Abstract
To improve the description of electrostatic interaction between QM and MM atoms when the QM is SCC-DFTB, we adopt a Klopman-Ohno (KO) functional form which considers the finite size of the QM and MM charge distributions. Compared to the original implementation that used a simple Coulombic interaction between QM Mulliken and MM point charges, the KO based QM/MM scheme takes charge penetration effect into consideration and therefore significantly improves the description of QM/MM interaction at short range, especially when the QM region is highly charged. To be consistent with the third-order formulation of SCC-DFTB, the Hubbard parameter in the KO functional is dependent on the QM charge. As a result, the effective size of the QM charge distribution naturally adjusts as the QM region undergoes chemical transformations, making the KO based QM/MM scheme particularly attractive for describing chemical reactions in the condensed phase. Together with the van der Waals parameters for the QM atom, the KO based QM/MM model introduces four parameters for each element type. They are fitted here based on microsolvation models of small solutes, focusing on negatively charged molecular ions, for elements O, C, H and P with a specific version of SCC-DFTB (SCC-DFTBPR). Test calculations confirm that the KO based QM/MM scheme significantly improves the interactions between QM and MM atoms over the original point charge based model and it is transferable due to the small number of parameters. The new form of QM/MM Hamiltonian will greatly improve the applicability of SCC-DFTB based QM/MM methods to problems that involve highly charged QM regions, such as enzyme catalyzed phosphoryl transfers.
I. INTRODUCTION
Hybrid QM/MM simulations have become an important tool for the investigation of reaction mechanisms in complex environments, such as in water and biomolecules19,21,33,38,60,66. Numerous careful studies have indicated that, once carefully calibrated, QM/MM methods are able to produce reliable results for not only reaction free energy profiles but also other experimental observables, such as infrared spectra29 and kinetic isotope effects22. Nevertheless, additional developments are needed to further increase the efficiency and robustness of QM/MM methods, especially for highly heterogeneous and flexible systems such as biomolecules.
In the last decade, a promising QM/MM approach based on an approximate density functional theory, the Self-Consistent-Charge Density-Functional-Tight-Binding (SCC-DFTB) method15, has emerged. In terms of computational efficiency, SCC-DFTB is comparable to the widely used semi-empirical methods such as AM1 and PM3, i.e., being 2–3 orders of magnitude faster than popular DFT methods. In terms of accuracy, fairly extensive benchmark calculations have indicated that it is particularly reliable for structural properties, while energetics are generally comparable to AM1 and PM342,50,64. There are several recent developments of SCC-DFTB12,23,77 for metal ions5,13,46,80 and a few other elements that require d orbitals for a reliable description (e.g., phosphorus78). Although there are still many areas for improvements, due to its reasonable balance of efficiency and speed, SCC-DFTB has become an attractive choice as the QM method in many QM/MM simulations of biomolecules2,7,11,14,29,31,57,60,73,76.
We have successfully applied the SCC-DFTB based QM/MM simulations to a fairly broad set of enzyme problems. For reactions that involve highly charged species, such as the hydrolysis of phosphate mono esters, we have demonstrated that the conventional SCC-DFTB/MM approach tends to significantly overestimate the interaction between QM and MM groups, leading to considerable errors in the reaction free energy profiles78. This observation motivated us to reexamine the Hamiltonian used to describe the interaction between the QM and MM regions in hybrid QM/MM simulations61.
The total Hamiltonian for the molecular system under consideration in a hybrid QM/MM framework is generally given by,
| (1) |
where ĤQM/MM describes the interaction between the QM and MM atoms governed by ĤQM and ĤMM, respectively. Typically, ĤQM/MM is written as the sum of electrostatic, van der Waals (vdW), and bonded components,
| (2) |
Here describes the electrostatic interactions between the QM electrons and nuclei with the MM partial charges (or higher moments55), which are variable if a polarizable MM model is used; with a simple fixed-charge MM model, it is given by,
| (3) |
where QA is the partial charge on the MM atom A and Zα is the nuclear charge for QM atom α. qualitatively describes the Pauli repulsion between the QM and MM atoms at short range and dispersion interactions at long range. In popular implementations, it is given as an empirical term that does not depend on the QM wave function; i.e.,
| (4) |
in which εAα and σAα are defined by the standard combination rules: εAα = (εAεα)1/2 and σAα = 1/2(σA + σα). The parameters for the QM atoms εα and σα are usually fitted empirically18,58 based on atom or element type and not allowed to change during a chemical reaction.
It should be emphasized that, due mainly to the approximate nature of MM force fields, the physical meaning of “electrostatic” and “van der Waals” QM/MM interactions is not as rigorously defined as in perturbative theories for intermolecular interactions68; i.e., it is usually not possible to directly compare the “electrostatic” and “van der Waals” QM/MM interaction energies to results from energy decomposition analysis35,47, unless a very physical30 and accurate MM model is used. Finally, the term is required when the QM/MM partitioning is across a covalent bond, such as between the Cα and Cβ of an amino acid16,20,56.
For reactions that occur in a polar environment, such as water and biomolecules, electrostatic interactions between the QM and MM atoms tend to dominate. Therefore, much consideration has been given to an accurate description of QM/MM electrostatics, both at short and long ranges. At short range, charge penetration effects68 becomes important and therefore treating the MM atom as a simple point charge tends to be problematic; smearing the MM charges into a continuous distribution (e.g., a Gaussian) has been proposed by several authors and shown to be effective10,72. At long range, both Ewald based48 (for periodic boundary condition) and reaction field based (for finite-size boundary condition) techniques have been adapted to describe QM/MM electrostatic interactions. Specifically for SCC-DFTB, we have focused on the treatment of long-range QM/MM interactions in the context of both Ewald59 and the Generalized Solvent Boundary Potential34,65 (GSBP) frameworks. In the current work, we examine the limitations of the originally implemented SCC-DFTB/MM8 electrostatic interactions at short range, which we believe are responsible for the significant overestimate of QM/MM interactions observed for systems that contain highly charged QM regions78. Specifically, we propose to revise the form of the SCC-DFTB/MM electrostatic Hamiltonian based on an approximation widely used in the semi-empirical QM literature69; we then show that the revised form considerably improves the accuracy of SCC-DFTB/MM interactions, especially for systems that feature high charges (e.g., phosphate esters).
Although the magnitude of the QM/MM van der Waals interactions tend to be substantially smaller than electrostatics for polar systems, the QM van der Waals parameters have a significant impact on the spatial distribution of the MM atoms around the QM atoms and thus indirectly influence the magnitude of QM/MM electrostatics58. Therefore, although error cancellation occurs in many cases, a critical consideration of the QM(/MM) van der Waals parameters is likely important when the QM region undergoes significant changes, such as during a chemical reaction. This issue has been addressed in the pioneering work of York and co-workers, who have developed a charge-dependent van der Waals model for QM/MM interactions27. In the reported model, the van der Waals term is decoupled from the determination of the QM wavefunction/density and computed in a post-SCF fashion; this complicates the computation of gradients, which are required in molecular dynamics simulations. Therefore, it is still valuable to develop alternative models, and here we briefly discuss the issue in the context of SCC-DFTB/MM simulations.
The paper is organized as follows: in Sect. II we present our new SCC-DFTB/MM interaction model and summarize the relevant computational details for parameterization and benchmark calculations. In Sect. III, we first present results for QM/MM interaction energies in simple cluster models, and then demonstrate the value of our new model using phosphate monoester dianion hydrolysis reactions in solution. Finally we draw several conclusions.
