Abstract
Pyrethroids are highly toxic to fish at parts per billion or parts per trillion concentrations. Their intended mechanism is prolonged sodium channel opening, but recent studies reveal that pyrethroids such as permethrin and bifenthrin also have endocrine activity. Additionally, metabolites may have greater endocrine activity than parent compounds. We evaluated the in vivo concentration-dependent ability of bifenthrin and permethrin to induce choriogenin (an estrogen-responsive protein) in Menidia beryllina, a fish species known to reside in pyrethroid contaminated aquatic habitats. We then compared the in vivo response to an in vitro assay: CALUX (Chemical Activated Luciferase Gene Expression). Juvenile Menidia beryllina exposed to bifenthrin (1, 10, 100 ng/L), permethrin (0.1, 1, 10 µg/L), and ethinylestradiol (1, 10, 50 ng/L) had significantly higher ng/mL choriogenin (Chg) measured in whole body homogenate than controls. While Chg expression in fish exposed to ethinylestradiol (EE2) exhibited a traditional sigmoidal concentration-response, curves fit to Chg expressed in fish exposed to pyrethroids suggest a unimodal response, decreasing slightly as concentration increases. While the in vivo response indicated that bifenthrin and permethrin or their metabolites act as estrogen agonists, the CALUX assay demonstrated estrogen antagonism by the pyrethroids. Our results, supported by evidence from previous studies, suggest that bifenthrin and permethrin, and/or their metabolites, appear to act as estrogen receptor (ER) agonists in vivo, and that the unmetabolized pyrethroids, particularly bifenthrin, act as an ER antagonists in cultured mammalian cells.
Keywords: choriogenin, CALUX, Menidia beryllina, ELISA, nonlinear regression
1. Introduction
Pyrethroid pesticide use has undergone a considerable increase as organophosphate pesticides are phased out due to concerns regarding mammalian toxicity [1, 2]. Pyrethroids, which are not acutely toxic to mammals at concentrations applied or found in the environment, are highly toxic to fish and aquatic invertebrates at parts per billion (ppb) or parts per trillion (pptr) levels [3, 4]. Although it has been argued that pyrethroid bioavailability is reduced in aquatic environments because these compounds are lipophilic and tend to bind to sediments instead of remaining dissolved, it has been shown that pyrethroids may remain in the water column for days to weeks after introduction [6] and that they are soluble enough to produce biological and toxic effects [3].
Pyrethroids disrupt the nervous system via prolongation of the opening of voltage-dependent ion channels, the consequences of which are convulsions, paralysis and eventual mortality [2, 3]. Sublethal neurotoxic effects include impaired swimming ability in both fish and invertebrates [7] and a reduced ability to avoid predators [8]. In addition to effects produced by the intended mechanism, recent results from in vitro assays reveal that some pyrethroids can act as estrogens, anti-estrogens and/or anti-androgens [9, 10]. Pyrethroid metabolites are reported to have even greater endocrine activity than their parent structures in vitro [11, 12, 13]. Pyrethroids also have considerable endocrine activity in vivo, particularly bifenthrin, permethrin and especially permethrin metabolites. These can induce estrogen-dependent egg proteins in male fish [13, 14]. Notably, permethrin metabolites induced relatively higher expression of such proteins than the parent compound in a recent study [13].
Permethrin and bifenthrin are two of the most frequently detected pyrethroid pesticides in aquatic ecosystems ([15, 16, 17, 18]. Both are used in agriculture, but the more toxic bifenthrin is also increasingly used for landscaping and structural pest control under the trade name Talstar™ [16]. Due to their increasing presence in aquatic ecosystems and their potential to cause endocrine disruption, here we describe studies examining the biochemical mechanisms and endocrine disrupting effects of low pyrethroid concentrations utilizing a combination of in vitro and in vivo approaches.
The first objective of our study was to evaluate the concentration-response to bifenthrin and permethrin in vivo, in Menidia beryllina, a fish species known to reside in pyrethroid contaminated aquatic habitats. Our second objective was to utilize a biomarker of estrogenic endocrine disruption that is potentially more sensitive than vitellogenin [19], the estrogen-dependent egg yolk protein typically measured following exposure to suspected endocrine disrupting compounds (EDCs) [20]. Although vitellogenin is a reliable marker of estrogenic endocrine disruption, we instead measured expression of choriogenin (Chg), an estrogen-dependent egg coat protein, using a Menidia specific antibody [21]. Chg, the precursor to the chorion or zona radiata, has been demonstrated to be more sensitive than vitellogenin (Vtg) [19, 22], and was shown recently to have increased mRNA expression in larval fish following bifenthrin exposure [23].
The third objective was to compare the response in fish to that in an estrogen-responsive cell bioassay (i.e. CALUX (Chemical Activated Luciferase Gene Expression)). In contrast to the widely utilized Yeast Estrogen Screen (YES), CALUX has increased sensitivity due to natively expressed estrogen receptors (ERs) and expresses mRNA for both ER alpha and beta [24]. To the best of our knowledge this is the first study to evaluate the concentration-response of an estrogen-responsive protein in fish to pyrethroid pesticides and is the first study to use CALUX to evaluate the estrogenic activity of pyrethroid pesticides.
2. Materials and Methods
2.1 Bioassay
We conducted a 14d static aqueous exposure (with daily water renewal) using 65–70d old juvenile Menidia beryllina (Aquatic Biosystems, Fort Collins, CO). Fish were exposed to three concentrations of bifenthrin (1, 10, 100 ng/L), permethrin (0.1, 1, 10 µg/L), or EE2 (positive control: 1, 10, 50 ng/L) spiked into laboratory control water. Control water consisted of distilled water and filtered sea water from Bodega Bay, CA (5 µm) mixed to achieve a salinity of 5 ± 1 parts per thousand, which is within the range of salinity in M. beryllina habitat [25]. Stock solutions of 1 µg/L for bifenthrin, permethrin and EE2 were made in methanol (MeOH) for all concentrations except 1 and 10 µg/L permethrin, for which a stock solution of 10 µg/mL was used. Methanol at a concentration equal to the highest amount used in bifenthrin and permethrin treatments (0.01%) was spiked into lab control water as the negative control. This concentration of methanol approved for use in toxicity testing with fish [26], therefore a non-methanol control was not included. Different ranges of concentrations (µg/L vs. ng/L) were used because bifenthrin and permethrin differ substantially in toxicity, the former being more toxic. The highest concentration used for permethrin (10 µg/L) was below the 96h LC50 of 27.5 µg/L for larval Menidia beryllina2], and this concentration has already been shown to induce estrogen-responsive proteins (vitellogenin) in a fish closely related to M. beryllina - Japanese medaka (O. latipes) [13]. The highest concentration for bifenthrin (100 ng/L) was well below the 144h LC50 in larval bluegill sunfish (Lepomis machrochirus - 350 ng/L) and two orders of magnitude below the 96h LC50 in larval sheepshead minnow (Cyprinidon variegatus – 17.8 µg /L), two species that have higher sensitivity to permethrin than Menidia beryllina2]. The highest concentrations of bifenthrin, permethrin, and EE2 were intended to induce maximal expression of Chg without causing significant mortality, while the lowest concentrations were intended to mimic environmentally realistic exposures. Mean survival in all treatments after 14 days was ≥ 80%, conforming to EPA chronic toxicity testing standards[27], with the exception of the 10 µg /L permethrin treatment in which mean survival was 70%. Permethrin (purity 99%) and ethinylestradiol (purity 99%) were obtained from Sigma Aldrich (St. Louis, MO), and bifenthrin (purity 99%) from the USGS Analytical Chemistry Laboratory (Sacramento, CA). Pyrethroids were 50/50 mixtures of isomers.
