Abstract
Immune recognition of tumors can limit cancer development, but antitumor immune responses are often blocked by tumor-mediated immunosuppression. Since microbes or microbial constituents are powerful adjuvants to stimulate immune responses, we evaluated whether intratumoral administration of a highly immunogenic but attenuated parasite could induce rejection of an established poorly immunogenic tumor.
We treated intradermal B16F10 murine melanoma by intratumoral injection of an attenuated strain of Toxoplasma gondii (cps) that cannot replicate in vivo and therefore is not infective. cps treatment stimulated a strong CD8+ T cell-mediated antitumor immune response in vivo that regressed established primary melanoma. cps monotherapy rapidly modified the tumor microenvironment, halting tumor growth, and subsequently, as tumor-reactive T cells expanded, the tumors disappeared and rarely returned. The treatment required live cps that could invade cells and also required CD8+ T cells and Natural Killer cells but did not require CD4+ T cells. Furthermore, we demonstrate that IL-12, IFN-γ and the CXCR3 stimulating cytokines are required for full treatment efficacy. The treatment developed systemic antitumor immune activity as well as antitumor immune memory and therefore might have an impact against human metastatic disease. The approach is not specific for either B16F10 or melanoma. Direct intratumoral injection of cps has efficacy against an inducible genetic melanoma model, and transplantable lung and ovarian tumors, demonstrating potential for broad clinical use. The combination of efficacy, systemic antitumor immune response and complete attenuation with no observed host toxicity demonstrates the potential value of this novel cancer therapy.
Introduction
Despite considerable progress using surgery, chemotherapy, and radiation to treat cancer, the 5 year survival rates for many cancers is still very low and not improving. There is currently a great deal of interest in developing therapies that stimulate effective immune responses against cancer in order to establish another major therapeutic option and improve outcomes. The immune system is stimulated by microorganisms, and since the studies of William Coley over 100 years ago (1), the possibility of using microorganisms as adjuvants to stimulate antitumor immunity has been recognized. However, despite frequent efficacy against what were deemed to be incurable, often metastatic cancers, “Coley’s toxins” were not accepted clinically. Since Coley’s time we have developed a detailed understanding of both the immune system response to tumors and the suppression of the immune system by tumors. Genetic manipulation enables the generation of microorganisms with reduced virulence that can still function as powerful immunologic adjuvants and this has led to progress in developing microorganisms as immune stimulating antitumor reagents. For example, the standard of care for treating superficial bladder cancer is instilling Bacillus Calmette-Guerin into the bladder (2), and a variety of other microorganisms such as Listeria monocytogenes, Salmonella typhimurium and multiple viruses are in various stages of development as antitumor vaccines and treatments (3–5). Importantly, while each of the most heavily studied organisms has efficacy in various models and some show promise in clinical trials, none of these organisms has been shown to eliminate an established, poorly immunogenic tumor.
Toxoplasma gondii is a single cell, obligate intracellular, eukaryotic parasite. cps, the strain utilized here, is a uracil auxotroph due to deletion of the carbamoyl phosphate synthetase II enzyme (6). Since the required enzyme is deleted, there is no potential for reversion. cps grows well in vitro in mammalian cells in uracil-supplemented medium. In vivo, cps is nonreplicative but efficiently invades cells and generates a strong adaptive immune response, characterized by activation of antigen presenting cells to stimulate CD8+ T cell maturation and expansion with associated generation of high levels of IL-12 and IFN-γ (7). Since such immune responses are associated with effective antitumor immunity, we evaluated whether cps could induce the specific antitumor immunity required to eliminate an aggressive, notoriously difficult to treat tumor.
B16F10 melanoma has been used extensively as a poorly immunogenic, highly aggressive model for murine tumor immunotherapy studies (8). Shrinkage of established B16F10 has not been achieved with an immune-based monotherapy. Established B16 tumors have been treated at low frequency by combination immunotherapies, such as adoptive transfer of antigen-specific transgenic T cells along with TLR agonist administration (9), or at high frequencies by combining adoptive transfer of antigen-specific transgenic T cells and recombinant viral infection and preconditioning the host through total body irradiation (10). These approaches are complex and will be difficult to accomplish in a widespread clinical context. However, they do provide encouragement for cancer immunotherapy. New approaches that are easy to apply and have high levels of efficacy and minimal side effects need to be developed in order to improve cancer immunotherapy outcomes.
Our hypothesis was that cps introduced into the tumor microenvironment would transform that environment from one that is predominantly immunosuppressive to an immunostimulatory environment in which the endogenous antitumor immune response would effectively eliminate the tumors. In the studies reported here we tested that hypothesis by using cps monotherapy to treat established B16F10 melanoma. We show that treatment of established B16F10 dermal melanoma by intratumoral injection of cps not only regresses the primary tumor but also establishes systemic and memory antitumor immune responses with significant ability to reject or slow the development of rechallenge. The efficacy of cps monotherapy against the primary tumor and development of systemic response and immune memory, in combination with its inability to replicate in vivo and associated lack of observed toxicity, establishes the cps strain of T. gondii as a potentially powerful reagent for immunotherapy of solid tumors.
Materials and Methods
Mice
C57BL/6 were purchased from the National Cancer Institute. IL-12p35−− (002692), IFN-γ−− (002287) and NOD/SKID/IL2Rγ−− (005557) mice were purchased from The Jackson Laboratory. Tumors were generated in Tyr-CreERt:BrafCA:Ptenlox/lox transgenic mice, on a mixed genetic background by intradermal (i.d.) injection of 4-hydroxy tamoxifen in the flank. Animal experiments were approved by the Institutional Animal Care and Use Committee at Dartmouth Medical School.
Tumor cell inoculations
The B16F10 mouse melanoma cell line was originally obtained from Isaiah Fidler (MD Anderson Cancer Center, Houston, Texas, USA) and passaged i.d. in C57BL/6 mice 7 times to ensure reproducible growth. Tumor cells were cultured in RPMI containing 7.5% FBS and inoculated into mice only if viability exceeded 96%. 1.25×105 live B16F10 cells were inoculated i.d. in the right flank. Tumor diameters were measured 3 times weekly, and mice were euthanized when tumor diameters reached 15 mm. To determine T cell recall capacity, B16F10 bearing mice were treated as seen in Fig. 1A and then euthanized on day 20 of tumor challenge, and CD8+ T cell responses were assessed by flow cytometry or ELISPOT as described below. The UpK10 tumor line was generated from C57BL/6 mice as described (27). 5×105 live UpK10 cells were injected i.d. Lewis lung tumors were generated by injecting 1×106 live cells, i.d. on the right flank.
