Abstract
OBJECTIVE:
Previous studies show that vasogenic cerebral edema (CE) occurs during diabetic ketoacidosis (DKA) treatment in children, but the role of intravenous fluids in contributing to CE is unclear. We used magnetic resonance diffusion weighted imaging to quantify subclinical CE in children with DKA randomized to 2 intravenous fluid regimens.
METHODS:
Children with DKA were randomized to receive fluids at a more rapid rate (n = 8) or a slower rate (n = 10), with all other aspects of DKA treatment kept identical. Children underwent diffusion weighted imaging 3 to 6 hours and 9 to 12 hours after beginning DKA treatment and after recovery from DKA (≥72 hours after beginning treatment). We calculated brain apparent diffusion coefficient (ADC) values as the average of measurements in the basal ganglia, thalamus, frontal white matter, and hippocampus and determined the mean brain ADC value during DKA treatment by averaging data from the 3- to 6-hour and 9- to 12-hour measurements. The difference in mean brain ADC between DKA treatment and postrecovery was used as an index of the severity of CE during DKA treatment.
RESULTS:
Mean brain ADC values during DKA treatment were significantly higher than postrecovery values, consistent with vasogenic CE (842 ± 38 vs 800 ± 41×10–6 mm2/second, P = .002). We did not detect significant differences in ADC elevation in children treated with more rapid versus slower rehydration (β coefficient 0.11 for 1 SD change in ADC, 95% confidence interval: –0.91 to 1.13).
CONCLUSIONS:
ADC changes during DKA treatment (reflective of vasogenic CE) do not appear to be substantially affected by the rate of intravenous fluid administration.
KEY WORDS: diabetic ketoacidosis, MRI, diffusion weighted imaging, cerebral edema, cerebral injury
What’s Known on This Subject:
Cerebral edema (CE) occurs frequently during treatment of diabetic ketoacidosis (DKA) in children. Severe, life-threatening CE occurs rarely, but subclinical CE is common. Whether the rate of infusion of intravenous fluids influences the occurrence or severity of CE is unknown.
What This Study Adds:
This study demonstrates that the rate of fluid infusion in children with DKA does not substantially affect MRI measures of CE. Studies assessing measures other than edema formation are necessary to determine whether fluid infusion rates influence DKA-related brain injury.
Optimal fluid therapy for children with diabetic ketoacidosis (DKA) is a topic of considerable debate. Much of this debate has centered on how to prevent cerebral edema (CE), the most feared complication of DKA in children. Investigators have proposed that DKA-related CE may be related to excessive rates of intravenous fluid infusion, particularly when associated with rapid declines in serum osmolality.1,2 According to this hypothesis, osmotic changes lead to fluid influx into brain cells. If this hypothesis is correct, DKA-related CE might be decreased by administering fluids at slower rates. Alternatively, DKA-related CE has been proposed to be caused by cerebral hypoperfusion and the effects of reperfusion during DKA treatment.3,4 The optimal rehydration strategy suggested by this hypothesis is less clear. More rapid rehydration could theoretically limit the duration of cerebral hypoperfusion, thereby reducing cerebral injury. Conversely, rapid rehydration might exacerbate vasogenic edema occurring during reperfusion, particularly if blood-brain barrier breakdown occurs. Thus, the optimal rehydration strategy for pediatric DKA remains unclear.
Clinically apparent CE occurs in only 0.5% to 1% of DKA episodes5–7; however, CE that is asymptomatic or associated with only minor mental status alterations occurs much more frequently.8–10 This subclinical CE can be demonstrated by using various techniques, including magnetic resonance diffusion weighted imaging (DWI).4,11 DWI quantifies the diffusion of water molecules in brain tissues and is reported as the apparent diffusion coefficient (ADC).12,13 During DKA treatment, children have elevated cerebral ADC values (suggesting vasogenic CE) that decrease after recovery.4,11 Comparison of ADC changes in children with DKA might therefore serve as an index of subclinical CE. In this study, we compared ADC changes in children with DKA randomly assigned to slower versus more rapid rehydration to determine whether rehydration rate affects CE formation.