II. THEORY AND METHODS
A. A simple but effective model for SCC-DFTB/MM electrostatics
In the original implementation of SCC-DFTB/MM8, the QM atoms interact with the MM sites electrostatically in the form of Mulliken partial charges,
| (5) |
where QA and ΔqB represent the MM partial charge and Mulliken partial charge, respectively. We note that although other definitions of charges37 in SCC-DFTB can in principle be used instead of the simple Mulliken charges, important parameters in SCC-DFTB (e.g., repulsive potentials) were optimized within the Mulliken framework.
Treating both QM and MM atoms as point charges is clearly a major approximation, especially when they approach each other and charge penetration becomes important68. The observation that QM/MM interactions are significantly overestimated (as compared to full QM calculations) when the QM region is highly charged further supports this consideration. Therefore, it is imperative to seek an alternative formulation that is physical and applicable for all ranges of QM/MM distances.
One possible avenue starts with reviewing the formulation of SCC-DFTB. The second-order expansion of the total energy takes the form,
| (6) |
With the monopole approximation and spherical charges,
| (7) |
the 2nd-order term becomes
| (8) |
To obtain explicit expressions for γαβ, Elstner et al. used Slater-like charge distributions to evaluate the approximate expression of the second-order term,
| (9) |
in which the exponent is related to the Hubbard parameter (Uα),
| (10) |
where ηα is the chemical hardness parameter for element α. In other words, the Hubbard parameter is closely related to the effective size of the atom (charge distribution). In recent studies12,23,77, it has been shown that including the charge dependence of the Hubbard parameter, which effectively extends the DFTB energy to third order in density (charge) fluctuation, significantly improves properties such as proton affinities. Physically this highlights that the size of an atom (charge distribution) changes considerably as it deprotonates.
In principle we can adopt γαβ for QM/MM interactions. However, its functional form is rather complex. As discussed in Ref.12, γαβ is closely related to other approximate expressions for two-center two-electron integrals developed in the NDDO literature41,69. For example, the Klopman-Ohno (KO) expression for the two-center two-electron integral, 〈μAνA|λBσB〉, is given as,
| (11) |
where the summations are over the multipole charges (Qi;j) used to mimic atomic orbitals, and di;j are parameters determined based on the condition that as the distance RAB approaches zero the expression reduces to known one-center two-electron integrals. For the interactions between two spherical charges (s orbitals), the expression can be used by setting the di;j as nothing but the corresponding Hubbard parameters.
Putting the two lines of thoughts together, it is evident that a physically motivated QM/MM Hamiltonian appropriate for SCC-DFTB is,
| (12) |
where we emphasize that Uα (Δqα) is dependent on the Mulliken charge of the QM atom; i.e., , in which the Hubbard derivative with respect to atomic charge ( ) is either calculated from atomic properties or fitted based on properties of interest23,77 (e.g., proton affinity23). The the MM “Hubbard” parameter (UA) can be taken from atomic electronic structure calculations or treated as a parameter similar to the width of the “Gaussian blur” in the approach introduced by Brooks and co-workers10, or simply set to ∞ (the point charge limit).
Adopting Eq. 12 for the SCC-DFTB/MM electrostatics has several advantages over the simple point charge model. First, it properly considers the damping of charge-charge interaction at short QM/MM distance and therefore avoids over polarization of the QM region in the presence of nearby MM atoms. In fact, in one of the first implementations of semi-empirical QM/MM methods16, the QM/MM electrostatic terms are described using essentially the same approach, i.e., using approximate < ss|ss > two-electron integrals. Second, using the charge-dependent Hubbard parameter naturally considers the the continuous variation of atomic (charge distribution) size during a chemical reaction. Although this does not replace including charge-dependence in the QM(/MM) van der Waals parameters (see below), it provides a more physical description of QM/MM electrostatics during a chemical transformation.
In this work, the KO functional form is slightly modified to introduce additional flexibility,
| (13) |
To avoid over fitting, the parameters aα and bα are set to be based on element rather than atom type; thus our model only introduces two extra parameters for each element. With the modified QM/MM electrostatic Hamiltonian, the SCC-DFTB Kohn-Sham matrix element also needs to be modified correspondingly; e.g., with UMM = ∞,
| (14) |
where μ ∈ C; υ ∈ D.
As discussed in Introduction, the QM(/MM) van der Waals interactions in principle should also be made charge dependent; both effective atomic radius and polarizability for the reactive site(s) are expected to change during a chemical transformation. In the framework of DFT, this can be accomplished by noting the relationship between atomic charge, effective atomic size and atomic polarizability24,51,53,54,77. For example, since the Hubbard parameter is directly related to the chemical hardness (Eq. 10), including charge dependence in the Hubbard parameter would also introduce charge-dependence into the chemical hardness, which is inversely related to atom size24,51,53,54,77. Therefore, it is possible to use this relationship to make the QM van der Waals radii to be charge dependent. Similar arguments can be made for the atomic polarizability52, which determines the effective well-depth for the QM(/MM) van der Waals terms; this is similar in spirit to the environment (density)-dependent dispersion coefficients introduced by several authors in the literature1,70. In practical terms, however, introducing those charge dependences into the QM van der Waals parameters in the framework of existing QM/MM programs would require considerable modification of the SCC-DFTB matrix elements and the SCF procedures; whether such increase in the computational cost is worthwhile for practical applications requires systematic analysis in the future. In the current work, we show that adopting the KO scheme for the QM/MM electrostatics along with a single set of optimized element-type dependent QM van der Waals parameters already leads to significant improvement over the original SCC-DFTB/MM model8 for the description of highly charged systems in polar environments.
B. Parameterization of the new KO models
To summarize, our new SCC-DFTB/MM model Hamiltonian contains four parameters for each element: aα, bα in Eq. 13 for QM/MM electrostatics and ε α, σ α for QM/MM van der Waals interactions. In principle, the MM Hubbard parameters can also be optimized to allow additional flexibility. In this work, we test two models: simply set the MM Hubbard parameters to ∞ (referred to as the KO-∞ model), or use the calculated Hubbard from atoms23 (referred to as the KO-MM model).
For parameterization of the KO models, small molecule-water interaction energy is used as the target property. Since we are mainly interested in condensed phase processes, the training and test sets contain small clusters that involve solutes interacting with multiple water molecules. Test calculations indicate that including multiple water molecules is essential to a successful parameterization; including only pair-wise models as in previous studies58 does not capture the complexity of interactions in the condensed phase and therefore does not lead to as transferrable parameters. The training set includes 23 molecules (see Table II) that mimic protein sidechains and phosphate species with various charge states; we focus on negatively charged solutes here because their charge distribution tends to be more diffuse and therefore essential to be described with a finite size. Each solute in the training set is first solvated with a water sphere of 25 Å radius, then 50 ps MD at 300 K is carried out with the solute treated as SCC-DFTB and water treated as TIP3P36; the original QM/MM Hamiltonian8 and QM van der Waals parameters58 are used for the sampling, and test calculations show that the optimized parameters are not very sensitive to this choice. Ten snapshots are taken from each trajectory, and water molecules beyond 4 Å from the solute are deleted, leaving about 15 water molecules surrounding the solute. The binding energies between the solute and water molecules at the full SCC-DFTB level are used as reference values for the parameterization; higher-level QM calculations can be used as references, but we leave this to future work following the completion of parameterizing DFTB3 for more element types. In this study, which mainly serves to demonstrate the value of the revised SCC-DFTB/MM Hamiltonian, we use a specific version of SCC-DFTB parameterized for phosphate hydrolysis (SCC-DFTBPR78). The parameters are optimized using a Genetic Algorithm (GA)28 in which the “fitness” (ξ) is defined as the inverse of a weighted sum of difference between binding energies determined from full SCC-DFTB and SCC-DFTB/MM calculation:
| (15) |
where i is the index of species in the training set and the sum is over all molecules in the training set. During optimization, a micro-GA technique with a population of 10 chromosomes that is allowed to operate for 500 generations with uniform crossovers; see Ref.Carroll for detailed descriptions and recommendations for GA options.
