Abstract
Chronic intermittent hypoxia (CIH) is a model of the arterial hypoxemia that accompanies sleep apnea and increases resting arterial pressure (AP). We examined the effects of 7 days exposure to CIH on arterial baroreflex control of renal sympathetic nerve activity (RSNA) and heart rate (HR) in rats. Sprague-Dawley rats (15 +/− 2 weeks old) were exposed to CIH (9% oxygen for 3min every 10 min, 8 h per day) for 7 days (n = 16) while control rats (n = 18) were maintained in normoxia. Baroreflex regulation of RSNA and HR were estimated in Inactin anesthetized and artificially ventilated rats during infusions of phenylephrine and nitroprusside to manipulate AP. After exposure to CIH, resting mean AP was higher in CIH than in control group (115 +/− 7 vs. 105 +/− 7, P<0.001). Resting HR did not differ between the two groups. Exposure to CIH shifted the AP-RSNA relationship rightward (approximately 10 mmHg, P<0.01). CIH did not alter maximum gain of the baroreflex control of RSNA (−2.6 +/− 0.6 vs. −2.5 +/− 0.6 arbitrary units (au)/mmHg) and HR (−1.8 +/− 0.6 vs. −1.8 +/− 0.7 bpm/mmHg, CIH vs. control). In addition, cardiac spontaneous baroreflex sensitivity in conscious rats (n = 8) also did not change during exposure to CIH. These results indicate that resetting of the sympathetic baroreflex control, rather than an impairment of its sensitivity, is associated with an onset of hypertension induced by CIH.
Keywords: sleep apnea, hypertension, sympathetic nerve activity, baroreflex
1. Introduction
Sleep apnea is an independent risk factor for developing hypertension (Peppard et al., 2000). Chronic intermittent hypoxia (CIH) has been used as a model of arterial hypoxemia that occurs during sleep apnea (Fletcher et al., 1992c). Exposure to CIH results in a sustained increase in arterial pressure (AP) that persists into the diurnal period (Fletcher et al., 1992c).
Several earlier studies (Gu et al., 2007; Lai et al., 2006; Lin et al., 2007; Peng et al., 2012; Yan et al., 2008; Zoccal et al., 2009) have examined baroreflex function after exposure to CIH. In rats (Gu et al., 2007; Yan et al., 2008) or mice (Lin et al., 2007), exposure to CIH for over 30 days induced a reduction in baroreflex control of heart rate (HR) that was associated with hypertension. Fletcher et al. (Fletcher et al., 1999) examined change of AP over a 35-day period during exposure to CIH in conscious rats and found that the elevation of AP at 7 days exposure was not different than the AP observed at 35 days exposure, indicating that the sustained increase in AP induced by CIH is maximal after 7 days exposure. However, baroreflex control of sympathetic nerve activity (SNA) after 7 days exposure to CIH remains unclear, although the AP elevation induced by CIH results from sympathetic activation (Fletcher et al., 1992b).
In previous studies, exposure to CIH for 10 days increased baroreflex sensitivity for HR despite an increasing AP (Zoccal et al., 2009). Lai et al. (Lai et al., 2006) examined changes over time in AP and spontaneous baroreflex sensitivity (SBRS) during CIH exposure. They demonstrated that AP was elevated in rats after exposure to CIH for 5 days, while a reduction in spontaneous baroreflex sensitivity was not observed until the 17th day of CIH. These findings suggest that an onset of hypertension induced by CIH may not be secondary to reductions in the baroreflex sensitivity. Accordingly, we hypothesized that 7 days exposure to CIH which results in a sustained increase in AP would reset baroreflex control of SNA rather than reduce its sensitivity. To test our hypothesis, we examined baroreflex stimulus-response curves of SNA in anesthetized rats after CIH exposure for 7 days. We also examined AP and cardiac SBRS before, during and after 7 days exposure to CIH in conscious rats.
2. Materials and methods
2.1 General
Experiments were performed using male adult Sprague-Dawley rats. All rats were given at least 1 wk to acclimate before being used for any procedures. The rats were randomly assigned either a CIH group (n = 8, 14±2 weeks old, 463±12 g) or a control group (n = 7, 14±2 weeks old, 498±25 g, at end of experiment) in conscious animal experiments. In anesthetized animal experiments, A separate group of rats from those used in the conscious animal experiments were randomly assigned to either a CIH group (n = 16, 15±1 weeks old, 450±11 g) or a control group (n = 18, 15±1 weeks old, 451±14 g). The Institutional Animal Care and Use Committee of the University of North Texas Health Science Center approved all experimental protocols.
