Abstract
Activating mutations in the receptor tyrosine kinase KIT, most notably KIT D816V, are commonly observed in patients with systemic mastocytosis. Thus, inhibition of KIT has been a major focus for treatment of this disorder. Here we investigated a novel approach to such inhibition. Utilizing rational drug design, we targeted the switch pocket (SP) of KIT which regulates its catalytic conformation. Two SP inhibitors thus identified, DP-2976 and DP-4851, were examined for effects on neoplastic mast cell proliferation and mast cell activation. Autophosphorylation of both wild type (WT) and, where also examined, KIT D816V was blocked by these compounds in transfected 293T cells, HMC 1.1 and 1.2 human mast cell lines; and in CD34+-derived human mast cells activated by stem cell factor (SCF). Both inhibitors induced apoptosis in the neoplastic mast cell lines and reduced survival of primary bone marrow mast cells from patients with mastocytosis. Moreover, the SP inhibitors more selectively blocked SCF potentiation of FcεRI-mediated degranulation. Overall, SP inhibitors represent an innovative mechanism of KIT inhibition whose dual suppression of KIT D816V neoplastic mast cell proliferation and SCF enhanced mast cell activation may provide significant therapeutic benefits.
Keywords: mastocytosis, switch pocket, KIT, KIT D816V, tyrosine kinase inhibitor, mast cell
Introduction
KIT is a member of the tyrosine kinase-containing family of growth factor receptors which is expressed on restricted hematopoietic cell lineages including mast cells.(1) Activation of KIT, following ligation by stem cell factor (SCF), is critical for mast cell growth, survival, differentiation and homeostasis and may contribute to mast cell homing to resident tissues.(2) The extent of antigen-mediated mast cell activation within these tissues also has the capacity to be up regulated by SCF-dependent KIT activation.(3) Hence inappropriate KIT activation profoundly influences mast cell-driven pathology.
Systemic mastocytosis is characterized by the pathologic accumulation of neoplastic mast cells in various tissues accompanied by activating mutations in KIT, most notably the aspartic acid to valine substitution at residue 816 (KIT D816V).(4) Clinically, this clonal accumulation often results in persistent mast cell mediator-related symptoms and, in rare cases, aggressive neoplastic overgrowth with end organ failure. In these latter presentations, lifespan is significantly reduced and cytoreductive therapy is required.(5) To date, tyrosine kinase inhibitors targeting the KIT D816V mutation have demonstrated only modest efficacy in advanced disease states and are often accompanied by significant side effects, likely the result of off-target kinase inhibition.(6–9)
Most tyrosine kinase inhibitors target the ATP binding pocket which is often conserved amongst kinases and subject to competition from high intracellular ATP concentrations. For reasons not clearly understood, certain ATP competitive inhibitors do not potently inhibit oncogenic forms of kinases which adopt aggressive, constitutively active conformations independent of normal regulatory mechanisms. KIT kinase can be aggressively activated in this manner and KIT mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients.(10)
KIT has an interior pocket located between the N- and C-lobes of the kinase which functions as a switch pocket (SP). KIT also has two pendent switch ligands which compete for occupancy of this SP.(11) If the inhibitory switch (exon 11 juxtamembrane domain) of KIT occupies this SP, the kinase adopts an inactive conformation. If the activating switch (exon 17 activation loop) occupies this SP, the kinase adopts a catalytically active conformation. Thus, changes in phosphorylation of both the inhibitory and activating switches govern the overall activity of KIT by regulation of the occupancy of its SP by its pendent inhibitory and activating switches.
Primary mutations and/or deletions in the exon 11 inhibitory switch render KIT constitutively active and such mutations drive the progression of gastrointestinal stromal tumors (GIST).(12) In mast cell leukemia/mastocytosis, the primary KIT mutation resides in the activating switch, and in particular, the D816V exon 17 mutation is most aggressive. Classical ATP competitive KIT inhibitors, including imatinib, sunitinib, sorafenib, regorafenib, do not block this aggressive mutant form of KIT. The SP inhibitors, DP-2976 and DP-4851, were developed by rational design to block access to the KIT SP and prevent KIT D816V from adopting the catalytically active conformation. Additionally, because SP inhibitors are not ATP competitive, they are resilient even to high 4–5 mM concentrations of cellular ATP.
Here we describe the ability of two KIT SP inhibitors, DP-2976 and DP-4851, to potently and selectively inhibit both wild type (WT) and KIT D816V activation and consequently proliferation of both neoplastic and non-neoplastic mast cell populations. Furthermore, these inhibitors effectively blocked the ability of SCF to enhance antigen-mediated mast cell activation. These studies demonstrate that by targeting the switch pocket, this class of KIT inhibitors possesses a novel mechanism of inhibition whose dual suppression of KIT D816V neoplastic proliferation and SCF enhanced mast cell activation advocate future clinical development.
Patients, Materials and Methods
Reagents and antibodies
DP-2976 and DP-4851 were synthesized within Deciphera Pharmaceuticals LLC (Lawrence, KS, USA). PKC 412 and imatinib were purchased from LC laboratories (Woburn, MA, USA). All compounds were prepared and stored as a 10 mM stock solution in DMSO at −20°C. The following antibodies were used for immunoblotting: mouse anti human KIT (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti pKit (Tyr-823) (Invitrogen, Carlsbad, CA), anti β– actin monoclonal antibody (Sigma-Aldrich, St. Louis, MO), horseradish peroxidase-conjugated anti-mouse (Sigma-Aldrich, St. Louis, MO) and anti-rabbit IgG (Amersham Biosciences, Piscataway, NJ).
In vitro kinase assays
KIT catalytic activity was determined by following the production of ADP from the kinase reaction through coupling with the pyruvate kinase/lactate dehydrogenase system.(13) The reaction mixture (100 µl) contained KIT (KIT residues T544-V976, 6 nM), polyE4Y (1 mg/ml), MgCl2 (10 mM), pyruvate kinase (~ 3 units), lactate dehydrogenase (~ 4 units), phosphoenol pyruvate (1 mM), and NADH (0.28 mM) in 90 mM Tris buffer containing 0.2 % octyl-glucoside and 1% DMSO, pH 7.5. Test compounds were incubated with KIT and other reaction reagents at 30 °C for 30 min before ATP (0.2, 1.2 or 4 mM) was added to start the reaction. The absorption at 340 nm was monitored continuously for 1 h at 30°C. Percent inhibition was obtained by comparison of reaction rate with that of a control (i.e. with no test compound).
