Abstract
Background
To evaluate racial variation in umbilical cord blood concentration of vitamin D and to explore its correlation with markers of the insulin-like growth factor axis (IGFs) and sex steroid hormones in white and black male neonates.
Methods
In 2004/2005 venous umbilical cord blood samples were collected from 75 black and 38 white male neonates, along with maternal and birth characteristics from two hospitals in Maryland, US. 25-hydroxyvitamin D [25(OH)D], and 1,25-dihydroxyvitamin D [1,25(OH)2D] were measured by radioimmunoassay (RIA), testosterone, estradiol and sex hormone binding globulin (SHBG) by immunoassay and IGF-1, IGF-2, and IGF-binding protein-3 (IGFBP-3) by ELISA. Crude and multivariable-adjusted geometric mean concentrations were computed.
Results
Mean 25(OH)D levels were lower in black than in white neonates (11.44; 95% CI 10.10–12.95 ng/mL vs. 18.24; 95% CI 15.32–21.72 ng/mL; p<0.0001). Black neonates were at higher risk of suboptimal vitamin D levels [25(OH)D < 20 ng/mL] than whites (84% vs. 63%). 25(OH)D concentrations varied by season in whites but not in blacks and were significantly inversely correlated with mother’s parity (number of live births) in blacks but not in whites. Mean concentration of 1,25(OH)2D did not differ by race. 25(OH)D and 1,25(OH)2D did not correlate with IGFs, sex steroid hormones and SHBG.
Conclusions
Suboptimal vitamin D levels were prevalent especially in blacks and influenced by mother’s parity and by season. The observed vitamin D differences between black and white neonates warrant further evaluation of the etiology of the disparity in chronic diseases in adulthood.
Keywords: Vitamin D, umbilical cord blood, black and white Americans
Introduction
Significant differences exist between black and white Americans in relation to the risk of chronic diseases such as cardiovascular disease and cancer [1–4]. Variations in the prevalence of exposure to factors thought to play a role in the etiology of these diseases may contribute to the observed health disparities. Vitamin D is a candidate to be studied. Suboptimal vitamin D level is thought to be associated with a range of diseases such as diabetes, cardiovascular disease and several types of cancer [5]. Due to their greater skin pigmentation, blacks have lower endogenous formation of vitamin D and, thus, often lower circulating levels than white individuals living at the same latitude [6].
It is thought that some chronic diseases have their origins in early life and even in utero, such that Barker et al. [7] suggested that fetuses react and adapt to their nutrient supply during prenatal growth with consequences for the risk of chronic diseases later in life. It is therefore plausible that vitamin D deficiency in utero or early childhood also affects the risk of these diseases [8].
The main circulating form of vitamin D is 25-hydroxyvitamin D [25(OH)D], which is metabolized into 1,25-dihydroxyvitamin D [1,25(OH)2D], the biologically active form of vitamin D. 1,25(OH)2D enters cells by passive diffusion and binds to the vitamin D receptor of the nucleus, which ultimately leads to changes in gene transcription [9]. In the fetus, 1,25(OH)2D has several functions; it is, for example, involved in glucose metabolism [10], which fits into Barker’s hypothesis [10,11].
During the last century, vitamin D fortification programs have largely eradicated health risks of vitamin D deficiency such as rickets and osteomalacia from western populations. However, the risk of vitamin D deficiency is again prevalent in industrialized nations [12–14]. So far, few studies examined vitamin D concentrations in black and white-American mothers and their neonates, e.g. [15–17,12]. Specifically, a Pittsburgh study reported higher concentrations of 25(OH)D in white than in black women and their neonates [16]. 25(OH)D is considered indicative of an individual’s vitamin D status, whereas 1,25(OH)2D is homeostatically controlled in the blood [18]. Few studies have examined racial differences in 1,25(OH)2D concentrations in cord blood of full-term births [15]. Insulin-like growth factor-1 (IGF-1) stimulates renal and placental 1,25(OH)2D production [19], but the association between these two markers has rarely been investigated in cord or maternal blood [20]. Even less is known about the relationship between vitamin D and sex steroid hormones during fetal development. So far, one in vitro study has shown that vitamin D is a physiological regulator of placental estradiol and progesterone secretion [21]. To the best of our knowledge, no one has yet examined racial variation in the correlation between vitamin D and these other hormones.
In the present study, we examined whether venous umbilical cord blood concentrations of 25-hydroxyvitamin D [25(OH)D], and 1,25-dihydroxyvitamin D [1,25(OH)2D] differed between 75 black and 38 white-American male neonates. In addition, the correlation of insulin-like growth factor-1 (IGF-I), and sex steroid hormones and sex hormone binding globulin (SHBG) with cord blood vitamin D levels was assessed by race.
