Abstract
The Programmed Death-1 (PD-1) pathway limits the function of virus-specific T cells during chronic infection. We have previously shown that blockade of the PD-1 pathway increases HIV-1 associated T cell function in vitro. However the effect of PD-1 blockade on HIV-1 disease progression in vivo has not been examined. As in humans, HIV-1 infected humanized Balb/c-Rag2−/−gc−/− (Rag-hu) mice express elevated levels of PD-1 on T cells during chronic infection. To examine the effect of PD-1 blockade on disease progression, Rag-hu mice with chronic HIV-1 infection were treated with a blocking monoclonal antibody (mab) directed against PD-L1, the ligand for PD-1. PD-L1 treated Rag-hu mice exhibited a progressive decrease in the HIV-1 plasma viral load with a 7 fold decrease by day 7, 20 fold by day 14, 178 fold by day 21 and 269 fold by day 28 post initiation of treatment. By day 7 the percentage of CD4+ T cells was statistically higher in the treated compared with the untreated group and this trend was sustained throughout the 28 day treatment period. Moreover, there was a strong inverse correlation between plasma viral load and the percentage of both CD4+ (r= −0.66; P<0.0001) and CD8+ (r=−0.64; P<0.0001) T cells in the treated mice but not the untreated mice. This study provides “proof of concept” that humanized mice can be used to examine the effects of immunotherapeutic interventions on HIV-1 infection. Furthermore, these data demonstrate for the first time that blockade of the PD-1 pathway reduces HIV-1 viral loads.
Introduction
Virus-specific T cells are functionally compromised during chronic infections. Although these T cells retain some functional attributes, their ability to proliferate and produce multiple cytokines (1) (2), both of which have been correlated with control of viral replication, are severely affected (3–5). It is now widely accepted that receptor-based inhibitory pathways limit the function of virus-specific T cells during chronic viral infection. Inhibitory receptors such as PD-1 are expressed at elevated levels on both CD4+ and CD8+ T cells in subjects with chronic HIV-1 infection and diminished function of these cells may contribute to ineffective control of HIV-1 replication (6–8). Disruption of the PD-1 pathway using monoclonal antibodies (mabs) that block PD-1/PD-L1 interaction increases the proliferative and cytokine producing capacity of HIV-1-specific T cells in vitro (6). Furthermore, blockade of the PD-1 pathway in vivo increased SIV-specific T cell function, decreased SIV viral loads and opportunistic infections and increased the life span of SIV infected macaques (9). These findings suggest that monoclonal antibodies that block the PD-1 pathway may have therapeutic benefit in HIV-1 infected subjects.
However, experimental studies designed to test the efficacy of PD-1 blocking reagents on HIV-1 disease progression, as defined by persistent HIV-1 viral loads and declining CD4+ T cell count, have been difficult to conduct due to the lack of suitable in vivo animal models. In this regard, recent advances in the development of new generation humanized mouse models for HIV-1 infection now make these studies possible (10). These new mouse models are constructed by injecting human CD34 hematopoietic stem cells into either Rag2 common gamma chain knockout or NOD scid gamma(NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice which are severely immunodeficient. Human CD34+ stem cells efficiently engraft in these mice and give rise to human T, B, and dendritic cells (DCS) and monocytes (11–13). Furthermore, CD4+ T cells, macrophages/monocytes and DCs capable of supporting productive HIV-1 infection in vivo are continuously generated and infected humanized mice exhibit many of the clinical manifestations such as plasma viremia and decreasing CD4+ T cell counts akin to that seen in HIV-1 infected humans (14, 15). In addition to acute infection we have shown that Rag-hu mice can also sustain chronic HIV-1 infection lasting more than a year. HIV can be experimentally transmitted to these mice via multiple routes including natural mucosal routes (16, 17).
These important attributes of next generation humanized mice have paved the way to dramatically expedite novel immunotherapeutic and immune reconstitution efficacy studies in vivo. Currently these studies are performed using nonhuman primate models or in human clinical trials both of which are expensive and laborious to conduct. In this proof-of-concept study we exploited HIV-1 infected Rag-hu mice with chronic viremia to evaluate the potential therapeutic effects of a proprietary human anti-PD-L1 monoclonal antibody on HIV-1 viral loads and CD4+ T cell counts in vivo. Our results demonstrate that HIV-1 infected Rag-hu mice treated with anti-PD-L1 antibody exhibited significantly lower viral loads and elevated/restored levels of CD4+ T cells in contrast to infected untreated control mice. Reduction in HIV-1 plasma viral loads correlated inversely with the percentage of CD4+ and CD8+ T cells in treated but not in untreated HIV-1 infected Rag-hu mice. This study is the first to show that humanized mice can be employed to evaluate novel antibody-based immunomodulation strategies for the treatment on HIV-1 infection.
Methods
Generation of humanized Rag2−/−γc−/− mice (RAG2-hu mice)
Humanized Balb/c-Rag2−/−gc−/− mice were prepared by engraftment with human fetal liver-derived CD34+ hematopoietic progenitor cells as previously described (14). Briefly, newborn mice were conditioned by irradiating with 3.5 Gy and then injected intrahepatically with 0.5–1×106 human CD34+ cells. Mice were screened for human cell engraftment at 10–12 weeks post-reconstitution. Peripheral blood was collected by tail bleed and red blood cells were lysed using the Whole Blood Erythrocyte Lysing Kit (R&D Systems, Minneapolis, MN). The white blood cell fraction was stained with antibodies against the human pan-leukocyte marker CD45 (Caltag) and analyzed by FACS to determine the levels of human cell engraftment as we previously described (14).
HIV-1 infection and measurement of viral loads
In these experiments mice with 70% or higher human cell engraftment levels were used. They were infected with HIV-1 by intraperitoneal (i/p) injection of CCR5 tropic strain BaL-1 (1.0 ×106 I.U.) at least 12 weeks after engraftment. Mice were observed daily and blood samples drawn weekly to assess plasma viremia. To detect HIV-1 in plasma of infected mice by QRT-PCR, RNA was extracted from 25–50ul of EDTA-treated plasma using the QIAamp Viral RNA kit (Qiagen, Valencia, CA). Q-PCR was performed using a primer set specific for the HIV-1 LTR sequence and a corresponding LTR specific probe as described previously (14).
