Abstract
Background
In 2008, the first clinic for women involved in high risk sexual behaviour was established in Kampala, offering targeted HIV prevention. This paper describes rates, determinants and trends of HIV incidence over 3 years.
Methods
1027 women at high risk were enrolled into a closed cohort. At 3-monthly visits, data were collected on socio-demographic variables and risk behaviour; biological samples were tested for HIV and other STIs. Hazard ratios (HR) for HIV incidence were estimated using Cox proportional hazards regression, among the 646 women HIV negative at enrolment.
Results
HIV incidence was 3.66/100pyr and declined from 6.80/100pyr in the first calendar year to 2.24/100pyr and 2.53/100pyr in the following years (P-trend=0.003). Socio-demographic and behavioural factors independently associated with HIV incidence were younger age, younger age at first sex, alcohol use (including frequency of use and binge drinking), number of paying clients in the past month, inconsistent condom use with clients, and not being pregnant. HIV incidence was also independently associated with M. genitalium infection at enrolment (aHR=2.28, 95%CI: 1.15-4.52), and with N. gonorrhoeae (aHR=5.91, 95%CI: 3.04-11.49) and T. vaginalis infections at the most recent visit (aHR=2.72, 95%CI: 1.27-5.84). The PAF of HIV incidence for alcohol use was 63.5% (95%CI 6.5%-85.8%), and for treatable STI/RTI was 70.0% (95%CI 18.8%-87.5%).
Conclusions
Alcohol use and STIs remain important risk factors for HIV acquisition, which call for more intensive control measures in women at high risk. Further longitudinal studies are needed to confirm the association between Mycoplasma genitalium and HIV acquisition.
Keywords: HIV incidence, Mycoplasma genitalium, STIs, alcohol, women at high risk, risk factors, Uganda
Introduction
Core groups involved in high risk sexual behaviour, such as female sex workers (FSW), play an important role both in the initial establishment of sexually transmitted HIV epidemics and in the later contracting phases, as HIV transmission again becomes more concentrated in high risk groups [1]. Uganda is currently undergoing this later stage epidemic, and risk reduction interventions for high risk groups are needed to prevent a resurgence of HIV in both core groups and the general population.
Several sub-Saharan African countries have successfully implemented HIV prevention interventions for FSW, combining improved access to treatment for sexually transmitted infections (STIs), condom promotion, and voluntary HIV counselling and testing (VCT) [2-5]. In low income countries, such focused interventions are effective in reducing HIV and STI acquisition [6], and are cost-effective in preventing new HIV infections [7].
In 2008, following a request from the Ugandan National AIDS Programme, the MRC Uganda Research Unit on AIDS recruited the first cohort of women involved in high-risk sexual behaviour in Kampala with the aim to study the size, determinants and dynamics of the HIV epidemic in this population. Women involved in sex work and/or employed in entertainment facilities were recruited from red-light-areas in Southern Kampala. A stand-alone clinic was established, offering a comprehensive package of HIV prevention interventions. Baseline data estimated HIV prevalence as 37% (95% CI 34%–40%) as well as high rates of other STIs, frequent unsafe sex with clients (40% inconsistent condom use in last month) and alcohol use (78%)[8,9].
The current paper presents the rates and determinants of HIV seroconversion as well as the changes over time in HIV incidence during follow-up in this high-risk cohort.
Methods
Study population and clinical procedures
Between April 2008 and April 2009, women likely to be involved in high risk sexual behaviour were recruited from red-light-areas in Southern Kampala. The study population, recruitment process and study procedures have been described in detail previously [8]. Briefly, self-reporting FSW and/or women employed in entertainment facilities were approached at night by our field workers. They were invited to visit the Good Health for Women Project (GHWP) clinic for screening and enrolment into the cohort. Among 1214 women screened, 79 were not eligible, 106 did not return for their enrolment visit and 2 declined consenting. Among the remaining 1027 women, 95% reported sex work. Enrolled women were scheduled for 3-monthly follow-up visits. At every scheduled visit, women were interviewed about their socio-demographic characteristics, sexual risk behaviour, alcohol use, intravaginal practices, reproductive health history and symptoms of STIs. VCT, family planning and antenatal care were offered. Blood was tested for HIV, HSV2 and syphilis. A speculum examination was performed and two endocervical specimens were collected, one for the diagnosis of Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) infection and one for diagnosis of Mycoplasma genitalium (MG). One high vaginal specimen was collected and inoculated for culture of Trichomonas vaginalis (TV), another to prepare a slide for the detection of bacterial vaginosis (BV) and of Candida infection. Women with symptomatic STIs were treated syndromically on the spot. Women with asymptomatic STIs were treated as soon as laboratory results became available. Health-education on HIV/STI, risk reduction counselling, and condom demonstration and promotion were provided.
After the first follow-up year genital specimens were collected and tested only 6-monthly. Due to financial constraints, testing for MG was limited to stored specimens from the baseline visit. Throughout the study, free primary health care was offered for participating women and their children aged less than 5 years old. All participants with confirmed HIV infection had their CD4-count measured: women eligible for antiretroviral therapy (ART) were referred to an HIV-care centre. Those not yet eligible were provided with cotrimoxazole prophylaxis and their CD4-count was measured at subsequent visits.