Cell culture
The HMC 1.1 and HMC 1.2 neoplastic mast cell lines were cultured as described.(14) Both cell lines carry an activating KIT V560G mutation (loss-of-function mutation in the exon 11 inhibitory switch). In addition, the HMC 1.2 cell line harbors the KIT D816V mutation (gain-of-function mutation in the exon 17 activating switch). The 293T cell line was cultured in Iscove’s DMEM containing 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (Mediatech, Manassas VA). Human mast cells (HuMCs) derived from CD34+ pluripotent blood progenitor cells were cultured as described.(15)
Cell transfections
pcDNA3-KIT (GNNK+ variant(16)) was a kind gift from Gunnar Nilsson (Karolinska Institutet, Stockholm, Sweden). The KIT D816V mutation was created using the QuickChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. KIT and KIT D816V open reading frames were cloned into the pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA) using standard molecular biology techniques.(17) These vectors were transfected into 293T cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. All transfections were carried out in DMEM medium containing 10% fetal bovine serum and 2 mM L-Glutamine. Three hundred thousand cells were plated in 6 well plates in 2 ml medium, and cultured overnight. The next day, the medium was replaced with 1 ml of fresh medium to which 1 µg of DNA and 5 µl of lipofectamine in 100 µl of Opti-MEM (Invitrogen, Carlsbad, CA) were added. Inhibitors (1 – 1000 nM in a final concentration of 0.1% DMSO) were added to the indicated wells. After 24 h, cells were washed twice in PBS and lysed using 100 µl of RIPA buffer (Thermo Fisher, Pittsburgh, PA). The protein concentrations were measured using Bradford assay (Bio Rad, Hercules, CA) and 20 µg of protein were used for immunoblot analysis as described.(18) Immunoreactive proteins were visualized with enhanced chemiluminesence (ECL) (Perkin Elmer Life Sciences, Waltham, MA) and the density of the appropriate bands was determined to quantitate the changes in phosphorylation.
Cell proliferation assay
HMC 1.1 and HMC 1.2 cells were plated at 5×104 cells/mL with the inhibitors (1 – 1000 nM) in a final concentration of 0.1% DMSO. After 72 h, an equal volume of 2X CyQuant direct detection reagent (Invitrogen) was added into the cells in culture. Following a 1 h incubation at 37°C with detection reagent, sample fluorescence was detected by using 492/535 nm wavelengths filter sets.
Apoptosis assay
HMC 1.1 and HMC 1.2 cells were plated at 1×105 cells/mL with 1000 nM of the inhibitors in DMSO (final concentration 0.1%). At 24, 48 and 72 h, annexin V staining using the Annexin V-FITC Apoptosis Detection Kit from BioVision (Mountain View, CA) was performed according to the manufacturer’s instructions. The samples were analyzed using a FACSCalibur (BD Biosciences, San Jose, CA) flow cytometer equipped with Cellquest (BD Biosciences) software.
Human mast cell degranulation assay
HuMCs were sensitized overnight with biotinylated-human IgE (100 ng/ml) in cytokine-free medium and rinsed with HEPES buffer (10 mM HEPES pH7.4, 137 mM NaCl, 27 mM KCl, 0.4 mM Na2HPO4.7H2O, 5.6 mM glucose, 1.8 mM CaCl22H2O, 1.3 mM MgSO4.7H2O) containing 0.04% bovine serum albumin.(19) Five thousand cells per well were plated in 96 well plates and preincubated in the presence and absence of inhibitors for 90 min at 37°C. The cells were then triggered with either 1 ng/ml streptavidin or 0.1 ng/ml streptavidin in the presence or absence of 10 ng/ml SCF. After 30 min, β–hexosaminidase (β-hex) activity in the supernatants and remaining cells was determined and degranulation was determined as the percentage of the total β-hex recovered from the supernatants.(20)
KIT phosphorylation
HuMCs were incubated overnight in growth medium without SCF and then washed 3 times in HEPES buffer containing 0.04% BSA. One million cells in 100 µl of HEPES buffer containing 0.04% BSA were pre-incubated with or without the inhibitors (1 – 1000 nM in 0.1% DMSO) for 90 min at 37°C and then the cells were incubated with 10 ng/ml SCF for 2 min. Cell lysates were prepared and 20 µl aliquots were loaded on to 4 – 12% NuPAGE Bis-Tris gels for electrophoretic separation and immunoblotting as described.(18) Immunoreactive proteins were visualized with enhanced chemiluminesence ECL (Perkin Elmer Life Sciences, Waltham, MA) and the density of the appropriate bands was determined to quantitate the changes in phosphorylation.
HMC 1.1 and HMC 1.2 cells were incubated for 3 h in Iscove’s DMEM media without FBS and then washed 3 times in HEPES buffer. Three hundred thousand cells in 100 µl of HEPES buffer containing 0.04% BSA were incubated with/without the inhibitors (1 – 1000 nM in 0.1% DMSO) for 90 min at 37°C. Cell lysate preparation and immunoblotting was performed in the same manner as above.
Patient samples and ex vivo mast cell experiments
Six patients with systemic mastocytosis were evaluated at the NIH between 2010 and 2011 as part of an Institutional Review Board-approved research protocol designed to study the pathogenesis and natural history of systemic mastocytosis (NCT00044122). All patients were diagnosed according to the World Health Organization (WHO) criteria.(5) The presence of the KIT D816V mutation was determined by PCR/RFLP as described.(21) Following informed consent, bone marrow mononuclear cells were isolated and incubated with the inhibitors (100, 1000 nM, 0.1% DMSO) for 7 d in the presence of SCF (100 ng/ml). Mast cell numbers were assessed by flow cytometry and normalized to numbers in the absence of inhibitor.(22)
Statistical analysis
Statistical analysis was performed using PRISM software, version 5 (GraphPad). Data were analyzed by a two tailed Student’s t test. The levels of significance were as follows: *, p<0.05; **, p<0.005; ***, p<0.0005. IC50 values were calculated using a nonlinear regression (curve fit)--sigmoidal dose-response equation.