PD-L1 monoclonal antibody treatment schedule
HIV-1 Bal-1 infected mice were monitored weekly to determine viremia. Consistent viremia was established in all infected mice by 4 weeks. For antibody treatment, seven viremic Rag-hu mice were injected with 200ug of BMS human anti-PD-L1 mab by i/p route once every three days for 4 weeks. Blood was drawn via tail vein weekly and viral load and CD4+ T cell percentages were determined during the 28 day treatment period and continued for several weeks after. Six untreated and 4 HIV-1 uninfected Rag-hu mice were also followed. The anti-PD-L1 is a fully human antibody derived by immunization of HuMab mice. Anti-PD-L1 is an IgG4 antibody with an S228P hinge mutation to prevent IgG4 exchange. The anti-PD-L1 monoclonal antibody binds with nanomolar affinity and a high degree of specificity to PD-L1. No binding to PD-L2 or other members of the related B7 family has been observed (18). The antibody blocks the binding of PD-L1 to PD-1 as well as the binding of PD-L1 to B7-1. Anti-PD-L1 promotes T cell activation as evidenced by increases in T cell proliferation and IFN-γ secretion upon addition of the antibody to cultures of T cells with allogeneic dendritic cells as compared to control IgG4 antibody.
Flow cytometry
Whole blood was collected and red blood cells lysed as reported previously (14, 19). White blood cells were stained with anti-human CD45-APC, CD3-PE, CD4-PECy5.5, CD8-Alexafluor 405, and PD-1-FITC. Cells were stained with the labeled antibodies and analyzed using a Coulter EPICS XL-MCL FACS analyzer (Beckman Coulter, Fullerton, CA) or BD LSRII (Becton Dickenson). CD4+ T cell levels were calculated as a ratio of the entire human CD45 population (i.e. CD45+CD4+CD3+). To establish the baseline CD4+ T cell levels, cells from all mice were analyzed prior to infection (Day 0). PD-1 expression was analyzed on CD45+CD3+CD4+ and CD45+CD3+CD8+ T cells and displayed as median fluorescence intensity using FlowJo software (TreeStar, Ashland OR). Maturation state and PD-1 expression levels were examined before an after treatment with anti-PD-L1. In order to obtain enough cells, blood from two groups (n=5 and n=6) of HLA matched, HIV-1 infected Raghu mice was pooled and stained with anti-human CD45-PE, CD3-BV 605, CD4-V500, CD8-Alexafluor 405, CD27-APCH7, CD45RA-PECy7 and PD-1-FITC and analyzed using a BD LSR II. FMO controls were used in all experiments.
Cytokine Analysis
Plasma samples from Raghu mice preinfection and pre and post anti-PD-L1 treatment was tested for cytokines using the Human Th1/Th2 Ultra-sensitive Cytokine kit (Meso Scale Development, Gaithersberg MD). TNF-α, IFN-γ, IL-13, IL-10, IL-5 and IL-12 P70 levels in the plasma were assayed. Plasma samples were stored immediately after collection at −80°C for this assay. Assays were performed per manufacturer’s instructions and analyzed using a Sector Imager 2400 (Meso Scale Discovery).
Statistical Analysis
Statistical significances in the viral load and the percentage of CD4+ T cells between PD-L1 mab treated and untreated mice were calculated by Mann-Whitney U test. Correlations between HIV-1 plasma viral load and the percentage of T cells were performed by Spearman test using Prism 3.0 software (GraphPad).
Results
PD-1 expression is elevated on T cells in HIV-1 infected Rag-hu mice
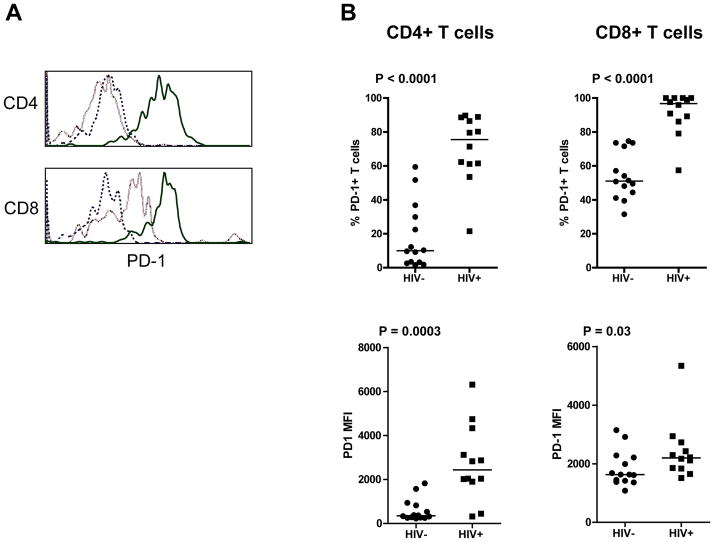
To determine if HIV-1 infection resulted in elevated expression of PD-1 in Rag-hu mice, its expression on total CD4+ and CD8+ T cells from the peripheral blood of twelve uninfected and fourteen HIV-1 infected Rag-hu mice 2 to 5 months post infection was assessed. As shown in Figure 1A and B, PD-1 expression (percent positive and median fluorescence intensity) was elevated on both CD4+ and CD8+ T cells in HIV-1 infected Rag-hu mice compared with uninfected control mice. The median percentage of PD-1+ CD4+ T cells was significantly higher (P<0.0001) on HIV-1 infected mice (75.5%, range 21.5–89.7%) than uninfected mice (10%, range 1.6–59.4). The same was true for CD8+ T cells where the median percentage of PD-1 positive cells was 96.7% (range 57.4–100%) on the HIV-1 infected mice and 51.1% (range 31.5–74.6%) on the uninfected mice (P < 0.00001). The PD-1 MFI was also significantly higher (P = 0.0003) on CD4+ T cells from HIV-1 infected Rag-hu mice (median 2436, range 318–6316) compared to uninfected controls (median 348, range 226–1824). PD-1 expression was also significantly higher (P = 0.03) on CD8+ T cells from HIV-1 infected Rag-hu mice (median 2195, range 1513–5339) compared to uninfected mice (median 1631, range 1079–3148). Furthermore, the expression of PD-1 on total CD4+ and CD8+ T cells from HIV-1-infected mice was similar. Of note however, the base-level PD-1 expression (MFI) on CD8+ T cells from uninfected Rag-hu mice was found to be higher, than on CD4+ T cells (over 4 fold higher) from the same animals. This may be due to a chronic low level xeno-reactivity of CD8+ T cells.