2.2 Chronic intermittent hypoxia exposure
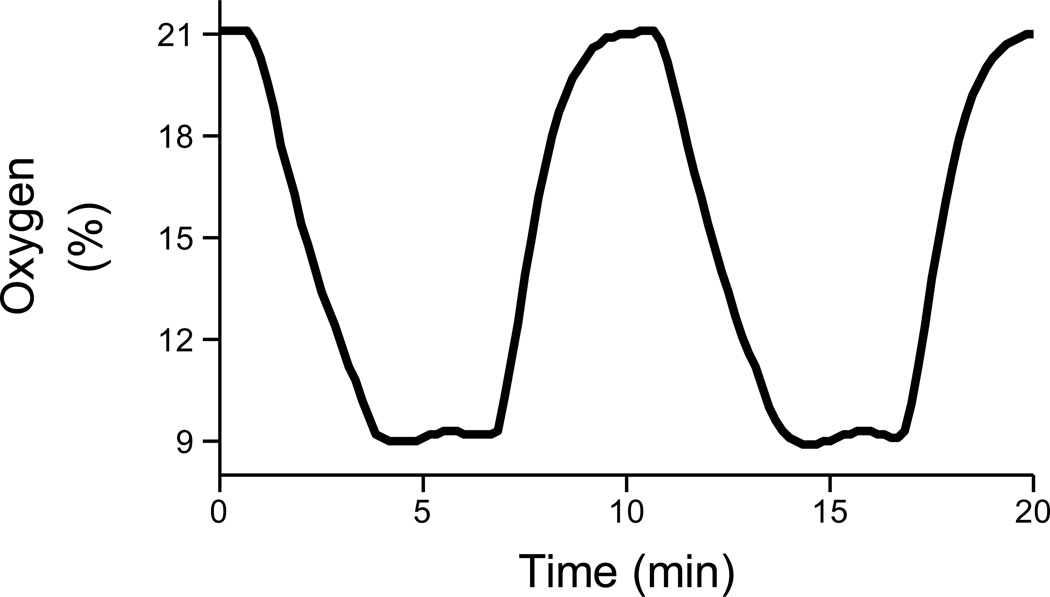
CIH rats were housed in hypoxia chambers with ad libitum food and water access as previously described (Zhang et al., 2008). O2 concentration in the chambers was controlled using 100% N2 and 100% O2 by a computerized system (Oxycycler, Biospherix, NY, USA). The intermittent hypoxia was set to cycle at 9% O2 for 6 min and 21% O2 for 4min. In this setting, O2 concentration of 9% was maintained for approximately 3 min in each hypoxia cycle (Fig. 1). Exposure to CIH occurred for 8 hrs during the light period (8:00 to 16:00) for 7 days. The control rats were housed in normoxic conditions. In experiments in anesthetized rats, the CIH rats were taken out of the intermittent hypoxia chamber in the morning following the last day of exposure. Therefore, the baroreflex curves were obtained at 20–22 h after the last exposure to intermittent hypoxia.
Figure 1.
Percentage of oxygen in the chronic intermittent hypoxia (CIH) chambers. Oxygen was maintained at 9 % for 3 min in each hypoxia cycle. Exposure to CIH occurred for 8 hrs during the light period (8:00 to 16:00) for 7 days.
2.3 Assessment of AP, HR, SBRS and respiratory frequency in conscious rats
A pressure transmitter (TA11PA-C40 Implant: DSI, MN, USA) was implanted in anesthetized rats (2 % Isoflurane). Seven days after implant surgery, mean AP (MAP), HR and respiratory frequency were recorded for 10 s every 10 min (144 times a day). In addition, AP was also recorded in a continuous manner for 1 h every 12 h (at 0:00 and 12:00) for estimation of SBRS.
2.4 Assessment of baroreflex function curves in anesthetized rats
2.4.1 Surgical preparations
The rats were anesthetized with the long-acting rodent anesthetic thiobutabarbital sodium (Inactin, SIGMA) at the initial dose of 110 mg/kg ip. Supplemental anesthetic was given in doses of 10 mg/kg ip as required to maintain a surgical plane of anesthesia (i.e., an absence of withdrawal to pinch of the hind paw and no evidence of fluctuations in AP in response to surgical manipulation or pinch of the hind paw following paralysis). Gallamine triethiodide (5 mg/kg/h) was infused intravenously to induce paralysis. Body temperature was maintained at approximately 38°C using a heating pad. The animals were intubated through a tracheotomy and mechanically ventilated with oxygen-enriched room air. A catheter was inserted into the abdominal aorta via the femoral artery and was used for the measurement of AP. HR was calculated from the AP waveform. Three catheters were inserted into the femoral vein for the infusion of drugs.