Fish were maintained in 3L glass jars with 1L test water in each (10 fish / jar). The experiment was maintained at a 14 hr /10 hr light/ dark cycle and temperature was controlled at 21 ± 2 °C. Each jar was aerated and dissolved oxygen, ammonia and pH were measured daily prior to water changes. Fish were fed live or frozen Artemia nauplii each day at least one hour prior to water change. For the bioassays, there were 4 replicates per treatment (except the positive control which had 2 replicates). Each of these replicates consisted of 10 individual juvenile fish (65–70d old at test initiation).
At test termination, fish were anesthetized on ice, immediately snap-frozen with liquid nitrogen and stored at −80 °C. Surviving whole fish (6–10 per replicate) were pooled and pulverized in ~5 mL of liquid nitrogen with a ceramic mortar and pestle. Once ground to a powder, ice-cold 50 mM Tris-HCl homogenization buffer with protease inhibitor (Roche COmplete Mini, Penzberg, Germany) was added at a ratio of 1 ml buffer:2g tissue and further homogenized with a Fisher Scientific Tissuemiser (ThermoFisher Scientific, Waltham, MA, USA) for 60 seconds. The homogenate was centrifuged for one hour at 20,800 ×g at 4 °C and the resulting supernatant was removed and recentrifuged at 14,850 ×g for 15 minutes to ensure removal of all particulates. The final supernatant was stored immediately at −80 °C and the protein concentration of each sample quantitated using the BCA protein assay (Pierce, Rockford, IL, USA).
2.2 ELISA
A Menidia polyclonal antibody, produced and optimized from Menidia chorion, was used to measure the relative amount of Chg expressed in whole body homogenate (WBH) via an indirect ELISA as previously described [21]. This methodology allows for accurate quantification of the amount of Chg in each sample relative to Menidia chorion levels expressed in the absence or presence of EE2.
2.3 CALUX assay
The estrogen receptor-based CALUX mammalian cell bioassay utilizes a human ovarian carcinoma (BG-1) cell line, which has been stably transfected with an estrogen-responsive luciferase reporter plasmid. This CALUX cell line responds to estrogenic chemicals with the induction of expression of firefly luciferase. The CALUX bioassay has advantages over the yeast-based YES bioassay commonly used to detect estrogenic chemicals in that it expresses mRNA for ER isoforms alpha and beta [24], in contrast to the YES assay that is usually only transfected with ER alpha. The CALUX bioassay was used to determine the concentration-dependent agonist (pesticide alone) and antagonist (pesticide in presence of estradiol) effects of pyrethroids used in these studies and chemical exposure and luciferase analyses was carried out as described in detail [28] (see Supplemental Methods / Results). The source of 17β estradiol used as a positive control in all CALUX bioassays was from Sigma Chemical Co. (St. Louis, MO, USA).
2.4 Pyrethroid extraction and analysis
Pyrethroid spiked water samples, both time zero and 24 hours, were sent to the USGS Analytical Laboratory (Sacramento, CA) for confirmatory chemistry. Water samples were filtered through 0.7 mm glass fiber filters, extracted onto Oasis® HLB solid phase extraction cartridges and analyzed by gas chromatography electron ionization mass spectrometry in selective ion monitoring mode. Methods were performed as described in Hladik et al. 2008 [29] (see Supplemental Methods / Results). All concentrations of bifenthrin and permethrin had acceptable recoveries (80%–110%).
2.5 Data Analysis
2.5.1 Choriogenin ELISA data
ELISA data were first quantified by comparison of absorbance values to a chorion standard curve to obtain Chg equivalents. Equivalents were then divided by the protein concentration per well to normalize the data as described in detail previously [21]. Then two types of statistical analyses were performed on Chg data: a traditional ANOVA followed by a Tukey HSD test to detect whether there were differences between Chg expression induced by different compounds (i.e. ethinylestradiol vs. bifenthrin), and nonlinear regression to fit concentration-response curves for each compound.
Because relationships between continuous variables, such as a concentration-response curve, cannot be properly described using ANOVA [30], we used nonlinear regression to evaluate the concentration-response for each compound (see Supplemental Methods / Results). The patterns of Chg expression in our experimental treatments did not appear to correspond to the sigmoidal concentration-response curve one would expect for increasing concentration of a toxic compound. Therefore, we used a model selection approach to choose the concentration-response curve that was best supported by our data, as done in a previous toxicological study with three concentrations each of the pyrethroids permethrin and cyfluthrin [4]. In general, concentration-response curves with more parameters (i.e., greater complexity) will afford a better fit to data. However, models with more parameters run the risk of overfitting: they may fit the random variation in a small data set well, but be unsuitable for predicting the underlying relationship or trend. We avoided overfitting by using information theory criteria to identify the most parsimonious model; that is, the model that affords the best fit with the fewest parameters [31]. We fit two different curves to each Chg dataset: a sigmoidal curve (three parameter logistic model) and a unimodal curve (a modified three parameter logistic model; [32]). Curves were fit by nonlinear least squares using the trust-region reflective algorithm in Matlab 7.11 (The Mathworks, Inc., Natick MA). The most parsimonious model was determined using Akaike’s Information Criterion corrected for small sample sizes (AICc), a method that is specifically intended to avoid overfitting small datasets [31], All curves were fit to log(x + 0.01) transformed concentrations data to avoid the problem of taking the log of concentration 0 (MeOH control).
2.5.2 CALUX
For the CALUX bioassays, an individual t-test was used to determine whether treatments were significantly different from the relevant positive control (estradiol), expressed as 100% cellular response. Resulting p-values were Bonferroni corrected to account for multiple comparisons. Differences among treatments in estrogen equivalents were tested using an ANOVA followed by a Tukey HSD test. All ANOVAs were performed with the statistical software R 2.11.1 [33].