Laboratory procedures
Serum specimens were tested for antibodies against HIV-1 (Abbott Determine HIV-1/2 with confirmation by two independent ELISA tests: Vironostika Uniform II plus O, Murex HIV 1.2.O), HSV-2 (IgG ELISA test, Kalon Biologicals Ltd, UK) and for syphilis (RPR Biotec and TPHA Biotec). NG and CT were diagnosed on endocervical specimens using the Amplicor PCR test (Roche Diagnostic Systems Inc., Branchburg, NJ) and TV was detected using the commercial culture kit (InPouch TV, BioMed Diagostics, White City, Oregon). Microscopy on a gram-stained vaginal specimen was performed to diagnose BV (using Nugent’s criteria) and Candida infection. Laboratory testing for all these infections was performed at the central laboratories of the MRC/UVRI Uganda Unit in Entebbe.
For the diagnosis of MG, endocervical specimens, collected using Cobas Amplicor STM collection tubes (Roche Diagnostic Systems Inc., Branchburg, NJ), were tested using a commercially available Real-TM PCR assay (Sacace Biotechnologies, Como, Italy) at the Centre for HIV and Sexually Transmitted Infections, National Institute for Communicable Diseases, National Health Laboratory Service in Johannesburg. Further details of this PCR test have been reported previously [9].
Statistical analysis
Data were double entered in Access and analysed using STATA 11.0 (Stata Inc., TX). Women were censored at the earliest of the date of HIV seroconversion, date last seen, or 31st March 2011. Cox proportional hazards regression with time-varying covariate approach was used to estimate hazard ratios (HR) for HIV seroconversion (assumed to take place midway between the last negative and first positive serology result). For time-changing exposures, the measurement at the most recent visit was used. MG results were only available from the baseline visit, while for other STIs the results at the most recent visit were used. Syphilis was categorized as no infection (RPR-TPHA-), past infection (RPR-TPHA+), low titre active infection (RPR+TPHA+ with RPR titre <8) and high titre active infection (RPR+TPHA+ with RPR titre ≥8).
Determinants of HIV seroconversion were first examined using age-adjusted analysis, as age was a strong a-priori confounder. The multivariable analysis was conducted using a hierarchical conceptual framework [10], grouping variables in three levels as sociodemographic, behavioral/reproductive health and clinical/biological. Sociodemographic factors associated in age-adjusted analysis (P≤0.10) were included in a multivariable model and retained in a core model if they remained independently associated with HIV seroconversion. Behavioral and reproductive factors were added to this model one by one and included in a multivariable model if P≤0.10. Factors remaining significant after adjustment for each other and the socio-demographic factors were retained. Factors describing alcohol use were treated separately to avoid co-linearity. The same steps were repeated with clinical and biological factors. Ordered variables were included as linear trends if there was no evidence of non-linearity using a likelihood ratio test.
Population attributable fractions (PAFs) of HIV acquisition for risk factors in the final model were estimated using the formula p(aHR-1)/aHR, with p=proportion of cases exposed and aHR= adjusted hazard ratio [11].
Ethical considerations
Informed consent was obtained from all study participants. The study was approved by the Science and Ethics Committee of the Ugandan Virus Research Institute, the Uganda National Committee for Science and Technology and the Ethics Committee of the London School of Hygiene and Tropical Medicine.
Results
HIV incidence
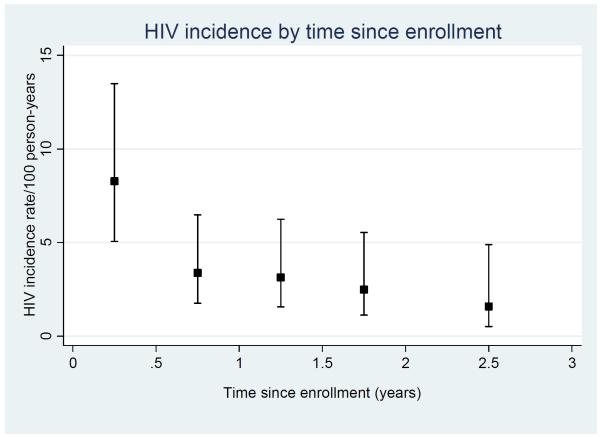
Of 1027 women enrolled, 646 were HIV-negative. The total follow-up time contributing to HIV seroconversion analyses was 1147 person-years (pyr), with median time in the cohort of 2.1 years (range 0.1-3.0 years). There was high retention in the cohort, with 77% of women attending the 12 month visit, and 74% attending the 18 month visit. Forty-two women seroconverted (HIV incidence rate: 3.66/100 pyr; 95%CI: 2.71-4.96/100 pyr). HIV seroconversion decreased substantially with time-in-study, from 8.26/100 pyr in the first 6 months to 2.01/100 pyr in the period 2.5-3 years after inclusion (age-adjusted p-value for trend=0.002, Figure 1). HIV seroconversion also decreased substantially with calendar time from 6.80/100 pyr in the period April 2008-March 2009, to 2.53/100 pyr in the period April 2010-March 2011 (age-adjusted p-value for trend=0.003).
Figure 1.
HIV incidence by time since enrolment
Factors associated with HIV seroconversion
In age-adjusted analyses, the only socio-demographic factor associated with HIV seroconversion was calendar time (Table 1), and age and calendar time were retained in the hierarchical model. Behavioural and reproductive factors independently associated with HIV seroconversion (after adjustment for age and calendar period as linear terms), were greater number of lifetime partners, younger age at first sex, alcohol use (including frequency of alcohol use, CAGE score and binge drinking), greater number of paying clients per month, inconsistent condom use with clients, and not being pregnant (Table 2). There was no evidence of an association with hormonal contraceptive use (either oral or injectable).
