Abstract
OBJECTIVE:
Exercise-induced wheeze (EIW) may identify a distinct population among asthmatics and give insight into asthma morbidity etiology. The prevalence of pediatric asthma and associated urgent medical visits varies greatly by neighborhood in New York City and is highest in low-income neighborhoods. Although increased asthma severity might contribute to the disparities in urgent medical visits, when controlling for health insurance coverage, we previously observed no differences in clinical measures of severity between asthmatic children living in neighborhoods with lower (3%–9%) versus higher (11%–19%) asthma prevalence. Among these asthmatics, we hypothesized that EIW would be associated with urgent medical visits and a child’s neighborhood asthma prevalence.
METHODS:
Families of 7- to 8-year-old children were recruited into a case-control study of asthma through an employer-based health insurance provider. Among the asthmatics (n = 195), prevalence ratios (PRs) for EIW were estimated. Final models included children with valid measures of lung function, seroatopy, and waist circumference (n = 140).
RESULTS:
EIW was associated with urgent medical visits for asthma (PR, 2.29; P = .021), independent of frequent wheeze symptoms. In contrast to frequent wheeze, EIW was not associated with seroatopy or exhaled NO, suggesting a distinct mechanism. EIW prevalence among asthmatics increased with increasing neighborhood asthma prevalence (PR, 1.09; P = .012), after adjustment for race, ethnicity, maternal asthma, environmental tobacco smoke, household income, and neighborhood income.
CONCLUSIONS:
EIW may contribute to the disparities in urgent medical visits for asthma between high- and low-income neighborhoods. Physicians caring for asthmatics should consider EIW an indicator of risk for urgent medical visits.
KEY WORDS: asthma, allergy, exercise, emergency department, exercise-induced bronchoconstriction
What’s Known on This Subject:
The prevalence of asthma and associated urgent medical visits vary dramatically across neighborhoods in New York City. Some, but not all, children with asthma wheeze when they exercise.
What This Study Adds:
Exercise-induced wheeze was more common for asthmatic children living in neighborhoods with higher versus lower asthma prevalence. Because exercise-induced symptoms indicate a propensity for rapid-onset symptoms, this increased prevalence may contribute to the observed increase in urgent medical visits.
Patterns of asthma symptoms are important to understanding the etiology and treatment of the disease.1,2 Exercise-induced bronchoconstriction (EIB) and exercise-induced wheeze (EIW) are common among asthmatics, with estimates that 40% to 90% of asthmatics have symptoms with exercise.3 With EIB, drying and cooling of the mucosa after brief exercise cause rapid bronchoconstriction through release of inflammatory mediators including histamine and leukotrienes.4,5 Although different entities, EIB may cause the symptoms that occur with EIW for many asthmatics. Although EIW is often described as a phenotype of asthma,2,6,7 it may be simply a discrete clinical expression of asthma.8 EIW among mild asthmatics has been implicated in >40 deaths during sporting activities between 1993 and 2000.9 Therefore, EIW may represent a clinically relevant asthma phenotype defined by a rapid onset of symptoms.
In New York City, the range in pediatric asthma prevalence (3%–19%) among neighborhoods is striking.10 The frequency of associated urgent medical visits varies even more dramatically; emergency department (ED) visits for asthma are up to 20 times more common in lower than in higher socioeconomic status (SES) neighborhoods.10,11 Differences in asthma severity could explain these differences in urgent medical visits. However, after controlling for health insurance coverage among a middle-income population of 7- to 8-year-old asthmatics, we observed no difference in clinical indicators of asthma severity (lung function, exhaled nitric oxide [NO], and frequency of wheeze) between children living in higher versus lower asthma prevalence neighborhoods (LAPNs).12,13 Although neighborhood SES was associated with urgent medical visits,13 we considered the possibility that an underlying biological determinant of these differences might also be involved. An asthma phenotype associated with rapid-onset exacerbations, like EIW, might increase a child’s risk for an urgent medical visit for asthma. We hypothesized that EIW would be associated with urgent medical visits, independent of clinical indicators of asthma severity, and with the prevalence of asthma in a child’s neighborhood. To minimize heterogeneity in potential confounders such as access to health care and household income, we tested this hypothesis among the children described previously,12–14 a group of middle-income asthmatic children living throughout New York City neighborhoods.
Methods
The NYC Neighborhood Asthma and Allergy Study is a case-control study of children with and without asthma described previously.12–14 Parents of 7- to 8-year-old children were recruited through the Health Insurance Plan of New York, a provider used primarily by a middle-income population. Neighborhoods were selected based on United Hospital Fund (UHF) level (several zip codes) asthma prevalence among 5-year-olds as reported by the New York City Department of Health and Mental Hygiene.10 All LAPNs (3%–9%) and higher asthma prevalence neighborhoods (HAPNs; 11%–19%) in the Bronx, Brooklyn, Queens, and Manhattan were selected for recruitment by mail.