Figure 1.
PD-1 expression is elevated on CD4+ and CD8+ T cells in the blood of HIV-1 infected Rag-hu mice. (A) Blood from HIV-1 negative (light dashed line) and HIV-1 positive (solid line) Rag-hu mice was stained with anti-human CD45, CD3, CD4, CD8 and PD-1. The fluorescence minus one (FMO) control is the dark dashed line. (B) The percentage (top) and median fluorescence intensity (bottom) of PD-1 on CD4+ and CD8+ T cells was assessed using flow cytometry. Statistical differences were determined using Mann Whitney T Test.
Anti PD-L1 treatment reduces HIV-1 viral load
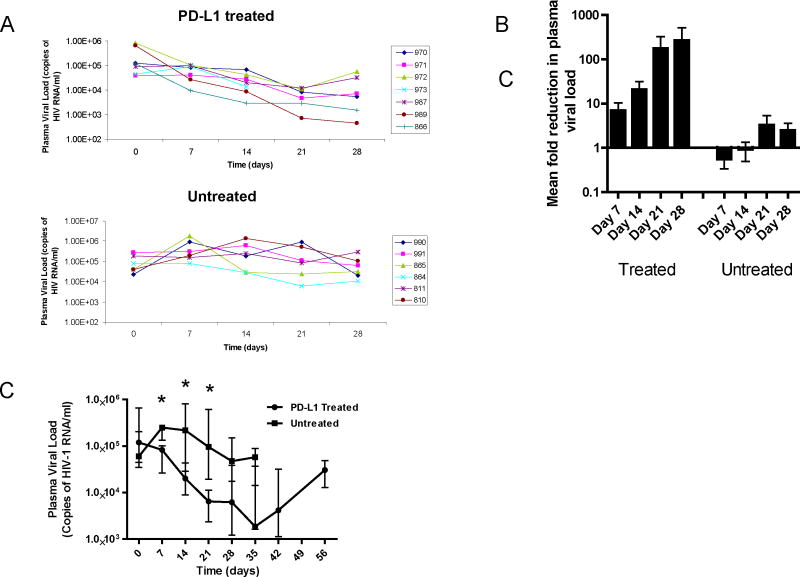
It has been shown that administering monoclonal antibodies that block the PD-1 pathway increases virus-specific T cell responses in vitro and decreases SIV and LCMV replication in vivo. However, the effect of PD-1 blockade on HIV-1 replication in vivo has not yet been examined. To determine the effect of PD-1 pathway blockade on HIV-1 replication we employed the Rag-hu mouse model of HIV-1 infection and used BMS humanized anti-PD-L1 monoclonal antibody. Data on individual treated and untreated mice are shown in Figure 2A. The median VL of both groups of mice pre treatment was not significantly different (P=0.29). Seven days post-treatment the plasma viral loads in 5 of 7 of the treated mice had decreased whereas it had only decreased in 1 of 6 untreated mice. By 21 days the viral load of all the treated mice had dropped below pre-treatment levels while 4 of the 6 untreated mice had higher viral loads than their pre-treatment levels. At 28 days all treated mice maintained lower viral loads relative to their pretreatment values while 3 of 6 untreated mice still had VL that were higher than their baseline VL. As shown in Figure 2B the treated Rag-hu mice had reductions of 7 fold at 7 days, 20 fold at 14 days, 178 fold at 21 days and 269 fold at 28 days in mean viral loads following the initiation of anti PD-L1 treatment. Conversely, untreated mice had a 0.5 and 0.9 fold increase in the mean VL at 7 and 14 days respectively and a 3 and 2 fold increase in mean VL at 21 and 28 days respectively compared to the pre-treatment levels. As shown in Figure 2C median HIV-1 plasma viral loads were significantly lower in the treated mice compared with untreated mice at 7 (P=0.014), 14 (P=0.022) and 21 (P=0.015) days post treatment. By 28 days, the viral load in the untreated mice started to decline. The decline in the VL in the untreated mice is attributed to the loss of CD4+ T target cells which were drastically reduced by this time point consistent with observations in previous studies. Accordingly, there is no statistically significant difference (P=0.064) in median VL between treated and untreated mice at this time point, although the levels in treated mice are still close to a log lower (6.2 × 103 vs. 4.7 × 104). The viral load was determined in treated mice for an additional 35 day period after cessation of treatment. During this time there was a relative increase in the VL, although it was still lower at day 56 than pre-treatment. HIV-1 plasma viral load was also examined in five Rag-hu mice treated with an isotype antibody (anti-diphtheria toxin mab). As expected, no reduction in HIV-1 plasma viral load was observed in isotype treated Rag-hu mice (data not shown).
Figure 2.
HIV-1 infected Rag-hu mice treated with anti-PD-L1 mab exhibited reduced HIV-1 plasma viral loads. Rag-hu mice with established chronic infection were injected I.P. with 200ug of Bristol Myer Squibb humanized anti-PD-L1 mab every three days for 4 weeks (day 3–28) and HIV-1 plasma viral load was determined by real-time PCR. (A) Plasma viral loads are shown for individual treated and untreated control Rag-hu mice. (B) Fold reduction in HIV-1 plasma viral load over pre treatment values are shown. (C) Median HIV-1 plasma viral load of treated (n=7) and untreated mice (n=6). This data is representative of 3 experiments. Asterisk indicates statistical significance (Mann Whitney T Test).