TABLE II.
Error (in kcal/mol) analysis of binding energies for the training seta
| Solute | ESCC | Unsigned Errorb
|
||
|---|---|---|---|---|
| KO-∞ | KO-MM | Point-charge | ||
| Propane | −0.6 | 4.3 | 3.8 | 3.4 |
| Isobutene | −1.6 | 5.4 | 5.0 | 4.6 |
| Butane | −1.1 | 5.4 | 4.7 | 4.5 |
| Toluene | −3.5 | 5.6 | 4.5 | 4.5 |
| 4-cresol | −10.5 | 2.6 | 2.5 | 2.4 |
| Methanol | −9.2 | 2.1 | 1.9 | 2.5 |
| Ethanol | −9.0 | 1.5 | 1.3 | 2.0 |
| Acetaldehyde | −6.7 | 1.3 | 1.1 | 1.8 |
| Methylacetate | −9.3 | 1.9 | 1.6 | 2.8 |
| Acetic acid | −7.6 | 1.5 | 1.2 | 2.7 |
| Propanic acid | −15.8 | 4.4 | 3.7 | 5.6 |
| Dimethyl ether | −4.2 | 1.6 | 1.3 | 2.0 |
| Methylphosphate | −21.9 | 7.4 | 5.3 | 8.6 |
| Dimethylphosphate | −15.9 | 2.8 | 3.3 | 5.2 |
| Acetate (−1) | −78.4 | 3.0 | 2.8 | 6.8 |
| Propanate (−1) | −88.3 | 2.5 | 2.7 | 2.1 |
| 4-cresol (−1) | −83.2 | 6.5 | 5.8 | 7.0 |
| Methoxide (−1) | −94.0 | 5.3 | 3.7 | 7.4 |
| Ethoxide (−1) | −95.5 | 6.7 | 4.1 | 10.0 |
| Hydroxide (−1) | −68.6 | 8.8 | 6.9 | 5.4 |
| Methylphosphate (−1) | −84.1 | 6.4 | 5.3 | 5.5 |
| Dimethylphosphate (−1) | −79.5 | 3.6 | 1.8 | 5.5 |
| Methyl phosphate (−2) | −249.6 | 7.6 | 5.6 | 8.6 |
|
| ||||
| Error Analysisc | ||||
| MUE | 4.3 | 3.3 | 4.8 | |
| MSE | −0.5 | −0.8 | −0.8 | |
The reference values (ESCC) are calculated with SCC-DFTBPR; for the different QM/MM schemes (see the footnote of Table 1 for notation), negative values indicate that the strength of interaction is overestimated relative to the reference.
The unsigned error is averaged over 10 snapshots for each solute;
MUE: mean unsigned error; MSE: mean signed error.
Finally, to test the transferability of the parameterization, a test set of 12 different clusters (see Table III) are constructed in a similar fashion. The KO-∞ and KO-MM models are compared to the original QM/MM interaction scheme8; for a fair comparison, we also reparametrize the QM van der Waals parameters for the original QM/MM interaction scheme with the same training set used for parameterizing the KO models.
TABLE III.
Error (in kcal/mol) analysis of binding energies for the test seta
| Solute | ESCC | Unsigned Error
|
||
|---|---|---|---|---|
| KO-∞ | KO-MM | Point-charge | ||
| Methane | −0.9 | 0.9 | 0.9 | 0.8 |
| Phenol | −6.3 | 4.8 | 4.2 | 4.7 |
| Propanol | −10.8 | 4.2 | 3.7 | 4.5 |
| Formaldehyde | −3.1 | 1.2 | 1.0 | 1.6 |
| Formic acid | −21.6 | 2.0 | 1.6 | 3.2 |
| Trimethyl phosphate | −21.0 | 7.2 | 6.4 | 12.0 |
| Formate (−1) | −93.6 | 5.6 | 1.7 | 10.8 |
| Benzoate (−1) | −94.1 | 10.7 | 5.3 | 15.1 |
| Propanoate (−1) | −96.5 | 6.8 | 3.1 | 10.9 |
| Dihydrogen phosphate (−1) | −105.4 | 2.6 | 2.6 | 9.0 |
| Methyl phenyl phosphate (−1) | −110.5 | 9.0 | 5.8 | 17.0 |
| Hydrogen phosphate (−2) | −271.5 | 2.1 | 5.9 | 17.1 |
|
| ||||
| Error Analysis | ||||
|
| ||||
| MUE | 4.7 | 3.5 | 8.9 | |
| MSE | −4.2 | −2.0 | −8.8 | |
See Table II for notations.
C. Potential of mean force (PMF) simulations for aqueous phosphate hydrolysis reactions
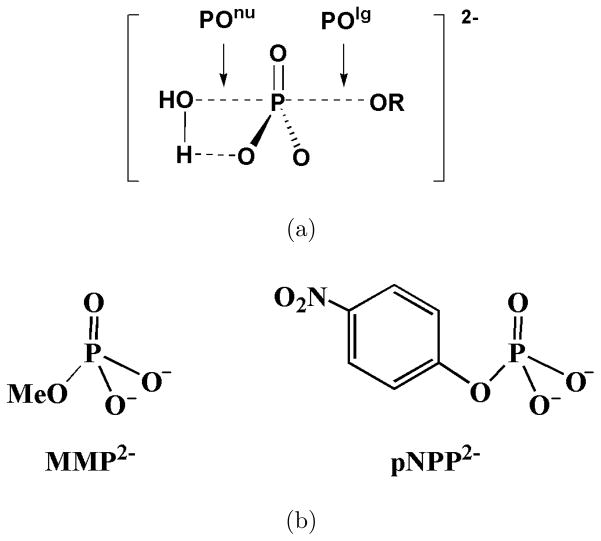
To explicitly evaluate the performance of different QM/MM interaction schemes for condense phase reactions, we study the aqueous hydrolysis reactions of two phosphate monoesters, methyl monophosphate2− (MMP2−) and p-nitrophenyl phosphate2− (pNPP2−) (see Fig. 1), with a water molecule as the nucleophile. These reactions serve as ideal benchmarks because there are extensive experimental44 and computational17,40 studies. In addition, these phosphate monoesters are the typical substrates of phosphatases,49,79 thus the results also provide important references for future enzyme studies.
FIG. 1.
The phosphate monoester dianions hydrolysis reactions studied in this work.
The solute (MMP2− or pNPP2−) is solvated by the standard protocol of superimposing the molecule with a water droplet of 25 Å radius and removing water molecules within 2.8 Å from any solute atoms. Water molecules are described using the TIP3P model36 with modified van der Waals parameters for hydrogen. The QM region includes the solute and the nucleophilic water. The generalized solvent boundary potential (GSBP)34,65 is used to treat long range electrostatics in MD simulations. To be consistent with the GSBP protocol, the extended electrostatic model67 is used to treat the electrostatic interactions among inner region atoms for which interactions beyond 12 Å are treated with multipolar expansions, including the dipolar and quadrupolar terms. The deformable boundary forces4 are added in the boundary region to constrain water molecules within the sphere. An additional weak GEO type of potential is added to the QM region to keep it in the center of the water sphere. An angular constraining potential is added to the nucleophilic water, the phosphate atom and the leaving group oxygen to favor the “in line attack” configuration. All bonds involving hydrogen in MM water are constrained using the SHAKE algorithm,63 and the time step is set to 1 fs.
