Abstract
Objective
To demonstrate proof-of-principle measurement for physiological change within an active myofascial trigger point (MTrP) undergoing trigger point release (ischemic compression).
Design
Interstitial fluid was sampled continuously at a trigger point before and after intervention.
Setting
A biomedical research clinic at a university hospital.
Participants
Two subjects from a pain clinic presenting with chronic headache pain.
Interventions
A single microdialysis catheter was inserted into an active MTrP of the upper trapezius to allow for continuous sampling of interstitial fluid before and after application of trigger point therapy by a massage therapist.
Main Outcome Measures
Procedural success, pain tolerance, feasibility of intervention during sample collection, determination of physiologically relevant values for local blood flow, as well as glucose and lactate concentrations.
Results
Both patients tolerated the microdialysis probe insertion into the MTrP and treatment intervention without complication. Glucose and lactate concentrations were measured in the physiological range. Following intervention, a sustained increase in lactate was noted for both subjects.
Conclusions
Identifying physiological constituents of MTrP’s following intervention is an important step toward understanding pathophysiology and resolution of myofascial pain. The present study forwards that aim by showing proof-of-concept for collection of interstitial fluid from an MTrP before and after intervention can be accomplished using microdialysis, thus providing methodological insight toward treatment mechanism and pain resolution. Of the biomarkers measured in this study, lactate may be the most relevant for detection and treatment of abnormalities in the MTrP.
Keywords: Massage, Headache, Myofascial pain, Complementary Medicine, Microdialysis, Lactate
The myofascial trigger point (MTrP) within skeletal muscle is an area of great interest within the study of myofascial pain syndrome (MPS). Recent investigations in tension-type headache have found active MTrP’s to exhibit a referral pain pattern in alignment with headache location 1, are of increased presence in headache populations2, and exhibit elevated local and referred pain compared to non-headache patients.3 Physiologically, the active MTrP also expresses disruption in biochemical activity. In work pioneered by Shah and colleagues, the interstitial milieu of active, latent and non-trigger point regions was assessed using a microanalytical procedure to sample microliter volumes of interstitial fluid.4, 5 Their work identified differences in the concentrations of inflammatory mediators, neuropeptides, catecholamines, and cytokines in the local biochemical milieu of subjects with active MTrPs versus latent MTrPs or without MTrPs in the upper trapezius muscle. While in-roads to the physiology of an MTrP have been made, what has not yet been shown is whether interstitial fluid sampling can successfully be conducted surrounding a treatment intervention.
Reduction in local and referred pain at an MTrP have been shown using techniques common to physical therapy. Massage techniques applied at MTrPs increase pain-pressure threshold locally and reduced referred pain fields, suggestive of physiological change at the MTrP as well as pain reducing effect. Trigger point release (formerly ischemic compression) is a massage technique that consists of moderate compression, typically applied with the fingers, to the MTrP. Application of this technique to an MTrP results in a positive effect on pain-pressure threshold.6 Physiology at the MTrP following intervention has not been reported, but is important to corroborate subjective reports of reduced pain sensitivity, to impart credibility to treatment techniques, and to provide insight into a mechanism of action.
In the present communication we describe use of microdialysis to sample the interstitial fluid of an active MTrP before and after trigger point release in two subjects with chronic headache. This study was undertaken to 1) determine feasibility of trigger point release at an active MTrP during interstitial fluid collection, and 2) to demonstrate proof-of-principle for physiological change of blood flow and carbohydrate metabolites within the active MTrP following trigger point release.
METHODS
Study procedures were approved by the Colorado Multiple Institutional Review Board at the University of Colorado at Denver.
Subjects
Two patients from the Pain Medicine Clinic at the University of Colorado Hospital were consented and enrolled in the study. The primary inclusion criterion was identification of an active myofascial trigger point in the upper trapezius muscle that reproduced a frequent headache complaint by the subject. Subjects reported to the study following breakfast, but did not eat during the study period. Subject #1 was a 51 year old male who presented with peripheral neuropathy with myalgia characterized by frequent headaches, degenerative disc disorder and fibromyalgia. Subject #2 was a 44 year old female diagnosed with thoracic neuritis, migraine, fibromyalgia, and depression. Three months prior to study Subject #2 received 1cc injection of 0.5% bivipocaine into trigger points of the right supraspinatus, bilateral paraspinatus, bilaterally around the iliac crest; no injection into the upper trapezius was performed. Physical therapy with massage was prescribed, but not undertaken before study participation. Exclusion criteria included self-report of cluster headache, injury-related onset of headache, cardiovascular disease, diabetes, and known bleeding disorder or consumption of anti-coagulants.