Table 1.
Socio-demographic factors associated with HIV seroconversion among 646 women at high risk in Kampala, Uganda
| N (%)(1) | HIV sero converters /pyr |
HIV incidence rate/100 pyr |
Age adjusted Hazard Ratio (95% CI) |
Adjusted Hazard Ratio(2) (95% CI) |
|
|---|---|---|---|---|---|
| Total | 42/1147 | 3.66 | |||
| Calendar period of follow-up | P-trend=0.02 | P-trend=0.02 | |||
| April 08-March 09 | 1281 (25) | 23/338 | 6.80 | 1 | 1 |
| April 09-March 10 | 1905 (38) | 11/492 | 2.24 | 0.27 (0.10-0.74) | 0.27 (0.10-0.74) |
| April 10- March 11 | 1862 (37) | 8/317 | 2.53 | 0.23 (0.06-0.94) | 0.23 (0.06-0.94) |
|
| |||||
| Attended scheduled visits (%) | P=0.10 | P=0.15 | |||
| 100% | 318 (49) | 22/692 | 3.18 | 1 | 1 |
| 75%-99% | 125 (19) | 8/252 | 3.17 | 0.93 (0.41-2.09) | 0.93 (0.41-2.09) |
| 50%-74% | 80 (12) | 6/141 | 4.26 | 1.05 (0.42-2.62) | 0.89 (0.35-2.25) |
| 25%-49% | 53 (8) | 3/55 | 5.45 | 1.49 (0.44-5.08) | 1.13 (0.32-3.94) |
| <25% | 70 (11) | 3/7 | 40.47 | 9.38 (2.55-34.49) | 7.66 (2.09 -27.97) |
|
| |||||
| Sociodemographic variables at enrolment | |||||
|
| |||||
| Age (y) | P-trend=0.01 | P-trend=0.009 | |||
| <25 | 291 (45) | 26/493 | 5.27 | 1 | 1 |
| 25-34 | 297 (46) | 15/549 | 2.73 | 0.52 (0.28-0.98) | 0.54 (0.28-1.02) |
| 35+ | 58 (9) | 1/104 | 0.96 | 0.18 (0.02-1.32) | 0.19 (0.02-1.37) |
|
| |||||
| Religion | P=0.17 | P=0.16 | |||
| Catholic | 264 (41) | 11/472 | 2.33 | 1 | 1 |
| Anglican | 182 (28) | 14/317 | 4.42 | 1.90 (0.86-4.18) | 1.88 (0.85-4.15) |
| Muslim | 177 (27) | 16/316 | 5.07 | 2.19 (1.02-4.72) | 2.18 (1.01-4.70) |
| Other | 23 (4) | 1/42 | 2.38 | 0.92 (0.12-7.15) | 0.83 (0.11-6.47) |
|
| |||||
| Education level | P=0.91 | P=0.83 | |||
| Primary completed | 363 (56) | 24/ 647 | 3.71 | 1 | 1 |
| Less than primary | 283 (44) | 18/500 | 3.60 | 1.04 (0.56-1.91) | 1.07 (0.58-1.98) |
|
| |||||
| Sociodemographic variables during follow-up | |||||
|
| |||||
| Marital status | P=0.81 | P=0.79 | |||
| Widow / Divorced | 3176 (63) | 26/720 | 3.61 | 1 | 1 |
| Currently married | 880 (17) | 5/185 | 2.71 | 0.74 (0.28-1.96) | 0.74 (0.28-1.95) |
| Never married | 992 (20) | 11/242 | 4.54 | 0.88 (0.42-1.84) | 0.86 (0.41-1.80) |
|
| |||||
| Having a regular partner | P=0.24 | P=0.22 | |||
| No | 1009 (20) | 11/230 | 4.79 | 1 | 1 |
| Yes | 4039 (80) | 31/917 | 3.38 | 0.65 (0.32-1.30) | 0.64 (0.32-1.28) |
|
| |||||
| Source of income(3) | P=0.24 | P=0.22 | |||
| Sex work alone | 1382 (27) | 14/326 | 4.29 | 1 | 1 |
| Sex work & other job | 2926 (58) | 26/666 | 3.90 | 0.87 (0.46-1.67) | 0.85 (0.44-1.63) |
| No sex work | 737 (15) | 2/154 | 1.30 | 0.33 (0.07-1.45) | 0.32 (0.07-1.42) |
|
| |||||
| Average price per sex act(4)(5) | P=0.42 | P=0.42 | |||
| <5000 UgSh | 689 (16) | 5/168 | 2.97 | 1 | 1 |
| >= 5000 UgSh | 3611 (84) | 35/821 | 4.26 | 1.47 (0.58-3.77) | 1.45 (0.56-3.70) |
Number of women for baseline variables (N= 646 women) or number of visits for time-varying variables (n=5048 visits)
Adjusted for age and calendar time
3 missing observations
Restricted to women involved in sex work; 11 missing observations
5000 Ugandan Shillings = 2 USD
Table 2.
