Abstract
Background and aims
Recent literature shows that accelerated Portland cement (APC) is a non-toxic material that may have potential to promote bone healing. The objective of this study was to histologically evaluate periodontal healing focusing on new bone regeneration following implantation of APC into intra-bony defects in dogs.
Materials and methods
Three-wall intra-bony periodontal defects were surgically created at the mesial aspect of the first molar in both sides of mandible in six dogs. One side was randomly filled with the material and other received a flap operation only. The animals were euthanized eight weeks post-surgery when block sections of the defect sites were collected and prepared for qualitative histological analysis.
Results
Compared to control group, stimulation of growth of new bone tissue in the cavity con-taining APC was significantly prominent in three of six cases, showing osteoid formation with osteoblastic rimming and new bone trabeculla. New bone formation was observed just close to cavity containing APC. Connective tissue proliferation and downgrowth of epithelium were signif-icantly less than those of control group.
Conclusion
Our results are encouraging for the use of APC as a bone substitute, but more comprehensive study are necessary before warranting clinical use.
Keywords: Accelerated Portland cement, bone substitutes, osteogenesis
Introduction
Mineral trioxide aggregate (MTA) is an endodontic material that was first used as a root-end filling material.1 The material has also been used for capping of pulp, root-end closure and repairing furcal perforations.2 , 3 In vitro studies have proven the biocompatibility of MTA.1 , 4 - 6, An in vivo study has also shown that MTA promotes both dental and bony regeneration in pulp and periradicular tissues.2 MTA and Portland cement have many similar physical and chemical properties. 1 , 7 - 10 Portland cement consists of the same elements as MTA except that MTA also contains bismuth.8 , 11 Wucherpfenning & Green10 reported that both materials have a very similar effect on the pulpal cells and that osteoblast-like cells (MG-63) have similar growth and matrix formation when in contact with either MTA or Portland cement. They suggested that Portland cement may be as ideal a filling material as MTA. Holland et al12 reported that MTA and Portland cement show similar comparative result when used in direct pulp protection after pulpotomy. Estrela et al8 studied the antimicrobial effect of MTA and Portland cement by means of the agar diffusion test and reported that both had similar antimicrobial activity. Holland et al13 investigated the rat subcutaneous connective tissue reaction to implanted dentin tube filled with MTA, Portland cement or calcium hydroxide and reported that results observed with MTA and Portland cement were similar and almost the same as those observed with calcium hydroxide. De Deus et al1 studied the cytotoxicity of MTA and Portland cement on human ECV304 endothelial cells and reported no statistically significant differences between any of experimental materials. They concluded that the cell reaction patterns were similar for MTA and Portland cement in all experimental time periods. Saidon et al9 evaluated the cytotoxic response to freshly prepared MTA and Portland cement and compared the tissue reaction to MTA and Portland cement in bone when implanted in the mandible of the guinea pig and reported that both materials have similar properties. They suggested that Portland cement has the potential to be used as a less expensive root-end filling material. Abdullah et al4 prepared the accelerated Portland cement (APC) with addition of CaCl2 to Portland cement for reducing the setting time of the cement. They investigated the biocompatibility of APC in vitro by observing the cytomorphology of SaOS2 (human osteosarcoma cells) in the presence of the material and the effect of the material on the expression of markers of bone remodeling. They resulted that APC is non-toxic and may have potential to promote bone healing. Ribeiro et al14 tested the genotoxicity and cytotoxicity of Portland cement on Chinese hamster ovary (CHO) cells in vitro and suggested that Portland cement is not genotoxin and is not able to induce cellular death. Braz et al15 evaluated the genetic damage in human peripheral lymphocytes exposed to Portland cement and summarized that exposure to Portland cement may not be a factor that increases the level of DNA lesions in human peripheral lymphocytes as detected by single cell gel (comet) assay. The aim of the present study was to evaluate periodontal healing with focus on new bone regeneration following implantation of APC into experimentally created intra-bony defects in dog mandibles.