We exposed the left renal sympathetic nerve retroperitoneally and attached a pair of Teflon-coated stainless steel wires to record renal SNA (RSNA). The nerve and electrodes were secured with silicone glue (Kwik-Sil, World Precision Instruments, FL, USA). The nerve signal was amplified (× 20,000–50,000) with the band-pass filters set between 100 and 3,000 Hz. The filtered signal was full-wave rectified and integrated using a time constant of 5 ms to quantify the nerve activity.
2.4.2 Protocols
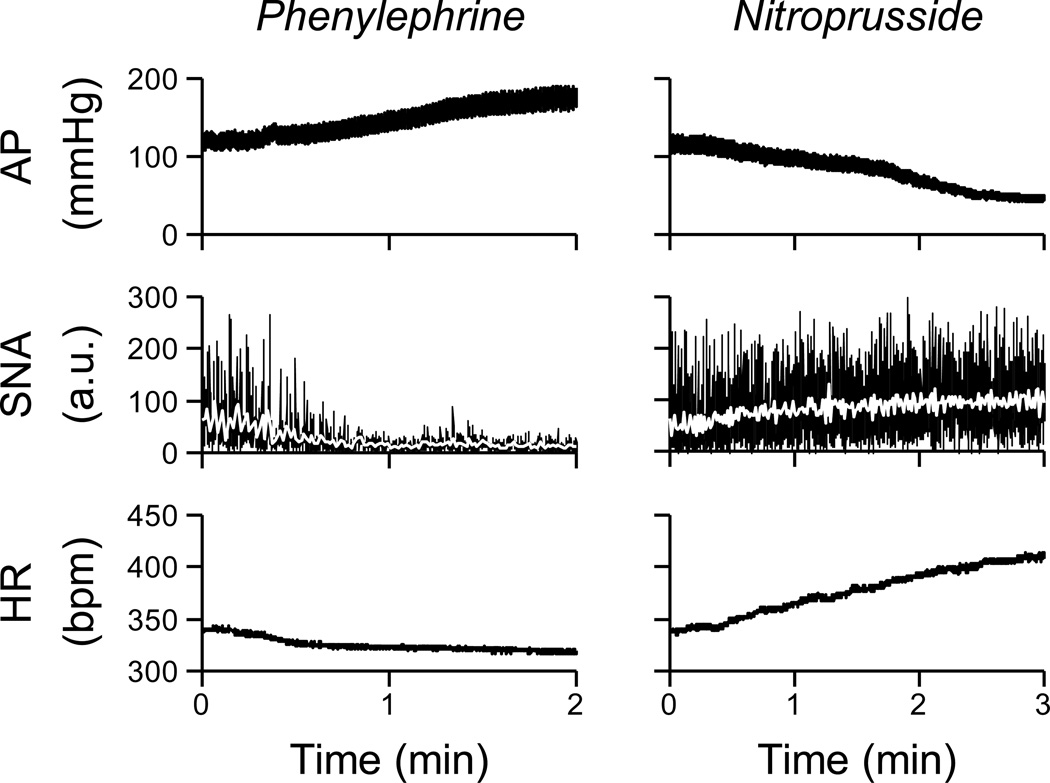
The experimental protocol was initiated at least 1 h after completion of the surgical procedures. After baseline AP and HR were obtained for 5 min, baroreflex function curves of RSNA and HR were determined during the infusion of phenylephrine HCI (100 µg/ml) to increase AP and sodium nitroprusside (100 µg/ml) to decrease AP. The rate of infusion was adjusted to produce continuous changes in AP at a rate of 1 mmHg/s (Fig. 2). The order of administration of phenylephrine and nitroprusside was randomized.
Figure 2.
Typical recordings of AP, renal sympathetic nerve activity [SNA; in arbitrary units (a.u.)], and HR obtained in anesthetized rat. The rate of infusion was adjusted to produce continuous changes in AP at 1 mmHg/s. Data in this figure were resampled at 10 Hz. White lines in the SNA panels were resampled at 1 Hz. Raw data was sampled at 1000 Hz.
2.5 Data analysis
2.5.1 MAP, HR, SBRS and respiratory frequency in conscious rats
To determine changes in MAP, HR, SBRS and respiratory frequency, we recorded AP at a sampling rate of 250 Hz. HR was calculated from pulse interval obtained from the AP waveform, respiratory frequency was estimated by fluctuation of the AP (Dataquest, DSI, MN, USA). MAP, HR and respiratory frequency were averaged in light period during CIH (8:00 to 16:00) and in dark period (19:00 to 7:00).
In the same group of animals, SBRS was evaluated by sequence analysis technique using the freely available software, HemoLab (Zoccal et al., 2009). SBRS was assessed by averaged slope of the linear regression between systolic AP and subsequent pulse interval pairs. A slope was obtained from progressive increases and decreases of four or more values of systolic AP that paralleled changes in pulse interval (3 beats of delay) with linear correlation higher than 0.8.
