Abstract
OBJECTIVE:
To examine temporal trends of adverse drug reactions (ADRs) associated with trimethoprim-sulfamethoxazole (TMP-SMX) use in children.
METHODS:
We performed a retrospective observational study to characterize TMP-SMX ADRs in children between 2000 and 2009. We completed a chart review at our institution by identifying children diagnosed with TMP-SMX ADRs. To compare local trends to comparable institutions, we estimated the frequency of hospitalizations for TMP-SMX ADRs at 25 tertiary pediatric hospitals utilizing the Pediatric Health Information System database. To determine whether changes in outpatient prescribing rates occurred, we used the National Ambulatory Medical Care Survey/National Hospital Ambulatory Medical Care Survey.
RESULTS:
At our institution, 109 children were diagnosed with a TMP-SMX ADR (5 cases from 2000 to 2004 as compared with 104 cases from 2005 to 2009). Fifty-eight percent had been treated for a skin and soft tissue infection (SSTI). A similar trend was observed nationally, where the incidence of TMP-SMX ADRs more than doubled from 2004 to 2009 at comparable pediatric hospitals (P < .001). Although national outpatient data revealed no change in overall TMP-SMX prescribing, the percentage of children prescribed TMP-SMX for SSTI sharply increased during the study period (0%–2% [2000-2004]; 9%–17% [2005–2009]).
CONCLUSIONS:
The majority of TMP-SMX ADRs at our institution occurred in conjunction with SSTI treatment. TMP-SMX ADRs have occurred more frequently coincident with increased prescribing for SSTI. Increased usage alone may explain the increasing trend of TMP-SMX ADRs in children; however drug–disease interaction may play a role and requires further investigation.
KEY WORDS: adverse drug reaction, trimethoprim-sulfamethoxazole, skin and soft tissue infections, pediatrics, physician practice patterns
What’s Known on This Subject:
Antimicrobials are a medication class frequently implicated in pediatric adverse drug reactions (ADRs). Trimethoprim-sulfamethoxazole (TMP-SMX) is long recognized as a contributor to the burden of these undesired and unpredictable events.
What This Study Adds:
TMP-SMX ADRs increased from 2000 to 2009, with the majority of children taking the antibiotic for skin and soft tissue infections. The significant increase in TMP-SMX prescribing for these infections may result in a continued increase of associated ADRs.
Adverse drug reactions (ADRs) represent a significant pediatric medical issue. A meta-analysis examining studies of pediatric ADRs demonstrated 9.5% of hospitalized children experience an ADR.1 Over half a million children in the United States seek outpatient medical care annually due to ADRs; more than half of these children are 0 to 4 years old, and antimicrobial agents are the most frequently implicated drug class for inflicting an undesired effect.2 An estimated 2% of all pediatric hospital admissions have been attributed to ADRs.3
Since approval in the United States in the early 1970s, trimethoprim-sulfamethoxazole (TMP-SMX) has long been associated with a variety of ADRs. Sulfonamides are one of the most frequently encountered antimicrobial agents resulting in ED visits secondary to undesired reactions.4 Although cutaneous reactions and gastrointestinal intolerance are the most commonly reported undesired effects, cytopenias and more severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis are associated with its use.5,6 A significantly higher incidence of TMP-SMX ADRs has been described in patients with HIV with up to 40% of HIV-infected children developing undesired drug reactions.7
The majority of published data concerning TMP-SMX ADRs in children are associated with usage of TMP-SMX for urinary tract infections (UTIs), otitis media, gastroenteritis, or the prevention/treatment of Pneumocystis jiroveci in children with HIV/AIDS.7–10 With a well-recognized increase of methicillin-resistant Staphylococcus aureus (MRSA) associated skin and soft tissue infections (SSTIs) over the past decade and relatively few oral antimicrobial therapeutic options,11–14 we hypothesized that the treatment of these SSTIs would be accompanied by an increase in TMP-SMX ADRs. Therefore, we sought to address 2 objectives. Our primary objective was to examine temporal trends in medical care sought for TMP-SMX ADRs at our institution. A secondary objective was to examine trends in TMP-SMX nationally and to evaluate prescribing patterns for TMP-SMX in ambulatory care, particularly in the context of the MRSA epidemic.