The 2D PMF calculations are carried out for the aqueous reactions. The system is energy minimized and slowly heated to 300 K and equilibrated for 50 ps. The reaction coordinate is defined as POlg-POnu and HOwat-HOpo; here Olg/nu indicates the oxygen in the leaving group/nucleophile, and Opo indicates the phosphate oxygen that the proton transfers to. The umbrella sampling approach71 is used to constrain the system along the reaction coordinates. In total, more than 250 windows are used for each PMF and 50 ps simulations are performed for each window. The first 10 ps trajectories are discarded and only the last 40 ps are used for data analysis. Convergence of the PMF is monitored by examining the overlap of reaction coordinate distributions sampled in different windows and by evaluating the effect of leaving out segments of trajectories. The probability distributions are combined together by the weighted histogram analysis method (WHAM)43 to obtain the PMF along the reaction coordinates.
As additional benchmarks that focus on the quality of the QM method rather than other technical details such as QM/MM coupling and sampling, we use a previously developed implicit solvent model32 to study these aqueous reactions of phosphate monoesters. In this model, the solute radii are dependent on the charge distribution and therefore the model is particularly useful for studying solution reactions that involve highly charged species; our previous benchmark calculations suggest that the method has comparable accuracy as the SM6 model for a fairly broad range of neutral and charged solutes39, while being much more efficient (due to the use of SCC-DFTB) and having only a small number of parameters. The energetics of the reactions are studied using adiabatic mapping and the reaction coordinates are the same as those in QM/MM PMF simulations. Each point in the 2D adiabatic map is obtained by starting the constrained optimization from several different initial structures and taking the lowest energy value. The initial grid size is 0.2 Å due to the large number of structures that need to be optimized. Later a finer grid size (0.1 Å) is used to scan the transition state (TS) region and to locate the TS structure. Finally, frequency calculations are carried out to confirm the nature of the stationary points and to compute the vibrational entropy and zero point energies to obtain approximate activation free energy; although using a harmonic approximation to estimate activation entropy is known to be of limited accuracy, previous studies of phosphate diester hydrolysis found that activation entropy does not differ much between different diesters62.
To account for intrinsic errors of SCC-DFTBPR energies, we explore corrections based on gas phase single-point energy calculations with MP2/6-311++G** at SCC-DFTBPR geometries. As discussed in the literature,9 such a simple correction may not always improve the energetics for semi-empirical methods given the errors in geometry; however, our previous tests31,32,78 indicated that this correction scheme appears useful for SCC-DFTBPR since the method gives fairly reliable structures, even for transition states.
III. RESULTS AND DISCUSSIONS
A. Solute-water binding energies in training and test sets
Since performance in the condensed phase is of main concern, we adopt the cluster type of models to explicitly sample multi-body interactions. As shown in Table II for the training set, the total interactions between the “first solvation shell” water and the solute cover a great range, from a few kcal/mol for aliphatic molecules to almost 250 kcal/mol for a methyl phosphate dianion. With the original point-charge based QM/MM electrostatic Hamiltonian, the error from full SCC-DFTB results is not overwhelmingly large once the QM van der Waals parameters are optimized (based on element type). The Mean Unsigned Error (MUE) is 4.8 kcal/mol for total solute-water interactions; even for charged solutes, such as ethoxide and methyl phosphate, the error is about 10 kcal/mol, which is merely a few percent of the total interaction. We note that using the van der Waals parameters optimized in Ref.58 based on solute-water dimers leads to substantially larger errors, with a MUE of 14.1 kcal/mol (data not shown). This is partly because the van der Waals parameters in Ref.58 were optimized for the second-order SCC-DFTB, while the SCC-DFTBPR78 used herein includes diagonal third-order contributions; more importantly, however, using the cluster models better captures the multi-body interactions featured in the condensed phase.
For the training set, the two KO models give slightly better results; the KO-∞ model has a MUE of 4.3 kcal/mol while KO-MM has a value of 3.3 kcal/mol. For all three QM/MM models, the interaction between non-polar solute and nearby water has notable errors of a few kcal/mol, which are comparable or even larger in magnitude than the total interaction at the full QM level. This is because the penalty function is largely dictated by charged molecules, which have much stronger interactions with water. Since for most applications the solute tends to be substantially charged, this is not expected to be a major issue in practice. Nevertheless, a more balanced description for broader classes of solutes is desirable and we leave this to future work in which we also plan to use higher level QM results as the reference for parameterization.
To examine the transferability of the parameterized models, we construct a test set that contains twelve molecules of biological relevance but not covered in the training set (see Table III). The performance of the KO models is quite comparable to that observed for the training set; the MUE is 4.7 kcal/mol for KO-∞ and 3.5 kcal/mol for KO-MM. With the original point-charge based SCC-DFTB/MM Hamiltonian8, however, the errors become quite large for several molecules, leading to a substantially larger MUE of 8.9 kcal/mol. Therefore, it seems that the KO models are substantially more robust than the original SCC-DFTB/MM Hamiltonian.
Finally, to examine the performance of the models for not only stable structures but also transition states, we have selected structures for ten model phosphate hydrolysis reactions in RNA from the QCRNA database established by the York group25. These include 16 stable states and 24 transition states. Solvated cluster models for these structures are constructed in the similar fashion as for the training and test sets and we again compare the solute-water interaction calculated by different SCC-DFTBPR/MM models to full SCC-DFTBPR results. As shown in Table IV, the errors are rather large with the original SCC-DFTB/MM Hamiltonian; the values are often about 10% of the total interaction energy. Fortunately, it appears that the errors are fairly comparable for the stable and transition states; the MUEs are 14.2 and 17.6 kcal/mol, respectively. This explains why SCC-DFTBPR/MM has been found to give fairly reasonable results for several phosphoryl transfer reactions in solution74 and enzymes31,75,76. With the KO-∞ model, the errors are smaller than the original SCC-DFTB/MM model by almost a factor two, with a MUE of 7.1 kcal/mol. Most impressively, the KO-MM model continues to perform well, the MUE is 4.3 kcal/mol, which is rather similar to both the training and test sets; the MUEs for stable states and transition states are also comparable, 3.5 and 4.8 kcal/mol, respectively. Therefore, the KO-MM model appears to be transferrable and gives robust QM/MM interactions for fairly broad set of polar and charged solutes.
TABLE IV.