A caregiver completed a detailed questionnaire on the child’s health, the family’s demographics, and environmental tobacco smoke (ETS) exposure. Parents were questioned about how many hours per week their child exercised or played sports. During the home visit, spirometry, fractional exhaled NO (FeNO) testing, and waist circumference measurements were conducted, serum was collected for total and allergen specific immunoglobulin E (IgE), and settled dust and airborne particulate matter were collected (details, online supplement). Columbia University’s Institutional Review Board approved this study.
Asthma Case Definition, Exercise-Induced Wheeze, and Frequent Wheeze
Asthma cases were defined by questionnaire, including the modules from the International Study of Asthma and Allergy in Childhood (ISAAC). Children were classified as asthmatic if their caregiver reported at least 1 of the following for the child in the past 12 months: (1) wheeze, (2) being awakened at night by cough without having a cold, (3) wheeze with exercise, or (4) report of medication use for asthma. Children not meeting the asthma definition were excluded from the current analyses.
The following outcomes also were reported for the previous 12 months. Exercise-induced wheeze (EIW) was defined as an affirmative response to questions about (1) wheezing after running or active play and/or (2) child’s chest sounding wheezy during or after exercise. Frequent wheeze was defined as ≥4 episodes of wheeze. Urgent medical visits were defined as a positive response to questions about taking the child either to the doctor in a hurry or to the ED due to breathing difficulties.
Neighborhood Variables
Children’s home addresses were geocoded and linked to a geographic information system (GIS) demographic database described previously.12,15 A child’s local neighborhood was assigned the demographic characteristics of households in the 500-m radius surrounding the home. Additional built environment variables related to air pollution sources are described in the online supplement. Each child was assigned a neighborhood asthma prevalence based on the UHF level asthma prevalence described earlier.10
Statistical Analysis
FeNO and total IgE were log-normally distributed; we used natural log-transformed values in the analyses. GIS variables were not normally distributed and thus were compared by using nonparametric tests. Prevalence ratios (PRs) were calculated by using generalized estimating equation (GEE) models with UHF used as a cluster variable because the neighborhood asthma prevalence was available at the UHF level. Variables conventionally considered as potential confounders were included in the multivariable models including gender, race, Hispanic ethnicity, maternal asthma, ETS, neighborhood income, and material hardship.16 Sensitivity analyses of the association between neighborhood asthma prevalence and EIW are described in the online supplement. Data were analyzed in SPSS version 17 (SPSS, Inc, Chicago, IL) and visualized in R version 2.14.0.
Results
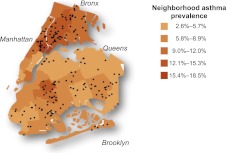
There were 195 children classified as asthmatic who had a blood sample, 95 and 100 in LAPN and HAPN, respectively (Fig 1). Among those, valid lung function and FeNO were available on 166 (eg, 85%) and 167 (86%) children, respectively. Waist circumference was not measured in the first 25 children and was thus available on 170 (87%) children. A complete set of data for multivariable models including lung function, household income, and waist circumference was available on 140 children.
FIGURE 1.
Map of New York City depicting study subjects’ places of residence overlaying neighborhood asthma prevalence. Neighborhood asthma prevalence was defined for 5-year-old school children as reported by the New York City Department of Health and Mental Hygiene.10 Participants were recruited from neighborhoods with lower (3%–9%) or higher (11%–18%) neighborhood asthma prevalence.
Asthmatics in the LAPN and HAPN had similar demographics, except for differences in Asian and mixed races, Hispanic ethnicity, report of seeing cockroaches or mice, and the presence of a smoker in their home (Table 1). As reported previously, there were no differences in clinical measures of asthma severity (lung function, FeNO, symptom frequency) or seroatopy (measureable IgE to at least 1 inhalant allergen) between asthmatics in the LAPN versus HAPN (Table 1).13 The local neighborhoods (500 m surrounding the homes) differed in the proportions of households reporting African-American race and Hispanic ethnicity and median household income.
TABLE 1.