3. Results
3.1 Differences between Treatments
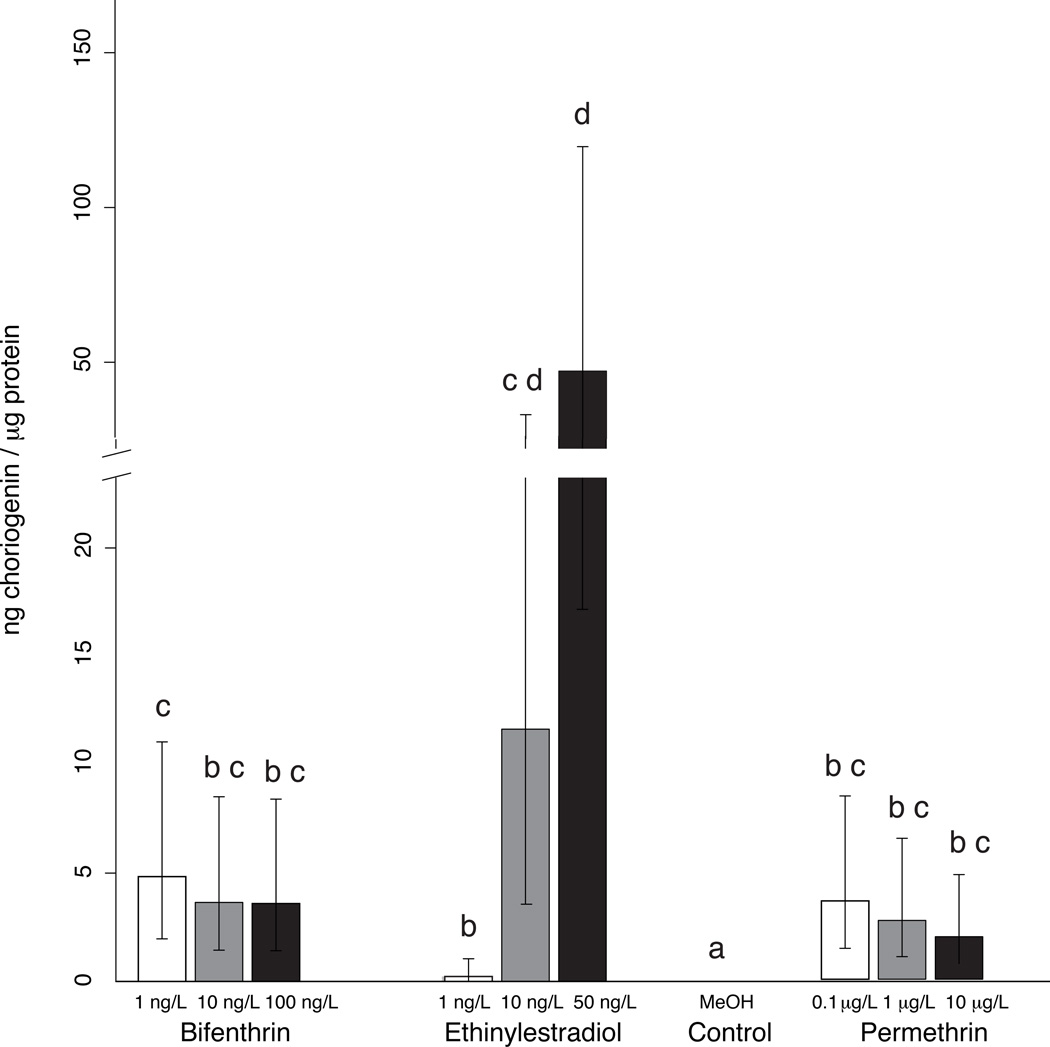
Juvenile Menidia beryllina exposed to all three concentrations of bifenthrin (1, 10, 100 ng/L), permethrin (0.1, 1, 10 µg/L), and EE2 (1, 10, 50 ng/L) have significantly higher relative ng/mL Chg expressed in whole body homogenate than the methanol control (Figure 1). None of the pyrethroid treatments were significantly different from one another when compared using ANOVA (p > 0.05), however a trend towards an inverse correlation between response and increasing concentration with both permethrin and bifenthrin is apparent (Figure 1). Average water quality parameters measured prior to daily renewal for temperature, pH, dissolved oxygen and ammonia were 21.0 ± 0.5, 7.5 ± 0.2, 8.5 ± 0.6, and 0.2 ± 0.1 respectively. The results pertaining to this trend will be further explained in the Concentration-Response section.
Figure 1. Effect of bifenthrin, permethrin and ethinylestradiol on choriogenin expression in juvenile Menidia beryllina.
Fish were exposed to the indicated concentration of each compound in water (with daily water changes) for 14 days followed by determination of choriogenin levels. Values represent the mean ± 95% confidence limits of whole body homogenate from 4 pooled replicates of 6–10 fish each (bifenthrin, permethrin) and 2 pooled replicates of 10 fish each (ethinylestradiol) as determined by ANOVA. Significant differences between treatments were determined via a Tukey test. Treatments that were not significantly different (p > 0.05) are indicated by the same letter and treatments that are significantly different from each other (p < 0.05) are indicated by different letters.
Permethrin and bifenthrin treatments were not significantly different from positive controls of 1 and 10 ng/L EE2 over a 100-fold concentration range, with the exception of 1 ng/L bifenthrin, which had a significantly higher relative Chg level than that produced by 1 ng/L EE2 (p = 0.022). However, all permethrin and bifenthrin concentrations trend towards having higher relative expression of Chg than the 1 ng/L EE2 treatment, and lower relative expression than the 10 ng/L EE2 treatment. A concentration-dependent increase in Chg levels was observed with EE2 exposure. The 1 ng/L EE2 treatment is significantly different from both 10 ng/L and 50 ng/L EE2. While the 10 and 50 ng/L EE2 treatments were not significantly different, the trend (mean values) was progressively higher with increasing concentration.
