Abstract
GATA-3, a C2C2 type zinc finger transcription factor, regulates many steps of T cell development and differentiation. It is also required for optimal production of type 2 cytokines by CD8+ T cells. However, its role in the development and function of this subset of T cells is still poorly characterized. Here we report that GATA-3 is required for MHC-mediated positive selection and final maturation of CD8 single positive thymocytes. Deficiency of GATA-3 mediated by a CD4cre transgene led to age-dependent lymphadenopathy partly due to abnormal expansion of CD8+ T cells driven by a cell-extrinsic mechanism. Paradoxically, GATA-3-deficient CD8+ T cells were hyporesponsive to antigen stimulation due to a defect in the maintenance/progression, but not initiation, of activation signals. More importantly, GATA-3-deficient CD8+ T cells were less efficient in killing antigen-bearing tumor cells in vivo. Taken together, our data further expand the role of GATA-3 in T cells.
Introduction
GATA-3, the third member of the C2C2 type zinc finger GATA family of transcription factors, was first cloned by its ability to bind a T cell-specific enhancer located in the TCR α gene (1, 2). Although GATA-3 is expressed in several types of cells, its expression is limited to cells of T lineage among hematopoietic cells (3). During the development of thymocytes, the expression of GATA-3 is induced after β-selection and MHC-mediated positive selection (4). The level of GATA-3 remains high in CD4 single positive thymocytes but is substantially reduced in CD8 single positive thymocytes. Among the functional subsets of peripheral Th cells, GATA-3 is highly enriched in Th2 cells and its expression has been used to define this subset of Th cells.
The function of GATA-3 in the development and differentiation of thymocytes and Th cells have been well characterized. GATA-3-deficient (G3KO) ES cells or hematopoietic stem cells failed to contribute to the T lineage in RAG-deficient or irradiated mice (5, 6), indicating that GATA-3 is indispensable for the thymic seeding or differentiation of early thymic progenitors. In the presence of Notch signals, the level of GATA-3 has to be “just right” in order to ensure the survival of DN1 and DN2 cells (7). Deficiency of GATA-3 at late DN2 stage results in a profound block in β-selection due to impaired production, but not re-arrangement of TCRβ chain (8). Deficiency of GATA-3 at early DP stage, however, resulted in a virtual absence of CD4SP thymocytes despite a near normal number of CD8SP cells (8, 9). How GATA-3 regulates the development of CD4SP is still largely unknown. Early TCR signaling cascades were apparently intact in GATA-3-deficient DP cells. Examination of positive selection of DP cells with various MHC class II-restricted TCR transgene found either no or only a modest defect in the absence of GATA-3. It has been shown that GATA-3 directly regulates the expression of Th-POK (9), a transcription factor that is also essential for the development of CD4SP cells (10, 11). However, a Th-POK transgene was unable to rescue the development of GATA-deficient CD4SP cells.
GATA-3 is also a critical transcription factor of Th2 cells (12, 13). It is preferentially expressed in Th2 cells compared to Th1 cells (14). Its expression in naive Th cells is induced by IL-4/Stat6 (15, 16), IL-2/Stat5 (17), and Notch signal pathways (18), all of which are important for the differentiation of Th2 cells. It can function as a chromatin-remodeling factor of the IL-4/IL-5/IL-13 locus as well as a direct transcription factor of IL-5 and IL-13 genes. It can also inhibit the activity of Stat4 and Runx3, thereby inhibiting Th1 transcription program (19). In agreement with these in vitro data, mice rendered deficient in GATA-3 only in mature T cells were unable to mount type 2 Th immune responses and were highly susceptible to parasitic infection (12, 13).
GATA-3 is expressed, albeit at a low level, in CD8SP thymocytes and CD8+ peripheral T cells (4, 20). Although deficiency of GATA-3 had little impact on the absolute number of polyclonal CD8SP thymocytes, GATA-3-deficient CD8+ peripheral T cells also displayed impaired production of type 2 cytokines even under optimal culture conditions (12). Despite these observations, the role of GATA-3 in regulating the development, homeostasis, activation, and effector function of CD8+ T cells is still poorly characterized.
In this report, we studied mice (G3KO), in which the deletion of GATA-3 was mediated by a CD4cre transgene. We show that G3KO CD8SP polyclonal thymocytes displayed a defect in down-regulation of CD24. In addition, G3KO mice developed age-dependent lymphadenopathy due to abnormal expansion of CD8+ T cells mediated by a cell-extrinsic mechanism. Paradoxically, G3KO CD8+ T cells were less efficient than control cells when undergoing homeostatic expansion in lymphopenic environments. Activated G3KO CD8+ T cells were also less efficient in killing antigen-bearing tumor cells in vivo. The impaired homeostatic expansion and in vivo antigen-specific killing was partly attributed to hyporesponsiveness to antigen stimulation. We further demonstrate that GATA-3 was essential for sustaining, but not initiating, activation signals after TCR engagement.
Materials and Methods
Mice
T cell-specific GATA-3-deficient (G3KO) mice have been described previously (8). OT-1 mice were obtained from The Jackson Laboratories and crossed with G3KO mice. The animals were housed under specific pathogen-free conditions, and all animal experiments were performed in accordance with the institutional guidelines for animal care at Dana-Farber Cancer Institute under approved protocols. Male or female mice aged 4–12 wk were used. In all experiments, littermates (FF) carrying the floxed GATA-3 allele but not the CD4cre transgene were used as controls.
Activation of CD8+ T cells
Naïve (CD62LhiCD44lo) CD8+ T cells were sorted from lymph nodes and spleens and activated with plate bound anti-CD3 (ranging from 1 µg/ml to 10 µg/ml) and soluble anti-CD28 (2 µg/ml) in the presence or absence of human IL-2 (100 unit/ml). In some experiments, sorted naïve CD8+ T cells were first stained with carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen) prior to stimulation.
FACS analysis and antibodies
The following clones of antibody were purchased from BioLegend (San Diego, CA) and used for cell surface staining: CD4(RM4-5), CD8(53-6.7), TCRβ(H57-597), CD62L(MEL-14), CD69(H1.2F3), CD25(PC61), CD24(M1/69), CD45.2(104), CD44(IM7), Qa2(695H1-9-9), KLRG1(2F1/KLRG1) Vβ5(MR9-4), FasL(MFL3). Flow cytometry was performed on a FACSCanto or FACSCanto II and analyzed with FlowJo software (Treestar, Ashland, OR).
Western blot and antibodies
In each sample, 5×105 naïve CD8+ T cells, either unstimulated or stimulated, were lysed in freshly prepared radioimmunoprecipitation assay buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 1% Triton x-100, 1% Sodium deoxycholate, and 0.1% SDS. Cell lysate was separated from debris by centrifugation at 12,000 rpm for 10 min. Lysate was loaded onto 9% polyacrylamide gels and transferred onto PVDF membrane (Polyscreen; Perkin Elmer). The membrane was subsequently blocked in 5% milk and probed with antibodies according to the manufacturer’s protocol. The following antibodies were used. Anti-ERK2 (C-14), anti-JNK (F-3), anti-p38α (C-20), anti-Lat (11B.12), and anti-Hsp90 were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Anti–Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), anti- Phospho-SAPK/JNK (Thr183/Tyr185), anti- Phospho-p38 MAPK (Thr180/Tyr182), and anti-p-Zap70(Y319) were purchased from Cell Signaling Technology, Danvers, MA. Anti-Zap70 (1E7.2) was purchased from BioLegend. Proteins were visualized using an ECL kit (PerkinElmer).