Results
DP-2976 and DP-4851 selectively inhibit WT and mutant KIT kinase activity at nanomolar concentrations
As part of drug discovery efforts, DP-2976 and DP-4851 were optimized for their inhibitory activity in KIT kinase assays. Low nanomolar IC50 values were observed in WT and mutated forms of KIT, including KIT D816V (Figure 1A). The inhibitory potencies were unaffected by increases in ATP concentrations up to 4 mM (Figure 1B), demonstrating that the ATP binding pocket was not targeted.
Figure 1. KIT SP inhibitors suppress WT and mutant KIT kinase activity at nanomolar concentrations via an ATP independent mechanism.
A. WT and mutant KIT kinase activity was measured in the presence of KIT SP inhibitors using the PK/LDH system. B. WT KIT kinase activity was measured in the presence of KIT SP inhibitors with varying concentrations (0.2, 1.2 or 4 mM) of ATP added to the reaction. IC50’s were calculated using the mean results of three separate experiments as shown for DP-2976 and DP-4851.
DP-2976 and DP-4851 inhibit intracellular KIT phosphorylation
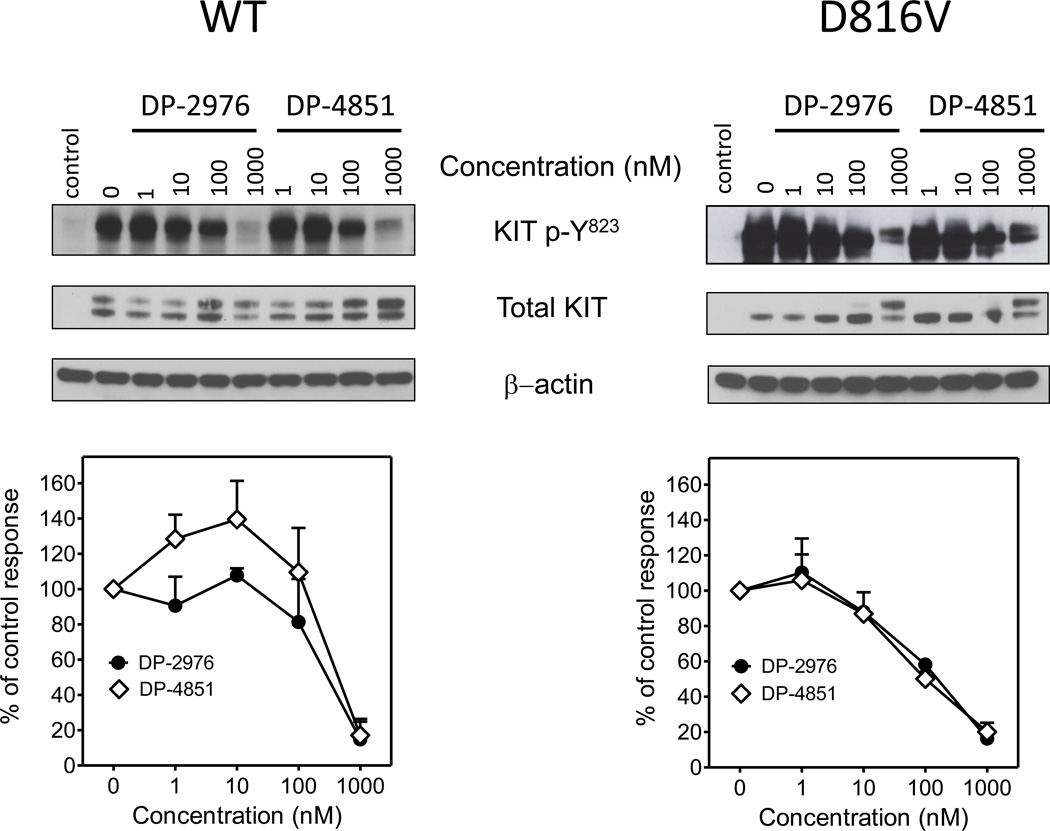
To explore the effects of the SP inhibitors on intracellular KIT activity, WT KIT and KIT D816V were expressed in 293T cells and the autophosphorylation status was assessed. Both DP-2976 and DP-4851 decreased the autophosphorylation of both wild type and KIT D816V in a concentration-dependent manner (Figure 2).
Figure 2. KIT SP inhibitors suppress KIT autophosphorylation in transfected 293T cells.
KIT and KIT D816V open reading frames were cloned into the pCDNA3.1 expression vector and transfected into 293T cells. The transfected cells were incubated with the KIT SP inhibitors (1 – 1000 nM, 0.1% DMSO) for 24 h. The cells were lysed and used for immunoblot analysis. The blots are representative of three separate experiments. The graphs were generated by scanning the blots of the three separate experiments and then normalizing to the SCF untreated condition. Data are presented as means ± SEM.
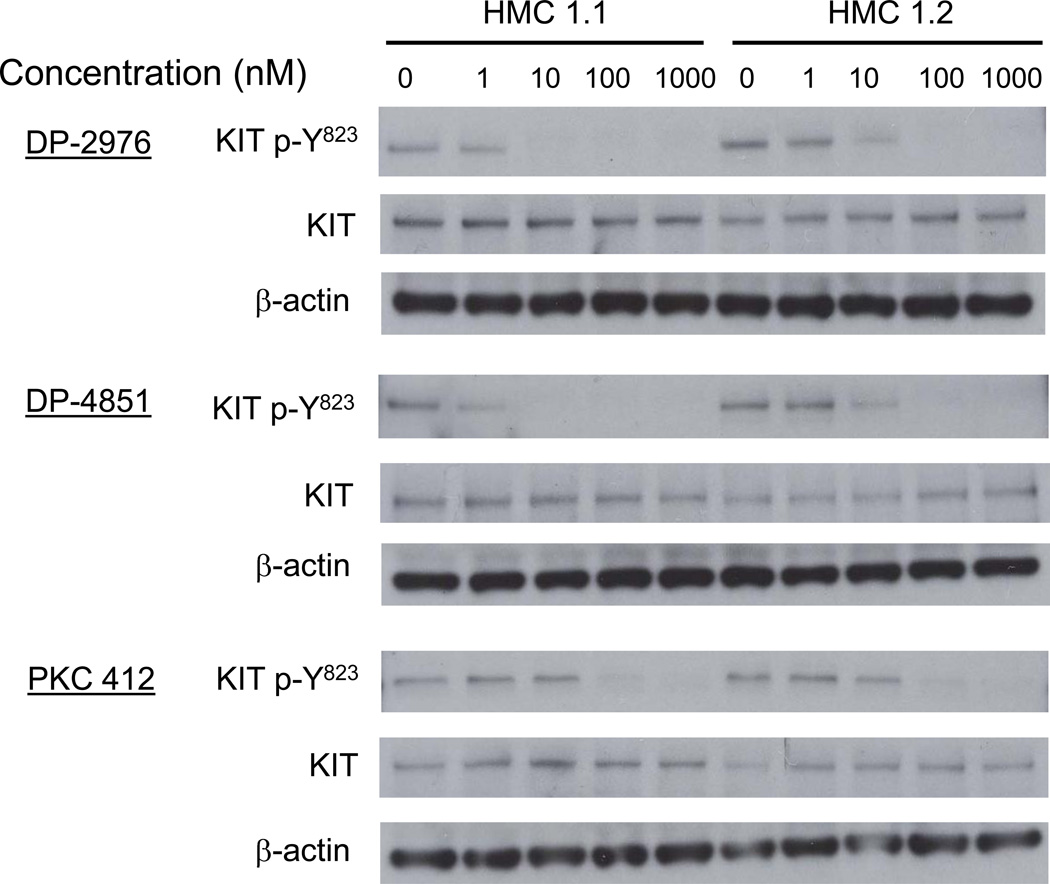
The effect of the inhibitors on KIT phosphorylation was also examined in the HMC 1.1 (negative for D816V mutation) and HMC 1.2 (positive for D816V mutation) neoplastic human mast cell lines. DP-2976 and DP-4851 inhibited KIT phosphorylation in a dose dependent manner. Furthermore, these compounds appeared more potent than PKC 412 in this model (Figure 3).