As shown in Figure 2A, there was a loss of one mouse (#973) in PD-L1 treated group before completion of the study. The CD4+ T cell counts and HIV-1 viral loads of this mouse were not significantly different from the same group of mice treated with anti-PD-L1 mab. It is unlikely that the loss of this mouse is due to the antibody treatment since none of the five uninfected Rag-hu mice treated with anti PD-L1 died (data not shown). Death of this animal is more likely due to the sporadic but well-documented spontaneous morbidity that occurs among severely immunocompromised mice (20).
Anti PD-L1 treatment increases the levels of human CD4+ T cells
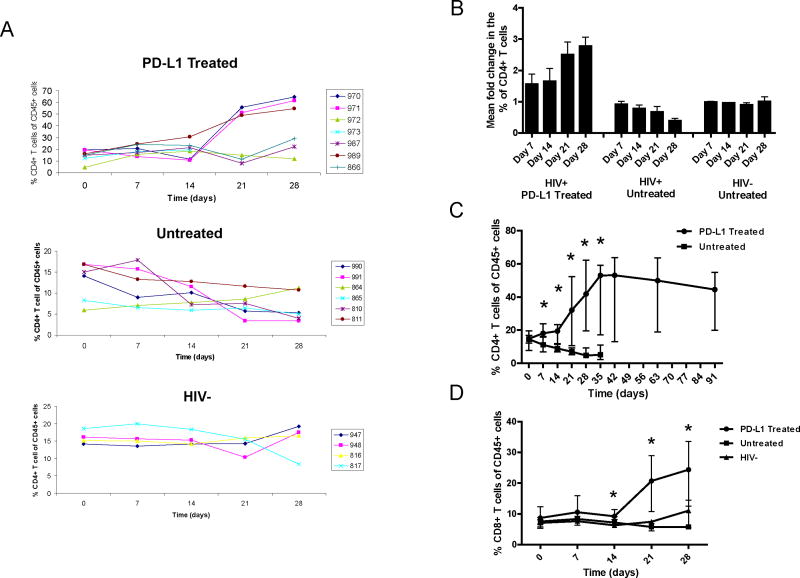
To determine if PD-L1 antibody has any effect on CD4+ T cell levels in HIV-1 viremic mice we monitored their levels on a weekly basis during and after the treatment. Our results showed that CD4+ T cell counts increased in HIV-1 infected Rag-hu mice treated with anti-PD-L1 mab. As shown in Figure 3A the percentage of human CD4+ T cells, normalized by human CD45, increased in all of the 7 treated mice during the 28 day treatment period. Of the 7 Rag-hu mice treated with anti-PD-L1, three (970, 971, and 989) had large increases and three (972, 987 and 866) had more modest increases in CD4+ T cell percentages. As mentioned above, one of the 7 HIV-1 infected Rag-hu mice (#973) died after 3 weeks of treatment although during this time the percentage of CD4+ T cells increased from 12% to 19%. Among the untreated HIV-1 infected Rag-hu mice all but one had a decline in the percentage of CD4+ T cells during the same 28 day period. As shown in Figure 3B the relative change in the percentage of CD4+ T cells at day 7 and 14 compared to pretreatment values were fairly static (1.4 and 1.5 fold higher respectively). However, by day 21 there was a greater than 2.5 fold and by day 28 it was almost 3 fold higher than pretreatment values. In contrast, the fold change in the untreated HIV-1 infected mice consistently decreased over the same period and by day 28 the mean percentage of CD4+ T cells had declined to half of the pretreatment levels. No change in the percentage CD4+ T cells was observed in four uninfected Rag-hu mice during the same time period (Figure 3A).
Figure 3.
Treatment of HIV-1-infected Rag-hu mice with anti PD-L1 mab increases the percentage of CD4+ and CD8+ T cells. (A) The percentage of CD4+ T cells normalized by human CD45 in the blood of PD-L1 treated HIV+, untreated HIV+ and untreated HIV− Rag-hu mice during the four week treatment period. (B) The fold change from pretreatment values for all three groups of Rag-hu mice. (C) The median percentage of CD4+ T cells for HIV-1-infected PD-L1 treated and untreated Rag-hu mice. (D) The median percentage of CD8+ T cells for all three groups. This data is representative of 3 experiments. Asterisks indicate statistical significance (Mann Whitney T Test).
To determine the impact of cessation of PD-L1 treatment on CD4+ T cell levels the PD-L1 treated group was followed until day 91, 63 days after the last anti-PD-L1 treatment. Surprisingly, as shown in Figure 3C the median percentage of CD4+ T cells remained elevated even 63 days after the treatment was discontinued. As seen in Figure 3C the percentage of CD4+ T cells in PD-L1 treated mice was significantly higher than in untreated Rag-hu mice at 7 (P=0.014), 14 (P=0.014), 21 (P=0.017), 28 (P=0.015) and 35 (P=0.015) days post initiation of treatment. Five HIV-1 negative Rag-hu mice were also treated with anti-PD-L1 and an increase the percentage of CD4+ T cells in the absence of HIV-1 infection was detected (data not shown) suggesting that some level of T cell exhaustion in HIV-1 uninfected Rag-hu mice exists. It is possible that an anti-xenograft response may lead to T cell exhaustion and limit the expansion of CD4+ T cells in the absence of HIV-1 infection. However, although PD-1 expression was elevated on CD8+ T cells in untreated mice, its level on CD4+ T cells was low and similar to what is seen on CD4+ T cells from uninfected humans.
The percentage of CD8+ T cells also increased in mice treated with anti-PD-L1. The median percentage of CD8+ T cells in HIV-1 infected PD-L1 treated and untreated and HIV-1 uninfected mice are shown in Figure 3D. As seen in the CD4+ subset, the percentage of CD8+ T cells also increased during the 28 day treatment period. The percentage of CD8+ T cells is significantly higher in the PD-L1 treated mice when compared with both untreated HIV-1 infected and uninfected Rag-hu mice at days 14 (P=0.001), 21 (P=0.004) and 28 (P=0.015). Although the increases were statistically significant they were not as dramatic as in the CD4+ T cell compartment. The lack of disparity may be due to the more subtle difference in PD-1 expression on CD8+ T cells from HIV-1 infected and uninfected Rag-hu mice.