Materials and Methods
Animals
Six 2-year-old mongrel dogs (approximately 20 kg in weight) with intact dentition and healthy periodontium were chosen. Animal selection, management, surgical protocol and preparation followed the routines of Experimental Animal Research Center at Shiraz University of Medical Sciences. Animals were fed a soft diet the first 10 days post-surgery. Thereafter, the animals received standard laboratory diet.
APC
Portland cement type II was used for preparation of APC. As described by Abdullah et al4 vials containing powder of APC with 10% CaCl2 (3 g Portland cement with 0.3 g calcium chloride) were prepared and subjected to a continuous ultraviolet light sterilization for a period of 24 hrs.
Surgical procedure
Surgical procedures were performed under general anesthesia induced by an intravenous sodium pentobarbital (20 mg/kg), and maintained on hallotan gas (2%) in 50% oxygen. The mandibular fourth premolar teeth were extracted prior to the experimental surgery, and the extracted sites were allowed to heal for 2 months.1 During the experimental surgery, buccal and lingual muccoperiosteal flaps were elevated and 3-wall intrabony defects (4×4×4 mm) were surgically created with water-cooled rotating diamond burs on the mesial aspect of first molars in both sides of the mandible. Following root planing, a reference notch was made with a round bur on the root surface at the base of the defect. The defect of one side, selected randomly, was filled with freshly mixed cement (APC powder mixed with sterile distilled water) and the other side received a flap operation only. The flaps were repositioned and sutured at presurgery position with resorbable chromic 2-0 cut gut. The animals were euthanized 8 weeks following the surgical procedure by an intravenous injection of concentrated sodium pentobarbital. Block sections including the surgical sites were removed at sacrifice. The sections were rinsed in sterile saline and fixed in 10% formalin for 1 week. After rinsing in water, the sections were decalcified in 10% nitric acid for 1 week and embedded in paraffin. Serial sections, 5 µm thick, were cut in a mesial–distal direction at intervals of 60 µm. The six most central sections from each block were stained with hematoxylin and eosin (H&E) and examined using light microscopy.
Results
In the control sites, proliferations of connective tissue and downgrowth of epithelium were observed and were prominent (Figure 1). There was no evidence of new bone trabeculla in any of control sites; however, osteoid bone formation of minimal amount was observed. There were no new cementum formations in controls.
Figure 1.
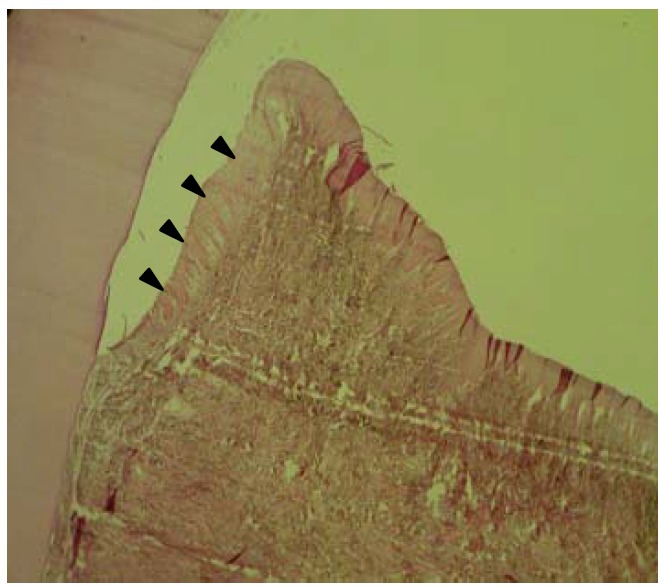
Histological section of one control site showing downgrowth of surface epithelium (►) and connective tissue proliferation that replaced the experimentally created bony defect (H&E, ×40).
In APC Samples, connective tissue proliferation and downgrowth of epithelium were minimal (Figure 2). Three of six cases of APC implanted samples showed osteoid formation with osteoblastic rimming and new bone trabeculla (Figure 3). There was new bone formation in immediate contact with the cavity containing the APC implants. Implanted materials were not detected due to the decalcification procedure. Osteoblast-like cells were arranged along the new bone surface (Figure 4). In one case, bone formation was separated from bony defect by a layer of connective tissue. In another case of APC samples, new cementum formation was observed.