Methods
Patients
Children presenting to the emergency department between 2008 and 2011 were eligible for participation if they were 8 to 18 years old, were diagnosed with type 1 diabetes, and had DKA (defined as serum glucose >300 mg/dL, venous pH <7.25, or serum bicarbonate <15 mEq/L, and a positive test for urine ketones). Children were excluded if they had dental hardware that could interfere with MRI or cognitive deficits that would limit ability to cooperate with imaging. Children transferred to the study center after beginning DKA treatment were also excluded. The study was approved by the hospital institutional review board.
Treatment Protocol
After obtaining written informed consent from guardians, patients were randomly assigned to 1 of 2 protocols by using a computer-generated random permuted block sequence. The protocols are summarized in Table 1. Clinicians and research personnel were made aware of the patient’s assignment by opening a sealed envelope after obtaining consent. Protocol assignment envelopes were pre-stocked in the ED by the principal investigators. Participants were not aware of their protocol assignments.
TABLE 1.
Summary of Fluid Protocols
| Protocol A | Protocol B | |
|---|---|---|
| Intravenous fluid bolus (0.9% saline) | 20 mL/Kg | 10 mL/Kg |
| Assumed fluid deficit | 10% of body wt | 7% of body wt |
| Rate of deficit replacement | Two-thirds over first 24 h; One-third over next 24 h | Evenly over 48 h |
| Urine output replacement | Half of urine vol replaced while serum glucose level is >250 mg/dL | None |
| Fluid type | 0.9% saline while serum glucose is >250 mg/dL, followed by 0.45% saline | 0.9% saline while serum glucose is >250 mg/dL, followed by 0.45% saline |
For both protocols, insulin was initiated after the first fluid bolus as a continuous infusion of 0.1 U/Kg/hour. Potassium was administered as an equal mixture of potassium chloride and potassium phosphate. To optimize patient safety, regardless of protocol assignment, additional fluid boluses could be administered if these were thought necessary based on circulatory status. Similarly, treating physicians were able to adjust fluid infusion rates if it was felt that the rate prescribed by the study protocol might compromise patient safety.
Vital signs were evaluated hourly. Neurologic status was assessed hourly by using an age-appropriate Glasgow Coma Scale (GCS)14 for all patients, and every 30 minutes for patients with altered mental status. Abnormal mental status was defined as a GCS score <14. Serum electrolyte concentrations, venous pH, and PCO2 were measured at presentation and every 3 hours, and blood glucose concentrations were measured hourly until the intravenous insulin infusion was discontinued. Effective osmolality was calculated as follows: 2[measured serum Na (mmol/L)] + [serum glucose (mg/dL) / 18].
Imaging Procedures
Patients underwent DWI at 3 time points: (1) 3 to 6 hours after the initiation of DKA treatment (defined by the administration of the first fluid bolus), (2) 9 to 12 hours after the initiation of DKA treatment, and (3) after recovery from DKA (≥72 hours after initiation of treatment). Methods for DWI were similar to those previously described11 and can be found in the Supplemental Information.
We recorded ADC values in 4 regions: the basal ganglia, thalamus, hippocampus, and frontal white matter. ADC measurements were obtained by a single radiologist who was blinded to the patients’ group assignments. The mean of ADC measurements on the right and left sides of the brain was used as the value for each region. Mean brain ADC values were determined by calculating the mean of ADC values measured in all 4 regions. Finally, to estimate brain ADC during the first 12 hours of treatment, ADC values at 3 to 6 hours and 9 to 12 hours were averaged. For patients who were unable to tolerate MRI at 1 of the time points (n = 4 at 3–6 hours, n = 2 at 9–12 hours), ADC data from just 1 time point were used. This approach was considered valid because ADC values at 3 to 6 hours and 9 to 12 hours were not significantly different (see Results). We avoided pharmacologic sedation during the imaging procedures whenever possible. When necessary, midazolam (≤0.1 mg/kg) was used.