2.5.2 Baroreflex function curves in anesthetized rats
We recorded AP, RSNA and HR at a sampling rate of 1000 Hz in Inactin anesthetized rats. Because the absolute magnitude of RSNA depended on recording conditions, RSNA was presented in arbitrary unit (au). Nerve activity after sacrifice was used to determine “0 au”. To determine “100 au” of RSNA, we used two different approaches as in our previous study of baroreflex regulation of RSNA in hypertensive rats (Vitela et al., 2005). In the first approach, the baseline RSNA in each animal was used to determine “100 au”. In the second approach, the maximum RSNA observed during the decrease in AP produced by the infusion of nitroprusside was used to determine “100 au”. The values for RSNA and HR during the reflex function curves were averaged into 5-mmHg bins of AP. The baroreflex function curves of the RSNA control (AP-SNA relationship) and the HR control (AP-HR relationship) were described using a four-parameter logistic function as follows (Kent et al., 1972):
| [1] |
where x and y denote the input (AP) and output (RSNA or HR), respectively; P1 is the response range of output; P2 is the slope coefficient; P3 is the midpoint pressure of input; and P4 is the minimum value of output. The maximum gain (Gmax) is –P1P2/4.
2.6 Statistical analysis
All data are presented as mean ± SE. The effects of CIH on MAP, HR, SBRS and respiratory frequency in conscious rats were tested by two-way ANOVA with repeated measurements. In the case of a significant F value, a post hoc test with Bonferroni method identified significant differences among mean values. Unpaired t tests were used to compare the baroreflex curve parameters between the control and CIH groups in anesthetized rats. Univariate regression and correlation analyses were used to analyze the relationship between resting MAP and midpoint pressure or maximum gain in the AP-SNA relationship. Differences were considered significant when P < 0.05.
3. Results
3.1 Responses to CIH in conscious rats
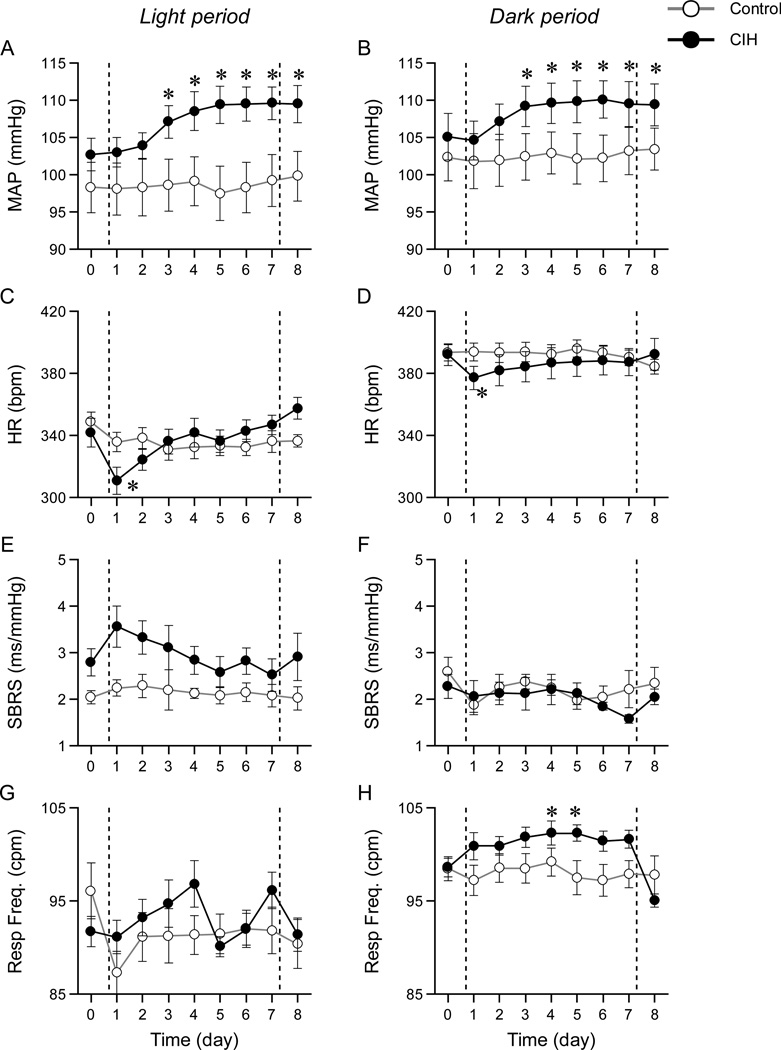
Figure 3 shows the MAP, HR, SBRS and respiratory frequency in the light period (A, C, E and G) and the dark period (B, D, F and H) in conscious rats before, during and after a 7 day exposure to CIH. Days 1–7, between the vertical dashed lines, represent the days the animals were exposed to CIH. The open and closed circles represent data points obtained from control and CIH groups, respectively. In MAP, two-way ANOVA indicated a significant interaction between group and time (day) in both the light period (A, P < 0.001) and the dark period (B, P < 0.001). MAP after exposure to CIH in the light period was higher than MAP before exposure in the CIH group. MAP in the dark period also increased after exposure to CIH. MAP remained elevated on the day after the last exposure to CIH (Fig. 3 A and B, day 8). There was a significant interaction in HR (C, P=0.001; D, P=0.001). Although HR decreased at first day of exposure to