Associations between behavioural and reproductive health factors and HIV seroconversion among 646 women at high risk in Kampala, Uganda
| N (%)(1) | HIV sero converters /pyr |
HIV incidence rate/ 100 pyr |
Age adjusted Hazard Ratio (95% CI) |
Adjusted Hazard Ratio(2) (95% CI) |
|
|---|---|---|---|---|---|
| Total | 42/1147 | 3.66 | |||
|
| |||||
| Behavioral factors | |||||
|
| |||||
| Number of lifetime partners | P-trend=0.06 | P-trend=0.02 | |||
| <20 | 95 (15) | 2/189 | 1.06 | 1 | 1 |
| 20-50 | 106 (16) | 7/195 | 3.59 | 3.38 (0.70-16.26) | 3.35 (0.68-16.37) |
| >50 or can’t remember |
445 (69) | 33/762 | 4.33 | 4.00 (0.96-16.67) | 4.78 (1.08-21.14) |
|
| |||||
| Age at first sex | P=0.01 | P=0.05 | |||
| <=14 | 220 (34) | 21/373 | 5.64 | 1 | 1 |
| 15-22 | 406 (63) | 18/740 | 2.43 | 0.44 (0.23-0.82) | 0.52 (0.27-0.99) |
| Can’t remember | 20 (3) | 3/34 | 8.73 | - | - |
|
| |||||
| Frequency of alcohol use (at baseline) | P=0.05 | P-trend=0.06 | |||
| Not using | 160 (25) | 4/276 | 1.45 | 1 | 1 |
| Less than once a week | 35 (5) | 1/71 | 1.40 | 1.00 (0.11-8.92) | 0.83 (0.09-7.60) |
| At least once a week | 297 (46) | 24/526 | 4.56 | 3.14 (1.09-9.06) | 2.72 (0.94-7.87) |
| Daily | 154 (24) | 13/273 | 4.76 | 3.26 (1.06-10.01) | 2.58 (0.81-8.20) |
|
| |||||
| CAGE (at baseline) | P=0.02 | P=0.06 | |||
| Not drinking | 160 (25) | 4/276 | 1.45 | 1 | 1 |
| Not problem drinking | 139(21) | 7/255 | 2.75 | 1.92 (0.56-6.56) | 1.65 (0.48-5.73) |
| Problem drinking | 347(54) | 31/616 | 5.03 | 3.45 (1.22-9.78) | 2.85 (0.99-8.17) |
|
| |||||
| Alcohol use in last 3 months (at follow-up) | P=0.002 | P=0.008 | |||
| No | 1559 (31) | 4/347 | 1.15 | 1 | 1 |
| Yes | 3489 (69) | 38/800 | 4.75 | 3.91 (1.39-10.97) | 3.36 (1.17-9.59) |
|
| |||||
| Binge drinking last 3 months (at follow-up) | P=0.006 | P=0.03 | |||
| Non drinker | 1559 (31) | 4/347 | 1.15 | 1 | 1 |
| Never binge | 2622 (52) | 26/595 | 4.37 | 3.63 (1.27-10.42) | 3.20 (1.10-9.31) |
| Ever Binge | 867 (17) | 12/205 | 5.85 | 4.72 (1.51-14.72) | 3.87 (1.20-12.52) |
|
| |||||
| Illicit drug use in last 3 months (at follow-up) | P=0.50 | P=0.32 | |||
| No | 3921 (78) | 31/894 | 3.47 | 1 | 1 |
| Yes | 112 (22) | 11/253 | 4.35 | 1.27 (0.64-2.53) | 1.44 (0.72-2.88) |
|
| |||||
| Number of paying clients in last month (at follow-up) (3) | P=0.05 | P=0.04 | |||
| <5 | 2632 (51) | 19/576 | 3.29 | 1 | 1 |
| 5-50 | 1983 (40) | 14/471 | 2.97 | 0.85 (0.42-1.70) | 0.85 (0.42-1.72) |
| >50 or can’t remember |
425 (8) | 9/101 | 9.27 | 2.47 (1.10-5.52) | 2.50 (1.12-5.57) |
|
| |||||
| Condom use with paying clients in last month (at follow-up) (4) | P=0.06 | P=0.07 | |||
| Consistent | 2163 (55) | 15/501 | 2.99 | 1 | 1 |
| Inconsistent | 1749 (45) | 22/408 | 5.39 | 1.91 (0.99-3.70) | 1.83 (0.95-3.54) |
|
| |||||
| Cleansing of the vagina (at follow-up) | P=0.65 | P=0.74 | |||
| Cleansing with soap | 2449 (49) | 21/565 | 3.71 | 1 | 1 |
| Cleansing without soap | 2291 (45) | 17/515 | 3.30 | 0.93 (0.49-1.77) | 0.96 (0.51-1.83) |
| No cleansing | 308 (6) | 4/66 | 6.01 | 1.60 (0.55-4.67) | 1.51 (0.52-4.42) |
|
| |||||
| Reproductive health | |||||
|
| |||||
| Pregnant (at follow-up) | P=0.02 | P=0.01 | |||
| No | 4515 (89) | 41/1015 | 4.04 | 1 | 1 |
| Yes | 531 (11) | 1/131 | 0.76 | 0.17 (0.02-1.23) | 0.16 (0.02-1.17) |
|
| |||||
| Hormonal contraceptive use in last 3 months (at follow-up) (5) | P=0.99 | P=0.95 | |||
| None or other | 2491 (55) | 23/559 | 4.11 | 1 | 1 |
| Oral | 611 (14) | 5/140 | 3.57 | 0.99 (0.38-2.62) | 0.90 (0.33-2.43) |
| Injectable (Depo- Provera) |
1415 (31) | 13/316 | 4.11 | 1.02 (0.52-2.02) | 0.88 (0.44-1.75) |
Number of women for baseline variables (N= 646 women) or number of visits for time-varying variables (n=5048 visits)
Adjusted for age, calendar time, number of lifetime partners, age at first sex, alcohol use during follow-up, number of paying clients in last 3 months, inconsistent condom use with paying clients and being pregnant
Missing for 8 women
For women with paying clients in the last month
For non-pregnant women only
As risky sexual behaviour may lie on the causal pathway between alcohol use and HIV seroconversion, we also estimated associations of alcohol and HIV not adjusted for behavioural variables (aHR=3.98, 95%CI 1.42-11.18 for alcohol use during follow-up; aHR=3.53, 95%CI 1.25-10.03 for problem drinking on the CAGE score vs not drinking; aHR=4.45, 95%CI 1.43-13.85 for binge drinking vs not drinking).