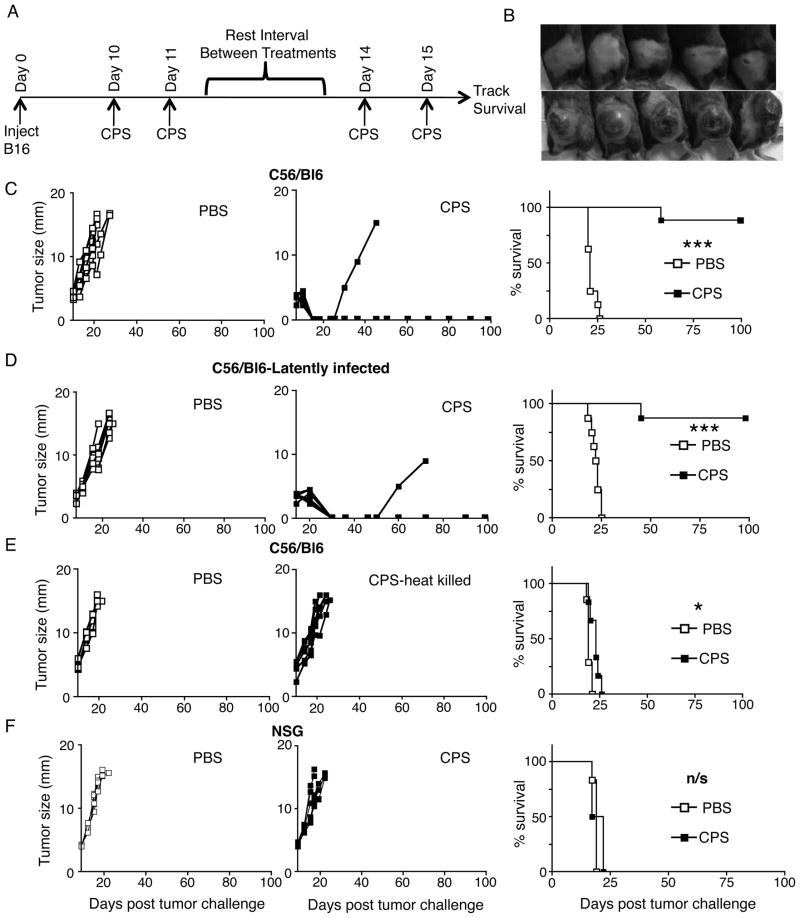
FIGURE 1.
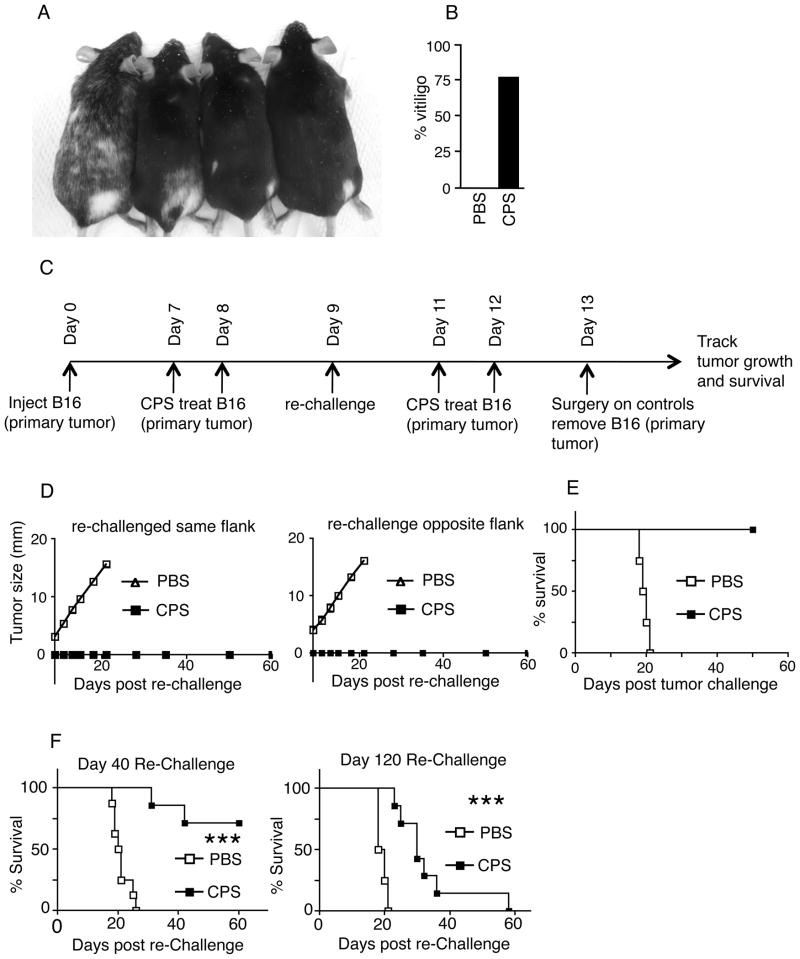
Live cps treatment causes regression of established dermal B16F10 melanoma by immune effects. (A) cps treatment strategy, each treatment was 1.5×107 tachyzoites of cps injected intratumorally that began when tumors reached 3.5–5mm diameter. (B) Photos of tumors 21 days post challenge. cps treated mice are top panel, PBS treated mice are bottom panel. (C) Individual mouse tumor growth kinetics (left) and survival curve (right) of established primary B16F10 tumor-bearing mice treated with cps or PBS. (D) Tumor growth and survival curve of B16F10 tumor-bearing mice treated with cps or PBS after established latent infection by Toxoplasma gondii (PRU). (E) Tumor growth and survival curve of primary tumor treatment with cps killed by heating at 65°C prior to injection. (F) Tumor growth and survival curve of tumors in NOD/SCID/IL2 gamma receptor KO mice. Error bars are SEM; P values: * < 0.05, ** < 0.01, *** < 0.001, n/s is not statistically significant, n= minimum of 6 per group, data are representative of at least 2 independent experiments.
Blocking antibodies
Depleting anti-CD4 (clone GK1.5) and anti-CD8 (clone 2.43) were produced as bioreactor supernatants and administered i.p. in doses of 250 μg one day prior to treatment and then once weekly. Greater than 95% depletion of target T cell populations was confirmed by flow cytometry. Depleting anti-NK1.1 (clone PK136) was produced similarly and administered i.p. in doses of 200μg for four treatments on days −2, 0, 2, and day 9 relative to initial cps tumor treatment. CXCR3 blocking antibody (clone CXCR3-173) was purchased from BioLegend and administered i.p. in doses of 100 ug beginning one day before cps treatment and continued every third day after for four total treatments. CXCR3 blockade was also performed at 200 μg yielding similar results.
Antibodies and gating strategies used for flow cytometry
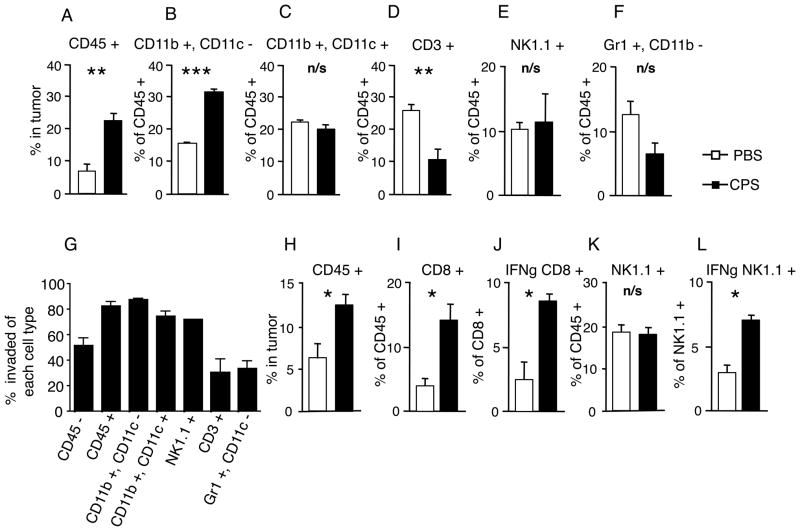
CD45 (30-F11), CD3ε (145-2C11), CD8b (YTS156.7.7), CD44 (IM7), CD11c (N418), CD11b (M1/70) were purchased from Biolegend. NK1.1 (14B11) and IFN-γ (XMG1.2) were purchased from eBioscience. Figure 2A–F flow cytometry samples were gated on CD45+ cells after the FSC and SSC gate was set. Then figure 2B was gated on CD11c− CD11b+ whereas figure 2C was gated on CD11c+ CD11b+ cell populations. Figure 2D was gated on CD3+ cells whereas figure 2E was gated on NK1.1 + populations and figure 2F was gated on Gr1 +, CD11b −. The initial gating strategy for figure 2G was the same strategy used for figure 2A–F, however a final gate was made which defined the population of cells infected. Infected cells were defined by the invaded presence of CSFE-stained cps parasites. Figure 2H–L samples were first gated on CD45+ cells after the FSC and SSC gate was set. Figure 2I was gated on CD3+ CD8+ cell populations and figure 2J was also gated on CD3+ CD8+ cell and then a histogram gate was made defining IFN-γ+ CD8 + cells. Figure 2K was gated on NK1.1 cells, figure 2L was first gated on NK1.1 and then gated on IFN-γ+ NK1.1
FIGURE 2.