Experimental design
Trigger point identification
An active myofascial trigger point was identified in the upper trapezius by the massage therapist and confirmed by the study physician7. Properties that identified the trigger point were a nodule associated with a taut band within the muscle, which elicited pain locally and referred pain upon palpation into the head that reproduced the patient’s typical headache.
Skin surface preparation
The skin surface above the trigger point was cleansed with isopropyl alcohol. A small volume of the short-acting anesthetic 2-chloroprocaine was injected into the dermal layer to minimize discomfort associated with microdialysis probe insertion; care was taken to ensure the needle did not pierce the upper trapezius muscle. 2-chloroprocain is rapidly metabolized (two minute half-life) in circulation making it unlikely that active compound could exert an anesthetizing effect on the MTrP through the circulatory system.
Microdialysis probe and placement
A CMA 20 microdialysis loop probe (10mm, 20kDa cutoff dialysis membrane, CMA Microdialysis, Solana, Sweden) was perfused at a flow rate of 5 μL min−1 with sterile Ringers solution supplemented with 10mM ethanol. Ethanol was added to detect qualitative changes in local nutritive blood flow (microvascular exchange) in the region surrounding the probe.8 The probe was disconnected from the perfusion pump and inserted into the identified MTrP under sterile conditions by the study physician (AB). The probe was slowly advanced with muscle penetration confirmed by increased tissue resistance; MTrP penetration was confirmed by observation of a local twitch response. The probe was then re-connected to the perfusion pump and the flow rate was set to 2 μL min−1. Sterile Tegaderm film was used to secure the probe to the skin surface.
Interstitial fluid sampling
Sample was discarded during the first 60 minutes following probe insertion to establish equilibrium of the microdialysis system and allow for dissipation of any trauma. Dialysate coming from the exit tubing of the microdialysis probe was collected continuously in sterile vials in 20-minute fractions. Three 20-minute samples were collected to establish baseline. Trigger point release was then administered with dialysate collected for another three 20-minute intervals.
Trigger point release
Intervention consisted of compression at the trigger point administered by a massage therapist with training in this technique. Up to five compressions of sufficient pressure to just elicit referred pain were applied at the trigger point using pincer grip. Duration for each compression was until the therapist detected a softening of the trigger point nodule, loss of referred pain phenomenon, or a maximum of 60 seconds. A 10 second rest was given between compressions to allow blood re-perfusion into the treatment site. Total duration of the intervention was approximately 6 minutes.
Sample processing
Dialysate samples were kept on ice during the procedures and kept at 4°C until processed for ethanol concentration. Ethanol in the dialysates was measured fluorometrically.9 The ethanol data were expressed as a ratio of the ethanol concentration in the outflowing dialysate and the inflowing perfusate solutions (ethanol outflow/inflow ratio), which is inversely related to blood flow. Dialysate samples were then frozen at −80°C and sent on dry ice to East Carolina University (Greenville, NC) for spectrophotometric measurement of glucose and lactate concentrations using an automated CMA600 Microdialysate Analyzer.a
RESULTS
Subjects reported either no or minimal pain sensation upon probe insertion into the MTrP following anesthetization of the skin. A local twitch response was observed by the MD upon needle insertion into MTrP for each subject confirming placement within the MTrP. Subject #2 reported some soreness at the treatment site at the end of the procedure (3 on a 0-10 scale). Trigger point release technique did not noticeably alter dialysate flow rate, volume collected, or sample appearance. Subject 1 received five ischemic compressions before resolution of the trigger point nodule; subject 2 received four repetitions. No sensation of the probe was detected by the massage therapist or subjects during the intervention. Disruption in the probe placement was not observed at study conclusion.