Methods
Study Design and Setting
A retrospective observational study was performed to characterize clinical and epidemiologic characteristics of patients seeking medical care for TMP-SMX ADRs. To address the primary objective of TMP-SMX ADRs at Children’s Mercy Hospital (CMH), we conducted a chart review of children identified as having a TMP-SMX ADR to assess clinical and laboratory features associated with the diagnosis. CMH is a 317-bed, tertiary care, free-standing children’s hospital in Kansas City, MO, that serves a 5-state, 100-county region with ∼15 000 admissions yearly. For the secondary objective, we used administrative data to retrospectively examine pediatric hospitalizations for TMP-SMX ADRs and prescribing trends for TMP-SMX in ambulatory care in the United States. The study protocol was approved by the CMH’s institutional review board.
Data Sources
TMP-SMX ADRs at CMH
We analyzed the health records of all children who presented to the ED or were admitted to CMH, between January 1, 2000, and December 31, 2009, with the diagnosis of a TMP-SMX ADR. Patients were identified on the basis of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) E codes including E931.9 (anti-infectives causing adverse effects in therapeutic use) and E931.0 (sulfonamides causing adverse effects in therapeutic use). ICD-9-CM codes were used because local ADR hospital surveillance data were not available during this time period and voluntary reporting of ADRs by the clinician to a national system (ie, the Food and Drug Administration’s MedWatch program) can be variable.15 A patient was included in the study if, in addition to the ICD-9-CM codes, there was supporting documentation by the care provider confirming the ADR and that the patient had been exposed to TMP-SMX within 2 weeks of developing the ADR. To enhance the specificity of the diagnosis, those patients with no documented history of TMP-SMX exposure were excluded from the study. Data were extracted manually from hospital charts by using a standardized data collection form. Medical records were reviewed to identify the following: setting (ED visit or inpatient admission), indication for TMP-SMX (UTI, SSTI, respiratory tract infection, Pneumocystis jiroveci, enteritis, other), age at presentation, race, clinical and laboratory history including dermatologic (rash), mucus membrane involvement (conjunctiva/mouth/genitalia), neurologic (fever, confusion), endocrine (hyperkalemia), gastrointestinal (nausea, vomiting, diarrhea), hematologic (thrombocytopenia, leucopenia, anemia), hepatitis, renal dysfunction (serum creatinine), angioedema/serum sickness, severe life-threatening reactions (Stevens-Johnson syndrome, toxic epidermal necrolysis, aplastic anemia, hepatic necrosis), laboratory testing confirming acute viral infection, and concurrent medications.
TMP-SMX ADRs at Tertiary Children’s Hospitals
We used the Pediatric Health Information System (PHIS) database, which is an administrative database maintained by the Children’s Hospital Association (CHA; formerly the Child Health Corporation of America) in association with over 40 member tertiary care, free-standing children’s hospitals across the United States. Member hospitals contribute patient level inpatient, ED, and outpatient data to a central repository maintained by Thompson Healthcare (CHA’s contracted data manager). Rigorous data quality and reliability measures exist to ensure the integrity of the stored data. Only hospitals with complete data during the selected study period were included in this analysis; 25 hospitals met this criterion. We examined the annual incidence of hospitalizations that included diagnosis of an ADR due to TMP-SMX. We identified hospitalizations with TMP-SMX ADRs based on the presence of a discharge diagnosis with the ICD-9-CM code for sulfonamides causing adverse effects in therapeutic use (E931.0). The study population included patients ≤18 years at CHA hospitals. We included data from the 25 hospitals with complete billing data for ED visits and hospital discharges from January 1, 2004, to December 31, 2009.
National Trends in Outpatient TMP-SMX Prescriptions
To evaluate national trends in TMP-SMX use for children in ambulatory care settings, we analyzed data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) and the National Ambulatory Medical Care Survey (NAMCS) from 2000 to 2009. The National Center for Health Statistics administers these surveys annually to collect data from a nationally representative sample of ambulatory visits to nonfederally funded, office-based physicians (NAMCS), EDs (NHAMCS), and hospital outpatient departments (NHAMCS). For each visit sampled in the NAMCS or NHAMCS, the National Center for Health Statistics provides a weight equal to the inverse probability of that visit being sampled. These visit weights allow for the generation of nationally representative estimates by using data collected in the NAMCS and the NHAMCS.