Methods
Study population
The current study, called the Hormones in Umbilical Cord Blood Study (HUB Study), was conducted as a pilot project as part of the partnership between the Howard University Cancer Center and the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, which was funded by the National Cancer Institute (U54 CA091409 and CA091431). The Institutional Review Boards of Prince George’s Hospital Center and the Johns Hopkins Bloomberg School of Public Health approved the joint study.
Umbilical cord blood samples were collected from neonates born at Prince George’s Hospital Center in Cheverly, MD (Hospital 1) and Johns Hopkins Hospital in Baltimore, MD (Hospital 2) between February 2004 and June 2005. The delivery room nurses collected venous umbilical cord samples and demographic data without identifying information. Inclusion criteria for the neonates were full term birth (38th to 42nd gestational week), birth weight between 2500 and 4000 g, no major birth defects, and singleton birth. We included mothers without pregnancy complications, such as gestational or chronic hypertensive disease, gestational or pre-gestational diabetes mellitus, thyroid disease; who did not use hormonal medications during pregnancy; and who had no known growth hormone deficiency. Furthermore, the baby’s mother and father were required to be of the same race, either black or white. Delivery room nurses drew fifteen milliliters of blood into two tubes containing sodium EDTA from the vein from each eligible baby’s umbilical cord. The samples were stored in a refrigerator and processed usually within 12 hours. After centrifugation for 15 min at 2400 rpm at room temperature, plasma, buffy coat, and red cells were aliquotted into cryovials and stored at −70°C. The nurses collected information on month and time (quadrant) of day of the birth, birth weight, placental weight, mother/child’s race, and mother’s age, parity (number of live births), and gravidity (number of pregnancies) using a standardized form. One hundred thirteen male specimens, 38 white and 75 black, were collected and eligible for the study. Details have been described previously [22].
Exposure assessment
25(OH)D and 1,25(OH)2D was measured at Heartland Assays, Inc. (Ames, USA). 25(OH)D and other hydroxylated metabolites were extracted from serum or plasma with acetonitrile. Following extraction, the treated sample was then assayed using an equilibrium radioimmunoassay (RIA) procedure. The RIA method is based on an antibody that is co-specific for 25(OH)D2 and 25(OH)D3. The 25(OH)D assay has a range of 2.5–100 ng/mL and intra- and inter-assay coefficients of variation (CVs) of 8.0% and 10.0%. Details of the assay have been described previously [23]. The assay for 1,25(OH)2D involved a preliminary extraction and subsequent purification of vitamin D metabolites from plasma using C18OH cartridges. Following extraction, the treated samples were then assayed using a competitive RIA procedure. The RIA method is based on a polyclonal antibody that is specific for both 1,25(OH)2D2 and 1,25(OH)2D3. The inter- and intra-assay CVs for this assay are 12.6% and 9.8% respectively. Details of the assay have been described previously [24]. The laboratory technicians were blinded to race and hospital.
Testosterone, estradiol, and SHBG were previously measured by competitive immunoassay (1010 Elecsys autoanalyzer, Roche Diagnostics, Indianapolis, IN) and androstanediol glucuronide by an enzyme immunoassay (Diagnostic Systems Laboratory, Webster, TX) in the laboratory of Dr. Nader Rifai, Children’s Hospital Boston [22]. IGF-1, IGF-2 and IGF binding protein (BP)-3 were previously measured by ELISA (Diagnostics Systems Laboratory, Webster, TX) in the laboratory of Dr. Michael Pollak, Jewish General Hospital [22].
Statistical analysis
We calculated geometric mean plasma concentrations of 25(OH)D and 1,25(OH)2D in the cord blood of the black and white male neonates because both analytes were not normally distributed, and tested for differences by race using the t-test. We also present the geometric means adjusted for season and mother’s parity estimated using generalized linear models because season is an important determinant of 25(OH)D concentration and mother’s parity was correlated with 25(OH)D levels in black neonates. We estimated the percentage of neonates with cord blood 25(OH)D concentrations < 20 ng/mL and < 12 ng/mL, cutpoints often used to define risk of suboptimal and risk of deficient vitamin D levels[25]. We examined the correlation of IGF-I, IGF-II, IGFBP-3, sex steroid hormones, and SHBG with concentration of 25(OH)D and 1,25(OH)2D by race using Spearman’s rank correlation coefficient. All analyses were conducted using SAS v. 9.1 (SAS Institute, Cary, NC).