cps treatment rapidly increased leukocyte infiltration of tumors and leukocytes were invaded by cps. (A–F) 4mm tumors were treated twice 24 hours apart and harvested 18hrs later to assess cell infiltration into tumor and assayed for: (A) percentage of CD45+ cells in the tumor, (B–F) the percentage of major leukocyte subsets within the CD45+ cells in the tumor, (B) CD11b+CD11c− cells, (C) CD11b+CD11c+ cells, (D) CD3+ cells, (E) NK1.1+ cells, (F) Gr1+CD11b−. White bars are control PBS-treated, black bars are cps-treated. (G) 1.5×107 CFSE-stained tachyzoites of cps were injected twice 24 hours apart into 4mm tumors that were harvested 18hr later. Cells were assayed for % of each ell type invaded by cps in the tumor. (H–L) 4mm tumors were treated as previously on 2 consecutive days and then harvested 3 days later to assess cell infiltration into tumors. White bars are PBS-treated and black bars are cps-treated. (H) % CD45+ cells in tumor, (I) % of CD45+ cells that are CD8+ cells, (J) % of CD8+ cells that are expressing IFN-γ, (K) % of CD45+ cells that are NK1.1+, (L) % of NK1.1+ cells that are expressing IFN-γ. Error bars are SEM; P values: * < 0.05, ** <0.01, *** < 0.001. n= minimum of 4 mice per group, data are representative of 2 independent experiments.
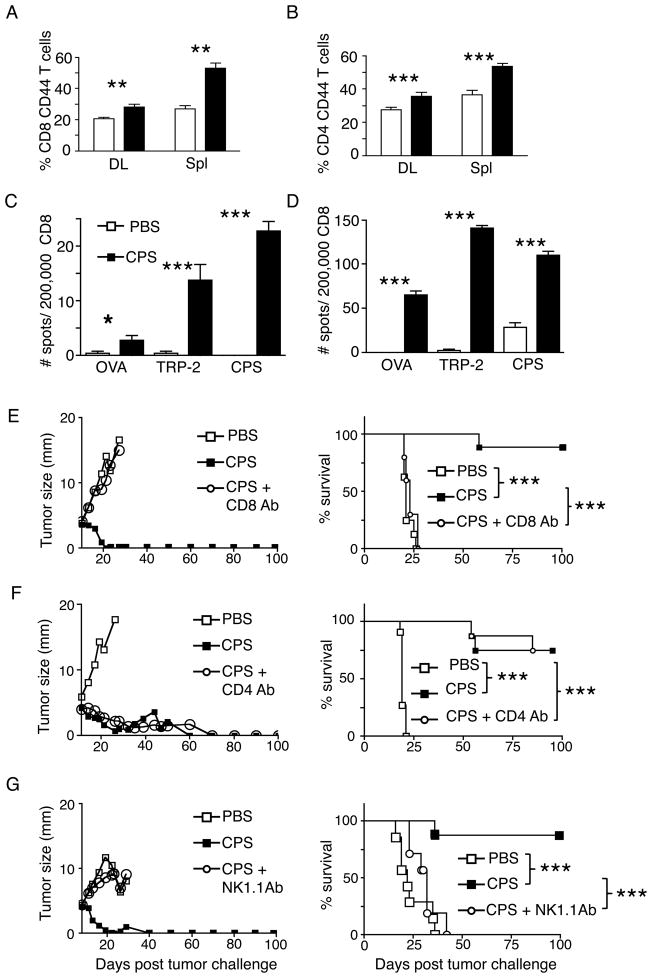
Figure 3A–B flow cytometry samples were first gated on CD45+ cells after the FSC and SSC gate was set. Then figure 3A was then gated on CD3+CD8+ cells followed by gating on the CD8+ CD44+ cell population. Figure 3B was gated on CD3+CD8− cells followed by gating on the CD8− CD44+ cell population.
FIGURE 3.
Treatment generates tumor antigen-specific responses and efficacy requires CD8+ T and NK cells. (A–C) Assays were done on day 20 post tumor challenge, 6–8 days past the final cps treatment. (A) % of CD8+ T cells that are antigen-experienced (CD44+) in draining lymph nodes (DL) and spleen (Spl). (B) % of CD4+ T cells that are antigen-experienced (CD44+) in draining lymph nodes (DL) and spleen (Spl). IFN-γ ELISPOT showing tumor antigen TRP-2 and T. gondii (cps) antigen-specific CD8+ T cells are enriched in draining lymph nodes (C) and spleen (D). OVA was an irrelevant peptide control. (E) B16F10 tumor bearing mice treated with cps or PBS, after CD8+ T cell depletion, left is average growth curves for the groups and right is survival curves. Similar for (F) CD4+ T cell depletion or (G) depletion of NK (NK1.1+) cells. Error bars are SEM; P values: * < 0.05, ** < 0.01, *** < 0.001. Growth curves show mean value with no error bars for visual clarity. n= minimum of 6 mice per group, data are representative of at least 2 independent experiments.
Cytokine Detection
Tumor explants were manually homogenized in T-Per Lysis Buffer (Thermo Scientific/Pierce Protein Research Products) with complete protease inhibitor cocktail (Roche Applied Science), and total protein was quantified using Life Technologies/Invitrogen Qubit Protein Assay kit and an Invitrogen Qubit 2 fluorometer. IFN-γ, CXCL9, and CXCL10 protein were quantified using specific ELISA kits (RND Systems) with SuperAquaBlue substrate (eBioscience); absorbance was measured using a BioTek Epoch micropate spectrophotometer. Cytokine and chemokine concentrations were calculated from a specific standard curve.
IFN-γ ELISPOT
CD8+ T cells were isolated from spleens or draining inguinal lymph nodes and purified using anti-CD8 MACS magnetic beads (Miltenyi Biotec). CD8+ T cells were then plated at a 10:1 ratio with irradiated EL-4 thymoma cell targets (ATCC) that had been pulsed with 1 μg/ml of MHC-I–restricted peptide epitopes TRP-2180–188, or OVA257–264, or heat killed cps.
Flow cytometry and cytokine assay
Flow cytometry was performed on a FACS-Canto system (BD Biosciences). Data were analyzed using FlowJo software (version 7.6). For survival experiments: Mice were i.d. injected with 1.25×105 B16 cells and treated when tumor size reached 3.5–5mm in diameter (unless otherwise stated). Tumors were injected 4 times with 1.5×107 tachyzoites of cps suspended in PBS as outlined in Fig. 1A. Mice were monitored daily and sacrificed when tumors reached 1.5cm in diameter.
Intracellular cytokine staining
Cells from tumors were cultured for 5 hrs at 37°C in RPMI containing brefeldin A (10 μg/ml). Following incubation, cells were washed and stained with antibodies against surface markers and then fixed, permeabilized, and stained intracellularly with anti-IFN-γ–PE (clone XMG1.2, BioLegend). Flow cytometry was performed as described above.