CIH in the light period and the dark period, HR on the day after the last exposure to CIH did not differ from HR before CIH. There were no significant interaction in SBRS in the light period (E, P = 0.64) and the dark period (F, P = 0.54), indicating that SBRS did not change over a 7day exposure to CIH. There was a significant interaction in respiratory frequency (G, P=0.01; H, P=0.001). Respiratory frequency was increased in the CIH compared to the control group on days 4 and 5 during the dark period. However this increased respiratory frequency returned to baseline level after CIH on day 8 even though MAP remained elevated, suggesting that the increase in MAP after exposure to CIH was independent of changes in respiratory frequency.
Figure 3.
Mean arterial pressure (MAP), heart rate (HR), spontaneous baroreflex sensitivity (SBRS) and respiratory frequency (Resp Freq) in the light period (A, C, E and G) and the dark period (B, D, F and H) in conscious rats. Between vertical dashed lines indicates during exposure to CIH. The open and closed circles represent data points obtained from control and CIH groups, respectively. MAP increased after exposure to CIH in the both light and dark periods. HR, SBRS and respiratory frequency did not differ between before and after exposure to CIH. *P<0.01 compared with before CIH within same group.
3.2 Baroreflex regulation of SNA in anesthetized rats
Baseline MAP and HR in the anesthetized rats are shown in Table 1. MAP was significantly higher in the CIH rats. HR did not differ between control and CIH rats.
Table 1.
Baseline and baroreflex parameters in anesthetized rats
| Control | CIH | |
|---|---|---|
| Baseline | ||
| MAP (mmHg) | 105 ± 2 | 115 ± 2* |
| HR (bpm) | 328 ± 6 | 342 ± 8 |
| SNA (baseline = 100 a.u.) | ||
| P1 (a.u.) | 159 ± 16 | 158 ± 15 |
| P2 (a.u./mmHg) | 0.12 ± 0.01 | 0.12 ± 0.01 |
| P3 (mmHg) | 99 ± 3 | 111 ± 3* |
| P4 (a.u.) | 23 ± 4 | 15 ± 3 |
| Gmax(a.u./mmHg) | −4.4 ± 0.3 | −4.6 ± 0.3 |
| SNA (maximum = 100 a.u.) | ||
| P1 (a.u.) | 85 ± 3 | 88 ± 2 |
| P2 (a.u./mmHg) | 0.12 ± 0.01 | 0.12 ± 0.01 |
| P3 (mmHg) | 99 ± 3 | 111 ± 3* |
| P4 (a.u.) | 15 ± 3 | 9 ± 2 |
| Gmax (a.u./mmHg) | −2.5 ± 0.1 | −2.6 ± 0.1 |
| HR | ||
| P1 (bpm) | 65 ± 6 | 73 ± 6 |
| P2 (bpm/mmHg) | 0.12 ± 0.01 | 0.10 ± 0.01 |
| P3 (mmHg) | 94 ± 3 | 101 ± 4 |
| P4 (bpm) | 307 ± 7 | 317 ± 4 |
| Gmax (bpm/mmHg) | −1.8 ± 0.2 | −1.8 ± 0.2 |
Values are mean ± SE (n = 18 in Control, n = 16 in CIH). CIH, chronic intermittent hypoxia; MAP, mean arterial pressure; HR, heart rate; SNA, sympathetic nerve activity; P1, the response range of output; P2, the slope coefficient; P3, the midpoint pressure of input; P4, the minimum value of output; Gmax, maximum gain.
P < 0.01 from control.
Figure 2 illustrates changes in AP, RSNA and HR during pharmacological manipulation of AP. The averaged velocity of changes in AP manipulations did not differ between control (0.6 ± 0.1 mmHg/s) and CIH (0.5 ± 0.1 mmHg/s) groups (P = 0.20).