Results
Table 1 shows the baseline characteristics of mothers and newborns. At birth, mothers of white male neonates were older than mothers of black male neonates and they were more often primipara. Mean birth weight was significantly higher in white than in black neonates. The relative percentage of births by season differed between blacks and whites, which is an artifact of the timing of the study in the two hospitals.
Table 1.
Maternal and birth characteristics by race, males, HUB study 2004–2005
| Blacks | Whites | p-value | |
|---|---|---|---|
| Number of cord blood samples (N) | 75 | 38 | |
| Mother’s age (year; mean ± SD) | 24.3 ± 6.1 | 28.7 ± 6.3 | 0.128a |
| Birth weight (g; mean ± SD) | 3,241 ± 346 | 3,470 ± 392 | 0.002a |
| Season (%) | |||
| Winter | 28 | 10.5 | |
| Spring | 53.3 | 68.4 | |
| Summer | 2.7 | 10.5 | |
| Fall | 16 | 10.5 | 0.049b |
| Mother’s parity (number of live births) (%) | |||
| 0 | 29.3 | 63.2 | |
| 1 | 36 | 21.1 | |
| 2 | 17.3 | 10.5 | |
| 3+ | 17.3 | 5.3 | 0.013b |
t-test
Chi-square-test
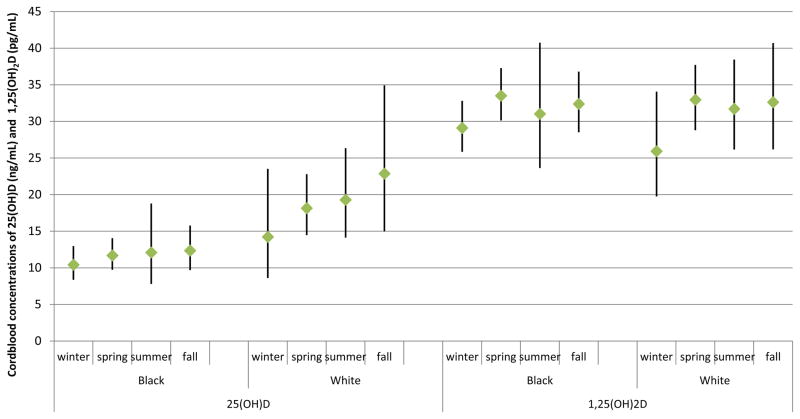
25(OH)D and 1,25(OH)2 D levels were not statistically significantly correlated with the age of the mother at birth of the child or with the birth weight of the neonates (table 2). 25(OH)D concentration was statistically significantly inversely correlated with the mother’s parity in black but not white male neonates. 25(OH)D concentrations varied among season of the year of birth in white (lowest levels during winter, highest levels in fall) but not in black neonates (figures 1). 1,25(OH)2D level varied by season of the year of birth in both black and white neonates similarly; the highest concentrations were seen in spring, the lowest in winter.
Table 2.
Partial Spearman correlation coefficients between maternal and birth characteristics and 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) concentrations in venous umbilical cord blood of black and white neonates, males, HUB study 2004–2005
| Blacks | Whites | ||||
|---|---|---|---|---|---|
| 25(OH)D | 1,25(OH)2D | 25(OH)D | 1,25(OH)2D | ||
| Birth weight | Spearman r | −0.063 | 0.148 | −0.081 | 0.021 |
| p-value | 0.59 | 0.21 | 0.63 | 0.9 | |
| Placental weight | Spearman r | −0.025 | 0.148 | −0.016 | 0.021 |
| p-value | 0.84 | 0.21 | 0.92 | 0.9 | |
| Mother’s parity (number of live | Spearman r | −0.283 | −0.146 | −0.071 | 0.205 |
| p-value | 0.01 | 0.21 | 0.67 | 0.22 | |
| Mother’s age | Spearman r | 0.115 | 0.146 | 0.15 | −0.069 |
| p-value | 0.33 | 0.21 | 0.38 | 0.69 | |
Figure 1.