3.2 Concentration-Response
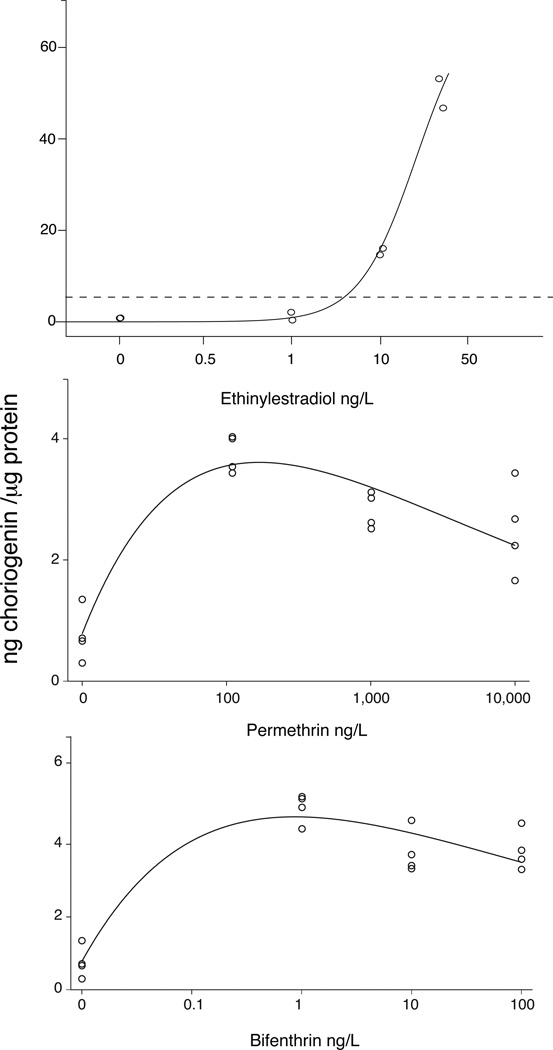
Concentration response curves for ethinylestradiol, permethrin and bifenthrin (Figure 2) were generated using nonlinear least squares regression and curve fit was evaluated using AICc ([31]; AIC adjusted for small sample sizes). As expected, a sigmoidal model had the most parsimonious fit to the EE2 response (Figure 2), with a delta AICc = 0 (Table A.1, Supplemental Methods / Results). The curve does not reach an asymptote likely because Chg induction may not have reached a maximum at our highest exposure of 50 ng/L.
Figure 2. Choriogenin concentration response of juvenile Menidia beryllina to ethinylestradiol, bifenthrin, and permethrin.
Most parsimonious curve fit to concentration-response data (sigmoidal or unimodal) using nonlinear regression. Each data point represents the combined whole body homogenate of 6–10 juvenile Menidia beryllina exposed for 14 days to the corresponding concentration. The horizontal dotted line across the top panel represents the maximum choriogenin response in pyrethroid-exposed fish.
In contrast to the results for EE2, the best (i.e. most parsimonious) concentration- response curve for induction of Chg by bifenthrin and permethrin is not sigmoidal, but a unimodal, or biphasic curve (Figure 2). A unimodal model, in which the response peaks and then begins to decrease as concentration increases, has a more parsimonious fit to the data (AICc = 0) than a linear or sigmoidal model despite having more parameters (delta AICc = 0.3 and 5.0, respectively).
3.3 CALUX assay
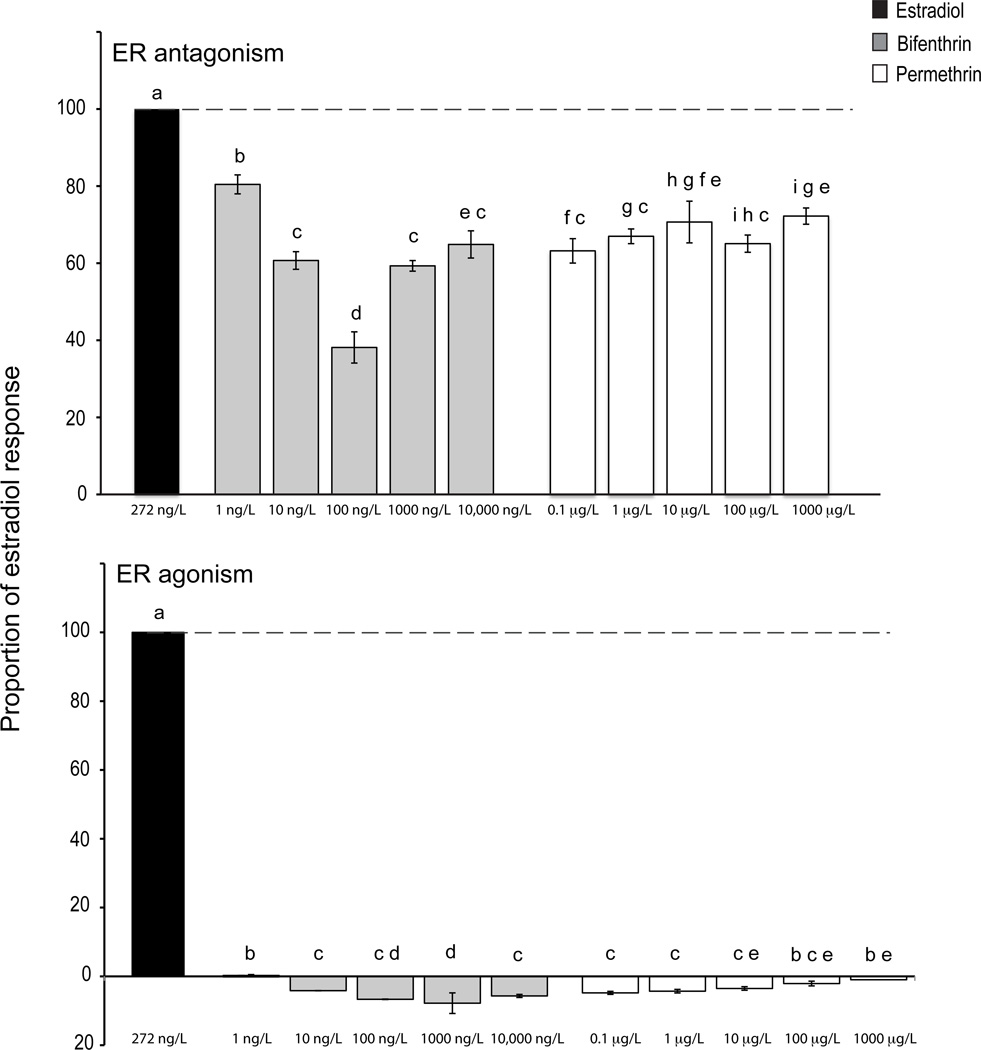
The CALUX assay did not detect ER agonism for any of the permethrin or bifenthrin concentrations tested (Figure 3). In the ER antagonism assay, however, an initial bifenthrin concentration-dependent decrease in the ability of E2 to induce ER-dependent reporter gene activity (1–100 ng/L bifenthrin) was observed with a recovery of estrogenic activity at bifenthrin concentrations greater than 100 ng/L (Figure 3). In contrast, although some permethrin-dependent reduction in estrogenic activity was observed (~30–40% of maximal estradiol activity), there was no concentration-dependence of this inhibitory effect.
Figure 3. Activation and inhibition of the ER CALUX by bifenthrin and permethrin.