In addition to examining TMP-SMX prescribing in all visits by children younger than 18 years old, we separately considered visits that were specifically for SSTI. Visits were characterized as for SSTI if any of 3 diagnosis fields contained an ICD-9-CM code consistent with skin or soft tissue infection (680–686, 035, 110–111, 704.8, 728.0, 611/771.5, 728.86). Among these visits, we determined the annual percentage in which TMP-SMX was prescribed. TMP-SMX was identified by using the Multum Lexicon drug code d00124 (sulfamethoxazole-trimethoprim).
Statistical Analysis
Descriptive statistics were constructed by using frequencies and proportions for categorical data elements and means for continuous variables. The Mann-Whitney U test, Pearson’s χ2 test, and Fisher’s exact test were used to examine differences in clinical characteristics and laboratory findings between Children’s Mercy TMP-SMX ADR patients seen in the ED and inpatient setting. Temporal trends for the incidence of TMP-SMX ADRs at children’s hospitals were evaluated by using a Mantel-Haenszel χ2 test, and trends in the frequency of national outpatient TMP-SMX prescriptions were examined by using logistic regression with year as a predictor variable. The incidence of TMP-SMX ADRs from the PHIS database was normalized to cases per 100 000 admissions to account for varying levels of patient loads from year to year.
Results
TMP-SMX ADRs at CMH
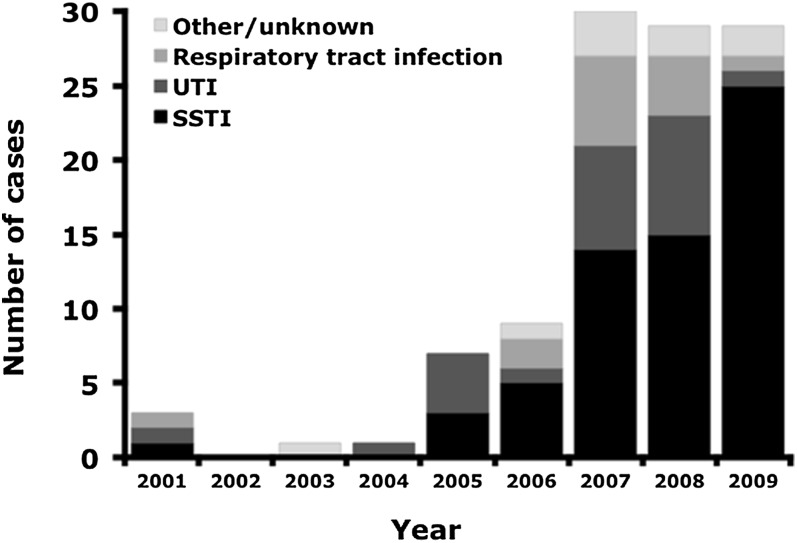
Of the 145 ADR cases identified by ICD-9-CM codes at our institution, 109 (75%) had a history confirmed by chart review of TMP-SMX exposure and symptoms attributed to TMP-SMX ADRs by a clinician. Of the 36 excluded, 34 had no discernable TMP-SMX exposure or had no documented ADR and 2 had history of exposure to a non-TMP-SMX sulfa containing medication. All but 1 of the subjects was identified by the ICD-9-CM code E931.0 (sulfonamides causing adverse effects in therapeutic use). All patients who were evaluated both in our hospital and the ED had been prescribed TMP-SMX before evaluation at our institution, consistent with outpatient use. From 2000 to 2004, only 5 TMP-SMX ADRs were identified as compared with 104 cases from 2005 to 2009. Fifty-eight percent (63/109) of patients with a documented TMP-SMX ADR had been treated for SSTI followed by 21% (23/109) for UTI (Fig 1). Of the patients with TMP-SMX ADRs, 37% (40/109) were hospitalized. Hospitalized patients more frequently had mucous membrane involvement, documented fever, vomiting, or diarrhea (P < .01) as compared with patients evaluated in the ED (Table 1).
