motivation for the present study arose from the recent finding from our laboratory (Vetri et al., 2012) that the total PKC activity in a rat model of type 1 diabetes mellitus was selectively increased in the glio-pial tissue, but not in cerebral cortex gray matter. In parallel with the PKC activity increase in glio-pial tissue, the efficiency of neurovascular coupling was reduced. That impairment was specifically related to two K+ channels, that are modulated by PKC, namely large conductance-Ca2+ operated (BKCa) and K+ inward rectifier (Kir) channels, and was completely reversed with the acute topical application of the general PKC inhibitor, Calphostin C (Vetri et al., 2012). Moreover, in the same study, we demonstrated that a PKC activator, phorbol 12,13-dibutyrate, was able to recapitulate the functional cerebrovascular alterations occurring in diabetic animals (Vetri et al., 2012). Therefore, the aim of our research was shifted toward identifying the PKC isoform(s) that may be responsible for the neurovascular coupling impairment seen in diabetic rats. We focused our study on PKC isoforms, both conventional, e.g. PKC-α, βII, and γ, and novel, e.g. PKC-δ and ε, that have been linked to either diabetes (Das Evcimen and King, 2007; Tickerhoof et al., 2003) or impaired neuro-vascular function (Geraldes et al., 2009; Lee et al., 1999).
PKC-α is expressed by virtually all cells in the brain. Its expression levels have been shown to be either upregulated (Ramakrishnan et al., 2004) or unchanged (Shiba et al., 1993) in different tissue regions of the type 1 diabetic brain. Our findings indicate divergent patterns of PKC-α expression changes when comparing glio-pial tissue (increase) to cortical gray matter (decrease) at 4 months from diabetes induction. There does not appear to be a common thread linking the studies cited above with the results reported in the present study, since different brain regions were examined in rats exposed to widely different durations of diabetes. Nevertheless, our data reveals that the pattern of PKC-α expression change is the closest, among the isoforms studied, to the total PKC activity changes seen in our recent paper (Vetri et al., 2012); where an increase in the glio-pial tissue, and a decrease in the cerebral cortex was found. Moreover, PKC-α has been linked to the inhibition of ionic channels important in neurovascular coupling, such as inward rectifier K+ channels (Park et al., 2006), and, less specifically, large conductance-Ca2+ operated (BKCa) K+ channels (Tian et al., 1999). Therefore, we speculate that the impairment of neurovascular coupling and cerebrovascular function, occurring in a rat model of type 1 diabetes mellitus, and revealed by our previous work, may be mechanistically linked to an increase in PKC-α expression and activity in pial vessels and/or the astrocytes in contact with them (glia limitans). The precise mechanisms involved, nevertheless, remain uncertain. Clearly, further experimentation is warranted.
PKC-βII has been classically linked to vascular alterations induced by hyperglycemia (Das Evcimen and King, 2007; Inoguchi et al., 1992; Koya and King, 1998), especially in the retina (Shiba et al., 1993). Yet, present and previous (Shiba et al., 1993) data did not reveal any significant changes in expression. Accordingly, one might conclude that PKC-βII is not involved in the cerebrovascular deleterious effects of diabetes. However, analysis of the expression of the scaffolding protein RACK1, which, among its multiple functions, has a major role in stabilizing the active form of PKC-βII (Ron et al., 1999), hints at a possible involvement of PKC-βII in diabetic cerebrovascular dysfunction. That is, RACK1 expression was significantly increased in the glio-pial tissue, but not in the cerebral cortex. This could imply that a localized increased activity of PKC-βII might contribute to the detrimental effects of diabetes on neurovascular coupling. However, further studies are needed.
We studied the expression of the γ isoform of PKC primarily because it modulates connexin 43 (Cx43) (Kuroki et al., 1998; Lin et al., 2007), which plays a very important role in the process of coupling increased sensorimotor activity to pial arteriolar dilation (Xu et al., 2008). In particular, autophosphorylation of PKC-γ on threonine 514 fully activates this isoform and, in turn, causes an inhibitory phosphorylation of Cx43 in the diabetic rat lens (Lin et al., 2007). Similarly, PKC-ε is responsible, in the diabetic rat heart, for a decrease in conductivity and suppression of cell-to-cell coupling, via an increased protein degradation of Cx43 (Lin et al., 2006). It was interesting, then, to study PKC-γ, pT514-PKC-γ and PKC-ε expression changes in our diabetes model. However, the expression of these PKC isoforms, and likely their activity, was decreased in the glio-pial tissue and generally unchanged in the cerebral cortex. We are not aware of any mechanism involving a decreased PKC-γ or -ε activity, which may account for a decreased efficiency in neurovascular communication.