Characteristics of the Study Participants and Their Neighborhood (n = 195)
| LAPN | HAPN | P | |
|---|---|---|---|
| Child’s demographics | |||
| Boy (%) | 59/95 (62.1) | 53/100 (53.0) | .20 |
| African-American race (%)a | 43/95 (45.3) | 55/100 (55.0) | .17 |
| Asian race (%)a | 16/95 (16.8) | 2/100 (2.0) | <.001 |
| White race (%)a | 17/95 (17.9) | 7/100 (7.0) | .021 |
| Other/mixed race (%)a | 15/95 (15.8) | 34/100 (34.0) | .003 |
| Hispanic ethnicity (%)b | 24/95 (25.3) | 45/100 (45.0) | .004 |
| Mother’s age (mean years) | 38.4 | 35.3 | <.001 |
| Maternal asthma (%) | 21/95 (22.1) | 27/100 (27.0) | .43 |
| Material hardship reported (%)c | 26/94 (27.4) | 29/100 (29.0) | .80 |
| Mother has college degree (%) | 37/94 (39.4) | 31/98 (31.6) | .26 |
| Household income (median)d | $50–60K | $40–45K | .077 |
| Smoker in the home (%) | 14/94 (14.9) | 26/99 (26.3) | .051 |
| Cockroach sightings not rare (%) | 19/95 (20.0) | 34/100 (34.0) | .028 |
| Mouse sightings not rare (%) | 6/95 (6.3) | 15/100 (15.0) | .051 |
| Clinical features | |||
| Frequent wheeze past 12 mo (%) | 18/95 (18.9) | 22/100 (22.0) | .60 |
| Controller medication past 12 mo (%) | 19/94 (20.2) | 31/100 (31.0) | .086 |
| Bronchodilator past 12 mo (%) | 52/94 (55.3) | 51/100 (51.0) | .55 |
| Seroatopy (%)e | 58/95 (61.1) | 61/100 (61.0) | .99 |
| Total IgE (IU/mL, GM [95% CI]) | 111 [85.2–146] | 98.9 [70.2–139] | .59 |
| FeNO (ppb, GM [95% CI])f | 10.4 [8.9–12.1] | 10.5 [9.0–12.4] | .92 |
| FEV1/FVC (median [25% to 75%])g | 0.88 | 0.87 | .60 |
| Waist circumference (cm, mean [95% CI])h | 63.1 [61–65] | 61.9 [60–63] | .32 |
| Neighborhood demographicsi | |||
| African-Americans, median, % | 28.8 | 45.9 | .040 |
| Hispanic, median, % | 14.1 | 42.8 | <.001 |
| Households below federal poverty income, median, % | 17.3 | 37.2 | <.001 |
| Median household income (median $ in 1000) | 40.4 | 21.3 | <.001 |
| Adult population with high school degree, median, % | 18.2 | 10.1 | <.001 |
95% CI, 95% confidence interval; GM, geometric mean.
Race was not reported for 4 (4.2%) and 2 (2.0%) of the children in the LAPN and HAPN, respectively.
Whether the child was of Hispanic ethnicity was not reported for 1 (1.1%) and 5 (5.0%) of the children in the LAPN and HAPN, respectively.
Mother reported that in the past 6 mo she and her family could not afford needed food, rent, clothing or medical care, or that gas/electricity was suspended because of bill nonpayment.30
There were 5 families in each group that did not report household income.
Seroatopy defined as serum IgE > 0.35 kIU to at least 1 of the tested allergens.
Missing data for FeNO on 17 (18%) and 15 (15%) children because of high ambient NO in the home at the time of testing.
Missing data for FEV1/FVC on 18 (19%) and 11 (11%) of children.
Missing data for waist circumference on 15 (16%) and 10 (10%).
Neighborhood variables are defined for each of the study participant based on 2000 US Census data for the households in the 500 m surrounding the participant’s home.
Wheeze, Urgent Medical Visits, and Medication Use
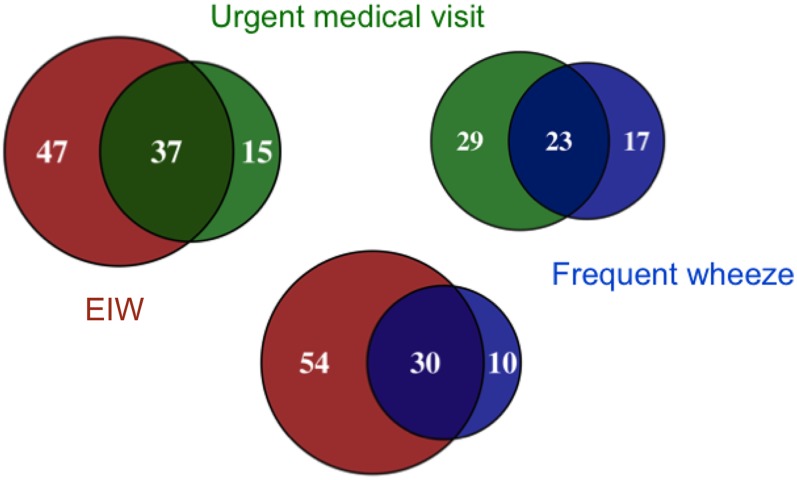
Among the children classified as asthmatic, 84/195 (43%) had a report of EIW, whereas frequent wheeze was less common (21%). Many but not all of the children with frequent wheeze also had EIW, whereas more than half of those children with EIW did not have frequent wheeze (Fig 2). Urgent medical visits for asthma in the past year were also common (26.7%) with 21.9% and 17.5% reporting taking their child to a doctor in a hurry and to the ED, respectively. In a multivariable model controlling for potential confounders, EIW (PR: 2.29 [1.13–4.63]; P = .021) and frequent wheeze (PR: 2.07 [1.12–3.84]; P = .020) were independently associated (same model) with urgent medical visits. In 2 similar models, EIW was associated with taking their child to the doctor in a hurry (PR: 2.62 [1.19–5.75]; P = .016) and borderline associated with ED visits for asthma (PR: 2.31 [0.95–5.63]; P = .066). Many asthmatics with EIW had used controller medications in the past year (42%) and a majority, but not all, had used short-acting bronchodilators (65%) in the past year. The association between EIW and urgent medical visits remained statistically significant (PR: 2.27 [1.11–4.63]; P = .024) after adjustment for use of preventative medications (1%, 16.5%, and 14.4% had taken long-acting β antagonist, inhaled or oral corticosteroids and leukotriene modifiers in the past year, respectively).