Energetics Benchmark Calculations for different QM/MM interaction schemes based on 10 phosphate reactions from the QCRNA databasea
| Reactions | Conf. ESCC | Errors | |||
|---|---|---|---|---|---|
|
| |||||
| Point-charge | KO-∞ | KO-MM | |||
| CH3O…P(O)(O)(OH)(OCH3) | ts12 | −255.0 | 28.0 | 14.0 | 2.4 |
| HO…P(O)(O)(OH)(OCH3) | ts12 | −252.3 | 25.8 | 11.8 | 4.2 |
| HO…P(O)(OH)(OH)(OCH3) | ts12 | −96.7 | 12.0 | 3.5 | 5.8 |
| min2 | −100.5 | 10.5 | 3.1 | 3.6 | |
| ts23 | −98.2 | 10.6 | 3.1 | 3.4 | |
| HOH…P(O)(O)(OCH3)(OCH3) | min1 | −99.3 | 11.2 | 3.6 | 5.2 |
| ts12 | −102.1 | 12.0 | 3.4 | 6.6 | |
| min2 | −98.7 | 12.0 | 3.9 | 7.3 | |
| ts23 | −100.2 | 10.8 | 2.8 | 6.2 | |
| min3 | −109.1 | 10.9 | 3.1 | 2.5 | |
| ts34 | −96.5 | 13.3 | 5.1 | 2.2 | |
| min4 | −98.4 | 11.3 | 3.8 | 2.0 | |
| ts45 | −98.6 | 14.4 | 6.4 | 1.9 | |
| min1 | −94.5 | 11.6 | 4.0 | 2.3 | |
| HO…P(O)(O)(OCH3)(OCH3) | ts12 | −257.6 | 23.0 | 7.6 | 2.1 |
| min2 | −274.0 | 26.3 | 11.4 | 2.3 | |
| ts23 | −267.2 | 24.5 | 10.4 | 1.7 | |
| CH3O…P(O)(O)(OCH3)(OCH3) | ts12 | −244.1 | 32.1 | 18.9 | 2.8 |
| CH3O…P(O)(OH)(OH)(OCH3) | ts12 | −102.0 | 13.1 | 4.8 | 1.9 |
| min2 | −93.3 | 13.2 | 4.9 | 3.9 | |
| ts23 | −99.6 | 14.4 | 6.0 | 2.6 | |
| CH3O…P(O)(OH)(OCH3)(OCH3) | ts12 | −94.8 | 16.6 | 9.1 | 3.3 |
| min2 | −98.1 | 13.2 | 5.2 | 6.0 | |
| ts23 | −107.4 | 16.1 | 7.6 | 4.0 | |
| min3 | −96.4 | 13.0 | 5.5 | 12.1 | |
| ts34 | −103.5 | 14.4 | 6.5 | 2.4 | |
| CH3O…P(O)(OCH3)(OCH3)(OCH3) | ts12 | −97.9 | 18.9 | 10.5 | 2.6 |
| min2 | −106.2 | 16.5 | 8.7 | 3.6 | |
| ts23 | −103.5 | 17.2 | 9.4 | 6.4 | |
| min3 | −99.4 | 16.9 | 9.0 | 2.6 | |
| ts34 | −92.0 | 17.4 | 10.0 | 4.5 | |
| min4 | −103.3 | 14.6 | 7.1 | 3.4 | |
| ts45 | −102.8 | 19.5 | 11.3 | 3.9 | |
| HO…P(O)(OCH3)(OCH3)(OCH3) | ts12 | −113.8 | 19.3 | 2.7 | 6.9 |
| min2 | −94.6 | 15.4 | 7.2 | 5.4 | |
| ts23 | −96.9 | 16.5 | 8.5 | 6.1 | |
| min3 | −101.0 | 14.6 | 6.3 | 5.6 | |
| ts34 | −96.6 | 13.8 | 6.0 | 6.7 | |
| min4 | −98.3 | 16.5 | 8.0 | 4.9 | |
| ts45 | −96.5 | 17.6 | 9.4 | 7.4 | |
|
| |||||
| Error Analysisb | |||||
|
| |||||
| Overall Performance | MUE | 16.2 | 7.1 | 4.3 | |
| Stable States Performance | MUE | 14.2 | 5.9 | 3.5 | |
| Transition States Performance | MUE | 17.6 | 7.9 | 4.8 | |
The unsigned error is averaged over 10 snapshots for each solute;
MUE: mean unsigned error.
B. PMF for phosphate monoester reactions
1. MMP2− hydrolysis reaction
MMP2− is a simple phosphate monoester and its solution reaction has been extensively studied by experimental and computational methods. In aqueous solution, the nucleophile has been determined as a water molecule and the experimental free energy barrier is 44.3 kcal/mol at 298K (converted from rate constants with transition state theory)44. The barrier was well reproduced as 47 kcal/mol at 312 K by B3LYP/COSMO model.40 The calculated transition state structure indicates that the water first transfers a proton to MMP2− to become a hydroxide, which in turn attacks the protonated phosphate monoester. The P-Olg and P-Onu bond lengths are 1.8 and 2.0 Å, respectively, in the rate-limiting transition state.
Before studying this reaction by different QM/MM interaction models, it is useful to establish the intrinsic error of the QM method. For this purpose, we use a recently developed implicit solvent model that combines SCC-DFTB method and Poisson-Boltzmann (PB) equation with a set of charge dependent radii.32 In the calculated potential energy surface shown in Fig. 2(a), the reactant state corresponds to the bottom left corner while the product state corresponds to the upper right corner. The first step involves an exothermic proton transfer from water to MMP2−, followed by the nucleophilic attack; this is consistent with the results from previous studies40. The calculated rate-limiting transition state has the reaction coordinate of POlg-POnu as 0.0 Å and the proton transfer coordinate as 1.1 Å. The POlg and POnu bond lengths are both 1.95 Å, also close to previous computational results. Rescanning the TS region by a finer grid leads to a reaction barrier of 30.5 kcal/mol. After including vibrational entropy and zero point energy corrections at 300 K, the free energy barrier is 39.5 kcal/mol; further including MP2 single point correction (see Table V) leads to the best estimated barrier of 45.7 kcal/mol. As shown in Fig. 2c, the overall potential energy surface after the MP2 correction remains similar to that at the SCC-DFTBPR/PB level, although the latter systematically underestimates the relative energy compared with infinitely separated reactants, especially for the upper left corner which corresponds to the proton transfer step. This is not unexpected because significant imbalance of proton affinities for phosphate and non-phosphate species remains in SCC-DFTBPR78. Our recent studies indicate that this deficiency is largely removed when the complete third-order contributions are included23 (M. Gaus and Q. Cui, work in progress).
FIG. 2.
Potential energy surface (PES, in kcal/mol) of MMP2− hydrolysis reaction in water. (a) 2D PES by SCC-DFTBPR/PB32; (b) 2D PES of the TS region with a finer grid size by SCC-DFTBPR/PB; (c) 2D PES that includes the gas-phase MP2/6-311++G** single point energy corrections over SCC-DFTBPR.
TABLE V.
Free energy barriers (kcal/mol) of phosphate monoester hydrolysis in water estimated by different methodsa
| Solute | Method | ΔG‡ |
|---|---|---|
| MMP2− | Expb | 44.3 (298K) |
| MP2/LDc | 43 (312K) | |
| SCC-DFTBPR/PBd | 39.5/45.7 | |
| Point-charge QM/MM | 55 | |
| KO-∞ | 41 | |
| KO-MM | 40 | |
|
| ||
| pNPP2− | Expe | 31.8 (298K) |
| SCC-DFTBPR/PBd | 29.3/27.0 | |
| KO-∞ | 33 | |
| KO-MM | 32 | |
All results are for 300 K unless noted otherwise;
Results taken from Ref.44;
Results taken from Ref.40;
The number before the slash is SCC-DFTBPR/PB result that includes entropic and ZPE corrections; the number after the slash further includes the gas phase MP2/6-311++G** single point corrections;
Results taken from Ref.45.
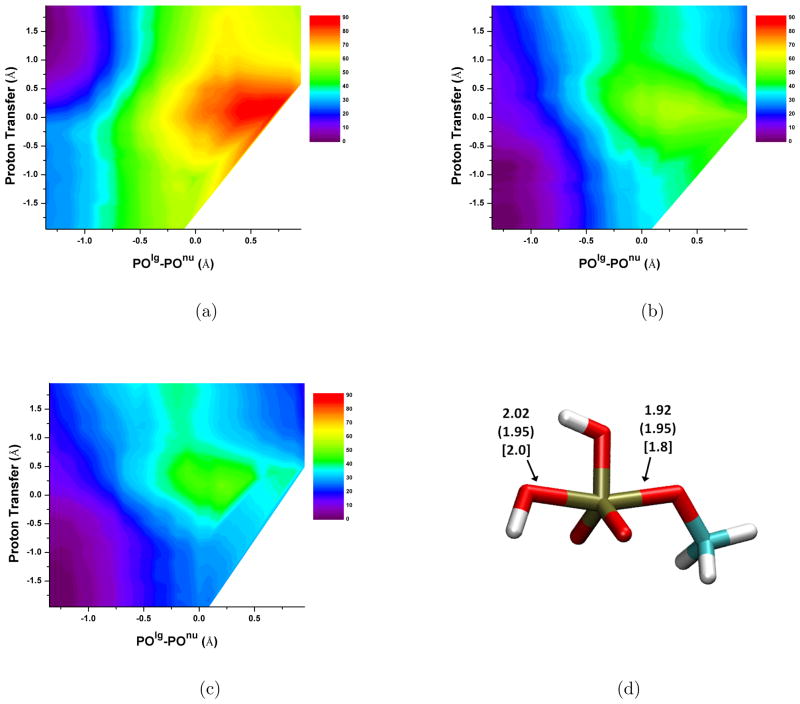
With QM/MM simulations, the results depend on the model for the QM/MM interactions. With the original point-charge based SCC-DFTB/MM Hamiltonian, the free energy barrier is grossly overestimated compared to both experiment and SCC-DFTBPR/PB results; the value of 55 kcal/mol is about 10 kcal/mol higher than the experimental result. With the KO models, the barriers are 41 (KO-∞) and 40 kcal/mol (KO-MM), respectively. These are close to the SCC-DFTBPR/PB results (without the MP2 correction). The transition state region calculated by the KO schemes is at reaction coordinate POlg-POnu slightly less than 0 Å and the proton transfer coordinate at 1.2 Å. The averaged POlg and POnu bond lengths are 1.94 and 2.04 Å, respectively, similar to those from previous calculations40 and the SCC-DFTBPR/PB model.