There was strong evidence for an association between HIV seroconversion and prevalent MG infection tested at the enrolment visit (aHR=2.19, 95%CI: 1.11-4.36), with NG (aHR=5.41, 95%CI: 2.76-10.57) and with TV infection (aHR =2.26, 95%CI: 1.03-4.93) (Table 3). Similar results were seen in a model in which the RTI/STIs were not adjusted for each other (results not shown).
Table 3.
Associations between STI and HIV seroconversion among 646 women at high risk in Kampala, Uganda.
| N (%)(1) | HIV sero converters /pyr |
HIV incidence rate/ 100 pyr |
Age adjusted Hazard Ratio (95% CI) |
Adjusted Hazard Ratio(2) (95% CI) |
|
|---|---|---|---|---|---|
| Total | 42/1147 | 3.66 | |||
|
| |||||
| Syphilis (3) | P=0.39 | P=0.34 | |||
| No infection | 4198 (84) | 35/937 | 3.63 | 1 | 1 |
| Past infection | 371 (7) | 1/105 | 1.91 | 0.39 (0.05-2.83) | 0.30 (0.04-2.25) |
| Active low titre syphilis (<8) | 332 (7) | 3/81 | 6.16 | 1.12 (0.34-3.66) | 0.72 (0.22-2.38) |
| Active high titre syphilis (8+) | 104 (2) | 3/19 | 5.32 | 2.42 (0.73-8.01) | 1.64 (0.48-5.67) |
|
| |||||
| HSV2 serostatus (4) | P=0.01 | P=0.29 | |||
| Negative | 1196 (24) | 6/289 | 2.08 | 1 | 1 |
| Positive | 3848 (76) | 36/858 | 4.20 | 2.70 (1.13-6.49) | 1.59 (0.65-3.93) |
|
| |||||
| N. gonorrhoeae (5) | P<0.01 | P<0.001 | |||
| Negative | 4576 (94) | 27/1046 | 2.58 | 1 | 1 |
| Positive | 317 (6) | 15/64 | 23.37 | 7.58 (3.99-14.42) | 5.41 (2.76-10.57) |
|
| |||||
| C. trachomatis (5) | P=0.01 | P=0.15 | |||
| Negative | 4622 (94) | 34/1048 | 3.24 | 1 | 1 |
| Positive | 271 (6) | 8/63 | 12.73 | 3.03 (1.37-6.72) | 1.91 (0.83-4.38) |
|
| |||||
| T. vaginalis (6) | P=0.02 | P=0.06 | |||
| Negative | 4505 (92) | 33/1024 | 3.22 | 1 | 1 |
| Positive | 396 (8) | 9/88 | 10.24 | 2.74 (1.30-5.76) | 2.26 (1.03-4.93) |
|
| |||||
| Bacterial vaginosis (7) | P=0.18 | P=0.87 | |||
| Negative | 2092 (43) | 12/479 | 2.50 | 1 | 1 |
| Intermediate | 244 (5) | 3/56 | 5.32 | 1.96 (0.55-7.01) | 1.36 (0.36-5.12) |
| Positive | 2553 (52) | 27/575 | 4.69 | 1.82 (0.92-3.60) | 1.17 (0.58-2.36) |
|
| |||||
| Candida albicans (8) | P=0.91 | P=0.54 | |||
| Negative | 4545 (93) | 39/1032 | 3.78 | 1 | 1 |
| Positive | 346 (7) | 3/79 | 3.82 | 0.93 (0.29-3.03) | 1.46 (0.44-4.85) |
|
| |||||
| M. genitalium (baseline) (9) | P=0.009 | P=0.03 | |||
| Negative | 564 (88) | 30/1007 | 2.98 | 1 | 1 |
| Positive | 80 (12) | 12/136 | 8.80 | 2.63 (1.34-5.17) | 2.19 (1.11-4.36) |
|
| |||||
| Confirmed VDS (10) | P=0.55 | P=0.87 | |||
| No | 3080 (61) | 21/672 | 3.12 | 1 | 1 |
| Yes | 1967 (39) | 21/475 | 4.42 | 1.21 (0.65-2.24) | 1.06 (0.56-1.99) |
|
| |||||
| Confirmed GUD (9) | P=0.12 | P=0.32 | |||
| No | 4954 (98) | 39/1123 | 3.47 | 1 | 1 |
| Yes | 92 (2) | 3/23 | 12.93 | 3.01 (0.91-9.95) | 2.02 (0.56-7.20) |
|
| |||||
| Confirmed PID (10) | P=0.22 | P=0.59 | |||
| No | 4673 (93) | 35/1048 | 3.34 | 1 | 1 |
| Yes | 374 (7) | 7/98 | 7.14 | 1.75 (0.75-4.10) | 1.28 (0.53-3.06) |
Number of women for baseline variables (N= 646 women) or number of visits for time-varying variables (n=5048 visits)
Adjusted for age, calendar time, age at first sexual intercourse, number of lifetime sexual partners, use of alcohol in the past 3 months, number of paying clients in past 3 months, inconsistent condom use with paying clients in the past 3 months, current pregnancy, and N. Gonorrhoea, T. Vaginalis and M. Genitalium infections.