FIGURE 2.
Venn diagrams depicting overlap in frequent wheeze, EIW, and urgent medical visits for asthma. There were 89/195 asthmatics who had no report of EIW, frequent wheeze, or urgent medical visits and thus not depicted in this figure.
Comparing Clinical Features of EIW and Frequent Wheeze
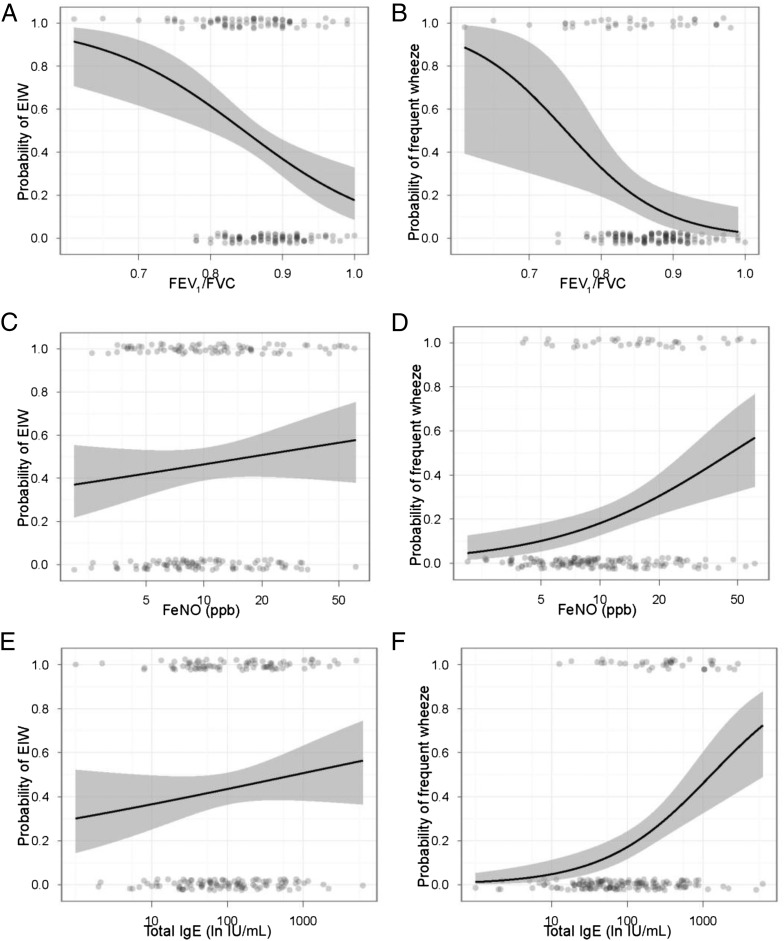
To validate EIW as distinct from frequent wheeze, we examined the clinical features of these phenomenon, first in bivariate analyses (Fig 3) and then in adjusted models. In multivariable analyses with individual models for each clinical variable, after controlling for gender, race, and Hispanic ethnicity, EIW was associated with forced expiratory volume in 1 second/forced vital capacity FEV1/FVC (as percent) (PR: 0.95 [0.92–0.99]; P = .010), but not with FeNO (PR: 1.21 [0.89–1.65]; P = .25) or total IgE (PR: 1.06 [0.92–1.22]; P = .43). In similar models, frequent wheeze was associated with FEV1/FVC (PR: 0.94 [0.89–0.99]; P = .025), FeNO (PR: 2.21 [1.40–3.49]; P = .001), and total IgE (PR: 1.61 [1.29–2.00]; P < .001). Seroatopy was associated with frequent wheeze (PR: 3.55 [1.48–8.54]; P = .005) but not EIW (PR: 1.12 [0.76–1.92]; P = .41).
FIGURE 3.
Logistic regression lines for univariate analyses are depicted in black with 95% confidence intervals in gray. P values are for the univariate logistic regressions. A, EIW with FEV1/FVC (P < .001); B, frequent wheeze with FEV1/FVC (P = .018); C, EIW with FeNO (P = .21); D, frequent wheeze with FeNO (P < .001); E, EIW with total IgE (P = .22); F, frequent wheeze with total IgE (P = .001). Probability of EIW (A, C, D) and frequent wheeze (B, E, F) with FEV1/FVC (A and B), FeNO (C and D), and total IgE (E and F).