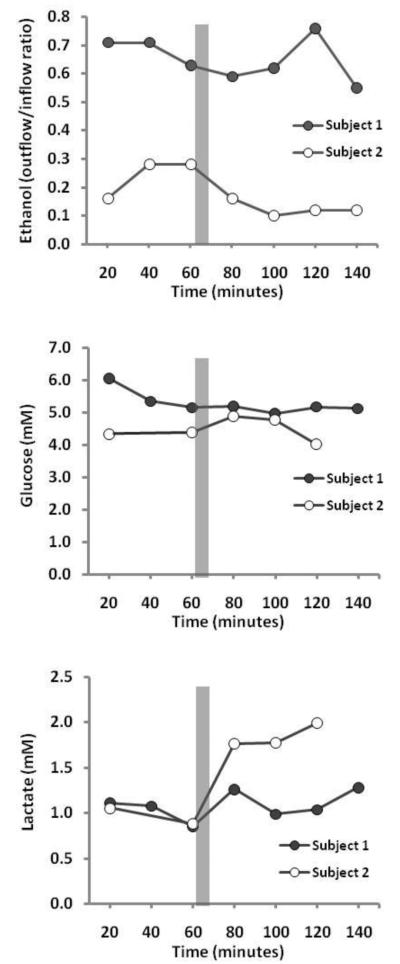
The physiological response to intervention at the MTrP yielded change in parameters for both subjects following intervention, with subject #2 exhibiting a more dramatic effect (figure 1). Blood flow, as measured by reduction in ethanol outflow/inflow ratio, increased during the 20-minute measurement immediately following intervention. This effect persisted for the remainder of the study for subject #2, but was more erratic for subject #1. Dialysate glucose concentration at the probe increased by 0.50mM immediately post-intervention for subject #2; minimal changes in glucose were observed for subject #1 (figure 1). Dialysate lactate concentration increased for both subjects at the MTrP following trigger point release, but was most dramatic for subject #2 where concentrations doubled from 0.88mM to 1.77mM and remained at that level for the duration of the study (figure 1). Due to technical problems, data for dialysate glucose and lactate from the 40-minute timepoint for subject 2 is missing.
Figure 1.
Blood flow as well as dialysate glucose and lactate concentrations within an MTrP across the study timeframe are presented. Blood flow is expressed as a ratio of the ethanol concentration in the outflowing dialysate and the inflowing perfusate solutions (ethanol outflow/inflow ratio), which is inversely related to blood flow. Grey bars indicate time when trigger point release was applied to the MTrP.
DISCUSSION
MTrP’s are sites associated with pain in MPS and are of great interest to researchers and clinicians. Multiple techniques to reduce trigger point pain have been tested, but have relied on subjective reporting to assess effectiveness10. Recently, sampling of the interstitial fluid at an MTrP has been achieved, yielding fascinating insight into the biochemical milieu of active and latent trigger points, suggestive of elevation in mediators of pain and inflammation.4 To date, no studies have reported on metabolic variables at an MTrP or changes in biochemical makeup at a trigger point following intervention. The data from the present case pair demonstrate that continuous sampling of interstitial fluid at an active MTrP is attainable throughout trigger point release (ischemic compression). Continuous sampling, as opposed to multi-site sampling, ensured that observed changes in measures resulted from alterations in local conditions rather than artifact from probe positioning or basal differences among MTrP’s. Interstitial glucose and lactate concentrations from the present study are within an expected physiological range for the upper trapezius muscle, conferring legitimacy to the findings.11-14
The present study provides preliminary findings that suggest changes in cellular metabolism occur abruptly and remain elevated within an active MTrP following trigger point release. Dialysate lactate, and to a lesser extent dialysate glucose, concentration increased in the MTrP in the 20 minutes following trigger point release Local nutritive blood flow (microvascular exchange) was similarly increased. Both lactate and blood flow remained elevated throughout the course of the study. Had the probe been incorrectly placed and/or trigger point release been administered to healthy tissue it is likely that any observed changes would quickly return to baseline following intervention due to the dynamic nature of healthy tissue.