Identifying cps infected cells
cps was stained in 7.5mM of CFSE for 5 mins at 37°C. After staining cps was washed in PBS then 1.5×107 tachyzoites were intratumorally injected into B16F10 tumor. Eighteen hours after second CSFE labeled cps injection, cells were harvested from the tumor by surgically removing the tumor and disassociating with a scalpel followed by flushing through a sterile cell strainer with 100μm pores (cat 22363549, Fisher Scientific). Strained cells were antibody stained using provider suggested staining protocols.
T. gondii lines and culture
cps is identical to cps2-1 (6). T. gondii strains were maintained as tachyzoites by serial passage in human foreskin fibroblast cell monolayers. To grow cps, tissue culture medium was supplemented with 0.2 mM uracil. For mouse injections with T. gondii, parasites were purified by filtration through a 3.0μm filter (Nuclepore; Steriltech Corp., Kent, WA) and washed with medium or phosphate-buffered saline (PBS).
Statistics and experimental repeats
All experiments were repeated at least 2 times with similar results between experiments. Survival experiments used between 4 and 9 mice per group. Figures denote statistical significance of p <0.05 as *, p <0.01 as **, and p<0.001 as ***. Statistical analysis was performed with Graph Pad Prism 4 software. p values less than 0.05 were considered significant. Survival experiments utilized log- rank Mantel Cox test for survival analysis. Data for bar graphs, including for the mean fluorescence of flow cytometry, is from a normal distribution applied to two independent groups, and the confidence interval was calculated using 2-tailed unpaired Student’s T test. Error bars represent standard error of the mean from independent samples assayed within the represented experiments. ELISPOTs utilized pooled cells from multiple mice and the values presented are from triplicate assay on the pools that were compared by one-way ANOVA.
Results
Live cps regresses established dermal B16F10 melanoma by immune mechanisms
B16F10 was inoculated intradermally into C57BL6 mice and palpable 3.5–5mm tumors (day 9–11 after inoculation) were treated with intratumoral cps injection (treatment schematic, Fig. 1A). cps-treated tumors initially stopped growing and then rapidly regressed and were undetectable within 12 days of the first treatment in 100% of the treated mice (Fig. 1B, C). In comparison, by day 21 at which all tumors had disappeared in cps treated mice, all mice treated with PBS had large tumors that constituted the experimental endpoint. The vast majority of the treated tumors (83%) did not return after the treatment (Fig. 1C) establishing cps treatment as the first immune-based monotherapy that can dependably regress established tumors of this very widely studied melanoma model.
Although cps does not express any tumor antigens, we also tested whether cps monotherapy could be therapeutic if injected into nontumor sites. cps was administered to mice with 3.5–5mm intradermal B16F10 both intraperitoneally (IP) and intravenously (IV). Although intratumoral cps injection regressed the tumor as expected, IV and IP cps injection did not slow tumor growth or improve survival in mice with established B16F10 melanoma (supplemental Fig. 1). This demonstrates that, for the treatment of a primary tumor to be effective, cps must be in the tumor microenvironment, since IV or IP applied cps would not have accumulated in the tumor.
T. gondii latently infects between 10–70% of people in most countries (11). In order to determine how latent infection with T. gondii affects treatment efficacy, we infected mice with the Prugniaud (PRU) strain of T. gondii to establish a latent infection in mice (12) and tested the efficacy of treating dermal B16F10 with cps in these latently infected mice. Latent infection with PRU did not interfere with the ability of cps treatment to elicit an effective anti-tumor response (Fig. 1D). These data indicate that the widespread latent infection of humans by T. gondii is not likely to affect the efficacy of cps as a clinical antitumor treatment.
Tumors were treated with heat-killed cps in order to determine whether live cps is required for efficacy, or whether cps constituents act by direct stimulation of the innate immune response through recognition of molecular patterns of pathogens. Using the same treatment regimen associated with regression, there was a slight delay in tumor growth and a modest increase in survival time (Fig. 1E, p<0.05). However, treatment with heat-killed cps failed to halt tumor growth or cause tumor regression in any mice, demonstrating that antitumor efficacy requires live organisms and suggesting that the organism must actively invade cells for efficacy.
A potential problem with live organism therapy is the possibility of establishment of active infection, especially in immunocompromised patients. In order to rigorously demonstrate that cps would be innocuous regardless of immune status, we administered the organism to NOD/SCID/IL-2 receptor gamma chain knockout mice (NSG). These mice lack B, T and NK cells (13). Intraperitoneal injection of 100,000 cps tachyzoites had no observable effect on immunocompromised mice (supplemental Fig. 2), despite the fact that 100 tachyzoites of the parental RH strain of T. gondii is lethal to wild type mice within 2 weeks (14). These studies demonstrate that even in an immunocompromised host, the cps strain of T. gondii poses no safety risk as a treatment modality.
A previous study using intraperitoneal injection of a nonattenuated strain of T. gondii demonstrated the ability to slow the growth of B16F10 and concluded that the affect was not adaptive immune system-mediated, as tumor growth reduction could be achieved in immunodeficient mice (15). To conclusively prove that immune effector function is required for cps-mediated elimination of established B16F10 tumors, we evaluated cps treatment of B16F10 in severely immunodeficient NSG mice. cps treatment failed to affect tumor growth in NSG mice (Fig. 1F), proving that cps-mediated tumor regression is mediated by the immune system.
cps treatment rapidly increased leukocyte infiltration of tumors and leukocytes were invaded by cps
Leukocyte distribution was assessed in untreated and cps-treated tumors by immunohistochemistry and flow cytometry. Both methodologies showed that cps treatment rapidly increased CD45+ leukocyte infiltration of the tumors by 2–3 fold after treatment (Fig. 2A). CD11b+CD11c− macrophages (M Φ) were significantly increased in roughly the same proportions as total CD45+ cells (Fig. 2A, B). CD11c-expressing myeloid dendritic cells (DCs), NK1.1-expressing NK cells and CD11b−Gr1+ neutrophils were not significantly changed as a proportion of CD45+ cells, although the increase in CD45+ cells means that the total cell numbers of these leukocytes did increase (Fig. 2C, E, F). Perhaps surprisingly, the percentage of CD3+ T cells in the tumor was significantly reduced in the early stages of the treatment. Overall this shows that the initial response to treatment is recruitment of myeloid but not lymphoid cells.
In order to determine what cells are being invaded by cps, cps was labeled with CFSE, injected intratumorally on consecutive days and assayed18 hours after the second cps injection. Flow cytometry revealed that roughly 50% of CD45− cells were CFSE positive and greater then 80% of leukocytes were invaded (CD45+ cells, Fig. 2G). This demonstrates that leukocytes are first rapidly recruited to the tumor by cps injection (Fig. 2A) and preferentially invaded by cps. Macrophages, DCs and NK cells were all 70–80% invaded but only 20–30% of T cells and neutrophils were invaded (Fig. 2G). This show that cps can invade a variety of nucleated cell types but preferentially invades phagocytes, as is consistent with wild type T. gondii.