Neighborhood Asthma Prevalence and EIW
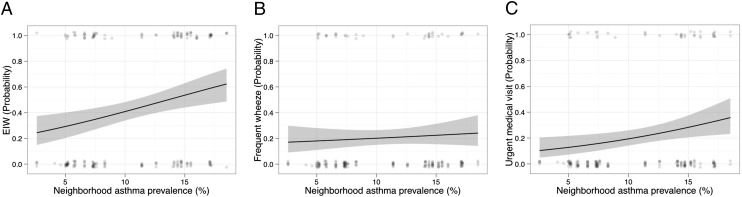
The prevalence of EIW increased with increasing neighborhood asthma prevalence in bivariate analyses (Fig 4A) and after adjustment for potential confounders (PR: 1.06 [1.003–1.13]; P = .040) including local neighborhood income. The prevalence of frequent wheeze was not associated with neighborhood asthma prevalence in bivariate analyses (Fig 4B) or in the multivariable model (PR: 0.93 [0.83–1.04]; P = .19). As we have described previously,13 the prevalence of urgent medical visits modestly increased with increasing asthma prevalence, although this was not statistically significant (Fig 4C). In the multivariable model, local neighborhood income (PR: 0.96 [0.93–0.998]; P = .039) and not neighborhood asthma prevalence (PR: 0.99 [0.90–1.09]; P = .84]) was associated with urgent visits and a similar association was observed with ED visits as the outcome (data not shown).
FIGURE 4.
Logistic regression lines for the univariate analyses in black with 95% confidence in gray. P values are for the univariate logistic regression. A, EIW (P = .011); B, frequent wheeze (P = .054); C, urgent medical visit (P = .098).
Stratification by Seroatopy, Sensitivity Analyses, and Report of Exercise
In multivariable models, neither lung function nor neighborhood asthma prevalence was associated with prevalence of EIW among the nonseroatopic asthmatics, although waist circumference trended toward a positive but not statistically significant association (Table 2). Among seroatopic children, both lung function and neighborhood asthma prevalence were independently associated with EIW. The association between neighborhood asthma prevalence and EIW was not altered (<10% change in PR) by stepwise inclusion of previously reported measures and estimations of allergens and combustion byproducts in or around the child’s homes (Supplemental Table 3). Median reported time spent exercising or playing sports did not differ significantly (P > .05) between children in HAPN versus LAPN or between children with or without EIW or urgent visits (all medians 4–5 hours per week). Also, the frequency of children with limited exercise or sports (<1 hour per week) did not differ by neighborhood or report of symptoms (data not shown).
TABLE 2.
Multivariable PRs for EIW
| Overalla (n = 140)b | Nonatopic (n = 52) | Atopic (n = 88) | |
|---|---|---|---|
| FEV1/FVC | 0.96 (0.92–0.997); P = .037 | 1.02 (0.92–1.13); P = .75 | 0.94 (0.89–0.99); P = .022 |
| Abdominal circumference | 1.01 (0.98–1.04); P = .40 | 1.05 (0.99–1.12); P = .11 | 1.01 (0.97–1.04); P = .67 |
| Neighborhood asthma prevalence | 1.09 (1.02–1.16); P = .012 | 1.09 (0.93–1.28); P = .27 | 1.09 (1.01–1.17); P = .024 |
PRs calculated by using a GEE model with community district used as a cluster variable, adjusted for gender, race, Hispanic ethnicity, maternal asthma, ETS, neighborhood income, and material hardship.
Sample size restricted based on complete information from cases.
Discussion
Among middle-income asthmatic children with the same health insurance provider, the proportion of asthmatics with a report of EIW increased with increasing asthma prevalence in the child’s neighborhood. These findings were robust in models adjusting for potential confounders and indicators of asthma severity. EIW and frequent wheeze symptoms were independently associated with urgent medical visits. These findings are intriguing because (1) EIW is an indicator of rapid-onset exacerbation, (2) there is an excess of urgent medical visits among asthmatics living in neighborhoods with higher asthma prevalence, and (3) urgent medical visits could be triggered by any rapid-onset exacerbation.
These results build on our previously observed associations between neighborhood income and urgent medical visits in this study population that reinforced the established dogma that urgent medical visits (eg, to the ED) for asthma in lower-income neighborhoods have socio-demographic determinants.13 Our findings also support a novel hypothesis: higher prevalence of an asthma phenotype, defined by rapid-onset symptoms, could provide an underlying biological mechanism for the higher frequency of urgent medical visits in HAPNs.