Human T cell levels are inversely correlated with HIV-1 plasma viral loads
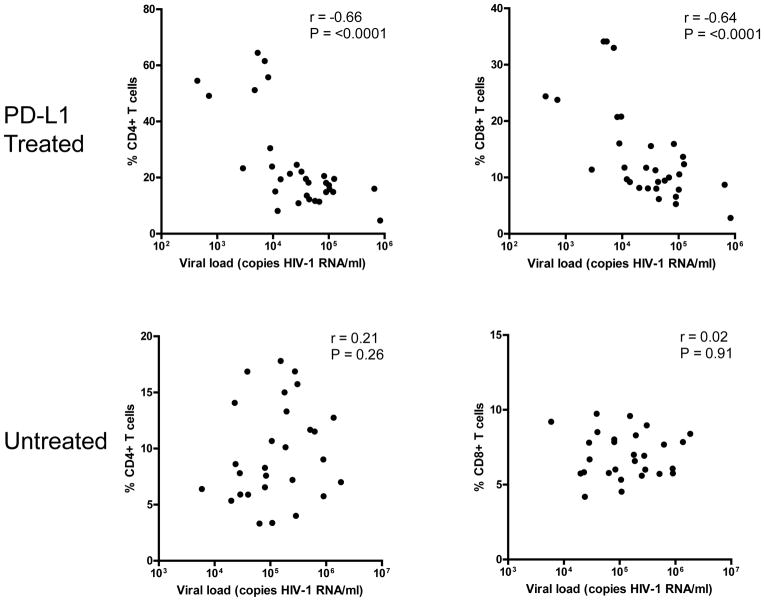
To determine if an association exists between T cells and viral replication, the percentages of CD4+ and CD8+ T cells and HIV-1 viral load were assessed. When the percentage of T cells and HIV-1 plasma viral load from all time points during the treatment period were examined, a strong inverse correlation between viral load and CD4+ (r= −0.66, P <0.0001) and CD8+ (r= −0.64, P <0.0001) T cells was found. However, when the HIV-1 viral load and CD4+ and CD8+ T cells in untreated mice were analyzed no such correlation could be found (r=0.21, P = 0.26 and r=0.02, P = 0.91 respectively) (Figure 4). Not surprisingly, this suggests that viral replication levels in untreated mice are directly correlated with the number of CD4+ T cells, implying that the more target CD4+ T cells there are to infect, the higher the plasma viral load. Overall, these data suggest that reinvigorated T cells in PD-L1 treated HIV-1 infected mice suppress HIV-1 replication.
Figure 4.
HIV-1 plasma viral load in PD-L1 treated mice is inversely correlated with the percentage of CD4+ and CD8+ T cells in treated (top) but not untreated (bottom) HIV-1-infected Rag-hu mice. Spearman correlation was performed to determine statistical significance.
PD-L1 treatment increases the percentage of naïve and central memory T cells
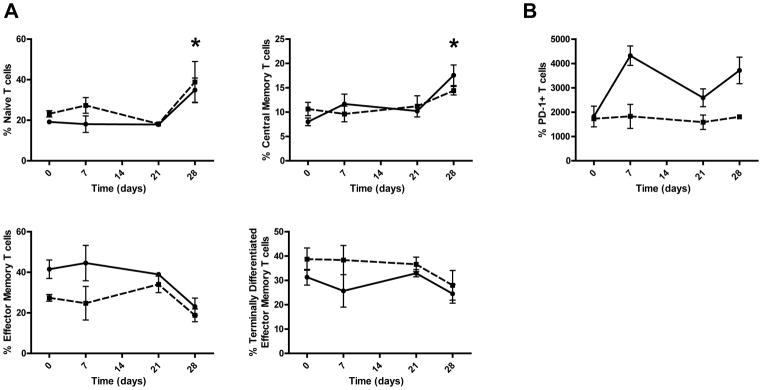
To better understand how anti-PD-L1 treatment increased CD4+ T cell counts and decreased HIV-1 plasma viral loads the expression of CD27 and CD45RA was used to determine the percentage of naïve (CD27+, CD45RA+), central memory (CD27+, CD45RA−), effector memory (CD27−, CD45RA) and terminally differentiated effector memory cells (CD27−, CD45RA+) CD4+ and CD8+ T cells. After 4 weeks of treatment the percentage of naïve CD4+ T cells increased from 19% to 35% while the naïve CD8+ T cells increased from 23% to 38% (Figure 5). In addition, there was an increase in central memory CD4+ T cells from 8% pretreatment to 17.5% 28 days post initiation of treatment. Accordingly, the central memory CD8+ T cells increased from 10% pretreatment to 14% 28 days post initiation of treatment. Conversely, effector memory CD4+ T cells decreased from 41.5 pretreatment to 23.0% 28 days post initiation of treatment as did CD8+ T cells which dropped from 27.4% to 18.7%. Terminally differentiated effector memory cells CD4+ T cells also dropped from 31.2% to 24.4% as did CD8+ T cells which went from 38.75 pretreatment to 27.9% twenty eight days post initiation of treatment. When CD4+ and CD8+ T cells are combined, naïve and central memory cells significantly elevated at 28 days compared with pretreatment (P = 0.02 and 0.03 respectively) while effector memory and terminally differentiated effector memory are treading lower (P = 0.08 and 0.3 respectively). PD-1 expression was also measured on CD4+ and CD8+ T cells before and during anti-PD-L1 treatment. PD-1 expression on CD8+ T cell stayed consistent over time while the mean fluorescence intensity of PD-1 on CD4+ T cells increased from 1,818 pretreatment to 3,714 at 28 days post initiation of treatment. These data suggest that naïve and central memory T cell expansion contributed to increasing T cell numbers and a consequent enhanced control of HIV-1 replication.
Figure 5.
Increased percentages of naïve and central memory T cells after anti-PD-L1 treatment. Blood was pooled from two groups of Rag-hu mice each engrafted from the same donor and stained with anti human CD45, CD3, CD4, CD8, CD27, CD45RA and PD-1. The percentage of naïve and memory subsets (A) and PD-1 expression (B) was determined pre and post anti-PD-L1 treatment on CD4+ T cells (solid line) and CD8+ T cells (dotted line). The asterisks indicate statistical significance (Mann Whitney T Test).