It is worth noting that the first proton transfer step is exothermic with the original SCC-DFTBPR/MM model but endothermic by about 10 kcal/mol with the KO models (see Fig. 3). Warshel and co-workers studied this step17 by MP2/LD method and obtained an endothermicity of ~ 9 kcal/mol. As noted above, the SCC-DFTBPR/PB model has notable errors in this region of the potential energy surface (Fig. 2) due to proton affinity errors in SCC-DFTBPR. These errors are partially cancelled by the remaining deficiencies in QM/MM interactions when the KO models are used, leading to fortuitous agreement with MP2/LD results. The substantial errors in the QM/MM interactions by the KO models for this region of the potential energy surface are not unexpected because the interaction between the nucleophile (hydroxide) and nearby water are difficult to describe with a QM/MM model (see Table II); the significant charge transfer between the hydroxide and nearby water in principle requires a full QM description.
FIG. 3.
2D PMF (in kcal/mol) of MMP2− hydrolysis reaction in water calculated with different SCC-DFTB/MM schemes. (a) The original QM/MM scheme8 with newly optimized QM van der Waals parameters (summarized in Table 1); (b) the KO-∞ scheme; (c) the KO-MM scheme; (d) the transition state structure. Numbers without parentheses are calculated with KO-MM, those with parentheses are calculated with SCC-DFTBPR/PB, those with brackets are taken from Ref.40.
2. pNPP2− hydrolysis reaction
In addition to MMP2−, we also study the hydrolysis of another phosphate monoester, pNPP2−, which has a rather different ester group. pNPP2− is an important substrate for phosphatase, therefore the aqueous results provide an important reference for enzyme studies. Similar to the MMP2− case, we first use SCC-DFTBPR/PB to estimate the inherent error in the QM method. The overall potential energy landscape (Fig. 4(a)) is similar to that of MMP2−; the phosphate non-bridging oxygen first abstracts a proton from the water and then the hydroxide acts as the nucleophile to attack the mono anionic phosphate. The free energy barrier is calculated to be 29.3 kcal/mol after adding entropic effects and zero point energy corrections; the value becomes 27.0 kcal/mol after adding MP2 single point energy corrections. The experimental value is 31.8 kca/mol at 298 K, slightly higher than our results. The transition state structure (Fig. 4(b)) has the reaction coordinate POlg-POnu as −0.3 Å and the proton transfer coordinate as 1.0 Å. The POlg bond length is 1.95 Å, similar to that for MMP2−; however, the POnu bond length increases to 2.26 Å and the overall transition state structure becomes looser than that for MMP2−. These observations are consistent with the trend found in previous studies that the transition state changes from associative to dissociative in nature as the pKa of the leaving group decreases.40 SCC-DFTBPR appears to be able to capture the substituent effect on the hydrolysis reaction.
FIG. 4.
2D potential energy surface (PES) and potential of mean force (PMF) of pNPP2− hydrolysis reaction in water calculated by SCC-DFTBPR/PB and SCC-DFTBPR/MM simulations, respectively. (a) 2D PES by SCC-DFTBPR/PB; (b) 2D PES for the transition state region with a finer grid size by SCC-DFTBPR/PB; (c) 2D PES including the gas phase MP2/6-311++G** single point energy corrections over SCC-DFTBPR; (d) 2D PMF by the KO-∞ scheme; (e) 2D PMF by the KO-MM scheme; (f) The transition state structure. Numbers without parentheses are calculated with the KO-MM scheme, those with parentheses are calculated with SCC-DFTBPR/PB.
With QM/MM simulations, the calculations are problematic with the original point-charge based SCC-DFTBPR/MM Hamiltonian; the grossly overestimated QM/MM interactions over polarize the QM region, leading to highly distorted structures and energetics. With the KO models, QM/MM PMF simulations are able to converge without much difficulty. Both lead to free energy barriers of 33 kcal/mol, similar to the SCC-DFTBPR/PB results; if the MP2 gas-phase correction estimated for SCC-DFTBPR/PB (~2 kcal/mol) is transferrable to the QM/MM results, the QM/MM barriers would be in good agreement with the experimental value. The rate-limiting transitions state structure from the QM/MM simulations is also consistent with the SCC-DFTBPR/PB results. The average POlg and POnu bond lengths are 1.84 and 2.14 Å, respectively, also in qualitative agreement with previous theoretical studies of Warshel and co-workers40.
IV. CONCLUDING REMARKS
QM/MM methods have become indispensable in the analysis of reaction mechanisms of complex systems. Once specific QM and MM models are chosen for a given application, the remaining factor that determines the accuracy of the calculation is the coupling between the QM and MM models. In the current work, we analyze and improve the QM/MM coupling Hamiltonian when SCC-DFTB is the QM method. In the original implementation8, the electrostatic interaction between SCC-DFTB and MM atoms is computed using a simple point charge model in which the QM charge density is represented as Mulliken charges. This becomes a poor approximation when the MM and QM atoms approach each other, making the original SCC-DFTB/MM method less reliable when the QM region is highly charged. Motivated by models used to approximate two electron integrals in semi-empirical QM methods, we propose to revise the electrostatic part of the SCC-DFTB/MM interaction to adopt a Klopman-Ohno (KO) functional form, which mimics the interaction between two spherical charges. Similar to the Gaussian-blur models10,72 for ab initio QM/MM, the KO model takes charge penetration effects into consideration and therefore significantly improves the description of charge-charge interactions at short range; at the same time, the computation remains efficient. To be consistent with the third-order formulation of SCC-DFTB23, the Hubbard parameter in the KO functional is dependent on the QM charge. In this way, the effective size of the QM charge distribution naturally adjusts as the QM region undergoes chemical transformations, making the KO based QM/MM scheme particularly attractive for describing chemical reactions in the condensed phase.
The KO based QM/MM scheme introduces two parameters for each element; together with the QM van der Waals parameters, there are only four QM/MM parameters for each element. As proof of concept, we have optimized these parameters for O, C, H and P using a specific version of SCC-DFTB (SCC-DFTBPR). Test calculations using microsolvation clusters indicate that the KO based QM/MM scheme significantly improves the interactions between QM and MM atoms over the original point-charge implementation8. Since the number of parameters is small, the parameterized model appears highly transferrable, as demonstrated by test calculations on molecules in the QCRNA database, which are rather different from the ones used for fitting the parameters. Finally, we apply the KO based QM/MM scheme to study the hydrolysis of two phosphate monoesters, MMP2− and pNPP2−. These were challenging to study using the original point-charge based SCC-DFTB/MM model, while the KO based SCC-DFTBPR/MM calculations lead to results consistent with experimental data and previous theoretical calculations using approximate solvent models.