The authors of other studies have examined the associations of specific triggers with slow- versus rapid-onset asthma exacerbations. Although viral infections are thought to trigger a majority of asthma exacerbations in children,17 upper respiratory infections have been associated with slower onset exacerbations.18 In contrast, allergen exposure and exercise have been associated with more rapid onset.19 An explanation for the rapid onset of symptoms with EIW lies in the mechanism of airway hyperresponsiveness, with release of bronchoconstrictive mediators from inflammatory cells upon rewarming and rehydration of the airway after exercise.5 A previous study in children demonstrated that an exercise challenge can increase subsequent airway hyperresponsiveness to allergens.20 Although we did not determine whether exercise was the trigger for the exacerbations leading to the urgent medical visits, our findings combined with those revealing susceptibility to allergen challenge after exercise20 suggest that a report of EIW may be an indicator of a rapid-onset phenotype that could be aggravated by other extrinsic exposures.
It is likely that the EIW phenotype and socio-demographic determinants could act together and lead to urgent medical visits. Repeat ED visits for asthma have been associated with families lacking confidence in the efficacy of asthma medications and not using criteria in deciding to seek emergency care.21,22 The proportion of children with EIW in this study who had taken controller medications in the past year (41%) may be consistent with current National Heart, Lung, and Blood Institute guidelines, or even high, given their relatively mild disease.6 However, one-third of children with an EIW episode in the past year had not used a bronchodilator, which is not in keeping with current guidelines. A rapid-onset exacerbation combined with a parent incorrectly treating their child’s symptoms could result in an urgent medical visit. Collectively, these findings suggest that physicians treating even mild asthmatics should consider EIW as a risk for urgent medical visits and provide appropriate education and medication.
The overall goal of the New York City Neighborhood Asthma and Allergy Study is to better understand neighborhood disparities in asthma prevalence and morbidity. Allergens and combustion byproducts are 2 types of exposures that we and others have hypothesized contribute to these disparities.23 In support of these hypotheses, we demonstrated the relevance of both cockroach allergen and airborne black carbon (BC) to allergy and airway inflammation among the children in this cohort.12,14 Other studies have revealed that exercise can modify the effect of air pollutants, including BC, on asthma outcomes.24,25 We examined whether allergen or combustion byproduct exposures partially explained the association between neighborhood and EIW (Supplemental Table 3). Despite testing a comprehensive set of both measured and modeled estimations of exposures, we were unable to show any associations with EIW or that exposures substantially diminished the association between neighborhood asthma prevalence and EIW. Future studies into the cause of the higher prevalence of EIW in HAPNs (eg, environment, stress) could lead to a better understanding of the etiology of urban asthma.
Despite overlap, EIW appeared to represent a phenotype with clinical outcomes that differed from those of frequent wheeze. Poorer lung function was associated with both outcomes, but FeNO and total IgE concentrations, traditional biomarkers of inflammation and atopy, although associated with frequent wheeze, were not associated with EIW. In our study, FeNO and total IgE were higher among asthma cases in general12 and among those cases with EIW (data not shown), than among controls, as others have observed.26 However, unlike frequent wheeze, FeNO and total IgE did not differ among asthmatics with and without EIW.
We did observe modest differences in risk patterns for EIW among children with and without seroatopy. Among the nonseroatopic asthmatics, there was an (statistically nonsignificant) association between EIW and waist circumference but no association with lung function. This lack of association among this group suggests that deconditioning may explain some of the dyspnea in the nonseroatopic asthmatic children, not EIW, which has been reported previously to be associated with obesity.27 But a recent study demonstrating associations between EIW and leptin and adiponectin in serum also lends support to the idea that EIW may have multiple etiologies.28 Neighborhood asthma prevalence was only a statistically significant predictor of EIW among the seroatopic asthmatics; however, whether the risk conferred by living in neighborhoods is really modified by seroatopy or if the relatively small sample size among the nonseroatopics led to type-2 error remains to be determined.
This study has several limitations. First, because the recruitment method was designed to reduce heterogeneity in health care access and household income, our participants are not fully representative of their neighborhoods. However, the health plan through which they were recruited has observed neighborhood differences in physician-diagnosed asthma among its members that are consistent geographically with those observed by the New York City Department of Health. Therefore, these children should be representative of middle-income children with employer-based health insurance. Second, we did not measure EIB. Although an objective measure of bronchoconstriction would have strengthened the findings, the ISAAC question assessing EIW has been shown to be predictive of EIB among 7-year-olds.27,29 Also, although our question about EIW came from the internationally validated ISAAC questionnaire to allow for comparison with the many other studies that have employed this questionnaire, we did not ask about other symptoms of bronchial hyperreactivity after exercise (eg, cough, chest tightness) that could manifest in the absence of wheeze. Therefore, we may have underestimated the prevalence of exercise-associated asthma symptoms among these children. Third, the cross-sectional design of the study did not allow us to evaluate the temporality of medication usage and urgent medical visits or the impact of previous controller medication use on EIW prevalence. Fourth, this population-based study included primarily asthmatics with only a few exacerbations in the previous year, preventing generalizability to asthmatic populations with more severe asthma. Finally, neighborhood differences in access to physical activity or limitation in physical activity because of previous exacerbations could have confounded our findings. However, we did not observe differences in the report of exercise by neighborhood or EIW symptoms, and the density of parks around the child’s home was not associated with EIW.