Th1 cytokines levels increase after PD-L1 treatment
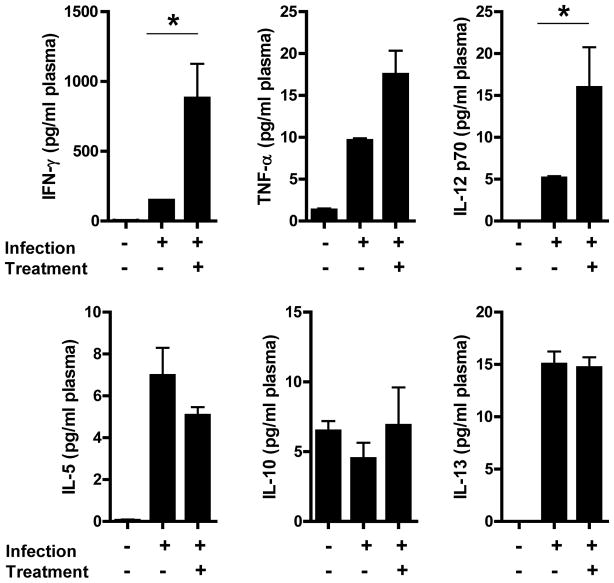
To further understand how PD-1 pathway blockade affects the course of HIV-1 infection in Raghu mice, peripheral blood was drawn from 11 mice before infection, just prior to the first dose and 7 days post the initial dose of anti-PD-L1 mab. The levels of human Th1 (IFN-γ, TNF-α, IL-12 P70) and Th2 (IL-13, IL-10, IL-5) cytokines in the plasma were measured (Figure 6). The levels of both Th1 and Th2 cytokines in uninfected mice were low to undetectable. After infection, but before anti-PD-L1 treatment, the levels of all cytokines examined were detectable and elevated in comparison with pre-infection levels, with IFN-γ being the highest. Seven days after initiation of the anti-PD-L1 treatment the level of all Th1 cytokines increased while there was little to no change in the level of Th2 cytokines. After treatment there was a 6 fold increase in the amount of IFN-γ (148 to 880 pg/ml) and 2 fold increase in TNF-α (9.6 to 17.5 pg/ml), both of which are produced by T cells (P = 0.002 and 0.002 respectively). Although there was a 3 fold increase in IL-12 (5.1 to 15.9 pg/ml), which is produced by monocytes/macrophages and dendritic cells, it was not statistically significant (P = 0.108). Conversely, there was no statistically significant change in the level of IL-10, IL-13 or IL-5 between pre and post anti-PD-L1 mab treatment. These data demonstrate that blockade of the PD-1 pathway induces the production of Th1 but not Th2 cytokines.
Figure 6.
Th1 cytokines are elevated after treatment with anti-PD-L1 mab. Plasma cytokines levels from 11 Rag-hu mice were measured. Plasma samples were obtained pre-infection with HIV-1 and directly before and 7 days after anti-PD-L1 treatment. The levels of Th1 cytokines (top) and Th2 (bottom) are shown. The asterisks indicate statistical significance between pre and post anti-PD-L1 treatment (Mann Whitney T Test).
Discussion
While the current antiretroviral therapies (ARTs) have achieved impressive suppression of HIV-1 viral loads and prolonged life, there is yet no complete cure. This goal can only be achieved by eliminating all the infected cells in the body by developing and testing novel and innovative methods such as strongly reinvigorating the impaired immune system resulting from long standing antigenemia associated with chronic viral infections. In these preliminary proof-of concept studies conducted towards this end, we have obtained strong in vivo evidence that interfering with the PD-1 pathway responsible for T cell exhaustion during chronic HIV-1 infection reduces viral loads and improves CD4+ T cell levels. The highlight of our present study is that the potential benefits of PD-1 blockade during HIV-1 infection are tested and verified in a physiologically relevant in vivo setting using a next generation humanized mouse model that mimics key aspects of chronic HIV-1 infection.
Until recently experimental studies centered on immune reconstitution and immuno-augmentation against HIV-1 have only been possible and carried out using non-human primate models infected with related viruses such as SIV/SHIV or in human clinical trials which are often expensive and time consuming. The recent advent of new mouse models that sustain continuous de novo multilineage human hematopoiesis have opened up many possibilities for in vivo experimentation. For example, these new mouse models have been used to evaluate HIV-1 gene therapy strategies (21), antiretroviral drugs (22, 23), topical microbiocides (24, 25), oral PrEP strategies (26), HIV-1 immune responses (27), anti-HIV-1 siRNAs (28, 29) and the dynamics of mucosal transmission (17). However, to date no studies examining the efficacy of immunomodulatory treatments involving receptor blockade have been performed using humanized mice.
Mounting evidence incriminated T cell exhaustion during chronic viral (HIV-1) infection as one of the mechanisms for the lack of an effective immune response and elimination of infected cells (30–35). Recent work from our group (7, 36) and others (6, 8) suggests that inhibitory pathways such as PD-1 play a major role in reducing the function of HIV-1-specific T cells. Thus, manipulation of these inhibitory pathways by blocking the binding of the receptor on the surface of T cells to it’s ligand on antigen presenting cells could possibly be used to reinvigorate virus-specific T cell immunity during chronic infection. To date, all the previous studies that have examined the effects of blocking inhibitory receptor on HIV-1 specific T cells have been done in vitro. These in vitro studies clearly show that blockade of the PD-1 pathway increases proliferation and cytokine production. However, the effect of PD-1/PD-L1 pathway blockade on HIV-1 replication and disease progression is unknown. In comparative studies using the LCMV mouse model (30) and SIV macaque model (9) of chronic infection it has been shown that blockade of PD-1 binding decreases viral replication.