Geometric mean 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) concentrations (and 95% CI) in venous umbilical cord blood of black and white neonates, by season of the year of birth, males HUB study 2004–2005
Unadjusted mean 25(OH)D concentrations were significantly lower in black (11.44, 95% CI 10.10–12.95 ng/mL) than in white neonates (18.24, 15.32–21.72 ng/mL; p<0.0001) (table 3). The same was true for means adjusted for mother’s parity and season of the year of birth. 38% of the newborns (32% black, 50% white) had 25(OH)D levels between 12 and < 20 ng/mL. 39% of the infants (52% black, 13% white) were at risk of vitamin D deficiency [25(OH)D < 12 ng/mL]. The percentages of neonates with suboptimal vitamin D levels [25(OH)D < 20 ng/mL] were much higher in black male neonates than in white male neonates (84% vs. 63%). Mean 1,25(OH)2D concentrations did not differ significantly between black (31.98, 95% CI 29.77–34.34 pg/mL) and white (31.94, 28.85–35.36; p=0.99; table 3) neonates. Concentrations of 25(OH)D and 1,25(OH)2D were statistically significantly correlated in white (Spearman correlation coefficient: 0.61, p<0.0001) and black (0.59, p<0.0001) neonates. We examined 1,25(OH)2D levels after stratifying into neonates with levels below and equal or above 20 ng/mL but did not observe statistically significant differences between black and white neonates when looking at low levels of 25(OH)D (black 30.00 pg/mL [95% CI 28.01–32.14]; white 29.87 pg/mL [26.66–33.47]; p-value 0.95) or at 25(OH)D levels above 20 ng/mL (black 44.33 pg/mL [95% CI 37.07–53.00]; white 36.40 pg/mL [30.66–43.22]; p-value 0.13)
Table 3.
Geometric mean (and 95% CI) 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) concentrations in venous umbilical cord blood of white and black neonates, males, HUB study 2004–2005
| Blacks | Whites | p-value | |||
|---|---|---|---|---|---|
| geometric mean | 95%CI | geometric mean | 95%CI | ||
| Unadjusted | |||||
| 25(OH)D (ng/mL) | 11.44 | 10.10–12.95 | 18.24 | 15.32–21.72 | <0.0001 |
| 1,25(OH)2D (pg/mL) | 31.98 | 29.77–34.34 | 31.94 | 28.85–35.36 | 0.986 |
| Adjusteda | |||||
| 25(OH)D (ng/mL) | 11.71 | 10.36–13.24 | 17.41 | 14.63–20.73 | 0.0003 |
| 1,25(OH)2D (pg/mL) | 32.22 | 29.99–34.63 | 31.45 | 28.35–34.89 | 0.712 |
|
| |||||
| Spearman correlation between 25(OH)D and 1,25(OH)2D (p- value) | 0.592 (p<.0001) | 0.61 (p<.0001) | |||
Adjusted for season of the year of birth and mother’s parity
After adjusting for season of the year of birth and mother’s parity, which was correlated with 25(OH)D levels in black neonates, cord blood levels of 25(OH)D and 1,25(OH)2D were not correlated with cord blood levels of sex steroid hormones and growth factors (IGFs) (table 4).
Table 4.
Partial Spearman Correlation Coefficientsa between 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) concentrations and sex steroid hormones and IGF axis concentrations in venous umbilical cord blood of black and white neonates, males, HUB study 2004–2005
| Blacks | Whites | ||||
|---|---|---|---|---|---|
| 25(OH)D | 1,25(OH)2D | 25(OH)D | 1,25(OH)2D | ||
| IGF-1 | Spearman r | −0.002 | −0.009 | −0.005 | −0.057 |
| p-value | 0.99 | 0.942 | 0.979 | 0.744 | |
| IGF-2 | Spearman r | 0.134 | 0.083 | 0.091 | 0.099 |
| p-value | 0.26 | 0.488 | 0.604 | 0.57 | |
| IGF binding protein-3 | Spearman r | −0.048 | 0.104 | 0.165 | 0.015 |
| p-value | 0.69 | 0.387 | 0.345 | 0.934 | |
| Testosterone | Spearman r | 0.167 | 0.11 | 0.103 | −0.056 |
| p-value | 0.16 | 0.358 | 0.555 | 0.748 | |
| Estradiol | Spearman r | 0.052 | −0.122 | 0.152 | 0.038 |
| p-value | 0.666 | 0.309 | 0.384 | 0.83 | |
| Sex hormone binding globuline | Spearman r | −0.051 | 0.019 | −0.038 | −0.057 |
| p-value | 0.672 | 0.873 | 0.827 | 0.746 | |
Adjusted for season of the year of birth and mother’s parity
Discussion
In the present study we observed that, in a group of 38 white and 75 black male full-term neonates born in Maryland, US, the percentage with suboptimal vitamin D levels [25(OH)D < 20 ng/mL] was much higher in black (84%) than in white (63%) male neonates. 25(OH)D concentration varied among the seasons in white but not in black neonates. 1,25(OH)2D cord blood level did not differ between races, but was correlated with 25(OH)D levels in both races. 25(OH)D and 1, 25(OH)2D levels were not correlated with IGFs, sex steroid hormones, or SHBG. Because of the small sample size, we cannot rule out chance as an explanation for our findings.