Conclusions
In a relatively homogenous, middle-income pediatric asthmatic population, EIW was more commonly reported for children living in New York City neighborhoods with higher versus lower asthma prevalence. EIW was significantly associated with urgent medical visits among these children, independent of measures of asthma severity or neighborhood income. Because exercise-induced symptoms indicate a propensity for rapid-onset exacerbation, the increased prevalence of EIW may be contributing to the observed increase in urgent medical visits. Therefore, the presence of EIW may be useful to pediatric practitioners to identify children at risk for urgent medical visits. Practitioners then may apply appropriate interventions, such as a primary care asthma evaluation, comprehensive asthma education, and medical treatment (eg, bronchodilators and leukotriene modifiers used before exercise, and/or other medications to improve asthma control).
Supplementary Material
Acknowledgments
This project would not have been possible without our collaborators at the Health Insurance Plan of New York; Beatriz Jaramillo, DrPH, was instrumental in the design and initial implementation of the study, and Michael Byrne, MA, has ensured the continued success of the recruitment for the study. We thank the New York City Neighborhood Asthma and Allergy Study field team for their hard work. We thank the families who have participated in the study.
Glossary
- BC
black carbon
- ED
emergency department
- EIB
exercise-induced bronchoconstriction
- EIW
exercise-induced wheeze
- ETS
environmental tobacco smoke
- FeNO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GEE
generalized estimating equation
- GIS
geographic information system
- HAPN
higher asthma prevalence neighborhood
- IgE
immunoglobulin E
- ISAAC
International Study of Asthma and Allergy in Childhood
- LAPN
lower asthma prevalence neighborhood
- NO
nitric oxide
- PR
prevalence ratio
- SES
socioeconomic status
- UHF
United Hospital Fund
Footnotes
Dr Mainardi contributed to the acquisition, analyses and interpretation of data, and was involved in drafting, revising, and final approval of the article; Dr Mellins contributed to the conception and design of the study, analyses and interpretation of data, and was involved in revising and final approval of the article; Dr Miller contributed to the conception and design of the study, analyses and interpretation of data, and was involved in revising and final approval of the article; Dr Acosta contributed to the acquisition, analyses and interpretation of data, and was involved in drafting, revising, and final approval of the article; Dr Cornell contributed to the interpretation of data and was involved in revising and final approval of the article; Dr Hoepner contributed to the acquisition, analyses and interpretation of data, and was involved in revising and final approval of the article; Dr Perera contributed to the conception and design of the study and was involved in revising and final approval of the article; Mr Quinn contributed to the acquisition, analyses and interpretation of data, and was involved in revising and final approval of the article; Dr Yan contributed to the acquisition, analyses and interpretation of data, and was involved in revising and final approval of the article; Dr Chillrud contributed to the conception and design of the study and was involved in revising and final approval of the article; Mr Olmedo contributed to the acquisition of data and was involved in revising and final approval of the article; Dr Goldstein contributed to the conception and design of the study, analyses and interpretation of data, and was involved in revising and final approval of the article; Dr Rundle contributed to the conception and design of the study, analyses and interpretation of data, and was involved in revising and final approval of the article; Dr Jacobson contributed to the conception and design of the study, analyses and interpretation of data, and was involved in revising and final approval of the article; and Dr Perzanowski contributed to the conception and design of the study, analyses and interpretation of data, and was involved in drafting, revising, and final approval of the article.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institute of Environmental Health Sciences (grant numbers R01 ES014400 and P30 ES09089), Housing and Urban Development Healthy Homes and Lead Technical Study (HUD NYHHU0003-11). Funded by the National Institutes of Health (NIH).