As future work, we will develop QM/MM-KO parameters for more elements following the complete parameterization of DFTB323, which is more robust and generally applicable than SCC-DFTBPR. For the new parameterization, solute-solvent interactions calculated at a higher level of QM method will be used as reference, rather than using SCC-DFTBPR as done here. Along this line, it is worth noting that SCC-DFTB is a minimal basis set method and therefore expected to have only limited polarizability26; as a result, to be fully consistent with high-level QM/MM calculations, it is likely that additional improvements such as including a response density contribution27 are necessary. Finally, it is worth exploring the practical benefit of better describing the van der Waals component of QM/MM coupling by making the QM atomic radii and polarizabilities fully consistent with the QM charge distribution. For reactions that involve highly charged species in a polar environment, it’s likely that the electrostatic component dominates although the contribution from the van der Waals component may still be non-negligible.
TABLE I.
Optimized parameters for different QM/MM interaction schemes
| Point-chargea | KO-∞b | KO-MMc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| εα (kcal/mol) | σα (Å) | εα (kcal/mol) | σα (Å) | aα − | bα (Å−1) | εα (kcal/mol) | σα (Å) | aα − | bα (Å−1) | |
| O | −0.18 | 1.92 | −0.03 | 2.05 | 0.068 | 0.017 | −0.06 | 1.88 | 0.042 | 0.021 |
| C | −0.06 | 2.15 | −0.07 | 2.11 | 0.046 | 0.059 | −0.05 | 2.15 | 0.026 | 0.069 |
| P | −1.23 | 2.36 | −0.52 | 2.39 | 0.060 | 0.001 | −0.26 | 2.42 | 0.054 | 0.001 |
| H | −0.02 | 0.82 | −0.04 | 0.76 | 0.211 | 0.055 | −0.02 | 0.81 | 0.066 | 0.053 |
The original point-charge based SCC-DFTB/MM Hamiltonian8 with newly optimized QM van der Waals parameters;
the Hubbard parameter for the MM atoms in the KO scheme (see Eq. 13) is set to ∞ ;
The Hubbard parameters for the MM atoms in the KO scheme are taken from the calculated values for the respective element23.
Acknowledgments
The work is supported in part by NIH (R01-GM084028) and by the WARF of UW-Madison. Computational resources from the National Center for Supercomputing Applications at the University of Illinois and the Center for High Throughput Computing (CHTC) at UW-Madison are greatly appreciated; computations are also supported in part by National Science Foundation through a major instrumentation grant (CHE-0840494).
References
- 1.Friesner RA, Guallar V. Annu Rev Phys Chem. 2005;56:389–427. doi: 10.1146/annurev.physchem.55.091602.094410. [DOI] [PubMed] [Google Scholar]
- 2.Gao JL, Ma SH, Major DT, Nam K, Pu JZ, Truhlar DG. Chem Rev. 2006;106:3188–3209. doi: 10.1021/cr050293k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riccardi D, Schaefer P, Yang Y, Yu H, Ghosh H, Prat-Resina X, K•onig P, Li G, Xu D, Guo H, Elstner M, Cui Q. J Phys Chem B. 2006;110:6458–6469. doi: 10.1021/jp056361o. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, Yang WT. Annu Rev Phys Chem. 2008;59:573–601. doi: 10.1146/annurev.physchem.59.032607.093618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senn HM, Thiel W. Angew Chem Int Ed. 2009;48:1198–1229. doi: 10.1002/anie.200802019. [DOI] [PubMed] [Google Scholar]
- 6.Kamerlin SCL, Haranczyk M, Warshel A. J Phys Chem B. 2009;113:1253–1272. doi: 10.1021/jp8071712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal P, Ghosh N, Phatak P, Clemens M, Gaus M, Elstner M, Cui Q. J Am Chem Soc. 2011;133:14981–14997. doi: 10.1021/ja201568s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao JL, Truhlar DG. Annu Rev Phys Chem. 2002;53:467–505. doi: 10.1146/annurev.physchem.53.091301.150114. [DOI] [PubMed] [Google Scholar]
- 9.Elstner M, Porezag D, Jungnickel G, Elsner J, Haugk M, Frauenheim T, Suhai S, Seifert G. Phys Rev B. 1998;58:7260–7268. [Google Scholar]
- 10.Kruger T, Elstner M, Schiffels P, Frauenheim T. J Chem Phys. 2005;122:114110. doi: 10.1063/1.1871913. [DOI] [PubMed] [Google Scholar]
- 11.Sattelmeyer KW, Tirado-Rives J, Jorgensen W. J Phys Chem A. 2006;110:13551–13559. doi: 10.1021/jp064544k. [DOI] [PubMed] [Google Scholar]
- 12.Otte N, Scholten M, Thiel W. J Phys Chem A. 2007;111:5751–5755. doi: 10.1021/jp0700130. [DOI] [PubMed] [Google Scholar]
- 13.Elstner M. J Phys Chem A. 2007;111:5614–5621. doi: 10.1021/jp071338j. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Yu H, York D, Cui Q, Elstner M. J Phys Chem A. 2007;111:10861–10873. doi: 10.1021/jp074167r. [DOI] [PubMed] [Google Scholar]
- 15.Gaus M, Cui Q, Elstner M. J Chem Theo Comp. 2011;7:931–948. doi: 10.1021/ct100684s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elstner M, Cui Q, Munih P, Kaxiras E, Frauenheim T, Karplus M. J Comput Chem. 2003;24:565–581. doi: 10.1002/jcc.10201. [DOI] [PubMed] [Google Scholar]
- 17.Cai Z, Lopez P, Reimers JR, Cui Q, Elstner M. J Phys Chem A. 2007;111:5743–5750. doi: 10.1021/jp071701m. [DOI] [PubMed] [Google Scholar]
- 18.Zheng GS, Witek HA, Bobadova-Parvanova P, Irle S, Musaev DG, Prabhakar R, Morokuma K. J Chem Theo Comp. 2007;3:1349–1367. doi: 10.1021/ct600312f. [DOI] [PubMed] [Google Scholar]
- 19.Moreira NH, Dolgonos G, Aradi B, da Roasa AL, Frauenheim T. J Chem Theo Comp. 2009;5:605–614. doi: 10.1021/ct800455a. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Yu H, York D, Elstner M, Cui Q. J Chem Theo Comp. 2008;4:2067–2084. doi: 10.1021/ct800330d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elstner M, Frauenheim T, Suhai S. J Mol Struct. 2003;632:29–41. [Google Scholar]
- 22.Elstner M. Theo Chem Acc. 2006;116:316–325. [Google Scholar]
- 23.Bondar AN, Smith JC, Elstner M. Theo Chem Acc. 2010;125:353–363. [Google Scholar]
- 24.Yang Y, Yu H, Cui Q. J Mol Biol. 2008;381:1407–1420. doi: 10.1016/j.jmb.2008.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riccardi D, Koenig P, Guo H, Cui Q. Biochem. 2008;47:2369–2378. doi: 10.1021/bi701950j. [DOI] [PubMed] [Google Scholar]