PKC-δ isoform expression was investigated on the basis of recent publications reporting an increase expression in diabetic retinal pericytes (Geraldes et al., 2009) as well as the ability of this isoform to interact with BKCa channels (Kim and Park, 2008). However, Western blot analysis failed to reveal any significant change in PKC-δ expression neither in the cerebral cortex nor in the glio-pial tissue. Therefore, we would tend to exclude PKC-δ phosphorylation products as possible mediators of vascular dysfunction in the diabetic brain
In the present study, we focused solely on the expression pattern of PKC isoforms. Although there is evidence suggesting that PKC activity and expression are tightly related (e.g. (Geraldes et al., 2009; Lin et al., 2007; Shiba et al., 1993), future studies should focus on the specific activities of the different PKC isoforms, in particular α and βII, as well as on specific pharmacologic interventions to treat and/or prevent cerebrovascular dysfunction in type 1 diabetes.
3.1 Conclusion
In conclusion, the pattern of PKC-α expression changes, increased in glio-pial tissue, and decreased in the cerebral cortex, is the closest, among the isoforms studied, to the total PKC activity changes seen in our recent in vivo study (Vetri et al., 2012). Therefore, we speculate that the impairment of neurovascular coupling and cerebrovascular function, occurring in the rat model of type 1 diabetes mellitus, may be mechanistically linked to an increase in PKC-α expression and activity in pial arterioles and/or the astrocytes in contact with them (glia limitans). Moreover, the upregulation of RACK1 in the glio-pial tissue does not allow one to exclude the participation of PKC-βII in the above mentioned cerebrovascular impairments. Finally, the fact that PKC inhibition can acutely reverse chronically elevated PKC activity and rapidly correct vascular dysfunction (Vetri et al., 2012) has clinical implications.
4. Experimental procedures
4.1 Diabetes model
Twenty-eight adult Sprague-Dawley female rats (4–6 month old, 250–350 g) were used for the experiments. Experimental procedures were approved by the Animal Care and Use Committee of the University of Illinois at Chicago. Type 1 diabetes was induced with a single i.v. streptozotocin (STZ) injection (50 mg/kg) to 2 month old rats (n = 14), around 16–18 weeks prior to the experiment. Animals were considered diabetic when blood glucose exceeded 350 mg/dL 3 days after STZ. Body weight and blood glucose were measured twice a week (Contour glucometer, Bayer, Mishawaka, IN) for the entire period before the study. Animals showing signs of infections or a body weight loss exceeding 20% compared to the other rats in the same batch were sacrificed and not used for the study. No insulin was administered to the diabetic or non-diabetic rats. The average blood glucose level and body weight for the non-diabetic rats at the time of sacrifice in the present study were 127±8 mg/dl and 311±11 grams, respectively. Diabetic rats had an average blood glucose of 538±32 mg/dl and body weight of 263±10 grams.
4.2 Western immunoblot analyses
PKC isoform-specific changes in protein expression in cerebral cortical gray matter and in glio-pial tissue, were monitored in diabetic (n = 14) and non-diabetic (n = 14) rats using Western immunoblot analysis. The procedure for preparation of cerebral cortical gray matter and glio-pial samples, as well as the rationale for performing such a differential protein analysis, are provided in a recent publication (Vetri et al., 2012).