FIGURE 1.
Diagnosis for which TMP-SMX was prescribed in patients with subsequent TMP-SMX ADRs at our institution.
TABLE 1.
Clinical and Laboratory Findings in Children With TMP-SMX ADRs at Our Institution
| ED (N = 69) | Hospitalized (N = 40) | P | |
|---|---|---|---|
| Age, y, mean | 8.36 | 9.41 | |
| Boy, n (%) | 21 (30) | 19 (48) | .08 |
| Clinical signs, n (%) | |||
| Rash | 66 (96) | 37 (93) | .49 |
| Mucous membrane involvement | 5 (7) | 19 (48) | <.01 |
| Documented fever | 4 (6) | 23 (58) | <.01 |
| Mental status changes | 0 (0) | 2 (5) | .13 |
| Vomiting | 4 (6) | 12 (30) | <.01 |
| Diarrhea | 0 (0) | 6 (15) | <.01 |
| Laboratory findings, n (%) | |||
| White blood cell count <4.5 | 4/7 (57) | 11/38 (29) | .20 |
| Hemoglobin level <10.5 | 0/7 (0) | 10/38 (26) | .32 |
| Platelet count <150 | 1/7 (14) | 11/38 (29) | .66 |
| Alanine aminotransferase >100 | 0/5 (0) | 9/31 (29) | .30 |
Trends in the Incidence of TMP-SMX ADRs at Children’s Hospitals
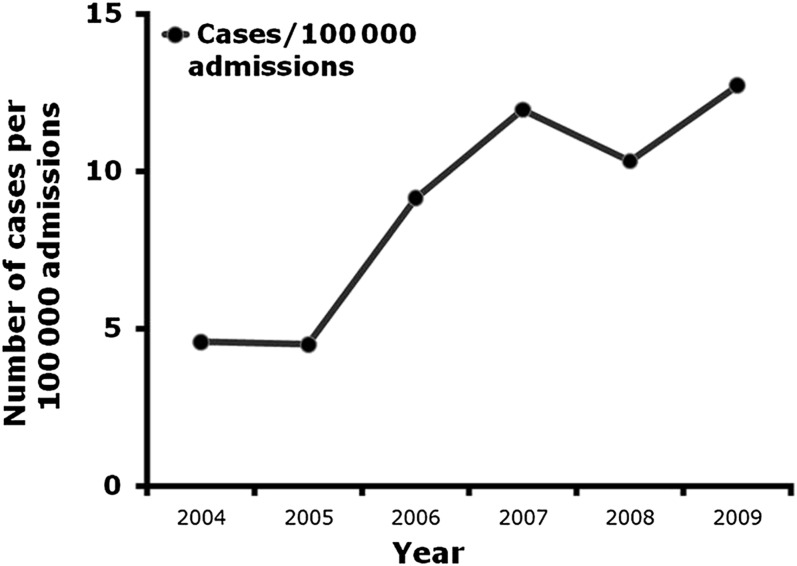
From 2004 to 2009, a total of 943 TMP-SMX ADR cases occurred. The incidence of TMP-SMX ADRs more than doubled in this 5-year period, increasing from 5 cases/100 000 admissions to 13 cases/100 000 admissions (P < .001; Fig 2).
FIGURE 2.
Number of cases of TMP-SMX ADRs in hospitalized children.