To further investigate the kinetics of the adaptive immune response, we performed 2 days of cps injection and then 3 days later analyzed the leukocyte populations in the tumor (Fig. 2H–L). Leukocytes (CD45+) were 2–3 fold higher in treated tumors (Fig. 2H, supplemental Fig. 3), CD8+ T cells increased 3 fold (Fig. 2I) and 8% of these CD8+ T cells were producing IFN-γ as compared to 3% in the controls, (Fig. 2J). Interestingly, while the fraction of NK cells were similar between treated tumors and the now much larger control tumors, there is a marked increase of NK cells producing IFN-γ in treated tumors (Fig. 2L). Together, these data show that cps treatment drives a rapid and durable leukocyte infiltration of the tumor (changes expressed in cell numbers rather than % are shown in supplemental Fig. 4). At this later stage of treatment there is an increase in CD8+ T cells and increased expression of IFN-γ by both CD8+ T cells and NK cells.
Treatment generates tumor antigen-specific responses and efficacy requires CD8+ and NK cells
To examine systemic changes of immune cells, we evaluated changes in draining lymph nodes and spleens at day 20, 11 days after treatment was begun and a time at which treated tumors were all visually eliminated. We detected a statistically significant increase in antigen experienced (CD44+) CD8+ and CD4+ T cells in both spleen and tumor-draining inguinal lymph nodes (Fig. 3A, B). cps does not carry tumor-specific antigens, so it was of interest to determine whether the treatment generated T cells that recognize specific tumor antigens in the peripheral lymphoid organs. This was assessed by ELISPOT assay of CD8+ IFN-γ producing T cells in response to presentation of the melanoma antigen TRP-2. There was a dramatic increase of IFN-γ secreting CD8+ T cells recognizing the Kb-restricted epitope TRP-2180-188 in both draining lymph nodes and spleens of treated versus untreated animals (Fig. 3C, D). These data demonstrate a systemic tumor antigen-specific CD8+ T cell response after cps treatment. Taken together with the results in Fig. 2, this indicates that cps monotherapy causes an early robust recruitment of innate immune leukocytes followed by generation of activated tumor-specific lymphocytes. We and collaborators have recently shown that cps induces a strong Th1 immune response and elicits a life-long CD8+ T cell immunity (16). Given that CD8+ T cell responses are capable of eliminating tumor masses, we next determined whether T cells were involved in tumor elimination.
In order to determine the relative roles of CD8+ and CD4+ T cells in tumor elimination, we evaluated cps-mediated tumor eradication and survival in conjunction with administration of depleting antibodies to remove CD4+ or CD8+ T cells. Interestingly, depletion of CD8+ cells, but not CD4+ cells, abrogated the antitumor efficacy of cps (Fig. 3E, F), demonstrating that CD8+ T cells are required for the success of cps monotherapy, but CD4+ T cells and associated T cell help are dispensable. The potential role of NK cells was also determined by administering antibodies to deplete NK1.1 expressing cells. As occurred with depletion of CD8+ T cells, tumor growth was similar in untreated animals and animals that were cps treated in combination with NK1.1 depletion, revealing a requirement for NK cells for successful cps monotherapy (Fig. 3G).
Treatment efficacy requires IFN-γ, IL-12 and CXCR3 signaling
Tumor-specific IFN-γ expressing CD8+ T cells are increased in the tumor following cps treatment (Fig. 2G). To determine the changes in IFN-γ levels after cps treatment, cytokines in tumor lysates were assayed at either 18 hours or 5 days after the initial cps treatment. IFN-γ is increased 5–10 fold at both time points (Fig. 4A). We therefore tested the functional importance of IFN-γ by cps treatment of B16F10 tumors in IFN-γ knockout mice (17). The treatment has no effect on the tumor in these mice (Fig. 4B), demonstrating a requirement for host IFN-γ for treatment efficacy.
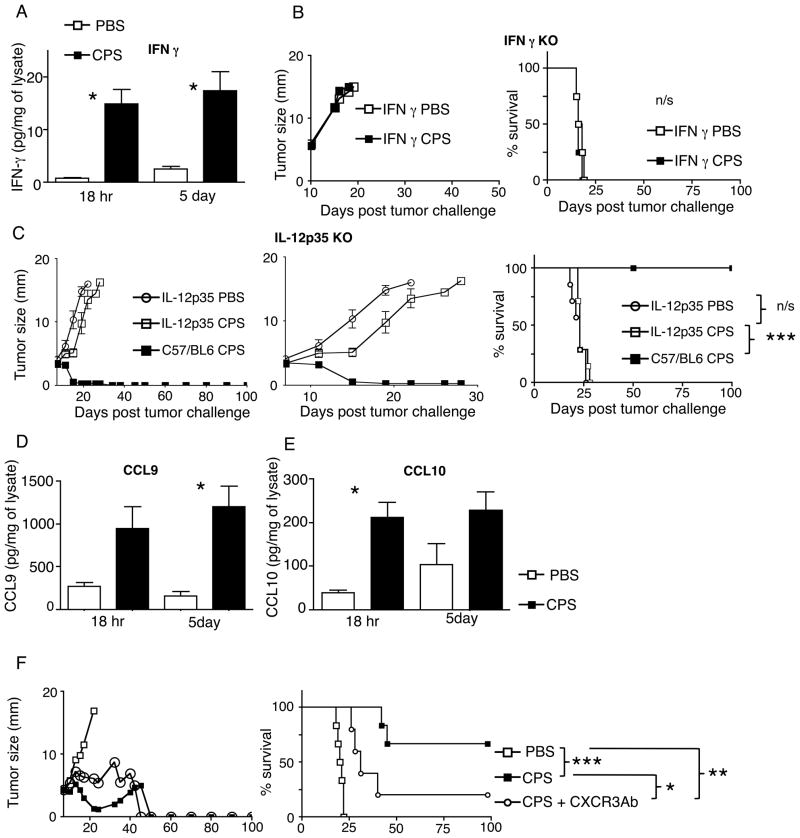
FIGURE 4.
Treatment efficacy requires IFN-γ, IL-12 and is supported by CXCR3. (A) Assay of intratumoral IFN-γ at 18 hr and 5 days after initial cps treatment. (B) Treatment of established B16F10 with cps or PBS in IFN-γ KO mice. (C) B16F10 tumor-bearing mice treated with cps or PBS in IL-12p35 KO mice, middle panel is an expanded view of day 5–30 of 100 day growth curves. (D and E) Assay of intratumoral CCL9 (D) and CCL10 (E) at 18 hr or 5 days after cps treatment. (F) Mean tumor size of B16F10 tumor-bearing mice treated with PBS, cps or cps + anti-CXCR3 blocking Ab. Error bars are SEM; P values: * < 0.05, ** < 0.01, *** < 0.001. n=3 for cytokine and chemokine, n= minimum of 4 per group for survival, data are representative of 2 independent experiments.
Along with IFN-γ, IL -12 is part of the cps-stimulated immune response (7) and is strongly associated with cell-mediated cytotoxic immune responses, including antitumor responses. A requirement for IL-12 for treatment efficacy was tested using IL12p35 knockout mice (18). cps treatment did not regress established tumors in IL-12 KO mice and the resultant tumor growth rate was not statistically different from controls over the full experimental course (Fig. 4C) demonstrating the clear requirement of IL-12 for the normal treatment response. Interestingly, the treatment did stall tumor growth during the early treatment phase when cps was likely still present in the tumor (see day 11, 16, Fig. 4C middle panel), a pattern not seen with IFN-γ KO mice or mice with CD8+ T or NK cell depletion. This shows that there are 2 distinct immunological phases of the treatment, an initial phase in which cps is abundant and does not require host IL-12, and a subsequent phase that no longer requires the presence of high levels of cps but does require IL-12, reflecting an ongoing antitumor adaptive immune response.