Statistical Analysis
This pilot study was aimed at determining whether clear and obvious differences in MRI measures of CE could be detected in patients hydrated with 2 different regimens. We aimed to enroll 10 patients into each arm providing an 80% power to detect a 1.3 SD difference in ADC change between DKA treatment and postrecovery. Data were examined yearly by an independent Data Safety Monitoring Board to monitor patient safety. No safety concerns were identified.
ADC values during DKA treatment were compared with postrecovery values by using the Wilcoxon signed-rank test. Differences between groups in ADC changes were compared by using the Wilcoxon rank-sum test. We used linear regression to evaluate the effects of group assignment on ADC change after adjusting for the patients’ risk for CE (high risk versus low risk). High risk was defined as the patient having either serum urea nitrogen (SUN) concentrations in the upper quartile or pH in the lower quartile of values for enrolled patients.6,15 Biochemical and demographic data for the groups were compared by using the Wilcoxon rank-sum test for continuous variables and the χ2 test for dichotomous variables. Finally, differences in rates of change of biochemical variables during treatment were evaluated by using linear mixed effects models for longitudinal data to estimate and compare average slopes (mean change in outcome per hour of treatment) for each group.
To provide more accurate graphic representations of trends in sodium and glucose concentrations, missing values (5% of sodium and 14% of glucose values) were imputed by assuming a linear trend in these measures. Missing values for pH (7%) and PCO2 (7%) were not imputed because trends in these variables were not consistently linear and were less predictable. We considered P values <.05 to indicate statistical significance and P values <.10 to represent a trend. Statistical analyses were performed by using Stata SE 11.1 (Stata Corp, College Station, TX).
Results
Eighteen patients were enrolled in the study. Two were enrolled twice, therefore the study involved 20 DKA episodes. Ten episodes were randomized to protocol A and 10 to protocol B. Two patients, both randomized to protocol A, were unable to complete the MRI. One patient had anxiety related to claustrophobia and was unable to remain sufficiently immobile to accomplish the studies. The other presented to the emergency department with abnormal mental status (GCS score of 9). Shortly after randomization, it was decided by the clinical team that the patient was not sufficiently stable for MRI studies. Both patients were withdrawn from the study and treated according to the institution’s standard DKA protocol. The final analytical data set therefore included 18 DKA episodes; 8 episodes treated with protocol A and 10 with protocol B.
All participants, including the 2 patients who were withdrawn, recovered fully from DKA without apparent neurologic or cognitive deficits. Of the patients included in the final data set, 1 (randomized to protocol A) was treated for suspected CE. This patient had a decline in GCS score to 13 ∼90 minutes after beginning treatment. Thirty minutes later, the patient’s GCS score declined to 12, and the patient was treated with mannitol. No improvement in mental status was observed after mannitol, and no additional treatment of CE was administered. The patient’s mental status spontaneously improved at hour 6 and returned to normal by hour 9. This patient was thought to be too unstable to undergo the first set of imaging studies. At the second time point (9–12 hours), imaging studies showed no findings indicative of overt CE. All other patients in the final database had GCS scores no lower than 14 throughout treatment.
Biochemical data describing the study groups are presented in Table 2. Although the volumes of initial fluid boluses were dictated by protocol, physicians were allowed to administer additional fluid boluses if this was felt necessary based on indicators of circulatory status. Patients in both groups received additional boluses beyond those dictated by the protocols. Group A received a mean of 32 ± 17 mL/kg as fluid boluses, and group B received 19 ± 10 mL/kg (P = .06). Two patients discontinued intravenous insulin and fluids before 12 hours, 1 after 8 and the other after 11 hours. Intravenous fluids received by both groups were therefore compared for the first 8 hours of treatment only. During this period, group A received a total of 61 ± 19 mL/kg and group B received 42 ± 7 mL/kg (P = .01). Note that the greater variability in fluid administration in group A is attributable to replacement of urine output volume as dictated by protocol A.
TABLE 2.