25(OH)D
Compared to the unadjusted mean values of 25(OH)D of 20.4 ng/mL in white and 13.4 ng/mL in black male neonates in our study, the vitamin D concentrations in the few other US studies ranged from 14.9 to 27 ng/mL in white and 9.5 to 15.7 ng/mL in black neonates [12,16,17,26]. The lowest levels were observed in cord blood samples taken in winter/early spring [12] [26], [16]. In comparison to the 84% of the black and 63% of the white male neonates with suboptimal levels of 25(OH)D < 20 ng/mL in our study, in the study by Merewood et al. in Boston [12], 67.4% of black and 37.5% of non-Hispanic white neonates had 25(OH)D levels of < 20 ng/mL. The percentage of neonates born in summer was much lower in our study than in their study (2.7% in blacks and 10.5% in whites compared to 25.8% in the study by Merewood [12]). The seasonal changes in 25(OH)D concentrations are well recognized [12,18,27]. In the present study, 25(OH)D concentration varied among the seasons in white but not in black male neonates. Our results are in line with the findings of a study by Basile et al. [28], in which vitamin D status was measured in neonates (including 20% preterm neonates) at latitude 32°72′ (southeastern US). The mean 25(OH)D level at birth in November–March compared to April–October was 11.3 ng/mL lower in white infants (from 29.0 to 17.7 ng/mL) and 3 ng/mL lower in black infants (from 13.1 to 10.1 ng/mL). Seasonality, while significant in both groups, had a greater impact on the vitamin D status of white newborns to recover to adequate levels, as in our study.
The vitamin D status of newborns is also closely related to maternal vitamin D status [29]. A secondary analysis of 928 pregnant and 5173 non-pregnant women aged 13–44 years from the National Health and Nutrition Examination Survey 2001–2006 [30] found that 13% of white and 80% of black pregnant women and 26% of white and 86% of black non-pregnant women were at risk of suboptimal vitamin D levels (25(OH)D < 50 nmol/L = < 20 ng/mL). In the present study, 25(OH)D concentration was inversely correlated with the mother’s parity in black but not in white neonates. We hypothesize that the increased need for vitamin D during pregnancy might deteriorate the suboptimal status of the mothers further, and multiparity may lead to an inability of the vitamin D status to recover to adequate levels between pregnancies. Overall the findings among the studies addressing this question are not consistent [31–35].
1,25(OH)2D
The assessment of serum 25(OH)D concentrations is generally considered to be the best approach for determining human vitamin D status [36]. 25(OH)D is biologically inert and must be converted to its biologically active form, 1,25(OH)2D. The production of 1,25(OH)2D is under stringent control [37,38]. Serum 1,25(OH) levels are often normal or even elevated in vitamin D deficiency [18]. A few epidemiological studies reported associations between blood levels of 1,25(OH)2D and chronic diseases [39,27,40,41]. In the present study, circulating levels of 1,25(OH)2D and 25(OH)D cord blood levels were highly correlated in both races. No differences of 1,25(OH)2D cord blood levels between black and white male neonates were observed. Only a few other studies examined differences in circulating levels of 1,25(OH)2D between different racial groups and, with one exception [42], no differences were observed [15,43].
IGFs and 25(OH)D and 1,25(OH)2D concentrations
Insulin-like growth factors (IGFs) are multifunctional peptides that regulate cell proliferation, differentiation, and apoptosis [44]. IGF-I stimulates renal and placental 1,25(OH)2D production [19]. In a previous analysis in the same sample of neonates, we observed lower cord blood IGF-1 and IGF-2 levels in black compared with white male neonates [22]. In the present study we found no associations between IGF growth factors and cord levels of 1,25(OH)2D or 25(OH)D. So far, one study reported a positive correlation between IGF-I and 1,25(OH)2D concentrations in serum of normotensive mothers but no significant correlation in the cord blood of their neonates. However, a statistically significant correlation was observed in cord blood of neonates of mothers with preeclampsia [20]. IGF-II is another growth factor, which is more important during fetal development than IGF-I [44], but whose association with vitamin D levels in cord blood has, to the best of our knowledge, not been analyzed in previous studies.