The increase in local blood flow could account for the observed increase in dialysate glucose following trigger point release because changes in dialysate glucose can be directly related to changes in local blood flow.9 However, increased blood flow is unlikely to account for the increase in dialysate lactate because lactate is produced by the muscle, released into the interstitial space and subsequently removed from the area by local blood flow. An increased blood flow would therefore be expected to remove lactate from the interstitium, thereby lowering dialysate lactate. Furthermore, lactate is produced from incomplete oxidation of glucose due to insufficient oxygen availability: interstitial and dialysate lactate would therefore not be directly increased by an increase in nutritive blood flow. Rather, the increased availability of glucose, likely from increased blood flow, provides increased substrate availability to muscle; glucose is metabolized to lactate via glycolysis increasing lactate concentration until oxidative systems (aerobic respiration) are re-established that can fully oxidize glucose and lactate. In keeping with the integrated hypothesis of a trigger point proposed by Simons, the zone around a MTrP is in an ischemic state resulting in a shortage of glucose and oxygen for metabolism.15 The current data support this hypothesis, in that upon relaxation of the trigger point nodule, nutritive blood flow to the tissue is enabled, allowing for increased substrate perfusion and oxygen delivery to skeletal muscle to meet cellular energy demands required to regain homeostasis.
In the present study we used anesthetic injected into the dermal layer to thwart pain associated with microdialysis probe insertion and placement. The use of anesthetic is controversial given the potential effect these agents have on MTrP’s 16, but is currently unavoidable for management of pain associated with correct microdialysis probe insertion. Shah et al were able to avoid anesthetic use owning to the small gauge needle (30 gauge) used in their studies4, 5; unfortunately, expertise required for that microdialysis technique is no longer available (personal communication, J.P. Shah). Both subjects tolerated the procedure used in the present study and reported minimal pain with anesthetic only injected into the dermal layer. In our opinion, it is unlikely that the anesthetic used could drain into the MTrP or reach it through general circulation and exert a confounding effect on biochemistry of the MTrP.
Study Limitations
While the present study was limited by its exploratory nature, several other aspects are worth noting. Assurance of probe placement into the MTrP was made by observation of a local twitch response by a medical doctor with extensive experience in trigger point injections. Recently, strides have been made to visualize MTrP’s using ultrasound 17, 18, which could potentially provide confirmation of probe placement. Along that same line, the microdialysis probe length used in the present study was 10mm, whereas trigger point diameters have been reported to be 3-4mm 18, thus sampling likely included interstitial fluid from regions outside of the MTrP. Lastly, assurance of trigger point release from treatment was made by the massage therapist sensing a softening of the trigger point nodule; additional verification of trigger point improvement, such as through algometry or visual analog scales, would be important to confirm effective treatment.
CONCLUSION
The findings from this brief investigation are intriguing for future research on MTrP’s and MPS. We found that upon MTrP release, dialysate lactate concentration and blood flow increased at the MTrP. Although more robust subject numbers are needed to confirm the current findings, this study opens the possibility for investigations of cellular metabolism and blood flow within the myofascial trigger point before, during, and following interventions designed to reduce MTrP contracture and pain.
Acknowledgments
The authors would like to thank Ann Mathews, CMT for providing the massage intervention, Rachel Van Pelt, PhD and Wendee Gozansky, MD for technical assistance, and the staff of the General Clinical Research Center at the University of Colorado Hospital for their assistance in conducting this study. This study was supported by a grant from the School of Nursing at the University of Colorado at Denver, and by the CCTSI, NIH Grant Number UL1 TR000154. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suppliers
Harvard Apparatus, 84 October Hill Road, Holliston, Massachusetts 01746.
Contributor Information
Albert F. Moraska, College of Nursing, University of Colorado Anschutz Medical Campus, Aurora, Colorado.
Robert C. Hickner, Departments of Kinesiology and Physiology, East Carolina University, Greenville, North Carolina.
Wendy M. Kohrt, Department of Medicine, Division of Geriatric Medicine, University of Colorado Anschutz Medical Campus, Aurora, Colorado.
Alan Brewer, Pain Medicine Clinic, University of Colorado Hospital, Denver, Colorado.