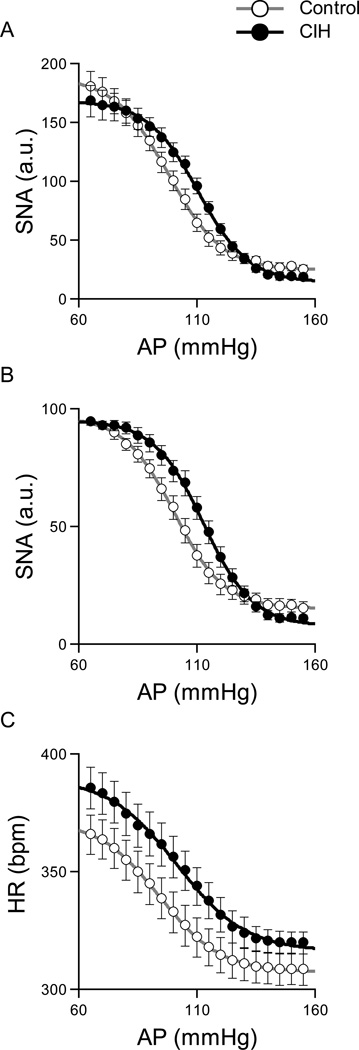
Figure 4 illustrates the baroreflex control of RSNA (A: baseline = 100 au, B, maximum = 100 au) and HR (C) obtained from the anesthetized rats. The AP-SNA relationship (A and B) showed a decreasing SNA response with an increase in AP. In both standardization approaches for RSNA, the midpoint pressure on the AP axis (P3) was significantly higher in the CIH rats (Table 1), indicating that CIH shifted the relationship rightward. The response range (P1), the minimum value (P4) of RSNA and the maximum gain did not differ in the CIH rats compared with the control rats. Although CIH tended to shift the AP-HR relationship upward, baroreflex parameters in the AP-HR relationship did not differ statistically between control and CIH groups (Table 1).
Figure 4.
Baroreflex control of SNA (A: baseline = 100 au, B: maximum = 100 au) and HR (C) averaged for the control (n = 18) and CIH (n = 16) rats in anesthetized rats. After exposure to CIH, the AP-SNA relationship shifted rightward (Table 1). Baroreflex parameters in the AP-HR relationship did not differ statistically between control and CIH rats.
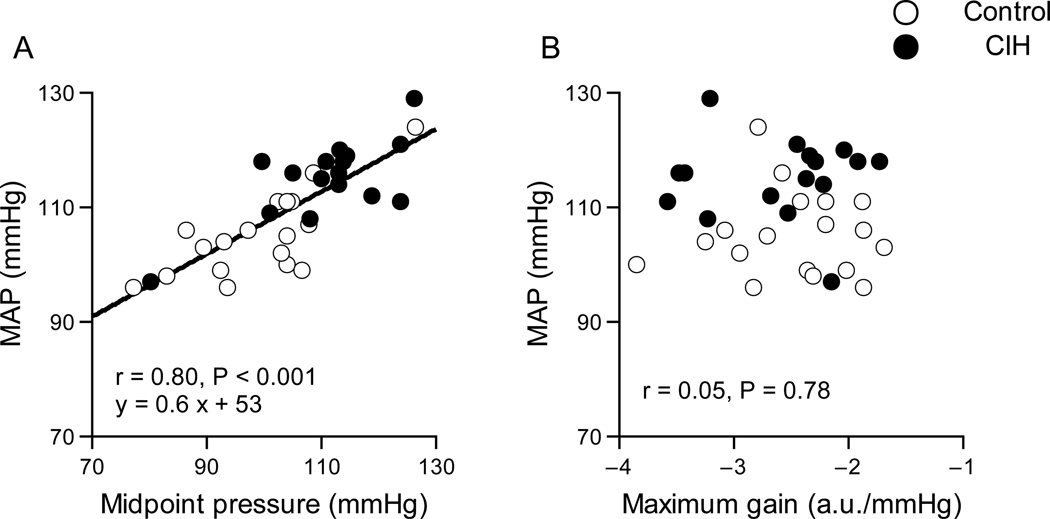
Figure 5 shows the relationships between MAP and midpoint pressure (A) or maximum gain (B) in AP-SNA relationship. The midpoint pressure correlated with resting MAP. The maximum gain did not correlate with MAP.
Figure 5.
Relationship between MAP and midpoint pressure (A) or maximum gain (B) in AP-SNA relationship. The midpoint pressure correlated with resting MAP. The maximum gain did not correlate with MAP.