Sex steroid hormones and 25(OH)D and 1,25(OH)2D concentrations
In the already mentioned previous analysis in the same sample of neonates we found a higher cord blood testosterone to SHBG ratio in black than in white male neonates after taking into account maternal and birth factors [22]. In the present study, no associations were observed between cord blood concentrations of 25(OH)D, and 1,25(OH)2D and testosterone, estradiol, and SHBG. A Finnish study observed no association between circulating 25(OH)D and testosterone and estradiol as well as other sex steroid hormones (progesterone, 17-hydroxyprogesterone, and androstenedione) among pregnant women [35]. An intervention in young nonpregnant women showed that per supplemented increase of 10 nmol/L of 25(OH)D, estradiol and progesterone decreased significantly by a factor of 3% and 10%, respectively [45].
Strengths and Limitations
Cord blood from black and white male neonates was sampled according to a standardized protocol during the same time period between 2004 and 2005 at two hospitals in the Baltimore-Washington, D.C. area. This reduces one source of vitamin D variation between blacks and whites as the mothers of the neonates lived at the same latitude. Given our inclusion and exclusion criteria, we sampled a relatively homogeneous group of neonates, excluding extremely large or small neonates, neonates born to mothers with pregnancy complications, and neonates with major birth defects. This allowed for elucidating racial differences in vitamin D cord blood levels and their association with IGF-I and sex steroid levels in healthy neonates. Many previous studies have, in contrast, been conducted among neonates small-for-gestational-age or born from mothers with pregnancy complications. Because this study was designed to collect anonymous cord blood specimens as discarded human tissue, we did not collect information from the mothers about their diet or outdoor behavior during the pregnancy. We also did not collect blood specimens from the mothers, so we were not able to correlate mother’s with baby’s circulating vitamin D concentrations or adjust for the mother’s behaviors affecting vitamin D status during pregnancy. Lastly, we measured vitamin D in our samples, which were collected 5 years ago and have been frozen at −70°C. However, 25(OH)D is extremely stable in samples frozen at −20°C for at least two years [46–48]. Furthermore, only slight deterioration of serum 25(OD)D was observed in a study by Bodnar et al. [49] after ≥ 40 years of storage.
Samples from black neonates were collected from two hospitals, whereas samples from white neonates were collected only in one [22]. However, vitamin D levels between black neonates from hospital 1 and hospital 2 were comparable as was birth weight of the neonates, and mothers did not differ with respect to parity and age at birth (data not shown), such that they were analyzed together.
Conclusions
Risk of suboptimal vitamin D levels (25(OH)D < 20 ng/mL) may be prevalent in US male newborns based on a sample from the Mid-Atlantic region, although our samples are small and not representative of the population. Black male neonates were substantially more likely to have low cord blood vitamin D levels. Their risk increased with mother’s parity, and in white male neonates if born in the winter. Vitamin D levels were not correlated with levels of sex steroid hormones, SHBG or IGFs. The observed vitamin D disparity between black and white male neonates warrants further evaluation in the etiology of the disparity in chronic disease risk in adulthood.
Acknowledgments
Financial support: The study has been supported by ISFE (Internationale Stiftung zur Förderung der Ernährungsforschung und Ernährungsaufklärung). This study was also supported by a National Cancer Institute U54 grant (Hopkins CA091409) and U54 (Howard CA091431). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
We thank Stacey Meyerer, laboratory manager, at the Johns Hopkins Bloomberg School of Public Health, for her assistance in the conduct of this study.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Kheirandish P, Chinegwundoh F. Ethnic differences in prostate cancer. Br J Cancer. 2011;105(4):481–485. doi: 10.1038/bjc.2011.273. bjc2011273 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiscella K, Winters P, Tancredi D, Hendren S, Franks P. Racial Disparity in Death From Colorectal Cancer Does Vitamin D Deficiency Contribute? Cancer. 2011;117(5):1061–1069. doi: 10.1002/Cncr.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman JS, Harper S, King NB. A More Complete Picture of Higher Cardiovascular Disease Prevalence Among Blacks Compared to Whites. American Journal of Medicine. 2011;124(5):E5–E6. doi: 10.1016/j.amjmed.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Grant WB, Peiris AN. Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J Am Med Dir Assoc. 2010;11(9):617–628. doi: 10.1016/j.jamda.2010.03.013. S1525-8610(10)00102-7 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Peiris AN, Bailey BA, Peiris P, Copeland RJ, Manning T. Race and vitamin D status and monitoring in male veterans. J Natl Med Assoc. 2011;103 (6):492–497. doi: 10.1016/s0027-9684(15)30363-1. [DOI] [PubMed] [Google Scholar]