Glio-pial tissue was harvested by stripping off pia mater and pial vessels from the whole cortical surface of the isolated brain with fine tip forceps (Vetri et al., 2012). Cerebral cortex samples were dissected, with a blade, from the parietal region. Tissue collection was performed shortly after the rat was sacrificed and trancardially perfused with PBS. Tissues were quickly immersed into a lysing buffer, at pH 7.4, containing in mM: 20 Tris, 2 EDTA, 2 EGTA, 1 PMSF, 1 NaVO4, 20 NaF; 1% (V/V) NP-40, and 2% (V/V) Sigma protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Samples were sonicated 3 times on ice for 10–15 sec, rocked for 1 h at 4º C, centrifuged at 100,000g for 1 h at 4º C, and stored in multiple small aliquots at −80º until used. These extraction conditions allowed us to extract proteins from cytosol, plasma membrane and nuclear membrane. Therefore, the total expression levels of the different PKC isoforms were monitored. Loading volumes were adjusted to obtain 10 μg of total protein in each lane. Proteins were separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, Ca) for 60 min and transferred on Immobilon FL (Millipore, Billerica, MA) PVDF membranes. The blots were hybridized with either mouse anti-PKC-α (1:400) (clone H-7, SantaCruz, Santa Cruz, CA), rabbit anti- PKC-βII (1:500) (clone C-18, SantaCruz), rabbit anti- PKC-γ (1:400) (clone C-19, SantaCruz), rabbit anti-pT514-PKC-γ (1:200) (Epitomics, Burlingame, CA), rabbit anti- PKC-δ (1:200) (clone C-17, SantaCruz), rabbit anti- PKC-ε (1:400) (clone C-15, SantaCruz), or mouse anti-RACK1 (1:200) (clone B-3, SantaCruz), and subsequently incubated with appropriate secondary antibodies conjugated with infrared dye (IRDye 680 or 800CW, LI-COR Biosciences, Lincoln, NE) diluted 1:5000 in 0.5X Odyssey blocking buffer and 0.1% Tween 20. Mouse α-tubulin was used as loading control (mouse monoclonal, 1:4000, Sigma-Aldrich, St. Louis, MO; goat or donkey anti-mouse, 1:10000, IRDye 680, LI-COR Biosciences). Blot membranes were then scanned using the LI-COR Odyssey Infrared Imaging System (LI-COR Biosciences). The protein levels in each brain sample (n = 5–7 in each group) were expressed as the ratio of the optical densities of the protein of interest over the housekeeping protein, normalized to the control average. Optical densities were calculated using the Odyssey software. To improve reliability, the average of the values from 3 different blots for each brain sample was used for statistical comparison between groups. Only one sample was derived from each animal, either the cerebral cortex or the glio-pial tissue.
4.3 Drugs
Streptozotocin was obtained from Sigma-Aldrich (St. Louis, MO).
4.4 Statistics
Statistical comparisons between groups were performed using an unpaired Student's t-test. Data are expressed as mean ± S.E.M. Statistical significance was set at P = 0.05; n refers to the number of samples/animals (only one sample derived from each animal).
Fig 7.
PKC-ε expression profile in diabetic vs non diabetic rat brains in cerebral cortex and glio-pial tissue. Western immunoblot protein analysis of PKC-ε in samples obtained from the cerebral cortex (left) or by stripping off pial tissue from the surface of the brain (right) in control and 4 month diabetic rats. Representative blots and quantitative protein expression data for PKC-ε and α-tubulin in control and diabetic rats are shown (*, P < 0.05; n = 5 in each group). See Materials and Methods for technical details.
Highlights.
The pattern of expression of several PKC isoforms was studied via Western blot
The tissues used for the analysis were cortical grey matter and glio-pial tissue
PKC-α expression was increased in glio-pial tissue, and decreased in the cerebral cortex,
PKC-α expression changes match the total PKC activity alterations seen in diabetic rats
Acknowledgments
This work was supported by NIH grant HL088259 to D.A. P., JDRF postdoctoral fellowship 3-2008-462 to F.V., and AHA grant 0635337N to H.L.X. A special thanks to Susan Anderson, Fulong Tan, and Kai Tan for their outstanding technical support.