Quantitative RNA analysis
Total RNA was purified using a Trizol Plus kit (Invitrogen, Carlsbad, CA). First-strand cDNA synthesis was performed with 200 ng of total RNA using the QuantiTect Reverse Transcription kit (QIAGEN, Hilden, Germany). Gene expression levels were determined by real-time PCR analysis performed using the Brilliant SYBR Green QPCR kit according to the manufacturer’s protocol (Stratagene, La Jolla, CA) on a MX-3000P apparatus (Stratagene) using the following cycling conditions: denaturation at 95 ° C for 30 s, annealing at 56 ° C for 60 s, and extension at 72 ° C for 30 s. Primer sets were designed using the Primer3 web utility (http://frodo.wi.mit.edu/). Levels of mRNA were adjusted for differences in β-actin expression. The following primers are used: c-fos (5’-gtagagcagctatctcctga; 3’-acgcagacttctcatcttc); c-Jun(5’-acagcttaagcagaaagtca; 3’-caaccagtcaagttctcaag); perforin (5’-gctgagaagacctatcagga; 3’- taggaggagatgagcctgt, actin primer (5’-ggctgtattcccctccatcg; 3’-ccagttggtaacaatgccatgt), IL-2 primer (5’-agcagctgttgatggaccta; 3’- cgcagaggtccaagttcat).
ELISA
Sandwich ELISA was performed using the following monoclonal antibody pairs (BD Biosciences, Franklin Lakes, NJ): anti–IL-2 (JES6-1A12)/biotin- anti–IL-2 (JES6-5H4), and anti–IFN-γ (R4-6A2)/biotin-anti–IFN-γ (XMG1.2).
Tumor rejection assay
OVA-transfected B16-derived melanoma cell line B16-OVA cells were kindly provided by Dr. Scott Gerber at University of Rochester Medical Center. B16-OVA cells were cultured in DMEM supplemented with 10% FCS and penicillin/streptomycin, trypsinized and washed twice in PBS. 1×105 cells were injected subcutaneously into shaved back of C57BL/6 mouse. Four days later, host animals were injected intravenously with 1×107 FF/OT-1 or G3KO/OT-1 CD8+ T cells that were pre-stimulated with SIINFEKL peptide derived from ovalbumin (OVA, 5 µg/ml, Invitrogen) in the presence of 100 unit/ml of IL-2 for 3 days. Host mice were sacrificed at day 16 and the tumor diameter was determined as the mean of the largest diameter and the diameter at right angle. Tumors were collected and grinded by cover slide. After passing through 70um filters, cells were recovered and stained with surface antibodies, collected through a FACS canto II, and analyzed by FlowJo software.
Statistical analysis
Student’s t-test was used for statistical analysis. *p<0.05; **p<0.005; ***p<0.0005.
Results
Abnormal MHC class I-mediated positive selection and impaired maturation of CD8SP thymocytes in the absence of GATA-3
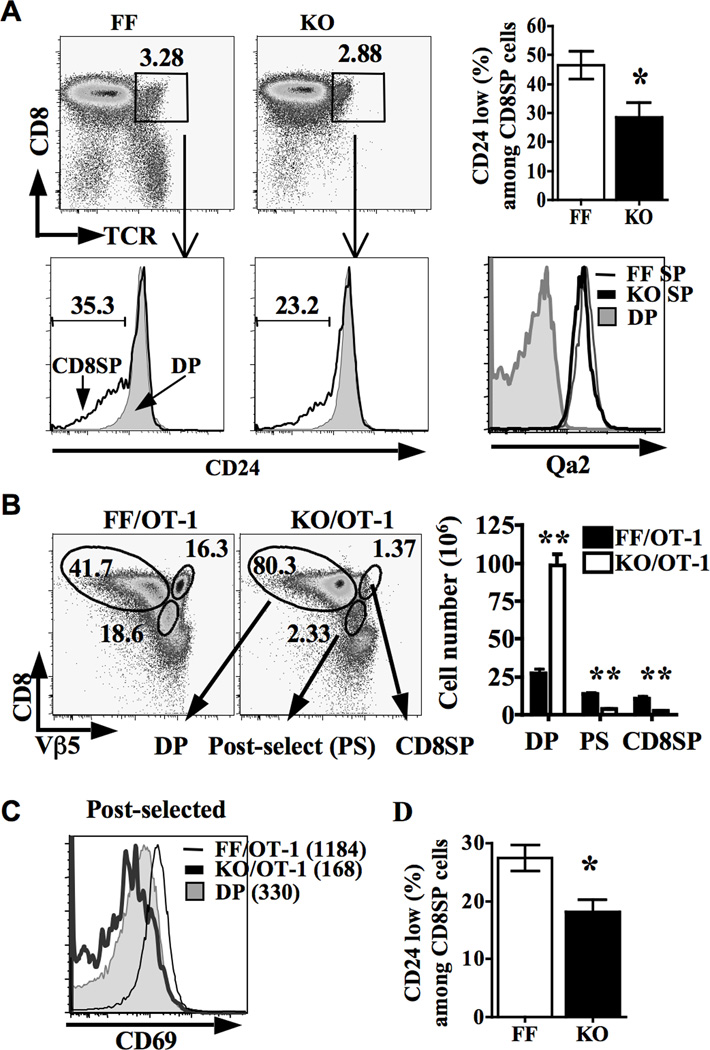
After positive selection and lineage commitment, both CD4SP and CD8SP thymocytes down-regulate CD24 prior to emigrating out of the thymus. We have previously shown that CD4cre-mediated deficiency of GATA-3 had little impact on the development of polyclonal CD8SP thymocytes despite the virtual absence of CD4SP cells. Although the total number of TCRhi CD8SP thymocytes was normal, we found that these mice contained significantly fewer mature TCRhiCD24lo CD8SP thymocytes and reciprocally more immature TCRhiCD24hi CD8SP cells (Figure 1A). The percentage of CD24lo cells among CD8SP subset was approximately 45% in FF mice but was only 25% in KO mice. Despite the defect in down-regulating CD24, G3KO CD8SP thymocytes did express a normal level of Qa2, another marker of mature SP thymocytes (the right lower panel of Figure 1A) (21). This data suggests that GATA-3 may be required for the final maturation step of polyclonal CD8SP thymocytes. To further examine the impact of GATA-3-deficiency on the development of CD8 lineage, we introduced OT-1 TCR transgene into G3KO mice. We found that the thymus of G3KO/OT-1 mice contained approximately 40% more cells than FF/OT-1 mice (data not shown). Eighty percents of G3KO/OT-1 thymocytes, compared to 40% of FF/OT-1 thymocytes, were DP (CD8hiVb5lo) cells, resulting in an approximately 4-fold increase in the number of DP cells in G3KO/OT-1 mice (Figure 1B). Despite the marked increase in the number of DP cells, there was a substantial reduction in the number of post-selected (CD8loVb5hi) and CD8SP (CD8hiVb5hi) thymocytes in G3KO/OT-1 mice. The residual post-selected G3KO/OT-1 thymocytes also did not upregulate CD69 (Figure 1C), an indirect readout of positive selection. The CD69 MFI in both FF/OT-1 and G3KO/OT-1 DP populations was very comparable (approximately 330). The CD69 MFI in FF/OT-1 post-selected cells expectedly increased to approximately 1184, whereas the CD69 MFI of G3KO/OT-1 post-selected cells actually decreased to approximately 168. These observations indicate that deficiency of GATA-3 results in a profound defect in the positive selection of OT-1 thymocytes. The remaining G3KO/OT-1 CD8SP thymocytes that survived positive selection still displayed a defect in the down-regulating CD24 (Figure 1D).
Figure 1. GATA-3 is required for the positive selection and final maturation of CD8SP thymocytes.