- 6.Loomis WF. Skin-pigment regulation of vitamin-D biosynthesis in man. Science. 1967;157 (3788):501–506. doi: 10.1126/science.157.3788.501. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ. A new model for the origins of chronic disease. Med Health Care Philos. 2001;4 (1):31–35. doi: 10.1023/a:1009934412988. [DOI] [PubMed] [Google Scholar]
- 8.Lucas RM, Ponsonby AL, Pasco JA, Morley R. Future health implications of prenatal and early-life vitamin D status. Nutr Rev. 2008;66(12):710–720. doi: 10.1111/j.1753-4887.2008.00126.x. NURE126 [pii] [DOI] [PubMed] [Google Scholar]
- 9.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. 80/6/1689S [pii] [DOI] [PubMed] [Google Scholar]
- 10.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, Marazita ML, Simhan HN. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr. 2010;140(5):999–1006. doi: 10.3945/jn.109.119636. jn.109.119636 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy-Marchal C, Czernichow P. Small for gestational age and the metabolic syndrome: which mechanism is suggested by epidemiological and clinical studies? Horm Res. 2006;65(Suppl 3):123–130. doi: 10.1159/000091517. HRE2006065S03123 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Merewood A, Mehta SD, Grossman X, Chen TC, Mathieu JS, Holick MF, Bauchner H. Widespread vitamin D deficiency in urban Massachusetts newborns and their mothers. Pediatrics. 2010;125(4):640–647. doi: 10.1542/peds.2009-2158. peds.2009-2158 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10 Suppl 2):S153–164. doi: 10.1111/j.1753-4887.2008.00100.x. NURE100 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollis BW, Pittard WB., 3rd Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59 (4):652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 16.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137(2):447–452. doi: 10.1093/jn/137.2.447. 137/2/447 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46(1):42–44. doi: 10.1177/0009922806289311. 46/1/42 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9(1):107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 19.Halhali A, Diaz L, Sanchez I, Garabedian M, Bourges H, Larrea F. Effects of IGF-I on 1,25-dihydroxyvitamin D(3) synthesis by human placenta in culture. Mol Hum Reprod. 1999;5 (8):771–776. doi: 10.1093/molehr/5.8.771. [DOI] [PubMed] [Google Scholar]
- 20.Halhali A, Tovar AR, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol Metab. 2000;85 (5):1828–1833. doi: 10.1210/jcem.85.5.6528. [DOI] [PubMed] [Google Scholar]
- 21.Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, Diaz L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol. 2007;103(3–5):529–532. doi: 10.1016/j.jsbmb.2006.12.097. S0960-0760(06)00463-8 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Rohrmann S, Sutcliffe CG, Bienstock JL, Monsegue D, Akereyeni F, Bradwin G, Rifai N, Pollak MN, Agurs-Collins T, Platz EA. Racial variation in sex steroid hormones and the insulin-like growth factor axis in umbilical cord blood of male neonates. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1484–1491. doi: 10.1158/1055-9965.EPI-08-0817. 18/5/1484 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39 (3):529–533. [PubMed] [Google Scholar]
- 24.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42 (4):586–592. [PubMed] [Google Scholar]
- 25.IOM, editor. Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington DC: 2011. [PubMed] [Google Scholar]
- 26.Dror DK, King JC, Durand DJ, Allen LH. Association of modifiable and nonmodifiable factors with vitamin D status in pregnant women and neonates in Oakland, CA. J Am Diet Assoc. 2011;111(1):111–116. doi: 10.1016/j.jada.2010.10.002. S0002-8223(10)01638-X [pii] [DOI] [PubMed] [Google Scholar]