Footnotes
STZ: streptozotocin
DAG: diacylglycerol
RACK1: receptor for the activated C kinase 1
BKCa: large conductance Ca2+ operated K+ channels
Kir: inward rectifier K+ channels
Cx4 : connexin 43
PKC: protein kinase C
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Adams DR, Ron D, Kiely PA. RACK1, A multifaceted scaffolding protein: Structure and function. Cell Commun Signal. 2011;9:22. doi: 10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav Brain Res. 2009;198:224–30. doi: 10.1016/j.bbr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992;89:11059–63. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JY, Park CS. Potentiation of large-conductance calcium-activated potassium (BK(Ca)) channels by a specific isoform of protein kinase C. Biochem Biophys Res Commun. 2008;365:459–65. doi: 10.1016/j.bbrc.2007.10.179. [DOI] [PubMed] [Google Scholar]
- 8.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 9.Kuroki T, Inoguchi T, Umeda F, Ueda F, Nawata H. High glucose induces alteration of gap junction permeability and phosphorylation of connexin-43 in cultured aortic smooth muscle cells. Diabetes. 1998;47:931–6. doi: 10.2337/diabetes.47.6.931. [DOI] [PubMed] [Google Scholar]
- 10.Lee IK, Koya D, Ishi H, Kanoh H, King GL. d-Alpha-tocopherol prevents the hyperglycemia induced activation of diacylglycerol (DAG)-protein kinase C (PKC) pathway in vascular smooth muscle cell by an increase of DAG kinase activity. Diabetes Res Clin Pract. 1999;45:183–90. doi: 10.1016/s0168-8227(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 11.Lin D, Harris R, Stutzman R, Zampighi GA, Davidson H, Takemoto DJ. Protein kinase C-gamma activation in the early streptozotocin diabetic rat lens. Curr Eye Res. 2007;32:523–32. doi: 10.1080/02713680701418124. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Ogawa K, Imanaga I, Tribulova N. Alterations of connexin 43 in the diabetic rat heart. Adv Cardiol. 2006;42:243–54. doi: 10.1159/000092573. [DOI] [PubMed] [Google Scholar]
- 13.Mayhan WG, Mayhan JF, Sun H, Patel KP. In vivo properties of potassium channels in cerebral blood vessels during diabetes mellitus. Microcirculation. 2004;11:605–13. doi: 10.1080/10739680490503410. [DOI] [PubMed] [Google Scholar]
- 14.Park WS, Kim N, Youm JB, Warda M, Ko JH, Kim SJ, Earm YE, Han J. Angiotensin II inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase Calpha. Biochem Biophys Res Commun. 2006;341:728–35. doi: 10.1016/j.bbrc.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Pelligrino DA, Albrecht RF. Chronic hyperglycemic diabetes in the rat is associated with a selective impairment of cerebral vasodilatory responses. J Cereb Blood Flow Metab. 1991;11:667–77. doi: 10.1038/jcbfm.1991.119. [DOI] [PubMed] [Google Scholar]
- 16.Pelligrino DA, Koenig HM, Wang Q, Albrecht RF. Protein kinase C suppresses receptor-mediated pial arteriolar relaxation in the diabetic rat. Neuroreport. 1994;5:417–20. doi: 10.1097/00001756-199401120-00011. [DOI] [PubMed] [Google Scholar]
- 17.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–12. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishnan R, Sheeladevi R, Suthanthirarajan N. PKC-alpha mediated alterations of indoleamine contents in diabetic rat brain. Brain Res Bull. 2004;64:189–94. doi: 10.1016/j.brainresbull.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–29. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 20.Ron D, Jiang Z, Yao L, Vagts A, Diamond I, Gordon A. Coordinated movement of RACK1 with activated betaIIPKC. J Biol Chem. 1999;274:27039–46. doi: 10.1074/jbc.274.38.27039. [DOI] [PubMed] [Google Scholar]
- 21.Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol. 1993;265:E783–93. doi: 10.1152/ajpendo.1993.265.5.E783. [DOI] [PubMed] [Google Scholar]
- 22.Tian L, Philp JA, Shipston MJ. Glucocorticoid block of protein kinase C signalling in mouse pituitary corticotroph AtT20 D16:16 cells. J Physiol. 1999;516(Pt 3):757–68. doi: 10.1111/j.1469-7793.1999.0757u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tickerhoof MM, Farrell PA, Korzick DH. Alterations in rat coronary vasoreactivity and vascular protein kinase C isoforms in Type 1 diabetes. Am J Physiol Heart Circ Physiol. 2003;285:H2694–703. doi: 10.1152/ajpheart.00394.2003. [DOI] [PubMed] [Google Scholar]
- 24.Vetri F, Xu H, Paisansathan C, Pelligrino DA. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK(Ca) and Kir channels. Am J Physiol Heart Circ Physiol. 2012;302:H1274–84. doi: 10.1152/ajpheart.01067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H622–32. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]