Clinical and Biochemical Characteristics of Treatment Groupsa
| Group A (n = 8) | Group B (n = 10) | P Value | |
|---|---|---|---|
| Age, y | 11.5 (9–14) | 15 (9–18) | .07 |
| Gender, % male | 38% | 60% | .34 |
| New-onset diabetes, % | 1 patient (12%) | 1 patient (10%) | .87 |
| Serum glucose, mmol/L | 34.5 (17.7–64.7) | 30.9 (19.2–54.8) | .59 |
| pH | 7.13 (6.93–7.2) | 7.12 (6.95–7.26) | .42 |
| PCO2 | 22 (21–36) | 21 (20–32) | .15 |
| Serum sodium, mmol/L | 131 (120–139) | 133 (132–149) | .11 |
| Serum potassium, mmol/L | 5.1 (3.9–6.3) | 5.0 (3.9–7.2) | .89 |
| Serum chloride, mmol/L | 97 (82–104) | 96 (90–101) | .79 |
| Serum bicarbonate, mmol/L | 9.5 (5–14) | 8.5 (5–12) | .47 |
| SUN, mmol/L | 7.5 (5.0–16.4) | 7.1 (5.0–10.7) | .53 |
Data presented as median (range).
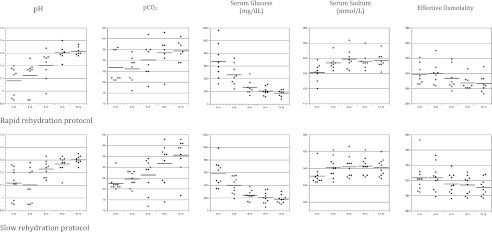
Rates of change in biochemical values during treatment were similar in both groups (Fig 1). A decline in pH during the first several hours of treatment occurred more frequently in group B (4 of 9 patients) than in group A (1 of 8 patients), but these differences did not reach statistical significance (P = .15).
FIGURE 1.
Changes in biochemical values during DKA treatment with rapid fluid infusion versus slow fluid infusion. Slope (95% confidence interval [CI]) for group A, slope (95% CI) for group B, and P value for each variable: pH: 0.021 (0.015 to 0.028), 0.017 (0.012 to 0.023), P = .39. PCO2: 0.75 (0.28 to 1.21), 1.05 (0.64 to 1.47), P = .33. Glucose: –41 (–55 to –29), –33 (–45 to –21), P = .35. Sodium: 0.56 (0.31 to 0.81), 0.35 (0.13 to 0.57), P = .23. Effective osmolality: –1.2 (–1.8 to –0.7), –1.1 (–1.6 to –0.6), P = .80. Note: Average slopes are the maximum likelihood estimates of the treatment group specific fixed effect of time from mixed effects linear regression models for longitudinal data. Models were specified with fixed effects for time of treatment, group, and the interaction of group and time and random effects for slopes and intercepts.
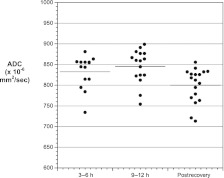
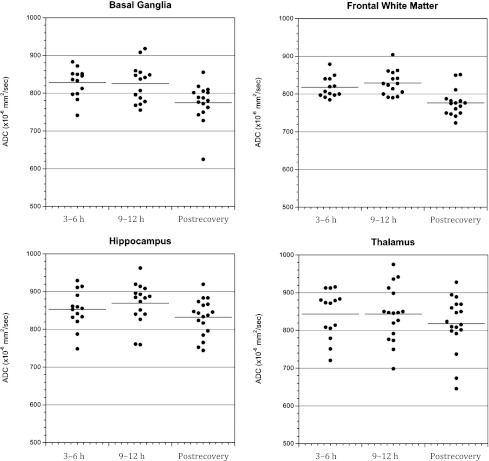
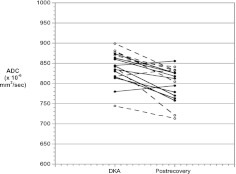
In both groups, mean brain ADC values were similar at 3 to 6 hours and 9 to 12 hours (Fig 2, P = .75). ADC values at both time points in both groups were significantly higher than after recovery, consistent with vasogenic CE. Similar trends were observed in all 4 brain regions, although ADC values were more variable in the thalamus than the other 3 regions (Fig 3). Because no significant differences were observed between the 3- to 6-hour and 9- to 12-hour points in mean brain ADC, these values were averaged to calculate overall mean ADC values during DKA treatment. This overall mean ADC was used for comparisons of ADC changes between the 2 groups. Comparison of the mean brain ADC changes during DKA treatment did not detect significant differences between groups (Fig 4). Because some patients received additional fluid boluses beyond those prescribed by protocol, we also compared ADC changes based on actual fluid volumes received. In this subanalysis, there were no significant differences in ADC change between patients who received >50 versus <50 cc/kg of intravenous fluid during the first 8 hours of treatment (mean ADC change 36 ± 31 vs 45 ± 37 mm2/sec, P = .58).