In the current in vivo study we determined the effects of administering a human anti-PD-L1 mab, which blocks the PD-1 pathway, on HIV-1 disease progression. A clinically relevant, human anti-PD-L1 mab (obtained from Bristol-Myers Squibb) which is currently being used in human clinical trials for melanoma and various hematologic malignancies is tested in our studies (18). We first established that CD4+ and CD8+ T cells from HIV-1 infected Rag-hu mice had elevated expression of PD-1. Our results are consistent with previous studies that used a humanized BLT mouse model in which it was shown that HIV-1-specific T cells during chronic HIV-1 infection exhibited elevated PD-1 expression (27). Exploiting this feature for testing, here we demonstrate for the first time that PD-L1 mab treatment reduced HIV-1 replication and increased the percentage of CD4+ T cells compared to pre-treatment values and in untreated HIV-1 infected Rag-hu mice. Interestingly, anti-PD-L1 treatment increased the percentage of naïve and central memory T cells. Increased naïve T cells are a marker of increased thymic output and are important for immune reconstitution after ART, and thus, it is likely that they fuel the expansion of CD4+ T cells after treatment with anti-PD-L1 (37, 38). Central memory cells have also been shown to be an early indicator of immune reconstitution during ART (38, 39). We also found a strong and statistically significant correlation between the percentage of CD4+ T cells and HIV-1 plasma viral loads in treated but not untreated Rag-hu mice. Reduction in HIV-1 plasma viral loads was dramatic in PD-L1 antibody treated mice. In mice showing the highest viral suppression, HIV-1 plasma viral loads decreased from over a million to less than a thousand copies of HIV-1 RNA/ml. The mean reduction level for the 7 HIV-1 infected treated mice was 269 fold. No such decrease in viral loads was seen in infected-untreated mice indicating that the decrease in HIV-1 replication is due to the blockade of the PD-1 pathway by the anti-PD-L1 mab.
Rag-hu mice as noted above support multilineage hematopoiesis with continuous generation of human T cells which mature in the mouse thymus. With regard to their immune-competence, Traggiai et. al. demonstrated that the human TCR Vβ repertoire in Rag-hu mice was normal and that these T cells were able to respond to Epstein Barr Virus in an HLA dependant manner (11). Furthermore, other investigators were also able to detect HIV-1-specific T cell responses using intracellular cytokine staining assays in pooled cells from the spleen and lymph node of infected mice (Lishan Su, Personal Communication). Our present observation that HIV-1 plasma viral loads decreased dramatically after anti-PD-L1 mab treatment suggests that reinvigorated HIV-1-specific T cell responses are exerting pressure on HIV-1 replication in vivo. In addition, when the antibody treatment was discontinued HIV-1 plasma viral loads rebounded, suggesting that PD-1 blockade was responsible for augmented HIV-1-specific T cell function resulting in control of HIV-1 replication. In addition, when the antibody treatment was discontinued HIV-1 plasma viral loads rebounded, suggesting that PD-1 blockade was responsible for augmented HIV-1-specific T cell function resulting in control of HIV-1 replication. Taken together, these data suggest that Rag-hu mice treated with anti-PD-L1 antibody mount effective HIV-1-specific T cell responses that suppress HIV-1 replication.
Control of HIV-1 replication in humans has been associated with a strong Th1 response (40, 41). This is the case in HIV-1 infected Raghu mice as well. Th1 cytokines but not Th2 cytokines were significantly elevated after the initiation of the anti-PD-L1 treatment. IFN-γ and TNF-α were elevated 7 days after the initiation of treatment and before an increase in T cell numbers demonstrating that anti-PD-L1 treatment enhanced Th1 cytokine production of anti-viral T cells. Interestingly, IL-12p70 levels were also elevated suggesting that the PD-1 blockade also induced Th1 promoting cytokine production by dendritic cells and monocytes/macrophages. Furthermore, an increase in central memory and decrease in effector memory CD4+ and CD8+ T cells was detected after treatment. Central memory CD8+ T cells have been shown to better control chronic LCMV infection in mice than effector memory because of their superior capacity to expand (42). Preservation of the central memory compartment has also been associated with long term control of HIV-1 and SIV replication (43, 44).
In the control infected-untreated mice, the high HIV-1 viral loads are indicative of establishment of the infection and the presence of available cell targets (CD4+ T cells) for infection. In the PD-L1 mab treated mice, while the percentage of CD4+ T cells increased significantly during the treatment period (thus providing more potential target cells for infection) the viral load actually decreased. In fact, in mice treated with the anti-PD-L1 mab the percentage of CD4+ T cells showed an inverse correlation with viral load. No such correlation existed in untreated Rag-hu mice. Although not statistically significant, there was a direct correlation between HIV-1 plasma viral load and CD4+ T cell count suggesting that higher percentages of CD4+ T cells in these mice provided more targets for infection which resulted in higher HIV-1 plasma viral loads as alluded to above. Not surprisingly, increased percentages of cytotoxic CD8+ T cells were inversely correlated with HIV-1 plasma viral load in PD-L1 treated but not untreated mice. Therefore, these data collectively suggest that re-energized HIV-1-specific T cells are responsible for subduing HIV-1 replication in Rag-hu mice when the PD-1/PD-L1 pathway is blocked.
In summary, this study shows for the first time that blockade of the PD-1 pathway reduces HIV-1 replication in vivo. No overt adverse effects were noted in these mice due to PD-L1 mab treatment, even when treated every 3 days for 4 weeks. These results also suggest that Rag-hu mice provide a good model to evaluate the immunomodulatory effects of receptor blockade in vivo on human cells and its effect on the progression of a chronic human viral disease. With regard to the clinical application of the antibody, this study opens up many new possibilities to answer important questions such as how long the therapeutic effect can be sustained and if antibodies to other co-inhibitory molecules like CTLA-4 will have additive therapeutic benefits.
Acknowledgments
This work was supported by NIH Grants R21AIO76161 (BEP), R21AIO76161-01A2S1 (BEP) and RO1AI073255 (RA).