National Trends in TMP-SMX Prescriptions
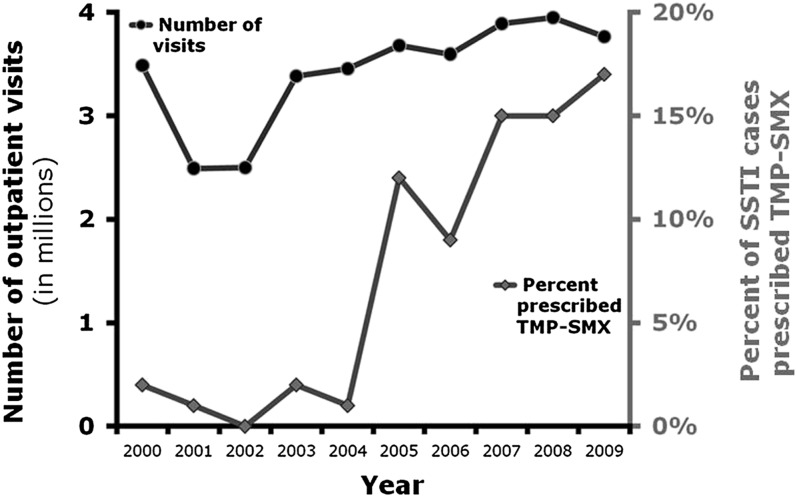
Between 2000 and 2009, TMP-SMX was prescribed in an estimated average of 2.2 million ambulatory pediatric visits per year in the United States. These 2.2 million visits per year were derived from 2655 visits sampled in the NAMCS and the NHAMCS. TMP-SMX was prescribed in 1% of ambulatory pediatric visits during the study period, and there was no time trend in proportion of visits in which TMP-SMX was prescribed (P = .16). SSTI was diagnosed in 13% of visits in which TMP-SMX was prescribed. The frequency of TMP-SMX prescribing for SSTIs increased substantially during the study period; whereas TMP-SMX was prescribed in 0% to 2% of visits for SSTI before 2005, TMP-SMX was prescribed in 17% of such visits in 2009 (Fig 3).
FIGURE 3.
Frequency of outpatient SSTI visits and TMP/SMX prescribing in children <18 years with a primary diagnosis of SSTI.
Discussion
We found a significant rise in the number of ADRs associated with TMP-SMX over a 10-year period at a single free-standing children’s hospital with a 20-fold increase in the number of cases of TMP-SMX ADRs diagnosed at our institution from 2000 to 2004 as compared with 2005–2009. The majority of these patients had been receiving TMP-SMX as outpatient therapy for SSTIs. Additional investigation indicates that this trend may be occurring at other children’s hospitals in the United States where we observed a threefold increase from 2004 to 2009. This is likely an underrepresentation of the true scope of the problem as both our local data and the PHIS data only represent tertiary care pediatric institutions and do not capture all potential cases evaluated at community hospitals or academic institutions not associated with this database. The observed increase may be due, at least in part, to greater use of TMP-SMX in pediatric ambulatory care settings for SSTIs.
Although there has been no change in the overall use of TMP-SMX among children in ambulatory care, our study demonstrates a significant increase in usage for patients with SSTI. It is somewhat expected that the percentage of TMP-SMX used in children with SSTI demonstrated a significant increase around 2005 as multiple studies have confirmed an increase in MRSA infections approximating that time period.11–13 With relatively few oral options for the empirical treatment of MRSA SSTI in the outpatient setting (clindamycin, TMP-SMX, tetracycline, and linezolid)14 and the increasing rate of clindamycin resistance being reported in areas of the United States,16 continued increases in the prescribing of TMP-SMX are likely. Such increased usage may be exacerbated by the limitations of alternative oral options (eg, contraindication of tetracyclines in children younger than 8 years of age or linezolid’s prohibitive cost). Clinicians should be more vigilant than ever for ADRs associated with TMP-SMX.
This observed association between an increase in TMP-SMX ADRs in the setting of SSTI therapy has not been investigated from a pathophysiologic standpoint to date. Existing studies examining TMP-SMX associated ADRs in the pediatric population have focused on patients treated for recurrent UTIs, otitis media, gastroenteritis, or in the setting of HIV infection as these were the populations most routinely prescribed TMP-SMX.7,9,10 HIV has historically been associated with a significantly higher rate of TMP-SMX ADRs, though the responsible mechanism remains unclear. The sulfonamide component of TMP-SMX has traditionally been implicated as the cause of severe ADRs. The proposed pathway suggests bioactivation of drug to a reactive metabolite that can lead to immunogen formation or cellular toxicity. Because the relationship between SSTI and TMP-SMX ADRs has not been critically examined, it is unclear if this observed increase is potentially related to dosing, length of therapy, repeat exposure due to recurrent infection, or disease–drug interaction.