FIGURE 2.
Mean brain ADC values in both treatment groups measured at 3 to 6 hours and 9 to 12 hours after beginning DKA treatment and after recovery from DKA. Data shown represent the mean of 4 regions of interest: basal ganglia, thalamus, frontal white matter, and hippocampus. P = .75 for comparison of 3- to 6-hour with 9- to 12-hour values, P = .002 for comparison of 3- to 6-hour with postrecovery values, P = .002 for comparison of 9- to 12-hour with postrecovery values.
FIGURE 3.
Brain ADC values in both treatment groups measured at 3 to 6 hours and 9 to 12 hours after beginning DKA treatment and after recovery from DKA in 4 regions of interest: basal ganglia, frontal white matter, hippocampus, and thalamus.
FIGURE 4.
Mean brain ADC values (average of 3- to 6-hour and 9- to 12-hour values) during DKA treatment and after recovery. Solid circles/solid lines: slow rehydration protocol. Open circles/dotted lines: rapid rehydration protocol. P < .001 for comparison of DKA ADC values versus postrecovery ADC values for all patients.
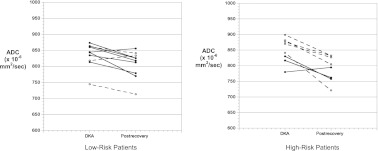
Because certain biochemical profiles (higher SUN concentrations and greater acidosis) are associated with higher risk of clinically evident CE as well as greater subclinical CE during DKA treatment,4,6,15 we divided patients into high- versus low-risk groups (high risk defined as SUN in the upper quartile [≥27 mg/dL] and/or pH in the lower quartile [≤6.97] for enrolled patients). Five patients in group A (62%) and 3 in group B (30%) met high-risk criteria (Fig 5). ADC changes were greater in the high-risk group (P = .04), regardless of protocol assignment. Among low-risk patients, there was a trend toward lesser ADC change in patients treated with more rapid rehydration (protocol A, P = .09), but there were no significant differences according to protocol assignment among the high-risk patients.
FIGURE 5.
Brain ADC values in patients at higher risk and lower risk of DKA-related CE. Solid circles: slow rehydration protocol; open circles: rapid rehydration protocol. P = .09 for comparison of ADC changes between groups in low-risk patients, P = .46 for comparison of ADC changes between groups in high-risk patients.
To investigate further the effects of treatment protocol on ADC change, we performed a multiple linear regression analysis. We examined the association of protocol assignment with ADC change, after adjusting for high- versus low-risk status. Group B served as the reference group. In this model, treatment group assignment was not significantly associated with ADC change (β coefficient 0.11 for 1 SD change in ADC, 95% confidence interval: –0.91 to 1.13).
Discussion
The pathophysiology of DKA-related CE is not well understood and debate has focused on the possible role of intravenous fluids in causing or contributing to CE. Clear associations between more rapid fluid infusion and greater risk of CE, however, have not been evident in studies that have controlled for DKA severity.6 In the current study, we demonstrate that MRI measures of CE (ADC change between treatment and recovery) appear similar in children with DKA, regardless of whether they are treated with more rapid or slower infusion of fluids. These findings suggest that the rate of fluid infusion may not be the main determinant of DKA-related CE or, alternatively, that more sensitive measures or larger studies are necessary to detect differences in CE or cerebral injury related to variations in fluid treatment.