In order to further dissect mechanisms by which cps treatment shrinks established tumors, we investigated chemokines by which lymphocytes could be recruited into the tumor following cps injection. Recruitment of effector T cells to tumor locations has been reported to involve interaction of the CXCR3 chemokine receptor expressed on T cells with its chemokines ligands, CXCL9, 10, or 11, generated in the tumor microenvironment (19). We assayed for expression of CXCL9 and CXCL10 in the tumors themselves 18 hours or 5 days after cps treatment was initiated and found that cps treatment generated robust increases of both cytokines at each time point (Fig. 4D, E). To determine whether the CXCR3 axis is functionally relevant in cps-mediated tumor treatment, we assessed the efficacy of cps treatment in the presence of CXCR3 blocking antibody. CXCR3 blockade reduced but did not eliminate the antitumor efficacy of cps treatment (p<0.05). Although survival of CXCR3-blocked animals was significantly better than untreated controls, only 20% of the treated mice exhibited complete regression of the primary tumor when CXCR3 was blocked (Fig. 4F). Taken together, these results indicate that cps monotherapy induces elevated levels of both CXCL9 and CXCL10, and this response is rapid (apparent at 18h) and durable (still present at 5 days). The presence of these cytokines is consistent with the partial requirement of CXCR3 signaling for efficacy.
Responses against T. gondii are reported to involve the TLR adaptor molecule Myd88 (20). The requirement for Myd88 for efficacy of treatment with cps was tested in mice lacking Myd88 (21). Surprisingly, tumors in Myd88 KO mice responded normally to the treatment (data not shown), and therefore Myd88 is not required for response to cps monotherapy. CCR5 is similarly thought to play an important role in the immune response to T. gondii by its involvement in stimulating IL-12 production (22, 23). Again, contrary to expectation, CCR5 knockout mice (24) responded identically to wild type animals to treatment of established B16F10 tumors with cps (data not shown), indicating that CCR5 signaling does not influence the therapy’s efficacy.
Mice treated by cps injection exhibit vitiligo, systemic antitumor immunity and antitumor immune memory
Across many individual experiments, 76% of wild type mice treated with cps for dermal B16F10 melanoma developed localized or disseminated vitiligo (Fig. 5A, B). This indicates that the immune response that eliminated the melanoma also initiated an immune response against normal melanocytes. The vitiligo suggested that the antitumor response was systemic and may be associated with system wide immunity and immunological memory against the tumor. To determine whether cps treatment of a primary tumor would impact a secondary tumor, mice were challenged with a secondary tumor inoculation on the day after the second injection of cps or PBS into the primary tumor. Secondary challenge inoculation was done into the skin of the flank with the primary tumor or the other flank, and treatment of the primary tumor was completed (Fig. 5C). cps-treated mice rejected the rechallenge in every case, while all tumors grew on PBS treated tumor-bearing mice (Fig. 5D, E) that had the primary tumor surgically removed on day 13 to better compare to standard clinical therapy and limit the size of the rapidly growing primary tumor.
FIGURE 5.
Regression of dermal melanoma by cps treatment stimulates vitiligo and supports systemic and memory antitumor immune responses. (A) Picture of mice exhibiting vitiligo after elimination of primary dermal B16F10 melanoma by cps treatment. (B) Graph of % of mice that developed vitiligo, 22 of 29 (76%) of cps treated mice got detectable vitiligo (among wild type mice with no other manipulation). (C) Treatment strategy for rechallenge during primary tumor treatment. (D) Growth curve of B16 intradermal rechallenge, same and opposite flank. (E) Survival curves for flank rechallenges. (F) dermal rechallenge on day 40 (left) or day 120 (right) after establishment of primary tumor. cps groups had the primary tumor regressed by cps, PBS-treated groups had the primary tumor surgically resected on day 13. Error bars are SEM; P values: * < 0.05, ** < 0.01, *** < 0.001. n= minimum of 5, data are representative of 2 independent experiments..
In order to demonstrate anti-tumor immune memory, we rechallenged mice that had successful treatment of the primary tumor 40 days or 120 days after inoculation of the primary tumor (20 days or 100 days after elimination of palpable primary tumor). The majority of these mice rejected the secondary tumor challenge at day 40 and the tumors that did grow in these mice grew more slowly than in the control mice whose primary tumor was surgically removed on day 12 (Fig. 5F). Although all secondary challenge tumors grew in the 120 day mice, they grew at a significantly slower rate than surgically treated control mice (Fig 5F).
Intratumoral injection of cps has efficacy against other tumors
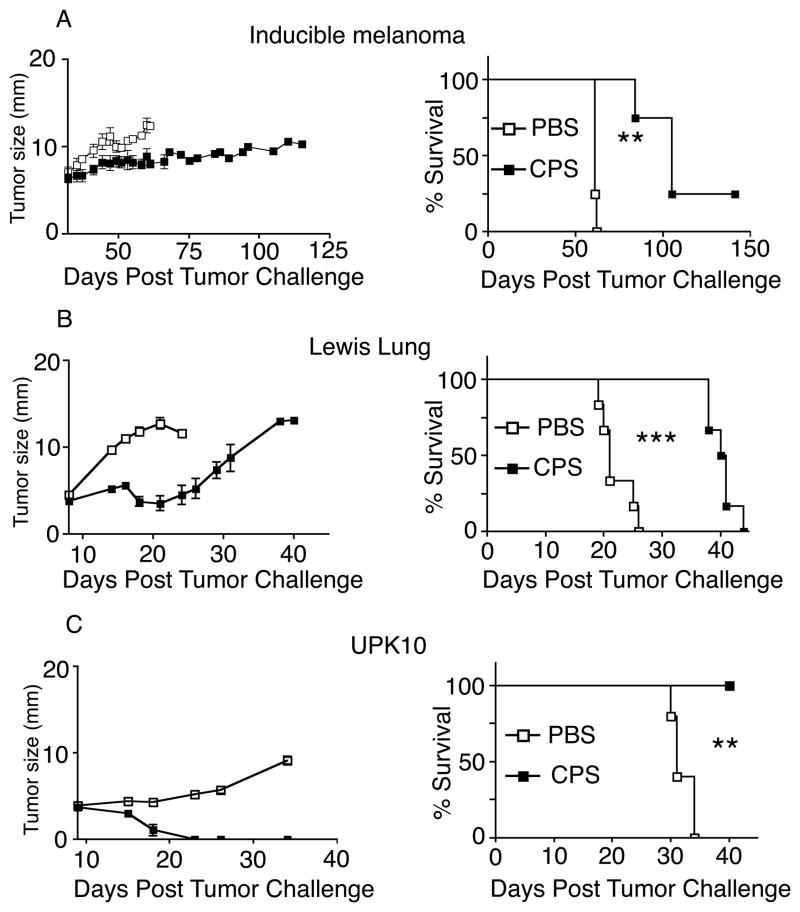
The ability of cps, which lacks melanoma-associated antigens, to mediate antitumor immunity against B16F10 suggests that cps treatment induces tumor-specific immune responses directed against tumor antigens. Therefore, we hypothesized that cps treatment would demonstrate efficacy against other tumors. The ability of cps to treat other tumors was tested using melanomas generated in an inducible genetic melanoma system that utilizes Braf mutation and Pten loss (25), syngeneic C57BL6 mouse derived Lewis Lung carcinoma (26), and syngeneic C57BL6 mouse-derived UpK10 ovarian cancer (27). Using a treatment approach similar to that described for B16F10, intratumoral cps administration either significantly slowed tumor growth or led to complete regression, depending on the model (Fig. 6A–C). Thus intratumoral cps injection is a generalizable approach that has efficacy against a variety of established tumors.
FIGURE 6.