43 missing
4 missing
155 missing
147 missing
159 missing
157 missing
2 missing
1 missing
Population Attributable Fractions (PAFs)
The estimated PAF of HIV seroconversion for alcohol use was 63.5% (95%CI 6.5%-85.8%). The PAFs for NG, MG and TV were 29.1% (95%CI 11.1-43.5%), 15.5% (95%CI 0%-30.3%) and 11.9% (95%CI 0%-25.0%) respectively. The PAF for any treatable RTI/STIs was 70.0% (95%CI 18.8%-87.5%).
Discussion
This study presents the first 3-years follow-up data from a large closed cohort of female sex workers who were attending a dedicated clinic offering HIV prevention interventions, in Kampala. The baseline HIV prevalence of the cohort was 37% and the HIV incidence rate was 3.66/100 pyr. These are 4-5 times higher than the most recent estimates of national HIV prevalence (7.5%) and incidence (0.94/100pyr) for women [12].
There was a substantial decline in the HIV incidence rate over the duration of the project. This is most likely because, in a closed cohort, women at highest risk seroconvert first leaving behind women at lower risk, and also because of the intervention efforts of the project.
Reported alcohol use was strongly associated with HIV incidence after adjusting for other behavioral factors. Due to the high prevalence of alcohol use in this cohort, we estimate that approximately 65% of new HIV infections in women at high risk could be attributed to alcohol use if the association is causal. Alcohol is a documented problem in Uganda, which ranked as the world’s leading consumer of alcohol in 2004, with an estimated annual average of 19.5 litres of recorded alcohol per capita consumption and an estimated 10 litres of unrecorded consumption [13]. A systematic review of 10 longitudinal studies in western countries found strong epidemiological evidence for an association between alcohol use and HIV incidence (summary adjusted risk ratio (RR)=1.77; 95%CI:1.43-2.19) [14], and similar results were found in a systematic review and meta-analysis of 11 studies from sub-Saharan Africa (summary adjusted RR=1.57, 95%CI 1.42-1.72) [15], which included two longitudinal studies, and these found a similar effect as in the meta-analysis [16,17]. Pathways for a possible causal link between alcohol use and HIV infection include: i) biological plausibility that heavy alcohol use increases susceptibility to HIV infection, through its effects on the liver and innate and acquired immune system functioning; ii) the direct effect of alcohol consumption on cognitive capacity and behaviour, which can affect behaviours such as consistent condom use or ability to negotiate safe sex; iii) the physical occupational co-location of sex work in places which sell alcohol.
In addition, there is co-occurrence of alcohol use and risky sexual behaviour among individuals with risk-taking personality characteristics [18]. A recent narrative review of studies in sub-Saharan Africa showed that alcohol consumption was associated with an increase in several risk behaviours that enhance risk of HIV incidence, including unprotected sex, sex with multiple partners, transactional sex and coercive sex [19]. Brief interventions (BI) including counselling to encourage people to alter their alcohol use and to be delivered by health care workers who do not specialize in alcoholism treatment, have been designed and a recent systematic review of alcohol studies in sub-Saharan Africa found that such BI gave promising short term results [20]. However, the only randomized controlled trial of an alcohol-reduction intervention conducted among female sex workers did not find a significant intervention effect [21]. Further research is urgently needed to develop and assess the effectiveness of alcohol reduction interventions adapted to population groups that are exposed to both HIV and alcohol because of the nature of their work.
STIs were also strongly associated with HIV incidence in this cohort. Women with MG infection at baseline were twice as likely to acquire HIV as those without. Baseline prevalence of MG in this cohort was 14% and we previously published evidence for an association with prevalent HIV infection at enrolment (OR=1.52, 95%CI: 1.07-2.17) [9]. As no specific treatment was given for this bacterial infection, MG may have persisted in some women, but not in all women, suggesting that the effect of this STI may be under-estimated in this analysis. Our findings suggest that there is an urgent need to improve screening for and treatment of MG infection, especially in HIV-burdened countries. A recently published case control study from Zimbabwe reported that MG infection increased the risk of HIV acquisition [22]. Additional studies are planned in our cohort to assess further the association with HIV incidence. NG and TV infections were also associated with HIV incidence as was also the case in similar studies in Kenya [23] and Zimbabwe [24].
The prevalence of BV is known to be high in sub-Saharan Africa [25 – 30]. As in clinical practice only symptomatic infections are treated, it is likely that many prevalent infections are persistent. Furthermore, in most women BV recurs quickly after treatment [31]. A recent two-year cohort study in rural Uganda, investigating weekly vaginal flora patterns, found that 59% of the enrolled women had at least 1 episode of BV over the follow-up time, with one third of women spending >50% of their time with BV [32]. We did not find significant evidence of an association between BV and HIV acquisition in this study. Nevertheless, we had relatively little power to look at this association and as confidence intervals were wide the data were not inconsistent with two recent meta-analyses of published HIV incidence studies showing evidence for an association between BV and HIV acquisition [33, 34]. Although the pathogenesis of BV is as yet poorly understood, its role as a risk factor for HIV and other STIs has recently attracted more interest [35]. Clinical trials are needed to investigate improved control strategies to reduce the prevalence and persistence of BV. There was no evidence of an association between intravaginal cleansing with water and soap and HIV acquisition in our study, although again our data are consistent with the modest increase in the risk of HIV seroconversion seen in a recent meta-analysis (pooled adjusted HR 1.24; 95%CI 1.01-1.53) [34].