Investigators have hypothesized that DKA-related CE may result from rapid declines in serum osmolality with infusion of intravenous fluids during DKA treatment.1,2 Conservative infusion of fluids has generally been advocated in pediatric guidelines.16–18 A large study using multivariable statistical methods to control for DKA severity, however, did not find associations between rates of fluid infusion and risk of CE.6 In addition, reports of severe and even fatal DKA-related CE before DKA treatment are difficult to explain based on osmotic fluctuations.19,20 Lack of consistent data to support osmotic change and/or excess fluid infusion as the cause of DKA-related CE suggest that other factors should be considered.
Recent data suggest that DKA-related CE may result from mechanisms more complicated than osmotic shifts. MRI studies in children indicate that edema developing during DKA treatment is vasogenic, with elevated ADC values and increased cerebral blood flow (CBF).11 These findings are not consistent with those anticipated to be caused by osmotic changes.21,22 Furthermore, animal studies suggest that the characteristics of cerebral perfusion, metabolism, and edema during DKA are similar to those observed during cerebral ischemia and reperfusion. For example, untreated DKA is characterized by low ADC values (suggesting cytotoxic edema) and low CBF.23,24 Brain lactate levels are elevated and levels of high-energy phosphates are decreased.25 These findings are similar to those observed during cerebral hypoxia or ischemia.26–29 During DKA treatment with insulin and saline, CBF is elevated, and vasogenic edema develops. Brain levels of high-energy phosphates and ratios of N-acetylaspartate to creatine (an indicator of neuronal health) decline during initial treatment with insulin and saline.25 These changes are similar to those observed during reperfusion after a hypoxic/ischemic insult.
Although data suggest >similarities between DKA-related cerebral injury and ischemia/reperfusion injury, it remains unclear how conditions present during DKA might reduce CBF to a degree sufficient to cause injury and/or how metabolic alterations during DKA might worsen injury caused by relatively mild cerebral hypoperfusion. Furthermore, if DKA-related brain injury is indeed similar to ischemia/reperfusion injury, the optimal treatment protocol to lessen such injury is unclear. More aggressive rehydration might lessen the duration of cerebral hypoperfusion and, because circulatory volume may decline during treatment as serum osmolality declines, more aggressive rehydration might prevent this decline, avoiding worsening of cerebral hypoperfusion. Conversely, because ischemia/reperfusion injury may be associated with blood-brain barrier breakdown, slower rehydration might decrease vasogenic edema later in the course of treatment. Therefore, either beneficial or adverse effects of various fluid protocols could be hypothesized. The current data do not indicate beneficial or adverse effects of either protocol, suggesting that larger studies using different or more sensitive measures of cerebral injury may be necessary.
The current study has several limitations. Because the exact time course of DKA-related CE is not known and the time of maximal edema formation may vary among patients, the optimal timing for imaging children to detect CE is unclear. To partially address this issue, we imaged children twice during DKA treatment. Nonetheless, it is possible that maximal edema formation occurring either early (before hour 3–6) or late in treatment (after hour 12) would not have been detected. Furthermore, although the current data suggest that large differences in MR measures of CE between children treated with the 2 fluid protocols are unlikely, our sample size was small, and we cannot exclude the possibility that differences of smaller magnitude may exist. This question could be addressed in a much larger study; however, given the variability in ADC among individuals and the complexity of the protocol (with multiple MRI sessions), completion of a study sufficiently powered to detect small differences would be difficult if not impossible. In addition, the current study used ADC changes as a measure of CE. Whether the ADC changes documented in the current study are indicative of risk of overt clinically apparent CE is uncertain. Because of the rarity of clinically apparent CE, however, a study of sufficient size to evaluate differences in rates of clinically apparent CE would not be feasible. Finally, in the current study, we evaluated 2 protocols that were within the standard of care for pediatric DKA. Although there was an ∼45% difference between study arms in fluid infusion rate, it is possible that a larger difference in fluid infusion may have been associated with greater differences in MRI measures of CE.