4. Discussion
The key new findings of the present study are as follows. Exposure to CIH for 7 days shifted the AP-SNA relationship rightward, while AP was elevated (Fig. 4 A and B and Table 1). The CIH did not attenuate baroreflex sensitivity of RSNA assessed by baroreflex maximum gain (Table 1). Midpoint pressure in sympathetic baroreflex function curve correlated with baseline MAP (Fig. 5 A). These results support our hypothesis that resetting of the sympathetic baroreflex is associated with the sustained increase in AP induced by 7 days exposure to CIH.
The present CIH protocol increased AP by approximately 7 mmHg in the conscious rats. We used the same CIH protocol prior to experiments in anesthetized rats and AP increased by approximately 10 mmHg compared with the control group. In a review of the physiological consequences of intermittent hypoxia by Fletcher (Fletcher, 2001), the increases in AP induced by CIH were 10, 13 and 10 mmHg at 14, 28 and 36 day of CIH, respectively. Thus, the present CIH protocol for 7 days was effective in elevating AP by the similar amount as observed after 14 – 36 days in the study by Fletcher.
Our data indicate that reduced baroreflex gain of RSNA does not contribute to the increased AP after a 7day exposure to CIH. On the other hand, the exposure to CIH reset the AP-SNA relationship rightward by approximately 10 mmHg. It is noteworthy that after exposure to CIH the rightward shift in the AP-SNA relationship is similar in magnitude to the increase in resting MAP. Furthermore, midpoint pressure in AP-SNA relationship significantly correlated with resting MAP. These findings suggest that a resetting of the sympathetic baroreflex control, rather than an impairment of the sympathetic baroreflex gain, is associated with a sustained increase in AP induced by the CIH.
Previous studies (Fletcher et al., 1992a; Peng et al., 2004) suggest that cardiorespiratory changes in response to CIH are mediated in part from augmented chemoreflex sensitivity to hypoxia. CIH-induced hypertension is prevented by carotid body denervation (Fletcher et al., 1992a), suggesting that intact peripheral chemoreceptors are necessary for the AP to increase in response to CIH. In addition, CIH augments the carotid chemoreceptor response to hypoxia (Peng et al., 2004), which in turn leads to reflex sympathetic activation. The activation of chemoreflex modulates baroreflex control of SNA (Malpas et al., 1996). Therefore, the resetting of baroreflex control of RSNA after CIH observed in the present study may be induced by tonic activation of the chemoreflex (Narkiewicz et al., 1998). Further studies are needed to verify the mechanisms that underlie the present findings.
Our results regarding baroreflex control of HR are consistent with recent studies. Zoccal et al. (Zoccal et al., 2009) demonstrated that exposure to CIH for 10 days did not attenuate baroreflex control of HR despite the elevated AP. Lai et al. (Lai et al., 2006) examined the time course changes in AP and SBRS during CIH exposure for 30 days. They demonstrated that AP was elevated after exposure to CIH for 5 days, while a reduction in spontaneous baroreflex sensitivity was not observed until the 17th day of CIH. The present study extended these results by directly measuring SNA over a wide range of APs and found that baroreflex control of RSNA was not attenuated after exposure to CIH for 7days. These results suggest that the onset of hypertension induced by CIH is not secondary to reductions in the baroreflex sensitivity. In addition, the present study provides new information that resetting of the sympathetic baroreflex control is associated with hypertension after 7 days exposure to CIH. Several studies (Gu et al., 2007; Lai et al., 2006; Lin et al., 2007; Yan et al., 2008) have demonstrated that baroreflex control of HR was attenuated after exposure to CIH for over 30 days. These results indicate a possibility that the baroreflex control of SNA may be reduced if a long term exposure to CIH is applied (Peng et al., 2012). Taken together, different mechanisms might occur between onset of hypertension and the long term sustained increase in AP induced by CIH.
Previous studies in rabbits (Ikeda et al., 1996; Yamamoto et al., 2008) and rats (Kawada et al., 2011; Sato et al., 2003) have indicated that the transfer function of the baroreflex neural arc from baroreceptor pressure input to efferent SNA exhibits “derivative” characteristics, which means that the dynamic gain of the SNA response to pressure perturbation becomes greater as the modulation frequency increases. In other words, the baroreflex gain for SNA becomes greater as the velocity of changes in AP increases. In the present study, we adjusted the rate of infusion to produce continuous changes in AP at within 1 mmHg/s (Fig. 3). Furthermore, the velocity of changes in AP manipulation did not differ between the control and CIH rats. Therefore, we believe that the dynamic characteristics of the baroreflex neural arc did not affect the results in the baroreflex control of RSNA in the present study.
Anesthesia might have modified the baroreflex control of RSNA and HR. In a previous study, exposure to CIH for 10 days increased cardiac baroreflex gain in conscious rats (Zoccal et al., 2009). In contrast, our results of baroreflex gain of both RSNA and HR in anesthetized rats did not differ between CIH and control groups. This did not seem to be an outcome of the anesthetic effect, because SBRS in the conscious condition was not altered after the present CIH protocol.