One also must recognize the challenges of accurately diagnosing ADRs in general. Although phenotype standardization of serious ADRs has been proposed,17,18 the diagnosis of an ADR can be challenging as the differential diagnosis can be broad, including infectious etiologies, autoimmune diseases, or dermatologic diseases. Currently, the diagnosis of TMP-SMX ADRs is based on history and clinical and laboratory findings, but no confirmatory test is available. Further investigation and development of potential biomarkers could aid in the diagnosis allowing for more precise categorization of these undesired reactions and potentially, identification of children at increased risk for more severe ADRs. Also, standardization of drug exposure information reporting (ie, dose, length of therapy, route) and recording a detailed clinical history in suspected ADR cases is important when establishing the diagnosis.
Finally, these results reiterate the fact that medications are not without risk. Adverse events can result in prolonged hospital stay, increased health care cost, and increased morbidity and mortality.19 The prescribing clinician should revisit the risks and benefits of medications with each patient encounter. For example, the indication for antimicrobial therapy in the management of MRSA skin and soft tissues varies. Incision and drainage of a simple, cutaneous abscess should serve as the primary treatment and adjunctive antimicrobial therapy is often not indicated.14,20,21 Bacterial culture and antimicrobial susceptibilities should be obtained when drainage is performed on recurrent or persistent abscesses to aid in guiding antimicrobial therapy if indicated.22 Due to the retrospective nature of this study, specific reasons for selecting TMP-SMX to treat an SSTI or other infection are unclear; however, the general overuse of antibiotics in children is well described.23 Because TMP-SMX is a well-recognized cause of ADRs, it is not unforeseen that these unexpected and undesired reactions will continue to occur more frequently both locally and nationwide with increasing use for treatment of SSTI. This information serves as a reminder to all clinicians that the risk of undesired and unpredictable ADRs is currently unavoidable; therefore, potential risks and benefits must be considered at each patient encounter in which a medication is being prescribed.
This study was limited because the chart review was retrospective in nature and the diagnosis of a TMP-SMX ADR was determined by the clinician without any specific inclusion criteria. The clinical information recorded in the charts varied by case, and detailed information on TMP-SMX dose, route of administration, and duration of exposure were not regularly recorded. A standardized clinical and physical history would be beneficial for describing and classifying ADRs. Future prospective studies may distinguish more clearly highly suspected ADRs from other causes of presenting symptoms. Furthermore, additional cases could have been overlooked if not coded properly. Regardless, the symptoms and concern of a TMP-SMX ADR resulted in seeking out additional medical care. Utilization of the PHIS database has recognized limitations as cases cannot be individually reviewed for accuracy and the diagnosis for which TMP-SMX was being prescribed could not identified. Also, few hospitals provided validated data before 2004, making the time period in which data could be examined limited in scope.
Conclusions
Because TMP-SMX is a well-recognized cause of ADRs, it is not unexpected that these undesired reactions have occurred more frequently with the observed increase in prescribing for SSTI. Over 15% of all children diagnosed with an SSTI in the United States in 2009 were prescribed TMP-SMX in the outpatient setting. If clindamycin resistance continues to increase in common MRSA strains, we anticipate a continued increase in TMP-SMX prescribing for SSTI. These findings demonstrate the long recognized but possibly forgotten undesired reactions associated with TMP-SMX. Consideration of the indication as well as the potential harm associated with prescribing TMP-SMX is recommended before the initiation of therapy.
Glossary
- ADR
adverse drug reaction
- CHA
Children’s Hospital Association
- CMH
Children’s Mercy Hospital
- ED
emergency department
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- MRSA
methicillin-resistant Staphylococcus aureus
- NAMCS
National Ambulatory Medical Care Survey
- NHAMCS
National Hospital Ambulatory Medical Care Survey
- PHIS
Pediatric Health Information System
- SSTI
skin and soft tissue infection
- TMP-SMX
trimethoprim-sulfamethoxazole
- UTI
urinary tract infection
Footnotes
Dr Goldman contributed to the concept and design, acquisition of data, analysis and interpretation of data, and drafting and revising the article; Dr Jackson contributed to the concept and design, interpretation of data, and article revision; Mr Herigon contributed to the concept and design, acquisition of data, analysis and interpretation of data, and article drafting and revision; Dr Hersh and Mr Shapiro participated in study concept, acquisition of data, data analysis, and article revision; and Dr Leeder contributed to the concept and design, analysis and interpretation of data, and article revision.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Goldman is supported by grant T32 HD069038 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Kearns, PI). Funded by the National Institutes of Health (NIH).