Figure 2 .
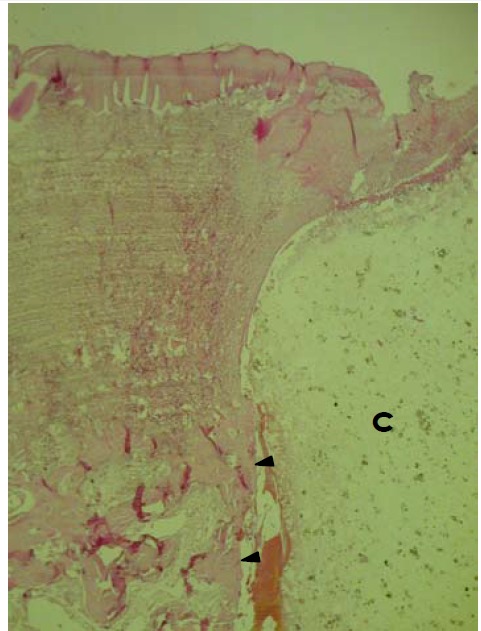
Histological section of site implanted with APC showing formation of new bone trabeculla (►) in direct contact with APC containing cavity (C). APC material is not seen due to decalcification procedure. Surface epithelium shows no evidence of downgrowth (H&E, ×100).
Figure 3.
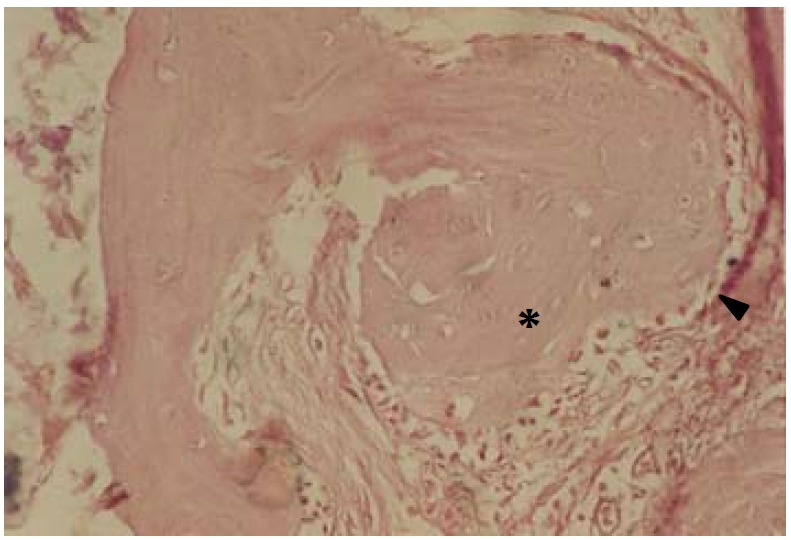
Histological section of site implanted with APC showing bony trabeculla (*) with osteoblastic rimming (►) in direct contact with APC containing cavity. APC material is not seen due to decalcification procedure (H&E, ×250).
Figure 4.