Intratumoral injection of cps has efficacy in treating other mouse tumor models. Growth and survival curves for cancers treated by cps using the same strategy as in figure 1. (A) inducible BRAF/PTEN genetic model of dermal melanoma, (B) Lewis lung carcinoma implanted intradermally, (C) UpK10 cell line from KRAS/p53 genetic model of ovarian cancer implanted intradermally. Error bars are SEM (some obscured by icons); P values: * < 0.05, ** < 0.01, *** < 0.001. n= minimum of, data are representative of 2 independent experiments.
Discussion
Intratumoral injection of cps is an effective immunotherapy with no toxicity
This report provides the first example of the use of cps for tumor immunotherapy and the first demonstration of high frequency successful immunological treatment of established B16F10 melanoma using either an attenuated pathogen or an immunological monotherapy. Importantly, cps accomplishes this with no infection-associated toxicity since it cannot replicate in vivo and cannot revert genetically. This combination of efficacy and lack of toxicity is the essential definition of a treatment with an outstanding therapeutic index.
Approximately 20% of the world population is latently infected with T. gondii, with latent infection much higher in some areas. Latent infection with T. gondii does not impact the efficacy of intratumorally-administered cps. Thus, this approach could be used to effectively treat patients, regardless of past exposure to T. gondii. This finding suggests that antibodies induced by infection with wild type T. gondii do not interfere with subsequent initial or repeated cps-based therapies. This is an advantage over viral vector-based cancer therapy strategies that often induce antibodies that block infectivity following repeated administrations (28).
T. gondii has been studied previously in connection with cancer therapy. Latent infection with T. gondii slowed tumor development in mice (29). Mouse studies using acute toxoplasmosis from IP injection to treat cancer retarded tumor growth, but unlike treatment with cps, it was not used to treat an established tumor, the adaptive immune system was not required, the primary tumors were not eliminated, and the approach itself was limited by toxicity of the infection (15). T. gondii extracts have been used to mature DCs in vitro to improve T cell adoptive transfer efficacy (30). This effect of T. gondii extracts is reflected in our studies using heat-killed cps, in which there was a discernable but modest slowing of tumor growth. The results reported here require live metabolically active cps and are not attributable to microbial constituents.
These studies show that leukocytes are preferentially but not exclusively invaded by cps and there is a highly effective specific immune response both against the tumors and against cps. Interestingly, efficacy was achieved even though cps does not express any tumor-antigens. While it is possible that there is a Toxoplasma antigen that crossreacts with an antigen in B16F10, the efficacy in lung and ovarian tumors argues against such a possibility. One explanation for apparent antigen spreading is that cps treatment and associated immunostimulation releases adaptive immune responses that are present but being suppressed by the tumors prior to treatment. In studies using Listeria monocytogenes (31–33) or Salmonella typhimurium (4) to stimulate antitumor immunity it was shown that expression of tumor antigens by pathogens significantly improves the antitumor immune response generated. cps can be engineered to express antigens to increase tumor-antigen specific responses which may further improve efficacy and immune memory development (34).
It is clear that treating dermal melanoma by immunotherapy is only superior to surgical resection if it stimulates systemic anti-tumor immune responses that can eliminate occult metastases. Vitiligo is generated by cps therapy and reveals an immune response against normal melanocytes. Development of vitiligo is a predictor of positive outcome in melanoma patients (37–39) and has been shown to be required for long-term T cell memory against B16F10 melanoma (40). The ability to reject or markedly impair subsequent challenges either during treatment of the primary tumor, or after the initial effector phase of the response is complete and the memory phase has begun, supports the potential for this approach to clinically impact early stage metastatic disease. This therapeutic approach may be effective in the crucial stage in the course of the disease when it may have metastasized but is not yet so disseminated as to be virtually incurable.
cps monotherapy orchestrates multiple immune pathways to achieve tumor regression
These studies demonstrate that treatment efficacy requires CD8+ T cells, which is not surprising, but less predictably shows that CD4+ T cells are not required for full efficacy. This demonstrates that the mechanism does not depend on classic T cell help. T cell help for cytotoxic T cells is associated with Th1 type helper T cells and the classic Th1 cytokines IFN-γ and IL-12, which are in fact both required for efficacy using cps. This implies that other cell types are taking over the role of Th1 CD4+ T cells when cps is present. It is likely that one of those cell types is NK cells, which are also required for efficacy and can be a major source of IFN-γ in cancer (35).
cps treatment efficacy required host-production of IL-12, and while T. gondii has been previously reported to increase IL-12 levels through CCR5 signaling (22, 23), CCR5 KO mice have normal efficacy with this treatment approach. Therefore, other pathways are manipulated by cps to increase IL-12 or the IL-12 is generated through the CCR5 pathway by the genetically normal B16F10 tumor cells. Such pathways may be functional normally or may require specific changes that are associated with the presence of tumors. Similarly, Myd88 signaling has been implicated in the immune response against T. gondii, but is not needed for treatment efficacy. It appears that this therapy is working through as yet unrecognized pathways of T. gondii interaction with the immune system. It should be noted that Myd88 is broadly expressed and since tumor cells are invaded by cps it is possible that Myd88 in tumor cells does play a role in the treatment.
Detailed examination of tumor growth curves from the IL-12 KO mouse studies shows that treatment initially inhibits growth of the tumor while cps is present. However, the tumor again overcomes the immune response once cps is less prevalent. By contrast, other conditions that block efficacy, such as elimination of NK or CD8+ T cells or treatment of IFN-γ KO mice, lost all treatment impact and were identical to PBS controls. This suggests that the early response, in which cps is present in the tumor, and the tumor growth halts, depends on different mechanisms than the later response during which the tumor is eliminated despite the fact that cps is no longer present. Potentially, the early response breaks immunosuppression and activates innate immune functions that support already existing but suppressed adaptive responses and the later response depends on expansion and recruitment of naive tumor-specific T cells.
The data show that the CXCR3 axis plays an important role in cps-mediated antitumor immune responses and is required for full treatment efficacy, but tumor growth is still significantly slowed when CXCR3 is blocked on host cells. While this suggests that the CXCR3 axis is not the only mechanism by which effector CD8+ T cells are recruited to the tumor, its importance is underlined by the finding that in humans, the presence of circulating tumor antigen-specific CXCR3+ CD8+ T cells was associated with enhanced tumor-free survival of melanoma patients (36).
cps treatment has efficacy as a monotherapy against an orthotopic poorly immunogenic tumor. Immunological monotherapy of any sort has rarely exhibited significant efficacy and most tumor immunotherapy approaches involve multiple, distinct immune manipulations. The demonstration of monotherapy effectiveness presented here is likely to be further improved by coupling with other immunological approaches such as adoptive T cell therapy and blocking of immune checkpoints that suppress T cells. Monotherapy cps treatment of B16F10 also stands out from many other immunotherapy approaches by relying totally on manipulation of the endogenous immune response in vivo.
The efficacy against established B16F10, lack of dependence on specific antigen expression that is subject to immunoediting, response regardless of preexposure to T. gondii, and systemic and memory responses establish cps monotherapy of tumors as a unique potential clinical therapy. Additionally, the simplicity, safety, and ability to effectively treat multiple solid tumor types suggest that cps can be developed into a valuable clinical tool for stimulation of therapeutic antitumor immunity.
Supplementary Material
Acknowledgments
This project was supported by funding from The Friends of the Norris Cotton Cancer Center and the National Cancer Institute, 5P30CA023108. NIH funding was provided by grants: CA134799 to DWM, AI041930 to DJB, CA120777 to MJT, U54 CA151662 to SF. Support for JRB was from the GAANN foundation and support for JRB and KTB was from the Dartmouth Immunology Training Program T32 AI007363.