References
- 1.Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109(suppl 2):362–367 [PubMed] [Google Scholar]
- 2.Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–360 [DOI] [PubMed] [Google Scholar]
- 3.McFadden ER, Jr, Gilbert IA. Exercise-induced asthma. N Engl J Med. 1994;330(19):1362–1367 [DOI] [PubMed] [Google Scholar]
- 4.Hallstrand TS, Henderson WR, Jr. Role of leukotrienes in exercise-induced bronchoconstriction. Curr Allergy Asthma Rep. 2009;9(1):18–25 [DOI] [PubMed] [Google Scholar]
- 5.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol. 2008;122(2):225–235, quiz 236–237 [DOI] [PubMed] [Google Scholar]
- 6.National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007:363–372 [Google Scholar]
- 7.Handoyo S, Rosenwasser LJ. Asthma phenotypes. Curr Allergy Asthma Rep. 2009;9(6):439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–813 [DOI] [PubMed] [Google Scholar]
- 9.Becker JM, Rogers J, Rossini G, Mirchandani H, D’Alonzo GE, Jr. Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol. 2004;113(2):264–267 [DOI] [PubMed] [Google Scholar]
- 10.Garg R, Karpati A, Leighton J, Perrin M, Shah M. Asthma Facts, 2nd ed. New York, NY: New York City Department of Health and Mental Hygiene; 2003 [Google Scholar]
- 11.NYC Government. 2005–2008 asthma emergency department (ED) visits tables and figures. Available at: www.nyc.gov/html/doh/downloads/pdf/asthma/asthma-emergency-dpt.pdf. Accessed March 21, 2012
- 12.Cornell AG, Chillrud SN, Mellins RB, et al. Domestic airborne black carbon and exhaled nitric oxide in children in NYC. J Expo Sci Environ Epidemiol. 2012;22(3):258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta LM, Miller RL, Goldstein IF, Rundle AG, Mellins RB, Cornell AG, Hoepner L, Andrews L, Lopez-Pintado S, Quinn JW, Chew GL, Jacobson JS, Perera FP, Perzanowski MS. Risks for asthma at age 7 differ by neighborhood and socio-economic status in New York City. J Allergy Clin Immunol. 2011;127(2):AB174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olmedo O, Goldstein IF, Acosta L, et al. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J Allergy Clin Immunol. 2011;128(2):284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 2009;31:7–20 [DOI] [PubMed] [Google Scholar]
- 16.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235 [DOI] [PubMed] [Google Scholar]
- 17.Heymann PW, Platts-Mills TA, Johnston SL. Role of viral infections, atopy and antiviral immunity in the etiology of wheezing exacerbations among children and young adults. Pediatr Infect Dis J. 2005;24(11):S217–S222, discussion S20–S21 [DOI] [PubMed] [Google Scholar]
- 18.Plaza V, Serrano J, Picado C, Sanchis J, High Risk Asthma Research Group . Frequency and clinical characteristics of rapid-onset fatal and near-fatal asthma. Eur Respir J. 2002;19(5):846–852 [DOI] [PubMed] [Google Scholar]
- 19.Barr RG, Woodruff PG, Clark S, Camargo CA, Jr. Sudden-onset asthma exacerbations: clinical features, response to therapy, and 2-week follow-up. Multicenter Airway Research Collaboration (MARC) investigators. Eur Respir J. 2000;15(2):266–273 [DOI] [PubMed] [Google Scholar]
- 20.Koh YY, Lim HS, Min KU. Airway responsiveness to allergen is increased 24 hours after exercise challenge. J Allergy Clin Immunol. 1994;94(3 pt 1):507–516 [DOI] [PubMed] [Google Scholar]
- 21.Wasilewski Y, Clark NM, Evans D, Levison MJ, Levin B, Mellins RB. Factors associated with emergency department visits by children with asthma: implications for health education. Am J Public Health. 1996;86(10):1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conn KM, Halterman JS, Fisher SG, Yoos HL, Chin NP, Szilagyi PG. Parental beliefs about medications and medication adherence among urban children with asthma. Ambul Pediatr. 2005;5(5):306–310 [DOI] [PubMed] [Google Scholar]
- 23.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125(3):540–544 [DOI] [PubMed] [Google Scholar]
- 24.McConnell R, Berhane K, Gilliland F, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359(9304):386–391 [DOI] [PubMed] [Google Scholar]
- 25.Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol. 2005;115(2):213–219, quiz 220 [DOI] [PubMed] [Google Scholar]
- 26.ElHalawani SM, Ly NT, Mahon RT, Amundson DE. Exhaled nitric oxide as a predictor of exercise-induced bronchoconstriction. Chest. 2003;124(2):639–643 [DOI] [PubMed] [Google Scholar]
- 27.Weiler JM, Anderson SD, Randolph C, et al. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology . Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010;105(suppl 6):S1–S47 [DOI] [PubMed] [Google Scholar]
- 28.Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011;107(1):14–21 [DOI] [PubMed] [Google Scholar]
- 29.Ponsonby AL, Couper D, Dwyer T, Carmichael A, Wood-Baker R. Exercise-induced bronchial hyperresponsiveness and parental ISAAC questionnaire responses. Eur Respir J. 1996;9(7):1356–1362 [DOI] [PubMed] [Google Scholar]
- 30.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26(3):373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perzanowski MS, Rosa MJ, Divjan A, Johnson A, Jacobson JS, Miller RL. Modifications improve an offline exhaled nitric oxide collection device for use with young children. J Allergy Clin Immunol. 2008;122(1):213–, author reply 214. [DOI] [PubMed] [Google Scholar]
- 32.Yan B, Kennedy D, Miller RL, et al. Validating a nondestructive optical method for apportioning colored particulate matter into black carbon and additional components. Atmos Environ. 2011;45(39):7478–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NYC Department of Health and Mental Hygiene. The New York City Community Air Survey. Results from Year One Monitoring 2008–2009. New York, NY: NYC Department of Health and Mental Hygiene; 2011
- 34.NYC Department of Health and Mental Hygiene. New York City Community Air Survey. Available at: www.nyc.gov/html/doh/html/eode/nyccas.shtml. Accessed February 25, 2011
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.