- 27.Brock KE, Huang WY, Fraser DR, Ke L, Tseng M, Mason RS, Stolzenberg-Solomon RZ, Freedman DM, Ahn J, Peters U, McCarty C, Hollis BW, Ziegler RG, Purdue MP, Graubard BI. Diabetes prevalence is associated with serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in US middle-aged Caucasian men and women: a cross-sectional analysis within the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Br J Nutr. 2011;106(3):339–344. doi: 10.1017/S0007114511001590. S0007114511001590 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basile LA, Taylor SN, Wagner CL, Quinones L, Hollis BW. Neonatal vitamin D status at birth at latitude 32 degrees 72′: evidence of deficiency. J Perinatol. 2007;27(9):568–571. doi: 10.1038/sj.jp.7211796. 7211796 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Greer FR. 25-Hydroxyvitamin D: functional outcomes in infants and young children. Am J Clin Nutr. 2008;88(2):529S–533S. doi: 10.1093/ajcn/88.2.529S. 88/2/529S [pii] [DOI] [PubMed] [Google Scholar]
- 30.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA., Jr Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202(5):436, e431–438. doi: 10.1016/j.ajog.2009.11.036. S0002-9378(09)02210-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28(1):7–12. doi: 10.1055/s-0030-1262505. [DOI] [PubMed] [Google Scholar]
- 32.Gannage-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res. 2000;15(9):1856–1862. doi: 10.1359/jbmr.2000.15.9.1856. [DOI] [PubMed] [Google Scholar]
- 33.van der Meer IM, Karamali NS, Boeke AJ, Lips P, Middelkoop BJ, Verhoeven I, Wuister JD. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84(2):350–353. doi: 10.1093/ajcn/84.1.350. quiz 468–359. 84/2/350 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Moghraby SA, Al Shawaf T, Akiel A, Sedrani SH, el Idrissy AT, Al-Meshari AA. Parity and vitamin D metabolites. Ann Trop Paediatr. 1987;7 (3):210–213. doi: 10.1080/02724936.1987.11748509. [DOI] [PubMed] [Google Scholar]
- 35.Toriola AT, Surcel HM, Husing A, Grankvist K, Lakso HA, Schock H, Lundin E, Lehtinen M, Lukanova A. Association of serum 25-hydroxyvitamin D (25-OHD) concentrations with maternal sex steroids and IGF-1 hormones during pregnancy. Cancer Causes Control. 2011;22(6):925–928. doi: 10.1007/s10552-011-9752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zittermann A, Schleithoff SS, Frisch S, Gotting C, Kuhn J, Koertke H, Kleesiek K, Tenderich G, Koerfer R. Circulating calcitriol concentrations and total mortality. Clin Chem. 2009;55(6):1163–1170. doi: 10.1373/clinchem.2008.120006. clinchem.2008.120006 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320(15):980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 38.Nomura AM, Stemmermann GN, Lee J, Kolonel LN, Chen TC, Turner A, Holick MF. Serum vitamin D metabolite levels and the subsequent development of prostate cancer (Hawaii, United States) Cancer Causes Control. 1998;9 (4):425–432. doi: 10.1023/a:1008875819232. [DOI] [PubMed] [Google Scholar]
- 39.Reis JP, von Muhlen D, Michos ED, Miller ER, 3rd, Appel LJ, Araneta MR, Barrett-Connor E. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207(2):585–590. doi: 10.1016/j.atherosclerosis.2009.05.030. S0021-9150(09)00423-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. 2009;6(10):621–630. doi: 10.1038/nrcardio.2009.135. nrcardio.2009.135 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47(2):87–91. doi: 10.1007/s00394-008-0700-4. [DOI] [PubMed] [Google Scholar]
- 42.Okonofua F, Menon RK, Houlder S, Thomas M, Robinson D, O’Brien S, Dandona P. Calcium, vitamin D and parathyroid hormone relationships in pregnant Caucasian and Asian women and their neonates. Ann Clin Biochem. 1987;24:22–28. doi: 10.1177/000456328702400103. [DOI] [PubMed] [Google Scholar]
- 43.Markestad T, Elzouki A, Legnain M, Ulstein M, Aksnes L. Serum concentrations of vitamin D metabolites in maternal and umbilical cord blood of Libyan and Norwegian women. Hum Nutr Clin Nutr. 1984;38 (1):55–62. [PubMed] [Google Scholar]
- 44.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21 (3):215–244. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 45.Knight JA, Wong J, Blackmore KM, Raboud JM, Vieth R. Vitamin D association with estradiol and progesterone in young women. Cancer Causes Control. 2010;21(3):479–483. doi: 10.1007/s10552-009-9466-0. [DOI] [PubMed] [Google Scholar]
- 46.Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem. 2004;41(Pt 4):272–281. doi: 10.1258/0004563041201464. [DOI] [PubMed] [Google Scholar]
- 47.Stamp TC, Round JM. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature. 1974;247 (442):563–565. doi: 10.1038/247563a0. [DOI] [PubMed] [Google Scholar]
- 48.Ellis G, Dixon K. Sequential-saturation-type assay for serum 25-hydroxyvitamin D. Clin Chem. 1977;23 (5):855–862. [PubMed] [Google Scholar]
- 49.Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr. 2009;101(2):278–284. doi: 10.1017/S0007114508981460. S0007114508981460 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]