Figure 3. KIT SP inhibitors suppress KIT autophosphorylation in HMC-1 mast cell lines.
HMC 1.1 and HMC 1.2 cells were incubated for 3 h in Iscove’s DMEM media without FBS. The cells were washed 3 times in HEPES buffer containing 0.04% BSA. Three hundred thousand cells in 100 µl were then pre-incubated with the inhibitors (1 – 1000 nM, 0.1% DMSO) for 90 min at 37°C and 20 µl of the cell lysate was used for immunoblot analysis. The blots are representative of three separate experiments.
DP-2976 and DP-4851 inhibit growth and induce apoptosis in HMC-1.1 and HMC1.2 mast cell lines at nanomolar concentrations
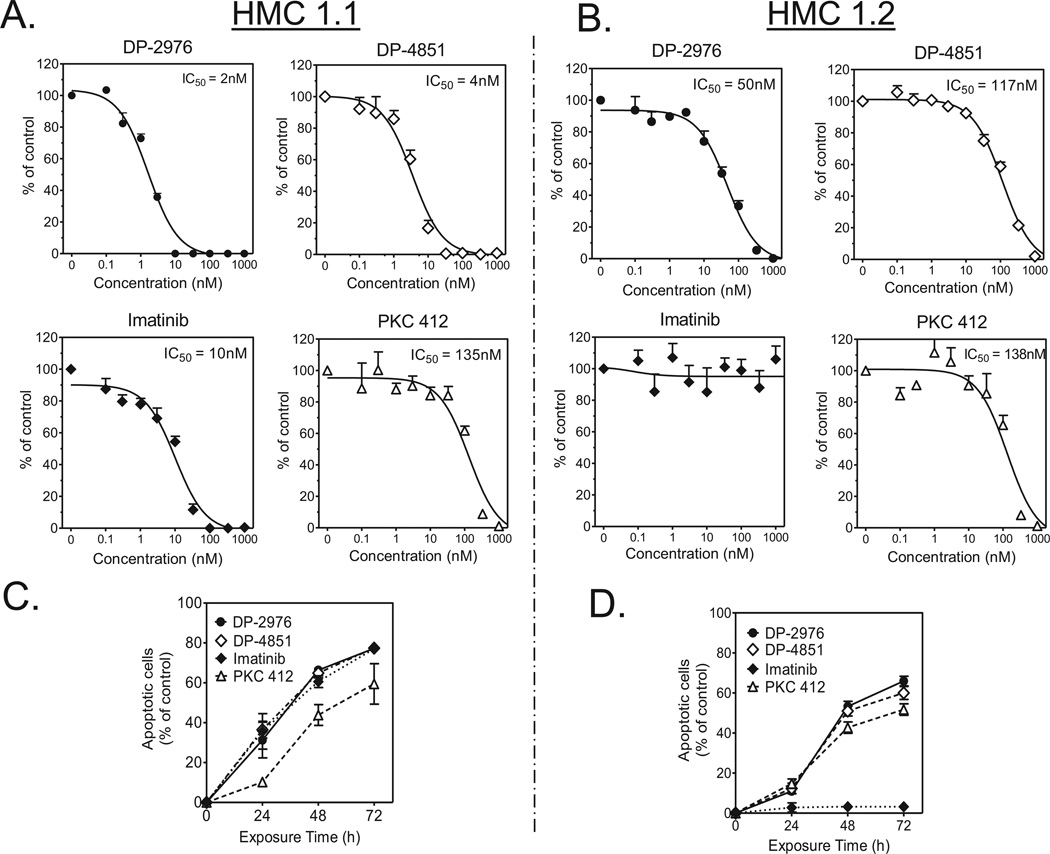
We next assessed the anti-neoplastic activity of the SP inhibitors in HMC 1.1 and HMC 1.2 cell proliferation assays. Both DP-2976 and DP-4851 potently inhibited HMC 1.1 proliferation with IC50 values of 2 nM and 4 nM respectively. These values were lower than that of imatinib (10 nM) or PKC 412 (135 nM) (Figure 4A). Similarly, when evaluated in HMC 1.2 cells bearing the KIT D816V mutation, both DP-2976 and DP-4851 inhibited cell proliferation with IC50 values of 50 nM and 117 nM respectively. Similar to previous reports, imatinib demonstrated no activity and PKC 412 demonstrated an IC50 of 138 nM (Figure 4B).(23)
Figure 4. KIT SP inhibitors suppress proliferation of, and induce apoptosis in, HMC-1 mast cell lines.