A. FACS analysis of thymocytes from 6–8 week-old FF or G3KO mice stained with the indicated fluorochrome-conjugated antibodies. The left four panels show representative CD8/TCR plots and CD24 histograms, and cumulated data from at least three experiments were shown in the right upper panel. The right lower panel shows a representative histogram of Qa2 level of CD8SP and DP thymocytes. B–D. FACS analysis of thymocytes from 6–8 week-old FF/OT-1 and G3KO/OT-1 stained with the indicated fluorochrome-conjugated antibodies. Gating strategy for various subsets is shown in representative CD8/Vβ5 plots in B. The numbers in the CD8/Vβ5 plots are the percentages of indicated subsets among total thymocytes. The absolute cell numbers of indicated subsets from at least three independent experiments are shown in the right panel of B. A representative histogram of CD69 level of post-selected and DP OT-1 thymocytes is shown in C. Percentages of CD24lo cells among CD8SP population from three pairs of FF/OT-1 and G3KO/OT-1 mice are shown in D.
G3KO mice develop age-dependent lymphadenopathy in part due to dysregulated expansion of CD8+ T cells
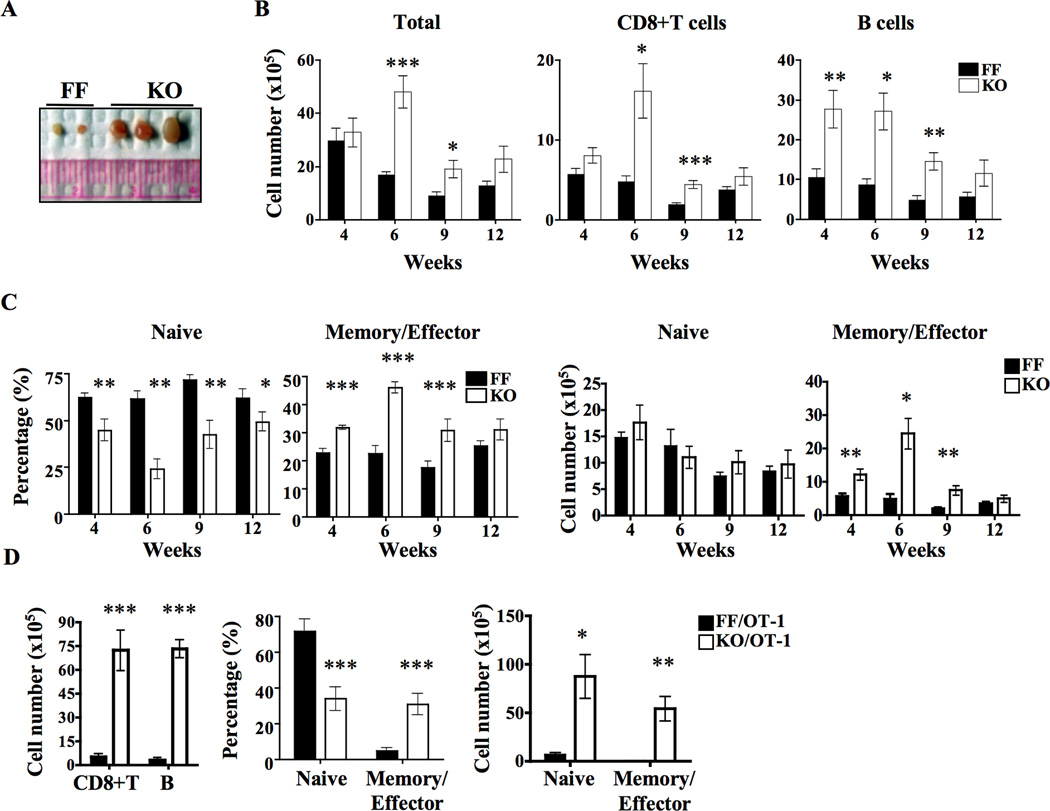
Despite the reduction in thymic output of mature CD8+ T cells and virtual absence of CD4+ T cells, the absolute number of peripheral CD8+ T cells of G3KO mice were comparable to that of control mice up to 4 weeks of age. Thereafter, G3KO mice gradually developed lymphadenopathy (Figure 2A), which peaked at approximately 6 weeks after birth. At that age, the number of total cells per inguinal lymph node of G3KO mice were approximately 3-fold more than FF mice and were made up of mainly CD8+ T and B cells (Figure 2B). While approximately 60% of FF lymph node CD8+ T cells displayed a naïve (CD44−CD62L+) phenotype, only 20% of G3KO CD8+ T cells were naïve cells. Reciprocally 50% of G3KO CD8+ T cells, compared to 20% of FF CD8+ T cells, were CD44+CD62L− “memory/effector” cells (the left two panels of Figure 2C). Thus, the abnormal expansion of CD8+ T cells was mainly due to memory/effector cells, while the number of naïve CD8+ T cells in lymph nodes was very comparable between FF and G3KO mice (the right two panels of Figure 2C). Interestingly, the lymphadenopathy and abnormal expansions of CD8+ T and B cells became less obvious 9 weeks after birth and almost fully resolved when the mice reached 12 weeks of age. The percentage of memory and naïve CD8+ T cells also nearly returned to normal. We found that 12-week-old G3KO mice still had very few CD4+ T cells and that their CD8+ T cells still had an undetectable level of GATA-3 protein (data not shown). Therefore, the resolution of lymphadenopathy was not due to repopulation by GATA-3-sufficient T cells. The lymphadenopathy and abnormal expansion of CD8+ T was not driven by autoantigens because this phenotype is even more striking in G3KO/OT-1 mice (Figure 2D). The number of CD8+ T cells and B cells in lymph nodes of G3KO/OT-1 mice was almost 7 times of that of FF/OT-1 mice. More than 35% of G3KO/OT-1 CD8+ T cells, compared to less than 10% of FF/OT-1 cells, displayed memory/effector features. In contrast to G3KO mice, the numbers of both naïve and memory/effector CD8+ T cells in lymph nodes were increased in G3KO/OT-1 cells (the right panel of Figure 2D), indicating that the abnormal expansion is not limited to memory CD8+ T cells. G3KO mice remained relatively healthy without any evidence of autoimmune features in SPF environment up to 12 months of age despite the age-dependent lymphadenopathy.
Figure 2. Age-dependent lymphadenopathy of G3KO mice.
A. A photo of inguinal lymph nodes from 6 week-old FF and G3KO mice. B. Absolutes numbers of lymph node cells, CD8+ T cells, and B cells per inguinal lymph node from FF or G3KO mice of indicated ages are shown. There were at least 5 mice in each group. C. The percentages (the left two panels) and numbers (the right two panels) of naïve (CD44loCD62Lhi) and memory/effector (CD44hiCD62Llo) cells among lymph node CD8+ T cells of FF and G3KO mice of indicated ages are shown. There were at least 5 mice in each group. D. The absolute numbers of CD8+ T cells and B cells per inguinal lymph node of 6 week-old FF/OT-1 and G3KO/OT-1 mice were counted and shown in the right panel. The percentages (the middle panel) and number (the right panel) of naïve and memory/effector populations among lymph node CD8+ T cells are shown in the right panel. There were 3 mice per group.