CALUX ER agonist and antagonist activity are presented as a proportion of the estradiol (272 ng/L = 1 nM) positive control (100%). Error bars represent 95% confidence intervals. With ER antagonism (n = 3 replicate wells), values below 100% indicate the occurrence of antagonism. With ER agonist activity (n = 3 replicate wells), values below 20% of the estradiol control indicate that bifenthrin and permethrin are not ER agonists at that concentration. Significant difference of all treatments from E2 controls in both assays was determined via Bonferroni corrected individual t-tests (p < 0.05) and is indicated with the letter “a.” Tukey test results are represented by letter codes “b – i” above each bar. Treatments with different letters are significantly different from each other (p < 0.05), treatments sharing letter codes are not significantly different (p > 0.05).
3.4 Dissolved pyrethroid concentrations
As was expected, there was a decrease in the dissolved pyrethroid concentrations over a 24-hour period. While initial (day 0) dissolved concentrations were within 90 – 111 % of nominal concentrations, concentrations measured after approximately 24 hours, prior to daily bioassay water changes had decreased to 64–73% of nominal (Table I). This decrease in pyrethroid concentration after 24 hours is likely due to adsorption of bifenthrin and permethrin to walls of the glass jars used [34, 35]. Levels of bifenthrin and permethrin were not measured in fish tissue since all of the samples were required for protein analysis.
Table 1. Chemical analysis of aqueous permethrin and bifenthrin concentrations.
Concentrations of bifenthrin and permethrin were measured via GC/MS in newly spiked laboratory control water and 24 hours later in water that had been used for the Menidia beryllina 14 day bioassay.
| compound | nominal | actual (new) | actual (24 hr) |
|---|---|---|---|
| bifenthrin | 1 ng/L | 0.898 ng/L | 0.733 ng/L |
| 10 ng/L | 9.514 ng/L | 6.890 ng/L | |
| 100 ng/L | 111 ng/L | 71 ng/L | |
| permethrin | 0.1 µg/L | 0.092 µg/L | 0.068 µg/L |
| 1 µg/L | 1.05 µg/L | 0.641 µg/L | |
| 10 µg/L | 9.12 µg/L | 6.52 µg/L |
4. Discussion
The first objective of our study was to evaluate the concentration-response to bifenthrin and permethrin in vivo, in a fish species known to reside in pyrethroid contaminated aquatic habitats. To achieve this goal we evaluated the effects of a range of bifenthrin and permethrin concentrations, some near or below those found recently in storm drain run-off (bifenthrin 73 ng/L and permethrin 66.1 ng/L – [16]) on Menidia beryllina (inland silverside) and close to the low ng/L concentrations frequently detected in the environment [2]. Our second objective was to utilize a biomarker of estrogenic endocrine disruption that is potentially more sensitive than vitellogenin [19], the estrogen-dependent egg yolk protein typically measured following exposure to suspected EDCs [20]. In lieu of vitellogenin we used a Menidia-specific antibody to detect Chg, an estrogen-dependent egg coat protein, via indirect ELISA [21]. The third objective was to compare fish response to an in vitro response by employing the CALUX (Chemical Activated Luciferase Gene Expression) assay. With the CALUX assay it was possible to examine the potential for additivity, antagonism, or synergism of pyrethroid pesticides with endogenous estrogen. To our knowledge this is first study to test the endocrine concentration-response of bifenthrin and permethrin at or near environmentally relevant concentrations. Additionally, this is the first study to use the CALUX assay, which express mRNA for both ER alpha and beta [24], to evaluate pyrethroid endocrine activity.
Comparability of response with other estuarine fish species is afforded by the use of Menidia [21]. Silversides are ubiquitous in and easily collected from estuarine, brackish and freshwater habitats [25], they are exposed to run-off containing pyrethroids throughout North America and in the Sacramento-San Joaquin Delta, and are commercially available for laboratory studies. Furthermore, the inland silverside has a higher published permethrin LC50 than many other fish species [2]. Although this may contribute to increased survival of silversides, it potentially renders them more susceptible to pyrethroid-induced endocrine disruption. Early life exposure to these low concentrations of pyrethroids may be particularly damaging to silverside populations, since the sex ratio of Menidia spp. has been shown to be susceptible to estrogen exposure during the larval period [36]. Notably, our study is the first to look at protein-level endocrine disrupting effects of pyrethroids on juvenile fishes, which are more sensitive to such environmental EDC perturbations than adults [14] and more likely to experience long-term developmental changes such as reduced fecundity and/or intersex when exposed to EDCs [37].
4.1 Responses in fish
The ability of single concentrations of permethrin and bifenthrin to induce egg yolk expression (vitellogenin) in male or juvenile fish, indirectly indicating ER binding and/or activation of the estrogen response element, has been demonstrated in a number of other studies [13, 38, 39]. Although vitellogenin is a reliable marker of estrogenic endocrine disruption, we instead measured expression of choriogenin (Chg) using a Menidia specific antibody [21]. Chg, which is a precursor to zona radiata, is an egg coat protein demonstrated to be more sensitive than vitellogenin (Vtg) in previous studies [1, 22, 40], and was shown recently to have increased mRNA expression in larval fish following bifenthrin exposure [23]. Like Vtg, this protein is normally only induced by endogenous estrogen in mature females [40], but is expressed in males and juveniles exposed to exogenous estrogens or estrogenic EDCs [22, 40].
Our study found that all concentrations (0.1, 1, 10 µg/L permethrin and 1, 10, 100 ng/L bifenthrin) significantly induced the expression of Chg in juvenile Menidia beryllina in comparison to methanol controls. Several of these concentrations are an order of magnitude lower than those shown in previous studies to induce estrogen-dependent mRNA or protein production. The concentrations used in our study decreased substantially over the 24-hour time course between water changes, indicating that fish were responding to decreasing concentrations than present at the start of dosing each day (at the water change). Notably, we observed that 1 ng/L bifenthrin induced significantly greater Chg expression than 1 ng/L EE2. This is alarming, considering that EE2 is a potent ER agonist that has even greater estrogenicity than endogenous estradiol [41]. This finding indicates that bifenthrin is estrogenic at the pptr levels that are regularly detected in aquatic ecosystems and also argues for future testing of concentrations below 1 ng/L. Considering that the current method detection limit for bifenthrin is 0.5 ng/L, the ability to test and detect concentrations below this presents a challenge to both toxicologists and chemists.