We examined RSNA as a representative of systemic SNA. The baroreflex control of SNA could vary in different sympathetic outflows. Although baroreflex stimulus-response curves of SNA are similar among renal, cardiac, and muscle SNAs (Kamiya et al., 2005), whether this holds true after exposure to CIH remains to be verified. Further studies are needed to understand the baroreflex control of SNA to different organs after exposure to CIH.
In conclusion, the present results indicate that exposure to CIH for 7 days shifts the baroreflex control of RSNA rightward in similar magnitude to the increase in resting MAP. In addition, there was the relationship between resting MAP and midpoint pressure of sympathetic baroreflex function curve in control and CIH group. These findings suggest that the onset of hypertension induced by CIH is associated with a resetting of baroreflex control of SNA.
Acknowledgments
This work was supported by HL-088052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Fletcher EC. Invited review: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992a;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992b;20:612–619. doi: 10.1161/01.hyp.20.5.612. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992c;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- Gu H, Lin M, Liu J, Gozal D, Scrogin KE, Wurster R, Chapleau MW, Ma X, Cheng ZJ. Selective impairment of central mediation of baroreflex in anesthetized young adult Fischer 344 rats after chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H2809–H2818. doi: 10.1152/ajpheart.00358.2007. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Kawada T, Sugimachi M, Kawaguchi O, Shishido T, Sato T, Miyano H, Matsuura W, Alexander J, Jr, Sunagawa K. Neural arc of baroreflex optimizes dynamic pressure regulation in achieving both stability and quickness. Am J Physiol. 1996;271:H882–H890. doi: 10.1152/ajpheart.1996.271.3.H882. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kawada T, Yamamoto K, Michikami D, Ariumi H, Miyamoto T, Uemura K, Sugimachi M, Sunagawa K. Muscle sympathetic nerve activity averaged over 1 minute parallels renal and cardiac sympathetic nerve activity in response to a forced baroreceptor pressure change. Circulation. 2005;112:384–386. doi: 10.1161/CIRCULATIONAHA.104.493338. [DOI] [PubMed] [Google Scholar]
- Kawada T, Shimizu S, Kamiya A, Sata Y, Uemura K, Sugimachi M. Dynamic characteristics of baroreflex neural and peripheral arcs are preserved in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R155–R165. doi: 10.1152/ajpregu.00540.2010. [DOI] [PubMed] [Google Scholar]
- Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol. 2006;100:1974–1982. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster R, Cheng ZJ. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol. 2007;293:H997–H1006. doi: 10.1152/ajpheart.01124.2006. [DOI] [PubMed] [Google Scholar]
- Malpas SC, Bendle RD, Head GA, Ricketts JH. Frequency and amplitude of sympathetic discharges by baroreflexes during hypoxia in conscious rabbits. Am J Physiol. 1996;271:H2563–H2574. doi: 10.1152/ajpheart.1996.271.6.H2563. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Zhang X, Wang N, Raghuraman G, Seagard J, Kumar GK, Prabhakar NR. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol. 2012;112:187–196. doi: 10.1152/japplphysiol.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. discussion 1196. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Sato T, Kawada T, Inagaki M, Shishido T, Sugimachi M, Sunagawa K. Dynamics of sympathetic baroreflex control of arterial pressure in rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R262–R270. doi: 10.1152/ajpregu.00692.2001. [DOI] [PubMed] [Google Scholar]
- Vitela M, Herrera-Rosales M, Haywood JR, Mifflin SW. Baroreflex regulation of renal sympathetic nerve activity and heart rate in renal wrap hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R856–R862. doi: 10.1152/ajpregu.00620.2004. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kawada T, Kamiya A, Takaki H, Shishido T, Sunagawa K, Sugimachi M. Muscle mechanoreflex augments arterial baroreflex-mediated dynamic sympathetic response to carotid sinus pressure. Am J Physiol Heart Circ Physiol. 2008;295:H1081–H1089. doi: 10.1152/ajpheart.00023.2008. [DOI] [PubMed] [Google Scholar]
- Yan B, Soukhova-O'Hare GK, Li L, Lin Y, Gozal D, Wead WB, Wurster RD, Cheng ZJ. Attenuation of heart rate control and neural degeneration in nucleus ambiguus following chronic intermittent hypoxia in young adult Fischer 344 rats. Neuroscience. 2008;153:709–720. doi: 10.1016/j.neuroscience.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1555–R1562. doi: 10.1152/ajpregu.90390.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol. 2009;94:972–983. doi: 10.1113/expphysiol.2009.048306. [DOI] [PubMed] [Google Scholar]