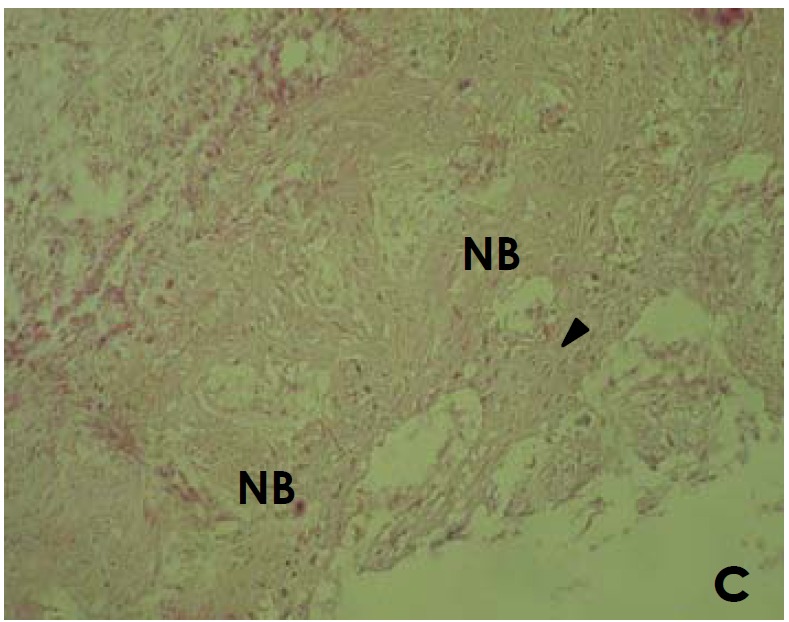
Histological section of site implanted with APC showing new bone (NB) and osteoid formation including osteoblastic cells (►) and matrix in direct contact with APC (C). APC material is not seen due to decalcification procedure (H&E, ×250).
Discussion
This study evaluated the bone healing following the implantation of APC in experimentally created intrabony defects in dogs. Abdullah et al4 reported that the improvement of setting time of Portland cement by the addition of CaCl2 did not interfere with the biocompatibility and osteoconductive property of the parent cement. Although the 8-week histological observations may not fully explain early healing events, an eight week healing interval has been considered useful to study periodontal regeneration in dog models.17 In addition, Choi et al1 reported that no differences in bone regeneration were noted between 8 and 24 weeks interval. Therefore, an 8-week healing period is sufficient to observe the initial healing process. Kim et al1 reported that one- and three-wall intrabony defects appear to be reproducible models to evaluate candidate technologies for periodontal regeneration. Critical size of the periodontal intrabony defects in dogs has not been defined; however, the size of 4×4 mm was used in similar studies.19 , 20 Although a histometric analysis was not used in the present study, the histopathological observation suggests a significant increase in bone regeneration following the implantation of APC in 50% of cases compared with controls. Stimulation of growth of new bone was also significantly prominent in three of six cases compared to control sites. Connective tissue proliferation and downgrowth of epithelium were significantly less than those of controls. This finding is in favor of new bone growth around the cavity and decreased secondary reaction of surface epithelium and submucosal connective tissue. In two cases, there were new bone formations in immediate contact with the remaining space of APC graft material. These results are in agreement with the results obtained by Saidon et al9 who reported that in more than 50% of cases there were new bone appositions in direct contact with Portland cement.
In conclusion, it seems that the positive histopathological results of this research are encouraging for the use of APC as a bone graft substitute. However, more comprehensive studies are necessary before warranting unlimited clinical use.