References
- 1.Alonso-Blanco C, Fernandez-de-las-Penas C, Fernandez-Mayoralas DM, de-la-Llave-Rincon AI, Pareja JA, Svensson P. Prevalence and anatomical localization of muscle referred pain from active trigger points in head and neck musculature in adults and children with chronic tension-type headache. Pain Med. 2011;12(10):1453–63. doi: 10.1111/j.1526-4637.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- 2.Couppe C, Torelli P, Fuglsang-Frederiksen A, Andersen KV, Jensen R. Myofascial trigger points are very prevalent in patients with chronic tension-type headache: a double-blinded controlled study. Clin J Pain. 2007;23(1):23–7. doi: 10.1097/01.ajp.0000210946.34676.7d. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-de-Las-Penas C, Ge HY, Arendt-Nielsen L, Cuadrado ML, Pareja JA. Referred pain from trapezius muscle trigger points shares similar characteristics with chronic tension type headache. Eur J Pain. 2007;11(4):475–82. doi: 10.1016/j.ejpain.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99(5):1977–84. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 6.Kostopoulos D, Nelson J, Arthur J, Ingber RS, Larkin RW. Reduction of spontaneous electrical activity and pain perception of trigger points in the upper trapezius muscle through trigger point compression and passive stretching. J Musculoskelet Pain. 2008;16(4):266–78. [Google Scholar]
- 7.Sciotti VM, Mittak VL, DiMarco L, Ford LM, Plezbert J, Santipadri E, et al. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain. 2001;93(3):259–66. doi: 10.1016/S0304-3959(01)00325-6. [DOI] [PubMed] [Google Scholar]
- 8.Hickner RC, Ekelund U, Mellander S, Ungerstedt U, Henriksson J. Muscle blood flow in cats: comparison of microdialysis ethanol technique with direct measurement. J Appl Physiol. 1995;79(2):638–47. doi: 10.1152/jappl.1995.79.2.638. [DOI] [PubMed] [Google Scholar]
- 9.Hickner RC, Rosdahl H, Borg I, Ungerstedt U, Jorfeldt L, Henriksson J. The ethanol technique of monitoring local blood flow changes in rat skeletal muscle: implications for microdialysis. Acta Physiol Scand. 1992;146(1):87–97. doi: 10.1111/j.1748-1716.1992.tb09396.x. [DOI] [PubMed] [Google Scholar]
- 10.Cummings M, Baldry P. Regional myofascial pain: diagnosis and management. Best Pract Res Clin Rheumatol. 2007;21(2):367–87. doi: 10.1016/j.berh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Ashina M, Stallknecht B, Bendtsen L, Pedersen JF, Galbo H, Dalgaard P, et al. In vivo evidence of altered skeletal muscle blood flow in chronic tension-type headache. Brain. 2002;125(Pt 2):320–6. doi: 10.1093/brain/awf029. [DOI] [PubMed] [Google Scholar]
- 12.Ashina M, Stallknecht B, Bendtsen L, Pedersen JF, Schifter S, Galbo H, et al. Tender points are not sites of ongoing inflammation-in vivo evidence in patients with chronic tension-type headache. Cephalalgia. 2003;23(2):109–16. doi: 10.1046/j.1468-2982.2003.00520.x. [DOI] [PubMed] [Google Scholar]
- 13.McIver KL, Evans C, Kraus RM, Ispas L, Sciotti VM, Hickner RC. NO-mediated alterations in skeletal muscle nutritive blood flow and lactate metabolism in fibromyalgia. Pain. 2006;120(1-2):161–9. doi: 10.1016/j.pain.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Rosendal L, Blangsted AK, Kristiansen J, Sogaard K, Langberg H, Sjogaard G, et al. Interstitial muscle lactate, pyruvate and potassium dynamics in the trapezius muscle during repetitive low-force arm movements, measured with microdialysis. Acta Physiol Scand. 2004;182(4):379–88. doi: 10.1111/j.1365-201X.2004.01356.x. [DOI] [PubMed] [Google Scholar]
- 15.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14(1):95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil. 1994;73(4):256–63. doi: 10.1097/00002060-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Sikdar S, Shah JP, Gebreab T, Yen RH, Gilliams E, Danoff J, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90(11):1829–38. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011;30(10):1331–40. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]