- 26.Xu DG, Guo H. J Am Chem Soc. 2009;131:9780–9788. doi: 10.1021/ja9027988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou GH, Cui Q. J Am Chem Soc. 2012;134:229–246. doi: 10.1021/ja205226d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakravorty DK, Wang B, Lee CW, Giedroc DP, Merz KM., Jr J Am Chem Soc. 2012;134:3367–3376. doi: 10.1021/ja208047b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riccardi D, Zhu X, Goyal P, Yang S, Hou G, Cui Q. Science China Chem. 2012;55:3–18. [Google Scholar]
- 30.Ponder JW, Wu CJ, Ren PY, Pande VS, Chodera JD, Schnieders MJ, Haque I, Mobley DL, Lambrecht DS, DiStasio RA, Head-Gordon M, Clark GNI, Johnson ME, Head-Gordon T. J Phys Chem B. 2010;114:2549–2564. doi: 10.1021/jp910674d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freindorf M, Gao JL. J Comp Chem. 1996;17:386–395. [Google Scholar]
- 32.Riccardi D, Li GH, Cui Q. J Phys Chem B. 2004;108:6467–6478. doi: 10.1021/jp037992q. [DOI] [PubMed] [Google Scholar]
- 33.Stone AJ. The theory of intermolecular forces; Oxford University Press; Oxford, UK: 1997. [Google Scholar]
- 34.Morokuma K. Acc Chem Res. 1977;10:294–300. [Google Scholar]
- 35.Jeziorski B, Moszynski R, Szalewicz K. Chem Rev. 1994;94:1887–1930. [Google Scholar]
- 36.Gresh N, Cisneros GA, Darden TA, Piguemal J-P. J Chem Theo Comp. 2007;3:1960–1986. doi: 10.1021/ct700134r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Field MJ, Bash PA, Karplus M. J Comp Chem. 1990;11:700–733. [Google Scholar]
- 38.Reuter N, Dejaegere A, Maigret B, Karplus M. J Phys Chem A. 2000;104:1720–1735. [Google Scholar]
- 39.Gao JL, Amara P, Alhambra C, Field MJ. J Phys Chem A. 1998;102:4714–4721. [Google Scholar]
- 40.Das D, Eurenius KP, Billings EM, Sherwood P, Chatfield DC, Hodoscek M, Brooks BR. J Chem Phys. 2002;117:10534–10547. [Google Scholar]
- 41.Wang B, Truhlar DG. J Chem Theo Comp. 2010;6:3330–3342. doi: 10.1021/ct1003862. [DOI] [PubMed] [Google Scholar]
- 42.Nam K, Gao JL, York DM. J Chem Theo Comp. 2005;1:2–13. doi: 10.1021/ct049941i. [DOI] [PubMed] [Google Scholar]
- 43.Riccardi D, Schaefer P, Cui Q. J Phys Chem B. 2005;109:17715–17733. doi: 10.1021/jp0517192. [DOI] [PubMed] [Google Scholar]
- 44.Im W, Bernèche S, Roux B. J Chem Phys. 2001;114:2924–2937. [Google Scholar]
- 45.Schaefer P, Riccardi D, Cui Q. J Chem Phys. 2005;123:014905. doi: 10.1063/1.1940047. [DOI] [PubMed] [Google Scholar]
- 46.Cui Q, Elstner M, Kaxiras E, Frauenheim T, Karplus M. J Phys Chem B. 2001;105:569–585. [Google Scholar]
- 47.Thiel W. Adv Chem Phys. 1996;93:703–757. [Google Scholar]
- 48.Giese TJ, York DM. J Chem Phys. 2007;127:194101. doi: 10.1063/1.2778428. [DOI] [PubMed] [Google Scholar]
- 49.Kalinowski JA, Lesyng B, Thompson JD, Cramer CJ, Truhlar DG. J Phys Chem A. 2004;108:2545–2549. [Google Scholar]
- 50.Kolb M, Thiel W. J Comp Chem. 1993;14:775–789. [Google Scholar]
- 51.Politzer P, Parr R, Murphy D. J Chem Phys. 1983;79:3859–3861. [Google Scholar]
- 52.Pearson R. Inorg Chem. 1988;27:734–740. [Google Scholar]
- 53.Ghosh D, Biswas R. Int J Mol Sci. 2003;4:379–407. [Google Scholar]
- 54.Politzer P, Murray J, Lane P. J Comp Chem. 2003;24:505–511. doi: 10.1002/jcc.10209. [DOI] [PubMed] [Google Scholar]
- 55.Politzer P. J Chem Phys. 1987;86:1072–1073. [Google Scholar]
- 56.Tkatchenko A, Scheffler M. Phys Rev Lett. 2009;102:073005. doi: 10.1103/PhysRevLett.102.073005. [DOI] [PubMed] [Google Scholar]
- 57.Becke AD, Johnson ER. J Chem Phys. 2005;123:154101. doi: 10.1063/1.2065267. [DOI] [PubMed] [Google Scholar]
- 58.Jorgensen W, Chandrasekhar J, Madura J, Impey R, Klein M. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 59.Goldberg DE. Genetic algorithms in search, optimization, and machine learning; Addison-Wesley; Reading, MA: 1989. [Google Scholar]
- 60.Carroll DL. [accessed Jan 2009]; http://cuaerospace.com/carroll/ga.html.
- 61.Lad C, Williams NH, Wolfenden R. Proc Natl Acad Sci USA. 2003;100:5607–5610. doi: 10.1073/pnas.0631607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Florián J, Warshel A. J Phys Chem B. 1998;102:719–734. [Google Scholar]
- 63.Klhn M, Rosta E, Warshel A. J Am Chem Soc. 2006;128:15310–15323. doi: 10.1021/ja065470t. [DOI] [PubMed] [Google Scholar]
- 64.O’Brie P, Herschlag D. Biochem. 2002;41:3207–3225. doi: 10.1021/bi012166y. [DOI] [PubMed] [Google Scholar]
- 65.Zalatan J, Fenn T, Brunger A, Herschlag D. Biochem. 2006;45:9788–9803. doi: 10.1021/bi060847t. [DOI] [PubMed] [Google Scholar]
- 66.Boorks C, Karplus M. J Chem Phys. 1983;79:6312–6325. [Google Scholar]
- 67.Steinbach P, Brooks B. J Comput Chem. 1994;15:667–683. [Google Scholar]
- 68.Brooks CL, Karplus M. J Chem Phys. 1983;79:6312–6325. [Google Scholar]
- 69.Rychaert J, Ciccotti G, Berendsen H. J Comput Phys. 1977;23:327–341. [Google Scholar]
- 70.Torrie GM, Valleau JP. J Chem Phys. 1977;23:187–199. [Google Scholar]
- 71.Kumar S, Bouzida D, Swendsen RH, Kollman PA, Rosenberg JM. J Comp Chem. 1992;13:1011–1021. [Google Scholar]
- 72.Hou GH, Zhu X, Cui Q. J Chem Theo Comp. 2010;6:2303–2314. doi: 10.1021/ct1001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly CP, Cramer CJ, Truhlar DG. J Chem Theo Comp. 2005;1:1133–1152. doi: 10.1021/ct050164b. [DOI] [PubMed] [Google Scholar]
- 74.Rosta E, Kamerlin SCL, Warshel A. Biochem. 2008;47:3725–3735. doi: 10.1021/bi702106m. [DOI] [PubMed] [Google Scholar]
- 75.Cui Q, Karplus M. J Phys Chem B. 2002;106:1768–1798. [Google Scholar]
- 76.Giese TJ, Gregersen BA, Liu Y, Nam K, Mayaan E, Moser A, Range K, Faza AN, Lopez CS, de Lera AR, Schaftenaar G, Lopez X, Lee TS, Karypis G, York DM. J Mol Graphics Modell. 2006;25:423–433. doi: 10.1016/j.jmgm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Cui Q. J Phys Chem B. 2009;113:4930–4933. doi: 10.1021/jp810755p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y, Cui Q. J Phys Chem A. 2009;113:12439–12446. doi: 10.1021/jp902949f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giese TJ, York DM. J Chem Phys. 2005;123:164108. doi: 10.1063/1.2080007. [DOI] [PubMed] [Google Scholar]
- 80.Lassila J, Zalatan J, Herschlag D. Annu Rev Biochem. 2011;80:669–702. doi: 10.1146/annurev-biochem-060409-092741. [DOI] [PMC free article] [PubMed] [Google Scholar]