A. HMC 1.1 and B. HMC 1.2 cells were cultured in the presence of inhibitors (1 – 1000 nM, 0.1% DMSO) for 72 h. A CyQuant cell proliferation assay was used to assess growth inhibition. Values represent the % viable cells as compared to untreated controls. The data are presented as means +/− SEM of three separate experiments each performed in triplicate. C. HMC 1.1 and D. HMC 1.2 cells were cultured in the presence of 1000 nM inhibitor. At various time points, the cells were double stained with annexin V-FITC and Sytox Green dye. Cells stained with annexin V were defined as apoptotic. Baseline values of annexin V positive cells in untreated cultures were subtracted from each treatment. The percent of apoptotic cells for each culture is plotted. The data are presented as the means ± SEM of three separate experiments, each performed in triplicate.
The HMC 1.1 and HMC 1.2 cells were then treated with the inhibitors (1000 nM) and evaluated for apoptosis over 72 h. A time dependent increase in apoptosis was induced by the SP inhibitors in both cell lines, with over 60% of the cells annexin-V positive at the 72 h (Figures 4C and 4D). Together, the SP inhibitors exhibited potent anti-neoplastic activity via apoptosis and outperformed current ATP competitive inhibitors in this system.
DP-2976 and DP-4851 inhibit survival of KIT D816V+ primary bone marrow mast cells
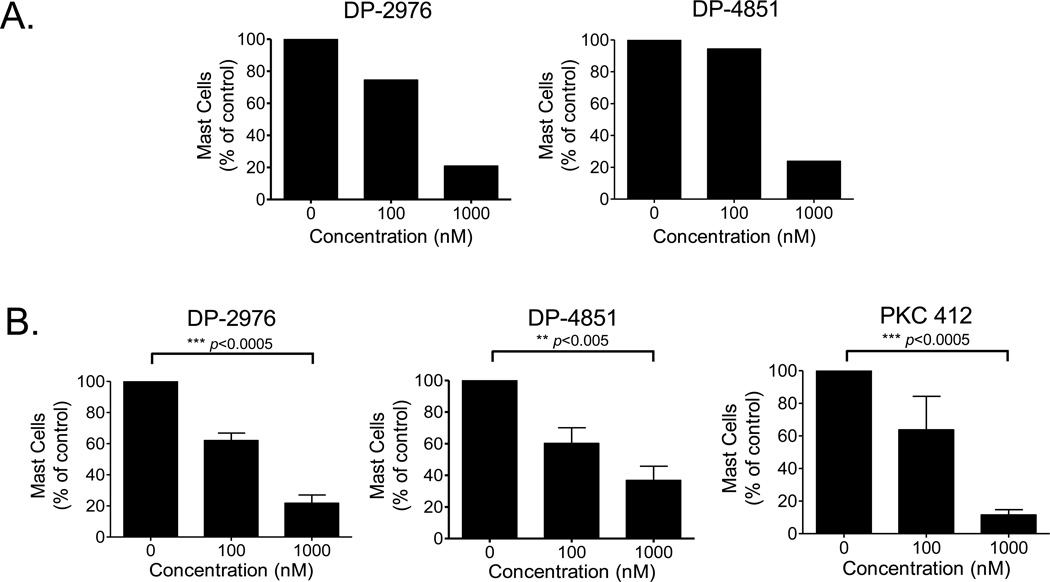
To determine if the in vitro observations translate clinically, we examined the efficacy of the SP inhibitors on neoplastic mast cells of patients with systemic mastocytosis. Patient characteristics are presented in Table 1. Patient #1 lacked the KIT D816V mutation and mast cells from this patient exhibited a dose dependent decrease in survival in response to the SP inhibitors (Figure 5A). Furthermore, DP-2976 (1 µM) produced a 78% reduction (p<0.0005) and DP-4851 a 63% reduction (p<0.005) in the viability of mast cells collected from the bone marrow of KIT D816V-positive systemic mastocytosis patients (n=5) (Figure 5B). A comparable level of reduction (88%) was seen with PKC 412 at 1000 nM (p<0.0005). No statistical difference in inhibition profiles was observed in the absence of SCF (data not shown). These results support that targeting the KIT switch pocket offers an innovative mechanism of inhibition with potential clinical application.
Table 1.
Characteristics of study subjects with Systemic Mastocytosis
| Patient | WHO Classification |
Age (years) |
Gender | Serum Tryptase (ng/mL) |
Bone Marrow Mast Cell Aggregates |
% Mast Cell Involvement of Bone Marrow |
CD2/CD25 Expression on Mast Cells |
KIT D816V |
|---|---|---|---|---|---|---|---|---|
| 1 | Indolent | 67 | F | 27 | + | 10 | − | − |
| 2 | Indolent | 65 | F | 118 | + | 10–15 | + | + |
| 3 | Indolent | 24 | F | 10 | − | <5 | + | + |
| 4 | Indolent | 24 | F | 80 | + | 10–15 | + | + |
| 5 | Smoldering | 55 | F | 119 | + | 20–30 | + | + |
| 6 | Smoldering | 48 | F | 166 | + | 50 | + | + |
Figure 5. KIT SP inhibitors decrease the survival of primary bone marrow mast cells from patients with systemic mastocytosis.
Bone marrow mononuclear cells from patients with systemic mastocytosis A. without the KIT D816V mutation (n=1) or B. with the KIT D816V mutation (n=5) were isolated and incubated with the inhibitors (0, 100, 1000 nM in 0.1% DMSO) for 7 d in the presence of SCF (100 ng/ml). Mast cell numbers were assessed by flow cytometry and normalized to values in the absence of inhibitor. The data in B are presented as the mean ± SEM of results from five patients with KIT D816V systemic mastocytosis.