Dysregulated expansion of G3KO CD8+ T cells is caused by a CD8+ T cell-extrinsic defect
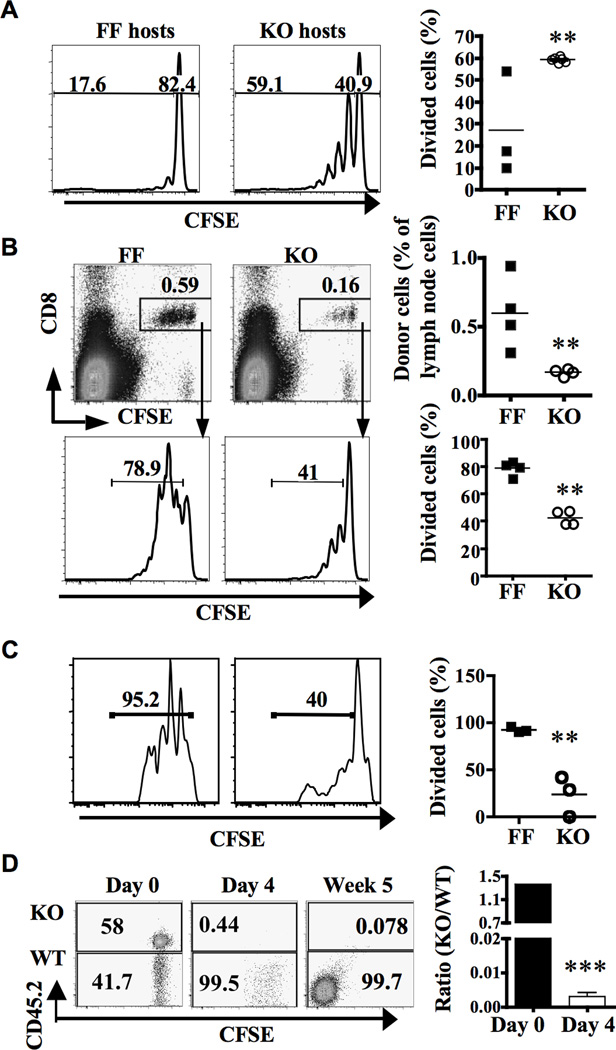
The abnormal expansion of CD8+ T cells observed in G3KO mice could be caused by a cell-intrinsic defect. The deletion of GATA-3 gene in G3KO mice is mediated by a CD4-driven Cre, which can be expressed in some non-T cells, including DC and lymphoid tissue-inducer cells. Some of these cells may also express a low level of GATA-3. Thus, the abnormal expansion of CD8+ T cells could also be mediated by a cell-extrinsic defect. To distinguish these two scenarios, we labeled wild type congenic CD45.1 CD8+ T cells with CFSE and transferred the cells into 4 week-old G3KO or FF mice. Host mice were examined two weeks later. Four week-old mice were chosen because the lymphadenopathy has yet to develop in this age. Most of the donor cells (more than 80%) recovered from peripheral lymph nodes of FF host mice remained undivided (Figure 3A). In contrast, approximately 60% of the donor cells recovered from G3KO host mice had undergone at least one division and their number per inguinal lymph node was twice of their counterparts (Figure 3A and data not shown). This data strongly suggests that the abnormal expansion of CD8+ T cells in G3KO mice was driven by a CD8+ T cell-extrinsic mechanism.
Figure 3. Cell-extrinsic and intrinsic defects in homeostasis of G3KO CD8+ T cells.
A. Congenic (CD45.1) WT naïve CD8+ T cells were labeled with CFSE and then transferred to 4 week-old FF or G3KO hosts (4 millions cells/host mouse) through a tail vein injection. Two weeks after the injection, donor cells were recovered from lymph nodes of host animals and analyzed for the content of CFSE. Representative histograms (left two panels) and cumulative percentages of divided cells in four pairs of mice (right panel) are shown. B & C. Naïve (B) and memory (C) FF and G3KO CD8+ T cells were purified, stained with CFSE, and separately transferred to RAG2KO mice (0.1 million cells/mouse). Four days after the transfer, lymph nodes cells of RAG2 hosts were subjected to FACS. Donor cells were identified on CD8/CFSE plots. The numbers in the CD8/CFSE plots are the percentages of donor cells among host lymph node cells. Divided donor cells were determined based on the content of CFSE as shown in representative CFSE histograms. Cumulative data from 4 pairs of mice are shown in the right panels. D. CD8SP thymocytes of G3KO mice and congenic (CD45.1) WT mice were mixed at 1:1 ratio (Day 0), stained with CFSE, and transferred into RAG2KO mice (0.1 million cells/mouse). At indicated time after transfer, donor cells within lymph nodes of host animals were analyzed for CFSE and CD45.2. Representative CD45.2/CFSE plots were shown. The numbers are the percentages of G3KO and WT cells within TCRhiCD8+ populations. The ratio between G3KO and WT donor cells from 4 host animals is shown in the right panel.
Deficiency of GATA-3 also leads to a cell-intrinsic defect in homeostatic expansion of CD8+ T cells
In a reciprocal experiment, we purified naïve G3KO or FF CD8+ T cells, labeled them with CFSE and adoptively transferred them into RAG2KO mice, allowing the cells to undergo homeostatic expansion. We then recovered donor cells from lymphoid organs of host animals four days after cell transfer. We found that FF donor cells constituted approximately 0.6% of lymph node cells (Figure 3B). More than 75% of the FF donor cells had undergone at least one round of division. We were surprised to found that less than 0.2% of lymph node cells from host animals that received G3KO CD8+ T cells were donor cells. Only 40% of those G3KO CD8+ T cells were proliferating (Figure 3B). This data is consistent with the notion that the abnormal expansion of CD8+ T cells seen in G3KO mice was not mediated by a T cell-intrinsic mechanism. Rather, G3KO CD8+ T cells demonstrated a cell-intrinsic defect in undergoing homeostatic expansion.
One possible explanation for the paradoxical result is that G3KO memory/effector cells expand more robustly than control cells through a cell-intrinsic mechanism. In disagreement with this explanation, we found that G3KO memory/effector (CD44+CD62L−) CD8+ T cells also proliferated poorly when transferred into RAG2KO mice compared to their FF counterpart (Figure 3C). Thus, G3KO memory CD8+ T cells also have a cell-intrinsic defect in undergoing homeostatic expansion.
The impaired homeostatic expansion was also observed with G3KO mature CD8SP thymocytes and was even more striking in the presence of competition. We isolated TCRhiCD24lo CD8SP thymocytes from G3KO and congenic (CD45.1) wild type mice, mixed them at 1:1 ratio, labeled them with CFSE, and transferred them into RAG2KO mice. When we examined the host animals four days later, we found virtually no G3KO cells in lymph nodes of host animals despite the presence and expansion of wild type donor cells (Figure 3D). The KO/WT ratio was reduced to less than 0.01 and declined further five weeks after cell transfer. The reduced number of G3KO cells in lymph nodes of host animals was not due to abnormal distribution of G3KO donor cells to other lymphoid organs because we detected very few donor cells, either G3KO or control, in spleen and peripheral blood of these animals (data not shown).
G3KO CD8+ T cells are hyporesponsive to TCR stimulation
Homeostatic expansion of T cells requires tonic stimulation through TCR and cytokines, such as IL-7 and IL-15. We found that G3KO CD8+ T cells expressed a normal level of CD127, the α subunit of IL-7R (data not shown). To examine their responsiveness to IL-7, we cultivated naïve G3KO and FF CD8+ T cells in vitro in the absence or presence of IL-7 for 4 days. In the absence of IL-7, only approximately 1–2% of cells remained alive (Figure 4A). The percentage of live cells increased to almost 25% in the presence of IL-7 and both G3KO and FF cells responded comparably. Similarly, G3KO CD8+ T cells responded normally to IL-15 stimulation.
Figure 4. G3KO CD8+ T cells are hyporesponsive to TCR stimulation.
A. Naïve FF and G3KO CD8+ T cells were left untreated or treated with IL-7 (20 ng/ml) or IL-15 (50 ng/ml) for three days. Percentage of live cells (gated on FSC/SSC plots) from one representative experiment is shown. B–D. Naïve FF and G3KO CD8+ T cells were labeled with CFSE and stimulated in vitro with indicated concentration of anti-CD3 and 2 µg/ml anti-CD28 for 4 days. Percentages of live cells (gated on FSC/SSC plots) from one representative experiment and cumulative data from more than 3 independent experiments are shown in B. The live cells in B were analyzed for the content of CSFE and expression of CD25. Representative CFSE/CD25 plots and percentage of activated cells (CFSEloCD25hi) from three independent experiments are shown in C. The concentration of IFN-γ and IL-2 in the supernatant of the stimulated cells in B was measured with ELISA and are shown in D. E. Naïve FF and G3KO CD8+ T cells were labeled with CFSE and stimulated with indicated concentration of anti-CD3 and anti-CD28 (2 µg/ml) in the presence of IL-2 (100 unit/ml) for 4 days; and analyzed as shown in B. Percentages of live cells and activated cells, concentration of IFN-γ and IL-2 in supernatant are shown.