Although the ANOVA showed no significant difference in Chg expression between pyrethroid concentrations, the best-fit curve for both pyrethroids suggests a biphasic or unimodal response. In contrast, EE2 displayed the expected sigmoidal response, previously confirmed by others [42]. While the unimodal fit as evaluated by AICc is not as parsimonious for bifenthrin as it is for permethrin, the graphical trend is the same suggesting that a unimodal curve is the best fit for both. The apparent difference in results between ANOVA and curve-fitting analyses is not surprising. ANOVA is designed to detect differences between categorical treatments (e.g., comparing bifenthrin to EE2 response), and so it is not ideal for detecting trends in data with a continuous predictor variable (e.g., increasing concentrations of same compound) – it effectively loses information by not accounting for the ordered relationship among treatments. Additionally, ANOVA has less statistical power than a curve-fitting approach to detect trends [30]. As such, curve fitting is a more powerful approach to detect trends such as the shape of a concentration-response curve, although ANOVA remains useful for detecting differences among treatments that do not have an ordered relationship, as we show in Figure 1.
Other studies have noted that some estrogenic compounds (i.e. Bisphenol A) induce an inverted “U” shaped or unimodal dose response due to low dose stimulation and higher dose inhibition, likely due to receptor-mediated responses that can saturate or vary depending on concentration [43, 44, 45]. Further studies are needed to determine whether the endocrine response to permethrin and bifenthrin is truly unimodal and to provide further information on the mechanisms underlying the trend observed here.
4.2 Responses in cell lines
Evidence for a difference in estrogenicity between the parent pyrethroid compounds and their metabolites is demonstrated by the results of the CALUX assay, which natively expresses the human estrogen receptor (in BG-1 cells). Using a cell line that expresses the human estrogen receptor is comparable to evaluating the fish ER response in vitro since there is high sequence identity between human and fish steroid receptors [46, 47]. As has been demonstrated in other studies with permethrin [9, 48], the CALUX assay results demonstrate that bifenthrin acts as an estrogen antagonist (Figure 3). The antagonism of bifenthrin increases with increasing concentration to a maximum of 100 ng/L, while permethrin’s antagonistic properties do not correlate with concentration tested. Thus, this inhibition is unlikely to result from a direct antagonistic effect of permethrin on the estrogen receptor or estrogen receptor-signaling pathway. This could indicate that toxicity is occurring via other mechanisms with the higher concentrations of permethrin relative to bifenthrin affecting the cells’ ability to respond.
Upon an examination of other research findings along with ours, it is possible that the lack of a consistent linear relationship between antagonism and concentration may be caused by bifenthrin and permethrin acting via different mechanisms at different concentrations or conditions. For example, whether permethrin blocks binding to the ER appears to depend upon the cell line used [9, 48]. Additionally, both bifenthrin and permethrin have been shown to activate expression of an estrogen-linked transcription factor in some studies [9, 49], but permethrin didn’t in others [48, 50]. Permethrin’s action at the ER is clearly dependent on the combination of cell line, concentration used and endpoint examined, and considering the results of our study bifenthrin appears to exhibit similar contradictory behavior.
Neither compound acted as an estrogen agonist in the CALUX assay at any concentration, a finding that is somewhat surprising considering results of previous research that indicate bifenthrin and permethrin operate as ER agonists via the classical nuclear receptor pathway [11, 38]. The difference seen may be accounted for by use of the CALUX assay, rather than the YES assay, which is a yeast cell line commonly used for testing estrogenic endocrine activity to date. Notably, unless modified to do so, the yeast YES assay does not discriminate well between agonists and antagonists [12], and the YES assay also does not express mRNA for both ER alpha and beta as the CALUX assay does [21], as it is normally only transfected with ER alpha [51]. As such, the mammalian cell CALUX assay may offer a more realistic assessment of receptor-dependent estrogenic or anti-estrogenic activity. Additionally, nearly all previous cell line studies conducted with pyrethroids used concentrations that were higher than those used in our study, by as much as an order of magnitude [11]. As mentioned earlier, the receptor-mediated responses evaluated here can be saturated or vary widely depending on concentration [45, 52], which may explain the difference in endocrine activity between the low concentrations used in our study with higher concentrations used in previous in vitro studies.
5. Conclusion
Our experimental evidence indicates that permethrin and bifenthrin are estrogenic in in vivo but anti-estrogenic in cells in vitro. The difference in results between the in vivo and in vitro assays used in our study may be due to the lack of appropriate metabolism in the CALUX cell line. This finding is corroborated by a number of studies which show that pyrethroid metabolites are more estrogenic than their parent compounds [11, 12, 13].
Future studies evaluating the activity of pyrethroid metabolites in the CALUX assay and studies that elucidate the action of permethrin, bifenthrin and their metabolites at each ER isoform (alpha, beta, gamma) are necessary. Furthermore, using an in vivo system to compare in vitro responses to when evaluating endocrine responses [53] and testing concentrations in the vicinity of those that have been detected in the environment are important considering our findings.
The estrogenic or anti-estrogenic activity exhibited by these compounds is likely to exacerbate their established toxicity in aquatic ecosystems. Furthermore, their ability to exert effects on the endocrine system of fishes at concentrations at or near those regularly detected in watersheds is cause for great concern, particularly with bifenthrin, especially when considering recent research that suggests changes in estrogen-dependent protein expression in fishes may precipitate population decline [54] and that the pyrethroid mixtures that occur in aquatic habitats likely have additive endocrine toxicity.
Supplementary Material
Acknowledgments
We would like to acknowledge the following funding sources that made this research possible: Delta Science (Pre-Doctoral Fellowship R/SF-27 and grant no. SCI-05-C111), the National Science Foundation (GK-12 Pre-Doctoral Fellowship, grant no. 0841297) and the UC Davis National Institutes of Environmental Health Sciences Superfund Research Program (ES04699). Financial support was also provided in part by the Sacramento Regional County Sanitation District. Thank you to Dr. Inge Werner for advice on experimental design and Dr. Doug Middaugh, Dr. Kathryn Kuivila, Dr. Carol Vines, Cherr Lab members and Bodega Marine Lab staff for assistance and support. Finally, we would like to extend our gratitude to Dr. Will White for advice on and assistance with data analysis. This work was performed under UC Davis IACUC protocol 13353.