DP-2976 and DP-4851 suppress KIT phosphorylation in primary human mast cells
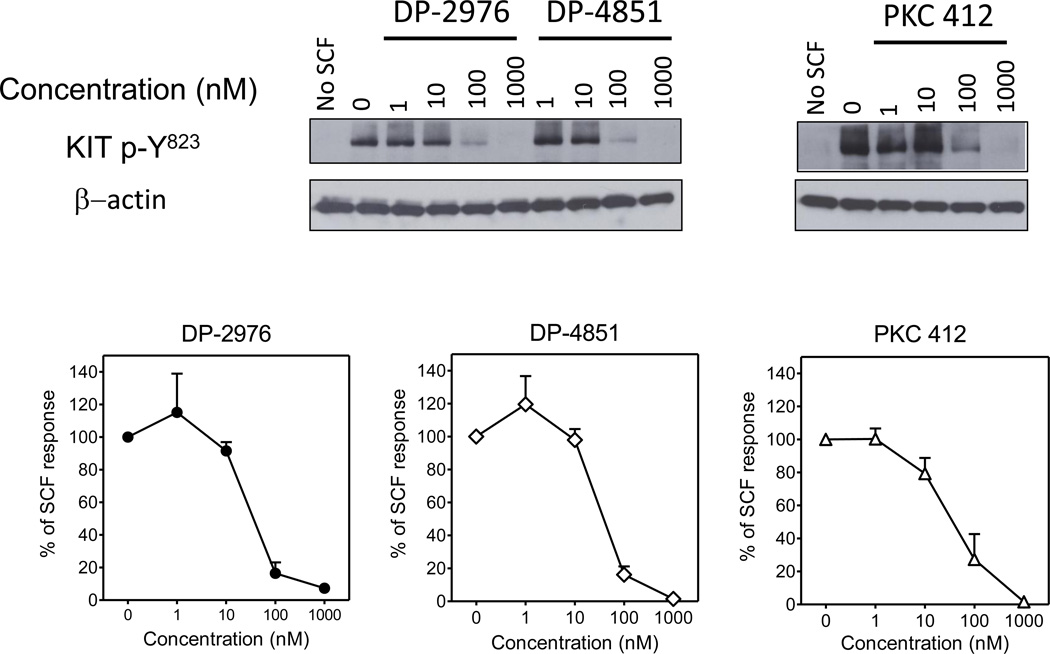
We next examined the effects of the inhibitors on WT KIT signaling in non-neoplastic HuMCs derived from peripheral blood progenitors, in response to SCF (10 ng/ml). In agreement with the transfection experiments (see figure 2), both DP-2976 and DP-4851 inhibited KIT phosphorylation in a concentration-dependent manner (Figure 6).
Figure 6. KIT SP inhibitors suppress KIT phosphorylation in primary HuMCs.
HuMCs were pre-incubated for 90 min with the inhibitors (1 – 1000 nM, 0.1% DMSO). The cells were then stimulated with SCF (10 ng/ml) for 2 min, lysed and proteins separated by gel electrophoresis for immunoblot analysis. The blots are representative of three separate experiments. The graphs were generated by scanning the blots of the three separate experiments and then normalizing to the untreated condition. The data are presented as the mean ± SEM.
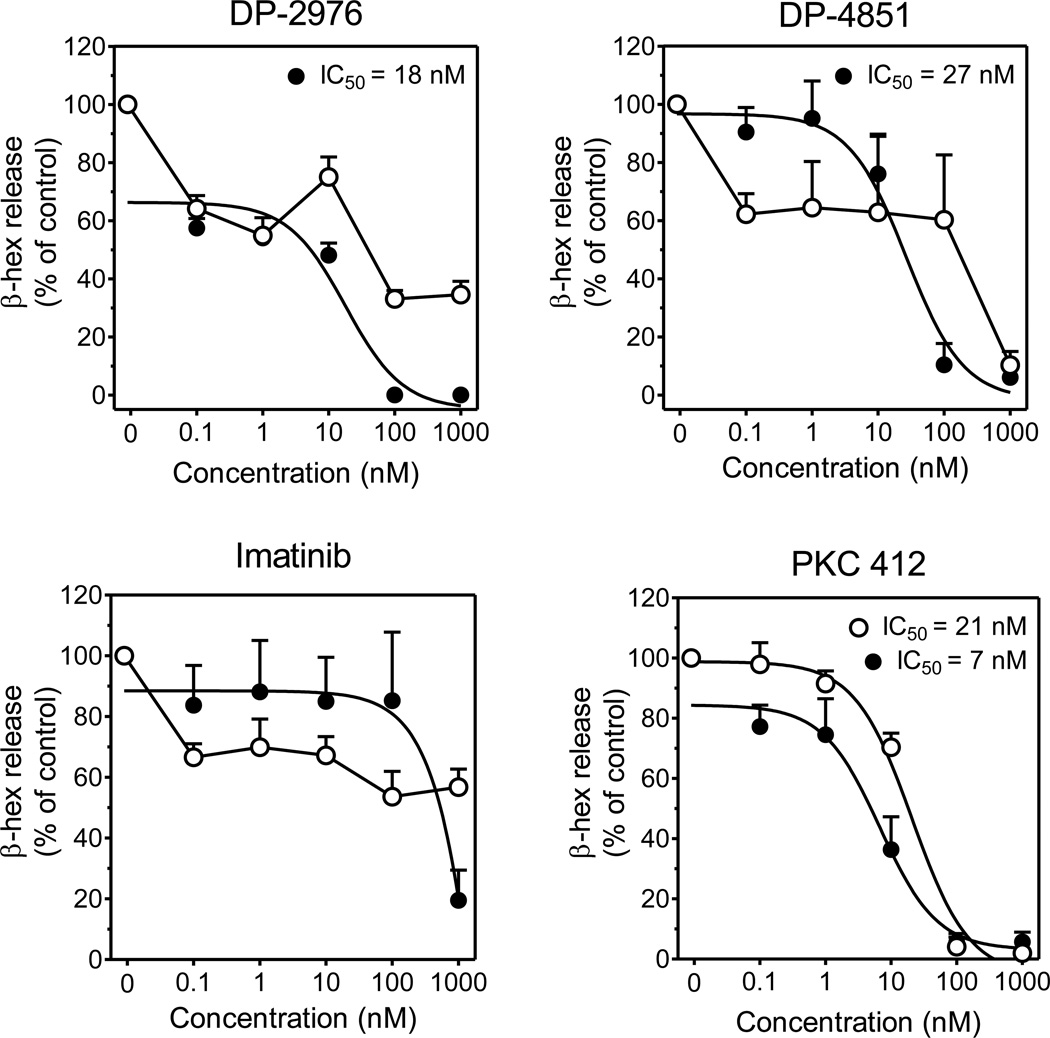
DP-2976 and DP-4851 inhibit SCF-enhanced mast cell activation
In addition to the end organ damage induced by neoplastic mast cell proliferation, patients with systemic mastocytosis suffer from mast cell activation and mediator-related symptoms. Enhanced mast cell degranulation via KIT activation is well described and targeting such may provide therapeutic benefit. With this in mind we evaluated the SP inhibitors for their effect on SCF enhanced mast cell degranulation. DP-2976, DP-4851 and imatinib had minimal effects on FcεRI mediated mast cell degranulation (Figure 7, open circles), with inhibition observed only at the highest concentration (1000 nM). SCF-enhanced degranulation (Figure 7, solid circles) was evaluated in tandem and as previously observed, imatinib inhibited SCF-enhanced degranulation at the highest concentration (1000 nM).(19) Additionally, both DP-2976 and DP-4851 also suppressed SCF-enhanced degranulation in a concentration-dependent manner with IC50 values of 18 nM and 27 nM respectively. In contrast, in addition to blocking SCF-enhanced degranulation (IC50=7nM), PKC 412 potently inhibited FcεRI-mediated degranulation (IC50=21nM). A similar reported observation suggested this to be the result of the inherent multi-kinase inhibition profile of PKC 412.(24)
Figure 7. KIT SP inhibitors suppress SCF-enhanced degranulation of primary HuMCs.