We then examined whether G3KO CD8+ T cells were hyporesponsive to TCR stimulation. We stimulated naïve G3KO and FF cells in vitro with anti-CD3 and anti-CD28. FF cells responded to anti-CD3 stimulation in a dose-dependent manner (Figure 4B). But the response of G3KO CD8+ T cells was markedly attenuated. Very few live G3KO CD8+ T cells were recovered after 4 days of stimulation and the remaining live cells expressed a low level of activation markers, such as CD25 and CD69, and proliferated poorly (Figure 4C and data not shown). Only approximately 25% of live G3KO T cells were CFSEloCD25hi, whereas almost 90% of live FF cells were activated and proliferating. In addition, very little IL-2 or IFN-γ was detected in supernatant of G3KO cells (Figure 4D). G3KO CD8+ T cells were also hyporesponsive to stimulation in an antigen-specific manner. We stimulated naïve G3KO and FF OT-1 cells with ova peptide in the presence of autologous APC. Again, we found that G3KO OT-1 cells also responded less robustly to ova peptide (Figure S1A). These results collectively indicate that deficiency of GATA-3 has a global impact on the activation/proliferation of CD8+ T cells.
This hyporesponsiveness could be partly corrected with exogenous IL-2. When stimulated with higher doses of anti-CD3 (5 µg/ml or higher) in the presence of IL-2, the percentage of live cells based on a FSC/SSC gate was near normal in the absence of GATA-3 (the upper left panel of Figure 4E). However, the percentage of activated/dividing (CFSEloCD25hi) cells and the production of IFN-γ and IL-2 were still much lower in G3KO populations (the other three panels of Figure 4E). One possible explanation for the partial rescue by exogenous IL-2 is that G3KO CD8+ T cells have an intrinsic defect in producing IL-2. This scenario is unlikely because the production of IL-2 by comparably activated G3KO CD8+ T cells (stimulated with 5 µg/ml anti-CD3 in the presence of exogenous IL-2) and FF cells (stimulated with 1 µg/ml anti-CD3 in the presence of exogenous IL-2) was very similar. In addition, G3KO CD8+ T cells were capable of maintaining the expression of CD25 in the presence of IL-2 after withdrawal from CD3 stimulation, suggesting that deficiency of GATA3 does not affect IL-2/STAT5 signaling (Figure S1B).
GATA-3 is required for the maintenance/progression but not initiation of activation signals
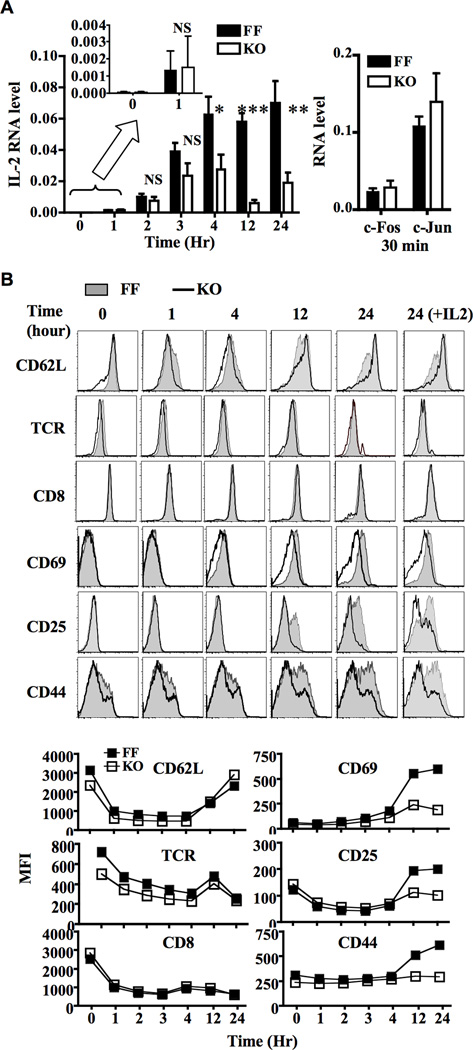
One logical explanation for the hypo-responsiveness of G3KO CD8+ T cells is that GATA-3 regulates the strength of TCR signals. We therefore examined the TCR signaling cascade in CD8+ T cells stimulated with 2 µg/ml anti-CD3 and 2 µg/ml anti-CD28 in the absence of exogenous IL-2, a condition that sufficiently activated FF but not G3KO cells. Surprisingly, we detected no or little impairment in the level and kinetics of tyrosine phosphorylation within 10 minutes and phosphorylation of Jnk and p38 within 30 minutes after stimulation (Figure 5A). NFAT phosphorylation/translocation and NF-κB nuclear translocation also progressed normally up to 4 hours after stimulation (Figure 5B). We did detect approximately 30% reduction in the level of p-Erk in G3KO cells (Figure 5A & 5C and Figure S2). This modest reduction in the level of p-Erk did not substantially affect the degradation of Bim, a known substrate of Erk (Figure 5C) (22), or induction of IL-2, c-Fos, and c-Jun within the first 3 hours after stimulation (Figure 6A). In addition, the earliest features of activation, including shedding of CD62L, internalization of TCR, and down-regulation of CD8 were undisturbed in the absence of GATA-3 (Figure 6B). These data indicated that G3KO CD8+ T cells were capable of initiating activation signals in response to stimulation. These observations also prompted us to look at signaling events beyond 4 hours after engagement of TCR. The initial wave of tyrosine phosphorylation died down by 4 hours in both FF and G3KO cells (Figure 5C). Subsequently, there was a resurgence of tyrosine phosphorylation in FF cells, starting 12 hours after stimulation and propagating up to 24 hours after stimulation. The level of p-Erk, p-Jnk, and p-p38, after the initial spike, dropped back to or below baseline by 4 hours and was either maintained or slightly enhanced in FF cells 24 hours after stimulation (Figure 5C and Figure S2). However, the “second wave” of tyrosine phosphorylation was markedly attenuated in the absence of GATA-3, and G3KO cells were unable to maintain the level of p-Erk, p-Jnk, and p-p38 (Figure 5D). Accordingly, more apoptotic cells were detected in G3KO population (Figure 5E).
Figure 5. GATA-3 is indispensable for the maintenance/progression, but not initiation, of activation signals.
A–E. Naïve FF and G3KO CD8+ T cells were stimulated with anti-CD3 (2 µg/ml) and anti-CD28 (2 µg/ml) for indicated amount of time. IL-2 (100 unit/ml) was added to some samples indicated in C. Whole cell extract (A, C, and D) or nuclear extract (B) were harvested and probed with indicated antibodies. pTyr stands for phosphorylated tyrosine. The protein levels shown in C were semi-quantitatively measured with densitometry, first normalized against the level of Erk2 of the same sample, then normalized against the normalized value of corresponding protein of FF cells at time 0, which was arbitrarily set as 1. The relative protein levels were plotted against time and shown in C. In order to show the differences between FF and G3KO cells, the relative levels of p-Erk, p-Jnk, and p-p38 at 24th hour after stimulation in G3KO cells were compared to those of FF cells, which were arbitrarily set as 100, and shown in D. A fraction of cells after stimulation for 24 hours in the absence of IL-2 were analyzed for the level of 7-AAD and Annexin-V and shown in E. The data shown in Figure 5 are representative of at least two experiments. Molecular weight markers (in kilo Dalton) are shown in A and C.