References
- 1.Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52(1):77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourgeois FT, Mandl KD, Valim C, Shannon MW. Pediatric adverse drug events in the outpatient setting: an 11-year national analysis. Pediatrics. 2009;124(4). Available at: www.pediatrics.org/cgi/content/full/124/2/e744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell AA, Lacouture PG, Sheehan JE, Kauffman RE, Shapiro S. Adverse drug reactions in children leading to hospital admission. Pediatrics. 1988;82(1):24–29 [PubMed] [Google Scholar]
- 4.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–743 [DOI] [PubMed] [Google Scholar]
- 5.Frisch JM. Clinical experience with adverse reactions to trimethoprim-sulfamethoxazole. J Infect Dis. 1973;128(suppl):607–612 [DOI] [PubMed] [Google Scholar]
- 6.Levi N, Bastuji-Garin S, Mockenhaupt M, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009;123(2). Available at: www.pediatrics.org/cgi/content/full/123/2/e297 [DOI] [PubMed] [Google Scholar]
- 7.Rieder MJ, King SM, Read S. Adverse reactions to trimethoprim-sulfamethoxazole among children with human immunodeficiency virus infection. Pediatr Infect Dis J. 1997;16(11):1028–1031 [DOI] [PubMed] [Google Scholar]
- 8.Karpman E, Kurzrock EA. Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J Urol. 2004;172(2):448–453 [DOI] [PubMed] [Google Scholar]
- 9.Gutman LT. The use of trimethoprim-sulfamethoxazole in children: a review of adverse reactions and indications. Pediatr Infect Dis. 1984;3(4):349–357 [DOI] [PubMed] [Google Scholar]
- 10.Uhari M, Nuutinen M, Turtinen J. Adverse reactions in children during long-term antimicrobial therapy. Pediatr Infect Dis J. 1996;15(5):404–408 [DOI] [PubMed] [Google Scholar]
- 11.Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis. 2009;49(1):65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frei CR, Makos BR, Daniels KR, Oramasionwu CU. Emergence of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections as a common cause of hospitalization in United States children. J Pediatr Surg. 2010;45(10):1967–1974 [DOI] [PubMed] [Google Scholar]
- 13.Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at Driscoll Children’s Hospital. Arch Pediatr Adolesc Med. 2005;159(10):980–985 [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Bayer A, Cosgrove SE, et al. Infectious Diseases Society of America . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55 [DOI] [PubMed] [Google Scholar]
- 15.Fontanarosa PB, Rennie D, DeAngelis CD. Postmarketing surveillance—lack of vigilance, lack of trust. JAMA. 2004;292(21):2647–2650 [DOI] [PubMed] [Google Scholar]
- 16.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40(12):1785–1791 [DOI] [PubMed] [Google Scholar]
- 17.Pirmohamed M, Friedmann PS, Molokhia M, et al. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharmacol Ther. 2011;89(6):896–901 [DOI] [PubMed] [Google Scholar]
- 18.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806–815 [DOI] [PubMed] [Google Scholar]
- 19.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277(4):301–306 [PubMed] [Google Scholar]
- 20.Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23(2):123–127 [DOI] [PubMed] [Google Scholar]
- 21.Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55(5):401–407 [DOI] [PubMed] [Google Scholar]
- 22.Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America . Practice guidelines for the diagnosis and management of skin and soft-tissue infections [published correction appears in Clin Infect Dis. 2006;42(8):1219]. Clin Infect Dis. 2005;41(10):1373–1406 [DOI] [PubMed] [Google Scholar]
- 23.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–1061 [DOI] [PubMed] [Google Scholar]