HuMCs were sensitized overnight and pre-incubated with inhibitors for 90 min. Cells were then stimulated with ○ Ag (1 ng/ml) or ● Ag (0.1 ng/ml) +SCF (10 ng/ml) for 30 min. Degranulation was determined as % release of β-hexosaminidase. The data are presented as the mean ± SEM of three separate experiments each performed in triplicate.
Discussion
Targeting kinase control switch pockets appears a promising approach to effectively inhibit oncogenic kinase activity. Recently, DCC-2036 was identified as a switch pocket inhibitor of ABL kinase.(25, 26) The gatekeeper mutant T315I represents an escape mutant of ABL and the oncogenic fusion protein BCR-ABL T315I is not readily controlled by currently marketed therapies.(27) DCC-2036 potently blocks ABL T315I, signifying that SP inhibitors may hold promise to suppress other kinases harboring oncogenic mutations. Indeed, this study demonstrates that the KIT SP inhibitors, DP-2976 and DP-4851, effectively inhibit the KIT D816V activating mutation and offer a novel mechanism to inhibit neoplastic mast cell proliferation and mast cell activation.
The inability of high ATP concentrations (4 mM) to blunt the inhibitory potencies of DP-2976 and DP-4851 further demonstrated that these KIT SP inhibitors function through an ATP-independent mechanism of action. Additionally, whereas ATP binding pockets are highly conserved throughout the kinome, switch pockets possess greater structural diversity. As a result, compounds targeting the switch pocket may inhibit kinase activity with higher selectivity. Indeed, the KIT SP inhibitors, DP-2976 and DP-4851, both displayed low nanomolar IC50 values in WT and KIT D816V kinase assays with modest off-target inhibition against other kinases (data not shown). Further, in our cell based assays, the KIT SP inhibitors displayed specific inhibition of KIT phosphorylation and KIT mediated (SCF) enhanced mast cell degranulation (Figures 2, 3, 6, 7).
The ATP-competitive kinase inhibitor which has demonstrated the most promise for advanced forms of systemic mastocytosis is PKC 412.(28, 29) PKC 412 is a multikinase inhibitor shown to not only inhibit neoplastic mast cell proliferation, but also antigen/IgE-dependent mediator release in human blood basophils and mast cells.(23, 24) Both DP-2976 and DP-4851 exhibited more potent inhibition profiles compared to PKC 412 in the HMC 1.1 (KIT D816V negative) and HMC 1.2 (KIT D816V positive) neoplastic mast cell lines and induced similar levels of apoptosis (Figure 4). Ex vivo studies examining the effects of the KIT SP inhibitors on neoplastic mast cells from patients with KIT D816V systemic mastocytosis displayed significant levels of inhibition comparable to PKC 412 (Figure 5). Overall, DP-2976 and DP-4851 inhibited neoplastic mast cell proliferation and/or survival at a level similar to (if not greater) than PKC 412, which is currently in a phase II clinical trial for systemic mastocytosis.
As our molecular understanding of advanced forms of systemic mastocytosis evolves, it has become apparent that cooperating genetic events may account for the diverse phenotypes and variable therapeutic responses observed in the disease.(30–32) Therefore, a multi-targeted therapeutic approach may be required.(33) Whether the KIT SP inhibitors produce a synergistic effect when used in combination with kinase inhibitors targeting the ATP binding site or KIT independent targets is an area of further investigation. The selectivity of the KIT SP inhibitors should be advantageous in a combinational therapeutic approach.
In summary, we have demonstrated that targeting the switch pocket of KIT offers a novel and selective method to effectively suppress KIT D816V neoplastic proliferation and SCF enhanced mast cell activation. Our studies provide a strong rationale for further clinical development of KIT SP inhibitors for mastocytosis and other KIT driven disorders.
Acknowledgements
We thank Dr. Cem Akin (Brigham and Women’s Hospital) for technical advice with the ex vivo studies and Sarka Smrzova (LAD) for technical support with human mast cell cultures. We thank all the subjects as well as the LAD clinical research staff for their contributions.
Funding: This study was supported in part by the Division of Intramural Research of the NIAID, NIH. Deciphera Pharmaceuticals supplied the study compounds and performed the in vitro kinase assays.
Footnotes
Authorship Contributions
Y.B., G.B., S.N.B., E.C.C., O.S., W-P.L. and T.M.W. performed the research. T.M.W., A.M.G., D.D.M., I.M., S.C.W., and D.L.F. designed the research. T.M.W. and D.D.M. recruited and cared for the patients. Y.B., G.B., E.C.C., O.S., I.M., S.C.W., D.L.F., D.D.M., and T.M.W. analyzed and interpreted the data. Y.B., G.B., A.M.G. and T.M.W. wrote the paper with edits from all authors.
Conflict of Interest
The NIH authors reported no potential conflicts of interest. W-P.L., S.C.W. and D.L.F. are employees of Deciphera Pharmaceuticals, which is the owner of DP-2976 and DP-4851, the compounds studied and reported in this work.