Figure 6. G3KO CD8+ T cells are unable to sustain TCR-induced activation.
A & B. Naïve FF and G3KO CD8+ T cells were stimulated with anti-CD3 (2 µg/ml) and anti-CD28 (2 µg/ml). Exogenous IL-2 (100 unit/ml) was added to some samples indicated in B. At indicated time points, RNA was collected from fractions of cells and the transcript levels of IL-2, c-Fos, and c-Jun were measured with real-time PCR, normalized against the level of actin, and are shown in A. The remaining cells were harvested and analyzed for surface expression of indicated markers. Representative histograms from one of at least three experiments are shown in B. MFI of each surface markers shown in B was plotted against time and was also shown.
In agreement with the late signaling defects, G3KO CD8+ T cells were unable to sustain the expression of IL-2 after 4 hours (Figure 6A). In addition, the induction of CD69, CD25, and CD44 that normally takes place after four hours of stimulation was marked reduced or did not occur (Figure 6B). In these analyses we gated only on “live” cells seen in FSC/SSC plots. More than 85% of the FF and G3KO “live” cells were negative for both 7AAD and Annexin-V 24 hour after stimulation (Figure S3). Therefore, the impaired expression of late activation markers in the absence of GATA-3 cannot be explained by contamination of apoptotic cells in G3KO population.
GATA-3 is essential for maintaining the level of a subset of signaling molecules
We then further investigated why G3KO CD8+ T cells could not maintain activation signals. As the levels of pTyr, p-Erk, p-Jnk, and p-p38 were markedly reduced in G3KO CD8+ T cells after 4th hour, we postulated that deficiency of GATA-3 had a global impact on signaling in CD8+ T cells. Indeed, we found that the levels of several signaling molecules, although normal prior to stimulation, were much lower in G3KO cells than those in FF cells at 12th hour or later time points (Figure 5C). Stimulation through TCR is known to rapidly induce transient downregulation of Lck (23). This effect is mediated at the level of transcription and RNA stability (24). In agreement with the finding that G3KO CD8+ T cells were able to initiate activation signals, we found that the down-regulation of Lck proceeded nearly normally in the absence of GATA-3. However, G3KO CD8+ T cells were unable to replenish Lck, a process started 12 hours after stimulation in FF cells. In addition, G3KO CD8+ T cells could not maintain the level of Sos, Lat, and Zap70 (Figure 5C and Figure S2). This reduction in the level of Lck, Sos, Lat, and Zap70 was not due to a global defect in gene expression because the level of Bcl2, Bim, and Erk was either normal or even slightly higher in G3KO CD8+ T cells at the same time points (Figure 5C and Figure S2). Furthermore, the re-appearance of CD62L, which occurred in FF cells 12 hours after stimulation, took place slightly earlier and reproducibly reached a higher level in G3KO CD8+ T cells (Figure 6B). Exogenous IL-2 partly restored the level of pTyr, Lck, and Sos in G3KO cells and modestly enhanced the level of CD25, a known target gene of STAT5, but still had no or little impact on the expression of CD69 and CD44 (Figure 5C and 6B). Taken together, our data indicate that GATA-3 was required for maintaining the expression of a subset of signaling molecules and was therefore indispensable for the maintenance/progression, but not the initiation, of activation signals. Such a defect eventually may lead to hyporesponsiveness to antigen stimulation in G3KO CD8+ T cells.
Genome-wide search for GATA-3 binding sites in naïve CD8+ T cells have been conducted using ChIP-seq technology (25). We searched the database for GATA-3 binding sites in the genes shown in Figure 5C and Figure S2. We found that in vivo binding of GATA-3 within promoter or gene body did not predict whether the expression of a given gene would be lost in G3KO cells. For example, heavy GATA-3 binding was detected in the loci of Bcl2 and Bcl2l11 (encoding Bim), whereas no GATA-3 binding was detected in the Sos1 locus. Therefore, GATA-3 very likely maintained the expression of Lck, Sos, and Lat by both direct and indirect mechanisms.
Impaired in vivo antigen-specific killing of G3KO CD8+ T cells
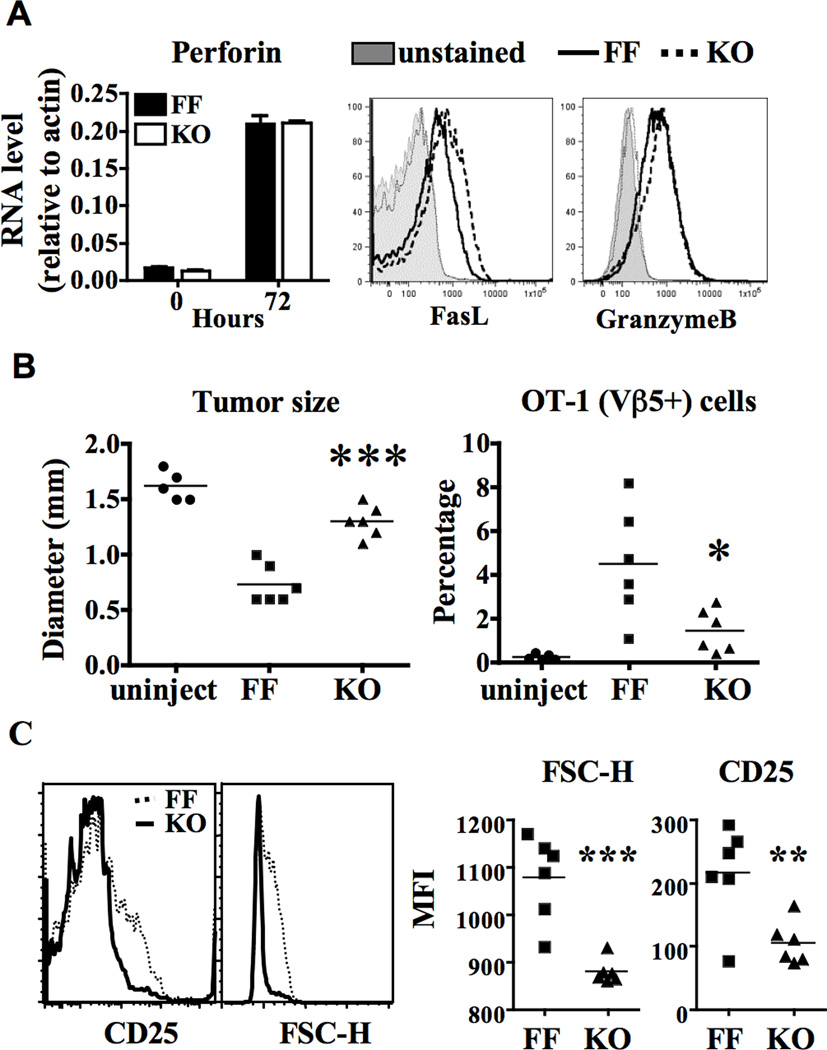
We then set to examine whether deficiency of GATA-3 would affect CTL activity of G3KO CD8+ T cells. We found that G3KO CD8+ T cells were capable of expressing a near normal level of FasL, granzyme B, and perforin when fully activated with a high dose of anti-CD3 (10 µg/ml) in the presence of IL-2 (Figure 7A). In addition, they were as efficient as FF cells in killing MHC-mismatched targets in vitro (Figure S4A). We then examined their antigen-specific CTL activity in an in vivo tumor model. We activated FF/OT-1 and G3KO/OT-1 cells in vitro with ova antigen at a dose that was high enough to activate both FF and KO cells comparably based on the value of FSC. The level of CD25 was, however, still slightly lower in G3KO cells even under the optimal condition. (Figure S4B). We then intradermally injected B16-OVA melanoma cells, which were engineered to express the ovalbumin antigen, into three groups of wild type C57BL/6 mice. The first group of mice received no additional cell transfer; the second group received pre-activated FF/OT-1 cells four days later; and the third group received pre-activated G3KO/OT-1 cells. In the absence of OT-1 cells, B16-OVA cells expanded quickly and formed tumors of approximately 1.6 cm in diameter by day 16 (the left panel of Figure 7B). FF/OT-1 cells were able to limit the growth of B16-OVA cells and the average tumor size in mice of the second group was 0.7 cm in diameter. However, G3KO/OT-1 cells were inefficient at killing B16-OVA cells. The average tumor size in the third group was 1.4 cm in diameter. There were significantly fewer G3KO/OT-1 cells, comparing to FF/OT-1, within tumors (the right panel of Figure 7B). Tumor infiltrating G3KO/OT-1 cells were smaller in size, judged by FSC-H, than infiltrating FF/OT-1 cells and still expressed a lower level of CD25, suggesting that they were less activated (Figure 7C). Taken together, our data indicate that G3KO CD8+ T cells, despite their nearly normal CTL activity in vitro, were unable to maintain their activation status and were less efficient in killing antigen-specific target cells in vivo.