Our study showed no evidence for an association between hormonal contraception (either oral or injectable) and HIV acquisition. Previous studies of HIV-1-acquisition risk related to contraceptive use have had inconsistent results, partly because of variable methodological quality [36]. Clinical studies have suggested that changes to vaginal structure, cytokine regulation, CCR5 expression, and cervicovaginal HIV-1 shedding may be possible mechanisms by which hormonal contraception could influence HIV-1 susceptibility and infectiousness [37], and a recent prospective HIV acquisition study in Africa found an overall association with hormonal contraception, especially with injectables (aHR=1.98, 95%CI:1.06–3.68) [38]. Following publication of these results, a WHO expert group was convened to review the evidence, and issued a statement that women using progestogen-only injectable contraception should be strongly advised to also always use condoms [39]. In our cohort, of the 337 HIV negative women at enrolment who were using injectable contraception, only 28% reported using male condoms.
In conclusion, our study showed that in this vulnerable group, despite regular screening, treatment and risk reduction education, STIs are important risk factors for HIV acquisition, which may call for more intensive control measures such as frequent presumptive treatment. The association between MG and HIV acquisition needs confirmation from longitudinal studies and more work is required to decide upon control measures of this emerging STI. Alcohol use is strongly associated with risk of HIV infection, and effective interventions adapted to population groups with a high prevalence of drinking are urgently needed.
Acknowledgements
We sincerely want to thank the study participants and the study team of the Good Health for Women Project in Kampala; the laboratory and data teams of the MRC/UVRI Unit in Entebbe; Women at Work International Uganda; Etienne Muller and David Lewis, Centre for HIV and Sexually Transmitted Infections, National Institute for Communicable Diseases, National Health Laboratory Service in Johannesburg.
The study was funded by the Medical Research Council (MRC), UK and the European and Developing Countries Clinical Trials Partnership (EDCTP).
Sources of financial support: Medical Research Council, UK and the European and Developing Countries Clinical Trials Partnership (EDCTP).
Footnotes
Conflicts of interest: none declared
References
- 1.Wasserheit JN, Aral SO. The dynamic topology of sexually transmitted disease epidemics: Implications for prevention strategies. J Infect Dis. 1996;174(suppl 2):S201–S213. doi: 10.1093/infdis/174.supplement_2.s201. [DOI] [PubMed] [Google Scholar]
- 2.Ghys PD, Diallo MO, Ettiègne-Traoré V, et al. Increase in condom use and decline in HIV and sexually transmitted diseases among female sex workers in Abidjan, Cote d’Ivoire, 1991-1998. AIDS. 2002;16:251–258. doi: 10.1097/00002030-200201250-00015. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Richardson BA, Martin HL, Jr, et al. Trends in HIV-1 Incidence in a Cohort of Prostitutes in Kenya: Implications for HIV-1 Vaccine Efficacy Trials. JAIDS. 2000;24:458–464. doi: 10.1097/00126334-200008150-00011. [DOI] [PubMed] [Google Scholar]
- 4.Alary M, Mukenge-Tshibakaa L, Bernier F, et al. Decline in the prevalence of HIV and sexually transmitted diseases among female sex workers in Cotonou, Bénin, 1993-1999. AIDS. 2002;16:463–470. doi: 10.1097/00002030-200202150-00019. [DOI] [PubMed] [Google Scholar]
- 5.Laga M, Alary M, Nzila N, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet. 1994;344:246–8. doi: 10.1016/s0140-6736(94)93005-8. [DOI] [PubMed] [Google Scholar]
- 6.Shahmanesh M, Patel V, Mabey D, et al. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Tropical Medicine and International Health. 2008;13:1–21. doi: 10.1111/j.1365-3156.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 7.Moses S, Plummer FA, Ngugi EN, et al. Controlling HIV in Africa; effectiveness and cost of an intervention in a high-frequency STD transmitter core group. AIDS. 1991;5:407–11. [PubMed] [Google Scholar]
- 8.Vandepitte J, Bukenya J, Weiss HA, et al. HIV and other sexually transmitted infections in a cohort of women involved in high-risk sexual behavior in Kampala, Uganda. Sex Transm Dis. 2011;38:316–323. [PMC free article] [PubMed] [Google Scholar]
- 9.Vandepitte J, Muller E, Bukenya J, et al. Prevalence and correlates of Mycoplasma genitalium infection among female sex workers in Kampala, Uganda. J Infect Dis. 2011;205:289–296. doi: 10.1093/infdis/jir733. [DOI] [PubMed] [Google Scholar]
- 10.Victoria CG, Huttly SR, Fuchs SC, et al. The role of conceptual frameworks in epidemiological analysis: A hierarchical approach. Int J Epidemiol. 1997;6:224–227. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 11.Greenland S, Newcombe R. Confidence limits made easy: interval estimation using a substitution method. Letters to Editor. Am J Epidemiol. 1999;149(9):884–885. doi: 10.1093/oxfordjournals.aje.a009906. [DOI] [PubMed] [Google Scholar]