Conclusions
In this pilot study, DKA treatment protocols using different rates of fluid infusion were not associated with large or obvious differences in MRI measures of CE. Whether these findings indicate that rates of fluid infusion do not influence cerebral injury during DKA or whether differences in subtle cerebral injury associated with fluid infusion rate might be detectable in a much larger study or by using other methods is unclear. Additional studies comparing the effects of variations in fluid infusion rate by using alternative methods to detect DKA-related cerebral injury are necessary.
Supplementary Material
Acknowledgments
The authors greatly appreciate the helpful assistance of Drs Joseph Barton, Sierra Beck, Selene Castrejon, Andrew Elms, Matthew Frances, Harlan Gallinger, Noami Halsey-McClure, Ann Juodakis, Irina Kalika, Kelly Owen, and Adam Pomerleau in enrolling and monitoring patients involved in this study. In addition, we appreciate the assistance of Mr Greg Davis in scheduling and coordinating the MRI studies.
Glossary
- ADC
apparent diffusion coefficient
- CBF
cerebral blood flow
- CE
cerebral edema
- DKA
diabetic ketoacidosis
- DWI
diffusion weighted imaging
- GCS
Glasgow Coma Scale
- SUN
serum urea nitrogen
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier R01NS048610).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health (grant R01 NS048610 to Dr Glaser). Funded by the National Institutes of Health (NIH).
References
- 1.Duck SC, Wyatt DT. Factors associated with brain herniation in the treatment of diabetic ketoacidosis. J Pediatr. 1988;113(1 pt 1):10–14 [DOI] [PubMed] [Google Scholar]
- 2.Harris GD, Fiordalisi I, Harris WL, Mosovich LL, Finberg L. Minimizing the risk of brain herniation during treatment of diabetic ketoacidemia: a retrospective and prospective study. J Pediatr. 1990;117(1 pt 1):22–31 [DOI] [PubMed] [Google Scholar]
- 3.Glaser N. Cerebral injury and cerebral edema in children with diabetic ketoacidosis: could cerebral ischemia and reperfusion injury be involved? Pediatr Diabetes. 2009;10(8):534–541 [DOI] [PubMed] [Google Scholar]
- 4.Glaser N, Marcin J, Wooton-Gorges S, et al. Correlation of clinical and biochemical findings with DKA-related cerebral edema in children using magnetic resonance diffusion weighted imaging. J Pediatr. 2008;153:541–546 [DOI] [PubMed] [Google Scholar]
- 5.Edge JA, Hawkins MM, Winter DL, Dunger DB. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001;85(1):16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaser N, Barnett P, McCaslin I, et al. Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics . Risk factors for cerebral edema in children with diabetic ketoacidosis. N Engl J Med. 2001;344(4):264–269 [DOI] [PubMed] [Google Scholar]
- 7.Lawrence SE, Cummings EA, Gaboury I, Daneman D. Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J Pediatr. 2005;146(5):688–692 [DOI] [PubMed] [Google Scholar]
- 8.Glaser NS, Wootton-Gorges SL, Buonocore MH, et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr Diabetes. 2006;7(2):75–80 [DOI] [PubMed] [Google Scholar]
- 9.Hoffman WH, Steinhart CM, el Gammal T, Steele S, Cuadrado AR, Morse PK. Cranial CT in children and adolescents with diabetic ketoacidosis. AJNR Am J Neuroradiol. 1988;9(4):733–739 [PMC free article] [PubMed] [Google Scholar]
- 10.Krane EJ, Rockoff MA, Wallman JK, Wolfsdorf JI. Subclinical brain swelling in children during treatment of diabetic ketoacidosis. N Engl J Med. 1985;312(18):1147–1151 [DOI] [PubMed] [Google Scholar]