Figure 7. Impaired in vivo antigen-specific killing of G3KO/OT-1 CD8+ T cells.
A. Naïve FF or G3KO CD8+ T cells were stimulated in vitro with CD3 (10 µg/ml) and CD28 (2 µg/ml) in the presence of IL-2 (100 units/ml) for 72 hours. The expression of perforin, FasL, and granzyme B was examined with real-time PCR (for perforin) or FACS. B. FF/OT-1 or G3KO/OT-1 splenocytes were stimulated in vitro with OVA peptide (5 µg/ml) and IL-2 (100 unit/ml) for 72 hours. The activated FF/OT-1 or G3KO/OT-1 OVA-specific CD8+ T cells were purified and injected intravenously (7 million cells/mouse) into B6 mice that received subcutaneous injection of B16-OVA cells (0.1 million cells/mouse) fours days prior to the T cell transfer. Tumor size and tumor infiltrating OT-1 cells were analyzed at day 16 after the transfer of T cells. C. FSC-H and CD25 expression level of tumor infiltrating OT-1 cells are shown in C.
Discussion
GATA-3 plays an important role in several steps during the differentiation of the T linage and in many subsets of T cells, including Th2 (19), NKT (26, 27), and most recently Treg cells (28, 29). Our data further expand the role of GATA-3 in T lineage and demonstrate that GATA-3 regulates many biological aspects of CD8+ T cells.
Single positive thymocytes down-regulate CD24 prior to emigrating out of thymus. The transcriptional regulation of this final maturation step, however, is poorly understood. A recent paper showed that deficiency of NKAP, a putative transcriptional repressor, led to impaired down-regulation of CD24 in mature SP thymocytes (30). One possible scenario is that GATA-3 and NKAP act sequentially in the same molecular pathway that suppresses the expression of CD24. We found that the transcript level of NKAP was normal in G3KO SP thymocytes and naïve CD8+ T cells (data not shown). In addition, other features of NKAP-deficient T cells are quite different from those of G3KO CD8+ T cells. For example, G3KO peripheral CD8+ T cells display a surface phenotype (CD24loQa2hi) indicating fully maturation in thymus (data not shown). This is in sharp contrast to NKAP-deficient naïve peripheral T cells, which are CD24hiQa2lo. Therefore, GATA-3 probably regulates the final maturation of SP thymocytes by a mechanism different from that mediated by NKAP. A similar defect in down-regulation of CD24 has also been observed in thymocytes defective in NK-κB signaling pathway and in OT-1/gld CD8SP thymocytes (31, 32). Further studies will be needed to elucidate how GATA-3 controls the down-regulation of CD24 in SP thymocytes and whether there is any link between GATA-3, NF-κB, and/or Fas/FasL signaling pathways. Others and we have previously shown that deficiency of GATA-3 had only a modest impact on MHC class-II mediated positive selection (8, 9). We were surprised to see the profound defect in the positive selection of G3KO/OT-I thymocytes (Figure 1). It remains to be determined whether this defect in positive selection can also be seen with other MHC class-I restricted TCR transgenes.
Our data revealed previously unknown role of GATA-3 in regulating CTL activity. Although adequately activated G3KO CD8+ T cells were competent in killing MHC-mismatched targets in vitro (Figure S4A), they were functionally impaired in killing antigen-expressing tumor cells in vivo. One possible explanation for the impaired in vivo killing is that G3KO CD8+ T cells have abnormal homing ability. There were indeed fewer tumor infiltrating G3KO/OT-1 CD8+ T cells than FF/OT-1 CD8+ T cells. We cannot completely rule out this possibility. However, we showed in Figure 3B and 3C that adoptively transferred G3KO CD8+ T cells or CD8SP thymocytes did home to secondary lymphoid organs appropriately. Given the observation that tumor infiltrating G3KO/OT-1 CD8+ T cells were relatively smaller and expressed a lower level of CD25 than FF/OT-1 cells, we speculate that activated G3KO/OT-1 CD8+ T cells could not maintain their status of activation and accordingly were unable to efficiently expand, survive, and kill antigen-bearing tumor cells in vivo. It remains unclear whether the intrinsic CTL function of G3KO CD8+ T cells is intact or impaired in vivo.
Unlike T cells that are deficient in known signaling molecules, the initial signaling cascade after antigen encounter proceeded nearly uneventfully in G3KO T cells but the activation signals could not be sustained in the absence of GATA-3. Very little is known regarding how activation signals, once initiated, are maintained at the level of transcription. GATA-3, to the best of our knowledge, is the first transcription factor that is demonstrated to be required for the maintenance/progression but not the initiation of activation signals. However, its mechanism of action is still unclear. Our data indicate that lack of IL-2/STAT5 signaling is not the cause. Instead, GATA-3 is required for maintaining the protein level of a subset of signaling molecules, such as Lck, Zap70, Lat, and Sos, after the initiation of TCR signals. While GATA-3 may directly control the expression of these signaling molecules, we seriously doubt this explanation for two reasons. First, the dependence on GATA-3 for maintaining the expression of these signaling molecules does not correlate with in vivo binding of GATA-3 to corresponding gene loci. Second, the level of these proteins is normal in naïve cells prior to stimulation. Therefore, their dependence on GATA-3 is temporally regulated and GATA-3 more likely indirectly controls the expression of these genes. More cells undergoing early apoptosis were detected in G3KO population before or immediately after stimulation, and the impaired activation we saw could be a consequence of enhanced apoptosis. In agreement with this scenario, several genes that are known to regulate the survival of T cells, including Mcl1, Fas, FasL, and Casp3, contain GATA-3 binding sites according to the ChIP-seq database (25). Although this scenario cannot be ruled out, we found that even the remaining healthy (7AAD−Annexin-V−) looking G3KO cells were still poorly activated. Therefore, we speculate that the inability to maintain activation signals could be independent from or even the cause of enhanced apoptosis. More studies will be needed to test these hypotheses.
Whether GATA-3 is also required for the maintenance/progression of activation signals in other subsets of T cells or at different developmental stages of T lineage is not known. It was recently shown that G3KO Treg cells were functionally impaired. These cells could not maintain a high level of Foxp3 and were prone to reverting to effector cells. One striking feature of G3KO Treg cells was that they had impaired homeostasis and were unable to compete with control cells in mixed bone marrow chimeric mice. This defect in homeostatic expansion probably contributed to their inability to protect RAGKO mice from developing colitis induced by naïve wild type Th cells (28, 29). This defect is reminiscent of the impaired homeostatic expansion of G3KO CD8+ T cells we observed in RAGKO mice and may be also due to a disruption in the maintenance/progression of activation signals. The impaired signal maintenance/progression in the absence of GATA-3 may also explain why G3KO mice have virtually no CD4SP thymocytes despite normal initiation of TCR signaling in DP cells and a near normal number of polyclonal CD4hiCD8lo post-selected cells expressing a near normal level of CD69 (8, 9), a marker of positive selection. It has been proposed that specification of CD4 lineage requires extended duration of TCR signals during MHC-mediated positive selection. It is logical to assume that impaired signal maintenance/progression caused by GATA-3 deficiency could lead to premature termination of TCR signals, thereby selectively interfering with the development of CD4SP cells.