References
- 1.Escribano L, Ocqueteau M, Almeida J, Orfao A, San Miguel JF. Expression of the c-kit (CD117) molecule in normal and malignant hematopoiesis. Leuk Lymphoma. 1998 Aug;30(5–6):459–466. doi: 10.3109/10428199809057558. [DOI] [PubMed] [Google Scholar]
- 2.Jensen BM, Akin C, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. Br J Pharmacol. 2008 Aug;154(8):1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004 Oct 15;104(8):2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 4.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horny H-P, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, Valent P. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffee ES, Pileri SA, Stein H, Thiele J, Varderman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edn. Lyon, France: International Agency for Research on Cancer; 2008. pp. 54–63. [Google Scholar]
- 6.Verstovsek S, Tefferi A, Cortes J, O'Brien S, Garcia-Manero G, Pardanani A, et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res. 2008 Jun 15;14(12):3906–3915. doi: 10.1158/1078-0432.CCR-08-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vega-Ruiz A, Cortes JE, Sever M, Manshouri T, Quintas-Cardama A, Luthra R, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009 Nov;33(11):1481–1484. doi: 10.1016/j.leukres.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreas H, Ottmann OG, Lauber S, Hughes T, Verhoef G, Schwarer AP, et al. A Phase II Study of Nilotinib, a Novel Inhibitor of c-Kit, PDGFR, and Bcr-Abl, Administered to Patients with Systemic Mastocytosis. Blood (ASH Annual Meeting Abstracts) 2006;108 abstract 2703. [Google Scholar]
- 9.Ustun C, DeRemer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res. 2011 Sep;35(9):1143–1152. doi: 10.1016/j.leukres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Gajiwala KS, Wu JC, Christensen J, Deshmukh GD, Diehl W, DiNitto JP, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proceedings of the National Academy of Sciences of the United States of America. 2009 Feb 3;106(5):1542–1547. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mol CD, Dougan DR, Schneider TR, Skene RJ, Kraus ML, Scheibe DN, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem. 2004 Jul 23;279(30):31655–31663. doi: 10.1074/jbc.M403319200. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher JA, Rubin BP. KIT mutations in GIST. Current opinion in genetics & development. 2007 Feb;17(1):3–7. doi: 10.1016/j.gde.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000 Sep 15;289(5486):1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 14.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12(4):345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 15.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999 Oct 1;94(7):2333–2342. [PubMed] [Google Scholar]
- 16.Caruana G, Cambareri AC, Ashman LK. Isoforms of c-KIT differ in activation of signalling pathways and transformation of NIH3T3 fibroblasts. Oncogene. 1999 Sep 30;18(40):5573–5581. doi: 10.1038/sj.onc.1202939. [DOI] [PubMed] [Google Scholar]
- 17.Iwaki S, Spicka J, Tkaczyk C, Jensen BM, Furumoto Y, Charles N, et al. Kit- and Fc epsilonRI-induced differential phosphorylation of the transmembrane adaptor molecule NTAL/LAB/LAT2 allows flexibility in its scaffolding function in mast cells. Cell Signal. 2008 Jan;20(1):195–205. doi: 10.1016/j.cellsig.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in Fc epsilon RI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. Journal of immunological methods. 2002 Oct 15;268(2):239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- 19.Jensen BM, Beaven MA, Iwaki S, Metcalfe DD, Gilfillan AM. Concurrent inhibition of kit- and FcepsilonRI-mediated signaling: coordinated suppression of mast cell activation. J Pharmacol Exp Ther. 2008 Jan;324(1):128–138. doi: 10.1124/jpet.107.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woolhiser MR, Okayama Y, Gilfillan AM, Metcalfe DD. IgG-dependent activation of human mast cells following up-regulation of FcgammaRI by IFN-gamma. Eur J Immunol. 2001 Nov;31(11):3298–3307. doi: 10.1002/1521-4141(200111)31:11<3298::aid-immu3298>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006 Jul 1;108(1):286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- 22.Akin C, Brockow K, D'Ambrosio C, Kirshenbaum AS, Ma Y, Longley BJ, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol. 2003 Aug;31(8):686–692. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 23.Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Bohm A, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006 Jan 15;107(2):752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 24.Krauth MT, Mirkina I, Herrmann H, Baumgartner C, Kneidinger M, Valent P. Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin Exp Allergy. 2009 Nov;39(11):1711–1720. doi: 10.1111/j.1365-2222.2009.03353.x. [DOI] [PubMed] [Google Scholar]
- 25.Eide CA, Adrian LT, Tyner JW, Mac Partlin M, Anderson DJ, Wise SC, et al. The ABL switch control inhibitor DCC-2036 is active against the chronic myeloid leukemia mutant BCR-ABLT315I and exhibits a narrow resistance profile. Cancer Res. 2011 May 1;71(9):3189–3195. doi: 10.1158/0008-5472.CAN-10-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan WW, Wise SC, Kaufman MD, Ahn YM, Ensinger CL, Haack T, et al. Conformational control inhibition of the BCR-ABL1 tyrosine kinase, including the gatekeeper T315I mutant, by the switch-control inhibitor DCC-2036. Cancer Cell. 2011 Apr 12;19(4):556–568. doi: 10.1016/j.ccr.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azam M, Seeliger MA, Gray NS, Kuriyan J, Daley GQ. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nature structural & molecular biology. 2008 Oct;15(10):1109–1118. doi: 10.1038/nsmb.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotlib J, Berube C, Growney JD, Chen CC, George TI, Williams C, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005 Oct 15;106(8):2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotlib J, DeAngelo DJ, George TI, Corless CL, Linder A, Langford C, et al. KIT Inhibitor Midostaurin Exhibits a High Rate of Clinically Meaningful and Durable Responses in Advanced Systemic Mastocytosis: Report of a Fully Accrued Phase II Trial. Blood (ASH Annual Meeting Abstracts) 2010;116(21) abstract 316. [Google Scholar]
- 30.Tefferi A, Levine RL, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. Frequent TET2 mutations in systemic mastocytosis: clinical, KITD816V and FIP1L1-PDGFRA correlates. Leukemia. 2009 May;23(5):900–904. doi: 10.1038/leu.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson TM, Maric I, Simakova O, Bai Y, Chan EC, Olivares N, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011 Mar;96(3):459–463. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sotlar K, Bache A, Stellmacher F, Bultmann B, Valent P, Horny HP. Systemic mastocytosis associated with chronic idiopathic myelofibrosis: a distinct subtype of systemic mastocytosis associated with a [corrected] clonal hematological non-mast [corrected] cell lineage disorder carrying the activating point mutations KITD816V and JAK2V617F. J Mol Diagn. 2008 Jan;10(1):58–66. doi: 10.2353/jmoldx.2008.070061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleixner KV, Mayerhofer M, Sonneck K, Gruze A, Samorapoompichit P, Baumgartner C, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica. 2007 Nov;92(11):1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]