- 12.Hladik W, Musinguzi J, Kirungi W, et al. The estimated burden of HIV/AIDS in Uganda, 2005-2010. AIDS. 2008;22:503–510. doi: 10.1097/QAD.0b013e3282f470be. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . Global Status Report on Alcohol 2004. Department of Mental Health and Substance Abuse, World Health Organization; Geneva: 2004. [Google Scholar]
- 14.Baliunas D, Rehm J, Irving H, et al. Alcohol consumption and risk of incident human immunodeficiency virus infection: a meta-analysis. Int J Public Health. 2010;55:159–66. doi: 10.1007/s00038-009-0095-x. [DOI] [PubMed] [Google Scholar]
- 15.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34:856–63. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 16.Kapiga SH, Lyamuya EF, Lwihula GK, et al. The incidence of HIV infection among women using family planning methods in Dar es Salaam, Tanzania. AIDS. 1998;12:75–84. doi: 10.1097/00002030-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Zablotska IB, Gray RH, Serwadda D, et al. Alcohol use before sex and HIV acquisition: a longitudinal study in Rakai, Uganda. AIDS. 2006;20:1191–6. doi: 10.1097/01.aids.0000226960.25589.72. [DOI] [PubMed] [Google Scholar]
- 18.Kalichman SC, Simbayi LC, Kaufman M, et al. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8(2):141–51. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- 19.Woolf-King SE, Maisto SA. Alcohol use and high-risk sexual behavior in Sub-Saharan Africa: a narrative review. Arch Sex Behav. 2011;40:17–42. doi: 10.1007/s10508-009-9516-4. [DOI] [PubMed] [Google Scholar]
- 20.Peltzer K. Brief intervention of alcohol problems in sub-Saharan Africa: A review. Journal of Psychology. 2009;19:415–22. [Google Scholar]
- 21.Wechsberg WM, Luseno WK, Lam WK, et al. Substance use, sexual risk, and violence: HIV prevention intervention with sex workers in Pretoria. AIDS Behav. 2006;10:131–7. doi: 10.1007/s10461-005-9036-8. [DOI] [PubMed] [Google Scholar]
- 22.Mavedzengea SN, Van der Pol B, Weiss HA, et al. The association between Mycoplasma genitalium and HIV-1 acquisition among women in Zimbabwe and Uganda. AIDS. 2012 doi: 10.1097/QAD.0b013e32834ff690. DOI:10.1097/QAD.0b013e32834ff690. [DOI] [PubMed] [Google Scholar]
- 23.Kaul R, Kimani J, Nagelkerke NJ, et al. Monthly Antibiotic Chemoprophylaxis and Incidence of Sexually Transmitted Infections and HIV-1 Infection in Kenyan Sex Workers. JAMA. 2004;291:2555–2562. doi: 10.1001/jama.291.21.2555. [DOI] [PubMed] [Google Scholar]
- 24.Van der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 25.Martin LH, Jr, Richardson BA, Nyange PM, et al. Vaginal Lactobacilli, Microbial Flora, and Risk of Human Immunodeficiency Virus Type 1 and Sexually Transmitted Disease Acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 26.Nagot N, Ouedraogo A, Defer MC, et al. Association between bacterial vaginosis and Herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm Infect. 2007;83:365–8. doi: 10.1136/sti.2007.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baisley K, Changalucha J, Weiss HA, et al. Bacterial vaginosis in female facility workers in north-western Tanzania: prevalence and risk factors. Sex Transm Infect. 2009;85:370–5. doi: 10.1136/sti.2008.035543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClelland RS, Richardson BA, Graham SM, et al. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sexually Transmitted Diseases. 2008;35:617–23. doi: 10.1097/OLQ.0b013e31816907fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;23:546–50. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 30.Taha E, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Hay P. Recurrent bacterial vaginosis. Curr Opin Infect Dis. 2009;22:82–6. doi: 10.1097/QCO.0b013e32832180c6. [DOI] [PubMed] [Google Scholar]
- 32.Thoma ME, Gray RH, Kiwanuka N, et al. The Short-term Variability of Bacterial Vaginosis Diagnosed by Nugent Gram Stain Criteria Among Sexually Active Women in Rakai, Uganda. Sex Transm Dis. 2011;38:111–116. doi: 10.1097/OLQ.0b013e3181f0bdd0. [DOI] [PubMed] [Google Scholar]
- 33.Atashilia J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med. 2011;8(2):e1000416. doi: 10.1371/journal.pmed.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwebke JR. Abnormal Vaginal Flora as a Biological Risk Factor for Acquisition of HIV Infection and Sexually Transmitted Diseases. J Infect Dis. 2005;192:1315–7. doi: 10.1086/462430. [DOI] [PubMed] [Google Scholar]
- 36.Baeten JM, Lavreys L, Overbaugh J. The influence of hormonal contraceptive use on HIV-1 transmission and disease progression. Clin Infect Dis. 2007;45:360–69. doi: 10.1086/519432. [DOI] [PubMed] [Google Scholar]
- 37.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet. 2011 doi: 10.1016/S1473-3099(11)70247-X. DOI:10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization (WHO) [Accessed at Feb 26, 2012];Hormonal contraception and HIV. Technical statement. WHO/RHR/12.08. Available at: www.who.int/reproductivehealth/topics/family planning/hormonal contraception_and_HIV.pdf. [PubMed]