Works Cited
- 1.Sudakin DL, Power LE. Organophosphate exposures in the United States: A longitudinal analysis of incidents reported to poison control centers. Journal of Toxicology and Environmental Health, Part A. 2007;70:141–147. doi: 10.1080/15287390600755224. [DOI] [PubMed] [Google Scholar]
- 2.Werner I, Moran K. Effects of pyrethroid insecticides on aquatic organisms. In: Gan J, Spurlock F, Hendley P, Weston DP, editors. Synthetic Pyrethroids: Occurrence and Behavior in Aquatic Environments. Vol ACS Symposium Series 991. Washington, D.C., USA: American Chemical Society; 2008. pp. 310–335. [Google Scholar]
- 3.Burr SA, Ray DE. Structure-activity and interaction effects of 14 different pyrethroids on voltage-gated chloride ion channels. Toxicological Sciences. 2004;77:341–346. doi: 10.1093/toxsci/kfh027. [DOI] [PubMed] [Google Scholar]
- 4.Brander SM, Werner I, White JW, Deanovic LA. Toxicity of a dissolved pyrethroid mixture to Hyalella azteca at environmentally relevant concentrations. Environmental Toxicology and Chemistry. 2009;28:1493–1499. doi: 10.1897/08-374.1. [DOI] [PubMed] [Google Scholar]
- 5.Leahey JP. The Pyrethroid Insecticides. London: Taylor & Francis Inc; 1985. p. 440. [Google Scholar]
- 6.Bondarenko S, Putt A, Kavanaugh S, Poletika N, Gan J. Time dependence of phase distribution of pyrethroid insecticides in sediment. Environmental Toxicology and Chemistry. 2006;25:3148–3154. doi: 10.1897/06-017r.1. [DOI] [PubMed] [Google Scholar]
- 7.Beggel S, Werner I, Connon RE, Geist JP. Sublethal toxicity of commercial insecticide formulations and their active ingredients to larval fathead minnow (Pimephales promelas) Science of The Total Environment. 2010;408:3169–3175. doi: 10.1016/j.scitotenv.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Floyd EY, Geist JP, Werner I. Acute, sublethal exposure to a pyrethroid insecticide alters behavior, growth, and predation risk in larvae of the fathead minnow (Pimephales promelas) Environmental Toxicology and Chemistry. 2008;27:1780–1787. doi: 10.1897/07-448.1. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Xiao J, Hu G, Zhou J, Xiao H, Wang X. Estrogenicity of organophosphorus and pyrethroid pesticides. Journal of Toxicology and Environmental Health, Part A. 2002;65:1419–1435. doi: 10.1080/00984100290071243. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Xu X-L, Xu L-C, Song L, Hong X, Chen J-F, Cui L-B, Wang X-R. Antiandrogenic activity of pyrethroid pesticides and their metabolites in a reporter gene assay. Chemosphere. 2007;66:474–479. doi: 10.1016/j.chemosphere.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 11.Tyler CR, Beresford N, Van der Woning M, Sumpter JP, Thorpe KL. Metabolism and environmental degradation of pyrethroid insecticides produce compounds with endocrine activities. Environmental Toxicology and Chemistry. 2000;19:801–809. [Google Scholar]
- 12.McCarthy AR, Thomson BM, Shaw IC, Abell AD. Estrogenicity of pyrethroid metabolites. Journal of Environmental Monitoring. 2006;8:197–202. doi: 10.1039/b511209e. [DOI] [PubMed] [Google Scholar]
- 13.Nillos MG, Chajkowski S, Rimoldi JM, Gan J, Lavado R, Schlenk D. Stereoselective Biotransformation of permethrin to estrogenic metabolites in fish. Chemical Research in Toxicology. 2010;23:1568–1575. doi: 10.1021/tx100167x. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Shu L, Huang F, Cao L, Sun L, Fu Z. Environmental cues influence EDC-mediated endocrine disruption effects in different developmental stages of Japanese medaka (Oryzias latipes) Aquatic Toxicology. 2011;101:254–260. doi: 10.1016/j.aquatox.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Weston DP, Holmes BW, You J, Lydy MJ. Aquatic toxicity due to residential use of pyrethroid insecticides. Environmental Science and Technology. 2005;39:9778–9784. doi: 10.1021/es0506354. [DOI] [PubMed] [Google Scholar]
- 16.Weston DP, Holmes RW, Lydy MJ. Residential runoff as a source of pyrethroid pesticides to urban creeks. Environmental Pollution. 2009;157:287–294. doi: 10.1016/j.envpol.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Phillips BM, Anderson BS, Voorhees JP, Hunt JW, Holmes RW, Mekebri A, Connor V, Tjeerdema RS. The contribution of pyrethroid pesticides to sediment toxicity in four urban creeks in California, USA. Journal of Pesticide Science. 2010;35:302–309. [Google Scholar]
- 18.Domagalski JL, Weston DP, Zhang M, Hladik M. Pyrethroid insecticide concentrations and toxicity in streambed sediments and loads in surface waters of the San Joaquin Valley, California, USA. Environmental Toxicology and Chemistry. 2010;29:813–823. doi: 10.1002/etc.106. [DOI] [PubMed] [Google Scholar]
- 19.Celius T, Walther BT. Differential sensitivity of zonagenesis and vitellogenesis in Atlantic salmon (Salmo salar L) to DDT pesticides. The Journal of Experimental Zoology. 1998;281:346–353. doi: 10.1002/(sici)1097-010x(19980701)281:4<346::aid-jez9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Rempel MA, Schlenk D. Effects of environmental estrogens and antiandrogens on endocrine function, gene regulation, and health in fish. International Review of Cell and Molecular Biology. Vol 267. International Review of Cell and Molecular Biology. 2008:207–252. doi: 10.1016/S1937-6448(08)00605-9. [DOI] [PubMed] [Google Scholar]
- 21.Brander SM, Cole BJ, Cherr GN. An approach to detecting estrogenic endocrine disruption via choriogenin expression in an estuarine model fish species. Ecotoxicology. 2012;21:1272–1280. doi: 10.1007/s10646-012-0879-2. [DOI] [PubMed] [Google Scholar]
- 22.Arukwe A, Celius T, Walther BT, Goksoyer A. Effects of xenoestrogen treatment on zona radiata protein and vitellogenin expression in Atlantic salmon (Salmo salar) Aquatic Toxicology. 2000;49:159–170. doi: 10.1016/s0166-445x(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 23.Beggel S, Connon R, Werner I, Geist J. Changes in gene transcription and whole organism responses in larval fathead minnow (Pimephales promelas) following short-term exposure to the synthetic pyrethroid bifenthrin. Aquatic Toxicology. 2011;105:180–188. doi: 10.1016/j.aquatox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Rogers JM, Denison MS. Recombinant cell bioassays for endocrine disruptors: development of a stably transfected human ovarian cell line for the detection of estrogenic and anti-estrogenic chemicals. In Vitro and Molecular Toxicology. 2000;13:67–82. [PubMed] [Google Scholar]
- 25.Middaugh DP, Hemmer MJ. Reproductive ecology of the inland silverside, Menidia beryllina (Pisces, Atherinidae) from Blackwater Bay, Florida. Copeia. 1992;1992:53–61. [Google Scholar]