We observed G3KO mice paradoxically developing age-dependent lymphadenopathy, despite a profound and cell-intrinsic defect in activation. One obvious and plausible explanation is lack of Treg cells. Unlike scurfy or Foxp3-deficient mice, the lymphadenopathy observed in G3KO mice is short-lived. It is possible that there still are few Treg cells present in young G3KO mice. This small Treg population then expands as G3KO mice age, thereby quenching the lymphadenopathy and abnormal expansion of CD8+ T cells in older mice. However, mice deficient in CD4, MHC class-II, or Th-POK have virtually no CD4+ T cells but are not reported to have age-dependent lymphadenopathy or abnormal expansion of CD8+ T and B cells. Thus, simply lacking CD4+ T cells, including Treg cells, is probably not the cause of the lymphadenopathy seen in G3KO mice. This phenotype is even more striking in G3KO/OT-1 mice and G3KO mice never develop autoimmune features even up to one year of age, arguing against an autoantigen-driven process. The deletion of GATA-3 is mediated by CD4cre in our G3KO mice. The expression of CD4 is also detected in non-Th cells, including CD11c+ DC and lymphoid tissue inducer cells (33–35). One intriguing scenario is that some of these cells may also express GATA-3 and that the deletion of GATA-3 in these cells may drive the age-dependent expansion of CD8+ T cells in G3KO mice. These possible scenarios are being investigated.
In conclusion, absence of GATA-3 in CD8+ T cells caused an unexpected defect in cell-intrinsic proliferation and defect in vivo killing. A lack of sustained signals via the T cell receptor may be responsible and indicates potential commonalities in the roles that GATA-3 plays in multiple sub-lineages of T cells.
Supplementary Material
Acknowledgements
ICH was responsible for the conception and design of the experiments, and writing of the manuscript. SYP was responsible for the design and execution of some of the experiments shown in Figure 1, 2 and S4A, and writing of the manuscript. TST was responsible for the design and execution of the experiments shown in other figures, and writing of the manuscript.
Footnotes
All authors declare no conflict of interest.
This manuscript was supported NIH grants AI054451 (ICH), AI097725 (ICH), and AI50601 (SYP).
References
- 1.Ho IC, Vorhees P, Marin N, Oakley BK, Tsai SF, Orkin SH, Leiden JM. Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO Journal. 1991;10:1187–1192. doi: 10.1002/j.1460-2075.1991.tb08059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko LJ, Yamamoto M, Leonard MW, George KM, Ting P, Engel JD. Murine and human T-lymphocyte GATA-3 factors mediate transcription through a cis-regulatory element within the human T-cell receptor delta gene enhancer. Molecular & Cellular Biology. 1991;11:2778–2784. doi: 10.1128/mcb.11.5.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nature reviews. 2009;9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendriks R, Nawijn M, Engel J, van Doorninck H, Grosveld F, Karis A. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. European journal of immunology. 1999;29:1912–1918. doi: 10.1002/(SICI)1521-4141(199906)29:06<1912::AID-IMMU1912>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Ting CN, Olson MC, Barton KP, Leiden JM. Transcription Factor Gata-3 Is Required For Development Of the T-Cell Lineage. Nature. 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 6.Hosoya T, Kuroha T, Moriguchi T, Cummings D, Maillard I, Lim KC, Engel JD. GATA-3 is required for early T lineage progenitor development. The Journal of experimental medicine. 2009;206:2987–3000. doi: 10.1084/jem.20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taghon T, Yui MA, Rothenberg EV. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nature immunology. 2007;8:845–855. doi: 10.1038/ni1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Wildt KF, Zhu J, Zhang X, Feigenbaum L, Tessarollo L, Paul WE, Fowlkes BJ, Bosselut R. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nature immunology. 2008;9:1122–1130. doi: 10.1038/ni.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 11.Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, Feigenbaum L, Galera P, Bosselut R. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nature immunology. 2005;6:373–381. doi: 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- 12.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci U S A. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J, Min B, Hu-Li J, Watson C, Grinberg A, Wang Q, Killeen N, Urban J, Guo L, Paul W. Conditional deletion of Gata3 shows its essential function in T(H)1–T(H)2 responses. Nature immunology. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 14.Zheng WP, Flavell RA. The Transcription Factor Gata-3 Is Necessary and Sufficient For Th2 Cytokine Gene Expression In Cd4 T Cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 15.Kurata H, Lee HJ, O'Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677–688. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 18.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 19.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. International immunology. 2011;23:415–420. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omori M, Yamashita M, Inami M, Ukai-Tadenuma M, Kimura M, Nigo Y, Hosokawa H, Hasegawa A, Taniguchi M, Nakayama T. CD8 T cell-specific downregulation of histone hyperacetylation and gene activation of the IL-4 gene locus by ROG, repressor of GATA. Immunity. 2003;19:281–294. doi: 10.1016/s1074-7613(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 21.Vernachio J, Li M, Donnenberg AD, Soloski MJ. Qa-2 expression in the adult murine thymus. A unique marker for a mature thymic subset. J Immunol. 1989;142:48–56. [PubMed] [Google Scholar]
- 22.D'Souza WN, Chang CF, Fischer AM, Li M, Hedrick SM. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181:7617–7629. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marth JD, Lewis DB, Wilson CB, Gearn ME, Krebs EG, Perlmutter RM. Regulation of pp56lck during T-cell activation: functional implications for the src-like protein tyrosine kinases. The EMBO journal. 1987;6:2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paillard F, Vaquero C. Down-regulation of lck mRNA by T cell activation involves transcriptional and post-transcriptional mechanisms. Nucleic acids research. 1991;19:4655–4661. doi: 10.1093/nar/19.17.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei G, Abraham BJ, Yagi R, Jothi R, Cui K, Sharma S, Narlikar L, Northrup DL, Tang Q, Paul WE, Zhu J, Zhao K. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35:299–311. doi: 10.1016/j.immuni.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim PJ, Pai SY, Brigl M, Besra GS, Gumperz J, Ho IC. GATA-3 regulates the development and function of invariant NKT cells. J Immunol. 2006;177:6650–6659. doi: 10.4049/jimmunol.177.10.6650. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Carr T, Xiong Y, Wildt KF, Zhu J, Feigenbaum L, Bendelac A, Bosselut R. The sequential activity of Gata3 and Thpok is required for the differentiation of CD1d-restricted CD4+ NKT cells. European journal of immunology. 2010;40:2385–2390. doi: 10.1002/eji.201040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, Paul WE, Bosselut R, Wei G, Zhao K, Oukka M, Zhu J, Belkaid Y. GATA3 controls Foxp3 regulatory T cell fate during inflammation in mice. The Journal of clinical investigation. 2012;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu FC, Pajerowski AG, Nelson-Holte M, Sundsbak R, Shapiro VS. NKAP is required for T cell maturation and acquisition of functional competency. The Journal of experimental medicine. 2011;208:1291–1304. doi: 10.1084/jem.20101874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 32.Boursalian TE, Fink PJ. Mutation in fas ligand impairs maturation of thymocytes bearing moderate affinity T cell receptors. The Journal of experimental medicine. 2003;198:349–360. doi: 10.1084/jem.20030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 34.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nature reviews. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 35.Mebius RE. Organogenesis of lymphoid tissues. Nature reviews. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.