References
- 1.De Deus G, Ximenes R, Gurgel-filho ED, Plotkowski MC, Coutinho Filho T. Cytotoxicity of MTA and Portland cement on human ECV 304 endothelial cells. Int Endod J. 2005;38:604–609. doi: 10.1111/j.1365-2591.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 2.Torabinejad M, Chivian N. Clinical application of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RS, Mauger M, Clement DJ, Walker III WA. Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc. 1999;130:967–75. doi: 10.14219/jada.archive.1999.0337. [DOI] [PubMed] [Google Scholar]
- 4.Abdullah D, Ford TR, Papaioannou S, Nicholson J, McDonald F. An evaluation of accelerated Portland cement as a restorative material. Biomaterials. 2002;23:4001–10. doi: 10.1016/s0142-9612(02)00147-3. [DOI] [PubMed] [Google Scholar]
- 5.Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997;37:432–9. doi: 10.1002/(sici)1097-4636(19971205)37:3<432::aid-jbm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell PJC, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide. Biomaterials. 1999;20:167–73. doi: 10.1016/s0142-9612(98)00157-4. [DOI] [PubMed] [Google Scholar]
- 7.Dammaschke T, Gerth HUV, Zuchner H, Schafer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dental Materials. 2005;21:731–738. doi: 10.1016/j.dental.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Estrela C, Bammann LL, Estrela CRA, Silva RS, Pecora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J. 2000;11:3–9. [PubMed] [Google Scholar]
- 9.Saidon J, He J, Zhu Q, Safavi K, Spangberg LSW. Cell and tissue reactions to mineral trioxide aggregate and Portland Cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:483–9. doi: 10.1067/moe.2003.20. [DOI] [PubMed] [Google Scholar]
- 10.Wucherpfenning AL, Green DB. Wucherpfenning AL, Green DBMineral trioxide vsPortland cement: two biocompatibile filling materials. J Endod. 2005;25:308 (abstract). [Google Scholar]
- 11.Islam I, Chng HK, Yap AUJ. X-ray diffraction analysis of mineral trioxide aggregate and Portland cement. Int Endod J. 2006;39:220–225. doi: 10.1111/j.1365-2591.2006.01077.x. [DOI] [PubMed] [Google Scholar]
- 12.Holland R, de Souza V, Murata SS, Nery MJ, Bernabe PFE, Otoboni Filho JA. Healing process of dog dental pulp after pulpotomy and pulp covering with Mineral Trioxide Aggregate or Portland Cement. Braz Dent J. 2001;12:109–13. [PubMed] [Google Scholar]
- 13.Holland R, de Souza V, Nery MJ, Faraco Junior IM, Bernabe PFE, Otoboni Filho JA. Reaction of rat connective tissue to implanted dentin tube filled with Mineral Trioxide Aggregate, Portland Cement or Calcium Hydroxide. Braz Dent J. 2001;12:3–8. [PubMed] [Google Scholar]
- 14.Ribeiro DA, Sugui MM, Matsumoto MA, Marques ME, Salvadori DM. Genotoxicity and cytotoxicity of mineral trioxide aggregate and regular and white Portland cements on Chinese hamster ovary (CHO) cells in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:258–61. doi: 10.1016/j.tripleo.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 15.Braz MG, Camargo EA, Salvadori DMF, Marques ME, Ribeiro DA. Evaluation of genetic damage in human peripheral lymphocytes exposed to mineral trioxide aggregate and Portland cements. J Oral Rehab. 2006;33:234–239. doi: 10.1111/j.1365-2842.2005.01559.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim CS, Choi SH, Chai JK, Cho KS, Moon IS, Wikesjo UME et. Periodontal repair on surgically created intrabony defects in dogs: influence of the number of bone walls on healing response. J Periodontol. 2004;75:229–235. doi: 10.1902/jop.2004.75.2.229. [DOI] [PubMed] [Google Scholar]
- 17.Haney JM, Zimmerman GJ, Wikesjo UME. Periodontal repair in dogs: evaluation of the natural disease model. J Clin Periodontol. 1995;22:208–213. doi: 10.1111/j.1600-051x.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 18.Choi SH, Kim CK, Cho KS, Huh JS, Sorensen RG, Wozney JM et. Effect of rhBMP-2/ACS on healing in 3-wall intrabony defects in dogs. J Periodontol. 2002;73:63–72. doi: 10.1902/jop.2002.73.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Song WS, Kim CS, Choi SH, Jhon GJ, Kim HY, Cho KS et. The effects of a bioabsorbable barrier membrane containing safflower seed extracts on periodontal healing of 1-wall intrabony defects in beagle dogs. J Periodontol. 2005;76:22–33. doi: 10.1902/jop.2005.76.1.22. [DOI] [PubMed] [Google Scholar]
- 20.Yeo YJ, Jeon DW, Kim CS, Choi SH, Cho KS, Lee YK et. Effects of chitosan nonwoven membrane on periodontal healing of surgically created one-wall intrabony defects in beagle dogs. J Biomed Mater Res B Appl Biomater. 2005;72B:86–93. doi: 10.1002/jbm.b.30121. [DOI] [PubMed] [Google Scholar]