- 11.Glaser NS, Wootton-Gorges SL, Marcin JP, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004;145(2):164–171 [DOI] [PubMed] [Google Scholar]
- 12.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217(2):331–345 [DOI] [PubMed] [Google Scholar]
- 13.Sener RN. Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph. 2001;25(4):299–326 [DOI] [PubMed] [Google Scholar]
- 14.Reilly PL, Simpson DA, Sprod R, Thomas L. Assessing the conscious level in infants and young children: a paediatric version of the Glasgow Coma Scale. Childs Nerv Syst. 1988;4(1):30–33 [DOI] [PubMed] [Google Scholar]
- 15.Edge JA, Jakes RW, Roy Y, et al. The UK case-control study of cerebral oedema complicating diabetic ketoacidosis in children. Diabetologia. 2006;49(9):2002–2009 [DOI] [PubMed] [Google Scholar]
- 16.Dunger D, Sperling M, Acerini C, et al. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004;113(2) Available at: www.pediatrics.org/cgi/content/full/113/2/e133 [DOI] [PubMed] [Google Scholar]
- 17.Wolfsdorf J, Glaser N, Sperling MA, American Diabetes Association . Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150–1159 [DOI] [PubMed] [Google Scholar]
- 18.Wolfsdorf J, Craig ME, Daneman D, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(suppl 12):118–133 [DOI] [PubMed] [Google Scholar]
- 19.Couch RM, Acott PD, Wong GW. Early onset fatal cerebral edema in diabetic ketoacidosis. Diabetes Care. 1991;14(1):78–79 [DOI] [PubMed] [Google Scholar]
- 20.Glasgow AM. Devastating cerebral edema in diabetic ketoacidosis before therapy. Diabetes Care. 1991;14(1):77–78 [DOI] [PubMed] [Google Scholar]
- 21.Righini A, Ramenghi L, Zirpoli S, Mosca F, Triulzi F. Brain apparent diffusion coefficient decrease during correction of severe hypernatremic dehydration. AJNR Am J Neuroradiol. 2005;26(7):1690–1694 [PMC free article] [PubMed] [Google Scholar]
- 22.Sevick EM, Jain RK. Effect of red blood cell rigidity on tumor blood flow: increase in viscous resistance during hyperglycemia. Cancer Res. 1991;51(10):2727–2730 [PubMed] [Google Scholar]
- 23.Lam TI, Anderson SE, Glaser N, O’Donnell ME. Bumetanide reduces cerebral edema formation in rats with diabetic ketoacidosis. Diabetes. 2005;54(2):510–516 [DOI] [PubMed] [Google Scholar]
- 24.Yuen N, Anderson SE, Glaser N, Tancredi DJ, O’Donnell ME. Cerebral blood flow and cerebral edema in rats with diabetic ketoacidosis. Diabetes. 2008;57(10):2588–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser N, Yuen N, Anderson SE, Tancredi DJ, O’Donnell ME. Cerebral metabolic alterations in rats with diabetic ketoacidosis: effects of treatment with insulin and intravenous fluids and effects of bumetanide. Diabetes. 2010;59(3):702–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine SR, Helpern JA, Welch KM, et al. Human focal cerebral ischemia: evaluation of brain pH and energy metabolism with P-31 NMR spectroscopy. Radiology. 1992;185(2):537–544 [DOI] [PubMed] [Google Scholar]
- 27.Levy RM, Berry I, Moseley ME, Weinstein PR. Combined magnetic resonance imaging and bihemispheric magnetic resonance spectroscopy in acute experimental focal cerebral ischemia. Acta Radiol Suppl. 1986;369:507–511 [PubMed] [Google Scholar]
- 28.Malisza KL, Kozlowski P, Peeling J. A review of in vivo 1H magnetic resonance spectroscopy of cerebral ischemia in rats. Biochem Cell Biol. 1998;76(2-3):487–496 [DOI] [PubMed] [Google Scholar]
- 29.Moseley ME, Cohen Y, Mintorovitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med. 1990;14(2):330–346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.