References
- 1.Palmer BE, Boritz E, Blyveis N, Wilson CC. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1- specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J Virol. 2002;76:5925–5936. doi: 10.1128/JVI.76.12.5925-5936.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. Journal of Experimental Medicine. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, Connors M. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:10611068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 6.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, Depierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 8.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8(+) T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 9.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berges BK, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 8:65–83. doi: 10.1186/1742-4690-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 12.Shultz LD, Brehm MA, Bavari S, Greiner DL. Humanized mice as a preclinical tool for infectious disease and biomedical research. Ann N Y Acad Sci. 1245:50–54. doi: 10.1111/j.1749-6632.2011.06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 14.Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gamma c−/−(RAG-hu) mouse model. Retrovirology. 2006;3:76–89. doi: 10.1186/1742-4690-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, Dewan MZ, Yu Z, Ito M, Morio T, Shimizu N, Honda M, Yamamoto N. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–218. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- 16.Berges BK, Akkina SR, Remling L, Akkina R. Humanized Rag2(−/−)gammac(−/−) (RAG-hu) mice can sustain long-term chronic HIV-1 infection lasting more than a year. Virology. 397:100–103. doi: 10.1016/j.virol.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berges BK, Akkina SR, Folkvord JM, Connick E, Akkina R. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2−/− gammac −/− (RAG-hu) mice. Virology. 2008;373:342–351. doi: 10.1016/j.virol.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akkina R, Berges BK, Palmer BE, Remling L, Neff CP, Kuruvilla J, Connick E, Folkvord J, Gagliardi K, Kassu A, Akkina SR. Humanized Rag1−/− gammac−/− mice support multilineage hematopoiesis and are susceptible to HIV-1 infection via systemic and vaginal routes. PLoS One. 6:e20169. doi: 10.1371/journal.pone.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foreman O, Kavirayani AM, Griffey SM, Reader R, Shultz LD. Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Vet Pathol. 48:495–499. doi: 10.1177/0300985810378282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph A, Zheng JH, Chen K, Dutta M, Chen C, Stiegler G, Kunert R, Follenzi A, Goldstein H. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol. 84:6645–6653. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, Powell DA, Payne D, Haase AT, Garcia JV. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhary SK, Rezk NL, Ince WL, Cheema M, Zhang L, Su L, Swanstrom R, Kashuba AD, Margolis DM. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/−{gamma}c−/− mouse. J Virol. 2009;83:8254–8258. doi: 10.1128/JVI.00580-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neff CP, Kurisu T, Ndolo T, Fox K, Akkina R. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS One. 6:e20209. doi: 10.1371/journal.pone.0020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denton PW, Othieno F, Martinez-Torres F, Zou W, Krisko JF, Fleming E, Zein S, Powell DA, Wahl A, Kwak YT, Welch BD, Kay MS, Payne DA, Gallay P, Appella E, Estes JD, Lu M, Garcia JV. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol. 85:7582–7593. doi: 10.1128/JVI.00537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neff CP, Ndolo T, Tandon A, Habu Y, Akkina R. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS One. 5:e15257. doi: 10.1371/journal.pone.0015257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, Tager AM. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83:7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, Smith DD, Swiderski P, Rossi JJ, Akkina R. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Sci Transl Med. 3:66ra6. doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang YG, Jeong JH, Lee KY, Kim YH, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee SK, Shankar P. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 31.Porichis F, Kaufmann DE. Role of PD-1 in HIV Pathogenesis and as Target for Therapy. Curr HIV/AIDS Rep. 9:81–90. doi: 10.1007/s11904-011-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, Pavlik DF, Rodriguez-Garcia M, Pereyra F, Freeman GJ, Kavanagh DG, Kaufmann DE. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 118:965–974. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 120:4546–4457. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golden-Mason L, Klarquist J, Wahed AS, Rosen HR. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol. 2008;180:3637–3641. doi: 10.4049/jimmunol.180.6.3637. [DOI] [PubMed] [Google Scholar]
- 35.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, Palmer BE. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol. 2010;185:3007–3018. doi: 10.4049/jimmunol.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schacker TW, Bosch RJ, Bennett K, Pollard R, Robbins GK, Collier AC, Gulick RM, Spritzler J, Mildvan D. Measurement of naive CD4 cells reliably predicts potential for immune reconstitution in HIV. J Acquir Immune Defic Syndr. 54:59–62. doi: 10.1097/QAI.0b013e3181c96520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz-Calleja C, Costantini A, Silvestri G, Butini L, Regnery CM, Mancini S, Montroni M. Highly active antiretroviral therapy induces specific changes in effector and central memory T cell sub-populations. Aids. 2001;15:1887–1897. doi: 10.1097/00002030-200109280-00022. [DOI] [PubMed] [Google Scholar]
- 39.Hua W, Jiao Y, Zhang H, Zhang T, Chen D, Zhang Y, Chen X, Wu H. Central memory CD4 cells are an early indicator of immune reconstitution in HIV/AIDS patients with anti-retroviral treatment. Immunol Invest. 41:1–14. doi: 10.3109/08820139.2011.576739. [DOI] [PubMed] [Google Scholar]
- 40.Clerici M, Balotta C, Meroni L, Ferrario E, Riva C, Trabattoni D, Ridolfo A, Villa M, Shearer GM, Moroni M, Galli M. Type 1 cytokine production and low prevalence of viral isolation correlate with long-term nonprogression in HIV infection. AIDS Res Hum Retroviruses. 1996;12:1053–1061. doi: 10.1089/aid.1996.12.1053. [DOI] [PubMed] [Google Scholar]
- 41.Vingert B, Benati D, Lambotte O, de Truchis P, Slama L, Jeannin P, Galperin M, Perez-Patrigeon S, Boufassa F, Kwok WW, Lemaitre F, Delfraissy JF, Theze J, Chakrabarti LA. HIV Controllers Maintain a Population of Highly Efficient Th1 Effector Cells in Contrast to Patients Treated in the Long Term. J Virol. 86:10661–10674. doi: 10.1128/JVI.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 43.Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, Colle JH, Urrutia A, Scott-Algara D, Boufassa F, Delfraissy JF, Theze J, Venet A, Chakrabarti LA. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol. 2007;81:13904–13915. doi: 10.1128/JVI.01401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawada M, Tsukamoto T, Yamamoto H, Takeda A, Igarashi H, Watkins DI, Matano T. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J Virol. 2007;81:5202–5211. doi: 10.1128/JVI.02881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]