We would like to acknowledge discussions with Jose Conejo-Garcia that contributed significantly to this project, tech support from Jennifer Fields, Sandra Warner and Laurie Horne, the Dartmouth Transgenic and Genetic Construct Shared Resource, the Dartmouth Immune Monitoring Shared Resource, the Dartmouth Flow Cytometry Shared Resource and the Dartmouth Center for Molecular Cellular and Translational Immunology (NIH-NCRR 1P30RR032136).
Literature cited
- 1.Coley W. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893 classical article. Clin Orthopaedics and Related Res. 1991;262:3. [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 3.Gravekamp C, Paterson Y. Harnessing Listeria monocytogenes to target tumors. Cancer Biol Ther. 2010:9. doi: 10.4161/cbt.9.4.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manuel ER, Blache CA, Paquette R, Kaltcheva TI, Ishizaki H, Ellenhorn JD, Hensel M, Metelitsa L, Diamond DJ. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res. 2011;71:4183. doi: 10.1158/0008-5472.CAN-10-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson Y, Guirnalda PD, Woo LM. Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin Immunol. 2010;22:183. doi: 10.1016/j.smim.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox BA, Bzik DJ. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature. 2002;415:926. doi: 10.1038/415926a. [DOI] [PubMed] [Google Scholar]
- 7.Gigley JP, Fox BA, Bzik DJ. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J Immunol. 2009;182:1069. doi: 10.4049/jimmunol.182.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516. [PubMed] [Google Scholar]
- 9.Amos SM, Pegram HJ, Westwood JA, John LB, Devaud C, Clarke CJ, Restifo NP, Smyth MJ, Darcy PK, Kershaw MH. Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol Immunother. 2011;60:671. doi: 10.1007/s00262-011-0984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, Rosenberg SA, Restifo NP. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zenner L, Foulet A, Caudrelier Y, Darcy F, Gosselin B, Capron A, Cesbron-Delauw MF. Infection with Toxoplasma gondii RH and Prugniaud strains in mice, rats and nude rats: kinetics of infection in blood and tissues related to pathology in acute and chronic infection. Pathol Res Pract. 1999;195:475. doi: 10.1016/S0344-0338(99)80051-X. [DOI] [PubMed] [Google Scholar]
- 13.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 14.Gross U, Muller WA, Knapp S, Heesemann J. Identification of a virulence-associated antigen Toxoplasma gondii by use of a mouse monoclonal antibody. Infect Immun. 1991;59:4511. doi: 10.1128/iai.59.12.4511-4516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter CA, Yu D, Gee M, Ngo CV, Sevignani C, Goldschmidt M, Golovkina TV, Evans S, Lee WF, Thomas-Tikhonenko A. Cutting edge: systemic inhibition of angiogenesis underlies resistance to tumors during acute toxoplasmosis. J Immunol. 2001;166:5878. doi: 10.4049/jimmunol.166.10.5878. [DOI] [PubMed] [Google Scholar]
- 16.Gigley JP, Fox BA, Bzik DJ. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J Immunol. 2009;182(2):1069. doi: 10.4049/jimmunol.182.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 18.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musha H, Ohtani H, Mizoi T, Kinouchi M, Nakayama T, Shiiba K, Miyagawa K, Nagura H, Yoshie O, Sasaki I. Selective infiltration of CCR5(+)CXCR3(+) T lymphocytes in human colorectal carcinoma. Int J Cancer. 2005;116:949. doi: 10.1002/ijc.21135. [DOI] [PubMed] [Google Scholar]
- 20.LaRosa DF, Stumhofer JS, Gelman AE, Rahman AH, Taylor DK, Hunter CA, Turka LA. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc Nat Acad Sci USA. 2008;105:3855. doi: 10.1073/pnas.0706663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim HM, Bannai H, Xuan X, Nishikawa Y. Toxoplasma gondii cyclophilin 18- mediated production of nitric oxide induces Bradyzoite conversion in a CCR5-dependent manner. Infect Immun. 2009;77:3686. doi: 10.1128/IAI.00361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, Reis e Sousa C, Fairlamb A, Ribeiro JM, Sher A. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat Immunol. 2003;4:485. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- 24.Sato N, Kuziel WA, Melby PC, Reddick RL, Kostecki V, Zhao W, Maeda N, Ahuja SK, Ahuja SS. Defects in the generation of IFN-gamma are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1 alpha-, or CCR2-deficient mice. J Immunol. 1999;163:5519. [PubMed] [Google Scholar]
- 25.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellmann K, Burrage K. Control of malignant metastases by ICRF 159. Nature. 1969;224:27305. doi: 10.1038/224273a0. [DOI] [PubMed] [Google Scholar]
- 27.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver JB, Fiering S, Conejo-Garcia JR. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JC. Melanoma vaccines. Cancer J. 2011;17:277. doi: 10.1097/PPO.0b013e3182325f72. [DOI] [PubMed] [Google Scholar]
- 29.Hibbs JB, Jr, Lambert LH, Jr, Remington JS. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J Infect Dis. 1971;124:587. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- 30.Motamedi M, Arab S, Moazzeni SM, Khamis Abadi M, Hadjati J. Improvement of a dendritic cell-based therapeutic cancer vaccine with components of Toxoplasma gondii. Clin Vaccine Immunol. 2009;16:1393. doi: 10.1128/CVI.00199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seavey MM, Maciag PC, Al-Rawi N, Sewell D, Paterson Y. An anti-vascular endothelial growth factor receptor 2/fetal liver kinase-1 Listeria monocytogenes anti-angiogenesis cancer vaccine for the treatment of primary and metastatic Her-2/neu+ breast tumors in a mouse model. J Immunol. 2009;182:5537. doi: 10.4049/jimmunol.0803742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seavey MM, Pan ZK, Maciag PC, Wallecha A, Rivera S, Paterson Y, Shahabi V. A novel human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA- A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res. 2009;15:924. doi: 10.1158/1078-0432.CCR-08-2283. [DOI] [PubMed] [Google Scholar]
- 33.Sinnathamby G, Lauer P, Zerfass J, Hanson B, Karabudak A, Krakover J, Secord AA, Clay TM, Morse MA, Dubensky TW, Jr, Brockstedt DG, Philip R, Giedlin M. Priming and activation of human ovarian and breast cancer-specific CD8+ T cells by polyvalent Listeria monocytogenes-based vaccines. J Immunother. 2009;32:856. doi: 10.1097/CJI.0b013e3181b0b125. [DOI] [PubMed] [Google Scholar]
- 34.Dzierszinski F, Pepper M, Stumhofer JS, LaRosa DF, Wilson EH, Turka LA, Halonen SK, Hunter CA, Roos DS. Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen- presenting cells. Infect Immun. 2007;75:5200. doi: 10.1128/IAI.00954-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. doi: 10.1155/2011/676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 37.Nordlund JJ, Kirkwood JM, Forget BM, Milton G, Albert DM, Lerner AB. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- 38.Bystryn JC, Rigel D, Friedman RJ, Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123:1053. [PubMed] [Google Scholar]
- 39.Quaglino P, Marenco F, Osella-Abate S, Cappello N, Ortoncelli M, Salomone B, Fierro MT, Savoia P, Bernengo MG. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 40.Byrne KT, Cote AL, Zhang P, Steinberg SM, Guo Y, Allie R, Zhang W, Ernstoff MS, Usherwood EJ, Turk MJ. Autoimmune melanocyte destruction is required for robust CD8+ memory T cell responses to mouse melanoma. J Clin Invest. 2011;121:1797. doi: 10.1172/JCI44849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.