- 26.ASTM. ASTM E1192-14. West Conshohocken, PA: 2008. Standard guide for conducting acute toxicity tests on aqueous ambient samples and effluents with fishes, macroinvertebrates, and amphibians. [Google Scholar]
- 27.USEPA. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 5th ed. Washington, D.C.: 2002. EPA-821-R-02-012. [Google Scholar]
- 28.Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DPY, Gee S, Hammock BD. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environmental Health Perspectives. 2008;116:1203–1210. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hladik ML, Smalling KL, Kuivila KM. A multi-residue method for the analysis of pesticides and pesticide degradates in water using HLB solid-phase extraction and gas chromatography-ion trap mass spectrometry. Bulletin of Environmental Contamination and Toxicology. 2008;80:139–144. doi: 10.1007/s00128-007-9332-2. [DOI] [PubMed] [Google Scholar]
- 30.Cottingham KL, Lennon JT, Brown BL. Knowing when to draw the line: designing more informative ecological experiments. Frontiers in Ecology and the Environment. 2005;3:145–152. [Google Scholar]
- 31.Burnham KP, Anderson DR. Model selection and inference: A practical information-theoretic approach. New York, NY USA: Springer-Verlag; 2002. [Google Scholar]
- 32.Brain P, Cousens R. An equation to describe dose responses when there is stimulation of growth at low doses. Weed Research. 1989;29:93–96. [Google Scholar]
- 33.R Core Development Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 34.Lee S, Gan J, Kabashima J. Recovery of synthetic pyrethroids in water samples during storage and extraction. Journal of Agricultural and Food Chemistry. 2002;50:7194–7198. doi: 10.1021/jf0258353. [DOI] [PubMed] [Google Scholar]
- 35.Wheelock CE, Miller JL, Miller MJ, Phillips BM, Gee SJ, Tjeerdema RS, Hammock BD. Influence of container adsorption upon observed pyrethroid toxicity to Ceriodaphnia dubia and Hyalella azteca. Aquatic Toxicology. 2005;74:47–52. doi: 10.1016/j.aquatox.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffy TA, McElroy AE, Conover DO. Variable susceptibility and response to estrogenic chemicals in Menidia menidia. Marine Ecology Progress Series. 2009;380:245–254. [Google Scholar]
- 37.Peters REM, Courtenay SC, Hewitt LM, MacLatchy DL. Effects of 17[alpha]-ethynylestradiol on early-life development, sex differentiation and vitellogenin induction in mummichog (Fundulus heteroclitus) Marine Environmental Research. 2009;69:178–186. doi: 10.1016/j.marenvres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Liu W, Yang C, Pan Z, Gan J, Xu C, Zhao M, Schlenk D. Enantioselectivity in estrogenic potential and uptake of bifenthrin. Environmental Science and Technology. 2007;41:6124–6128. doi: 10.1021/es070220d. [DOI] [PubMed] [Google Scholar]
- 39.Jin Y, Wang W, Xu C, Zhengwei F, Liu W. Induction of hepatic estrogen-responsive gene transcription by permethrin enantiomers in male adult zebrafish. Aquatic Toxicology. 2008;88:146–152. doi: 10.1016/j.aquatox.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Oppen-Berntsen DO, Hyllner SJ, Haux C, Helvik JV, Walther BT. Eggshell zona radiata-proteins from cod (Gadus morhua): extra-ovarian origin and induction by estradiol-17 beta. International Journal of Developmental Biology. 1992;36:247–254. [PubMed] [Google Scholar]
- 41.Metcalfe CD, Metcalfe TL, Kiparissis Y, Koenig BG, Khan C, Hughes RJ, Croley TR, March RE, Potter T. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes) Environmental Toxicology and Chemistry. 2001;20:297–308. [PubMed] [Google Scholar]
- 42.Thorpe KL, Cummings RI, Hutchinson TH, Scholze M, Brighty G, Sumpter JP, Tyler CR. Relative potencies and combination effects of steroidal estrogens in fish. Environmental Science and Technology. 2003;37:1142–1149. doi: 10.1021/es0201348. [DOI] [PubMed] [Google Scholar]
- 43.Calabrese EJ. Estrogen and related compounds: Biphasic dose responses. Critical Reviews in Toxicology. 2001;31:503–515. doi: 10.1080/20014091111785. [DOI] [PubMed] [Google Scholar]
- 44.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposuresIMechanisms for endocrine-disrupting chemicals with estrogenic Activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocrine Reviews. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pakdel F, Le Guellec C, Vaillant C, Le Roux MGl, Valotaire Y. Identification and estrogen induction of two estrogen receptors (ER) messenger ribonucleic acids in the rainbow trout liver: Sequence homology with other ERs. Molecular Endocrinology. 1989;3:44–51. doi: 10.1210/mend-3-1-44. [DOI] [PubMed] [Google Scholar]
- 47.Shyu C, Cavileer TD, Nagler JJ, Ytreberg FM. Computational estimation of rainbow trout estrogen receptor binding affinities for environmental estrogens. Toxicology and Applied Pharmacology. 2011;250:322–326. doi: 10.1016/j.taap.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim IY, Shin JH, Kim HS, Lee SJ, Kang IH, Kim TS, Moon HJ, Choi KS, Moon A, Han SY. Assessing estrogenic activity of pyrethroid insecticides using In Vitro combination assays. Journal of Reproduction and Development. 2004;50:245–255. doi: 10.1262/jrd.50.245. [DOI] [PubMed] [Google Scholar]
- 49.Zhao M, Chen F, Wang C, Zhang Q, Gan J, Liu W. Integrative assessment of enantioselectivity in endocrine disruption and immunotoxicity of synthetic pyrethroids. Environmental Pollution. 2010;158:1968–1973. doi: 10.1016/j.envpol.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 50.Go V, Garey J, Wolff MS, Pogo BGT. Estrogenic potential of certain pyrethroid compounds in the MCF-7 human breast carcinoma cell line. Environmental Health Perspectives. 1999;107:173–177. doi: 10.1289/ehp.99107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Routledge EJ, Sumpter JP. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environmental Toxicology and Chemistry. 1996;15:241–248. [Google Scholar]
- 52.Li L, Andersen ME, Heber S, Zhang Q. Non-monotonic dose-response relationship in steroid hormone receptor-mediated gene expression. Journal of Molecular Endocrinology. 2007;38:569–585. doi: 10.1677/JME-07-0003. [DOI] [PubMed] [Google Scholar]
- 53.Taxvig C, Olesen PT, Nellemann C. Use of external metabolizing systems when testing for endocrine disruption in the T-screen assay. Toxicology and Applied Pharmacology. 2011;250:263–269. doi: 10.1016/j.taap.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 54.Kidd KA, Blanchfield PJ, Mills KH, P PV, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proceedings of the National Academy of Science. 2007;104:8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.