Abstract
Infantile hemangiomas (IHs) are common neoplasms composed of proliferating endothelial-like cells. Despite the relative frequency of IH and the potential severity of complications, there are currently no uniform guidelines for treatment. Although propranolol has rapidly been adopted, there is significant uncertainty and divergence of opinion regarding safety monitoring, dose escalation, and its use in PHACE syndrome (PHACE = posterior fossa, hemangioma, arterial lesions, cardiac abnormalities, eye abnormalities; a cutaneous neurovascular syndrome characterized by large, segmental hemangiomas of the head and neck along with congenital anomalies of the brain, heart, eyes and/or chest wall). A consensus conference was held on December 9, 2011. The multidisciplinary team reviewed existing data on the pharmacologic properties of propranolol and all published reports pertaining to the use of propranolol in pediatric patients. Workgroups were assigned specific topics to propose protocols on the following subjects: contraindications, special populations, pretreatment evaluation, dose escalation, and monitoring. Consensus protocols were recorded during the meeting and refined after the meeting. When appropriate, protocol clarifications and revision were made and agreed upon by the group via teleconference. Because of the absence of high-quality clinical research data, evidence-based recommendations are not possible at present. However, the team agreed on a number of recommendations that arose from a review of existing evidence, including when to treat complicated IH; contraindications and pretreatment evaluation protocols; propranolol use in PHACE syndrome; formulation, target dose, and frequency of propranolol; initiation of propranolol in infants; cardiovascular monitoring; ongoing monitoring; and prevention of hypoglycemia. Where there was considerable controversy, the more conservative approach was selected. We acknowledge that the recommendations are conservative in nature and anticipate that they will be revised as more data are made available.
KEY WORDS: infantile hemangioma, propranolol, PHACE syndrome, hypertension, bradycardia, hypoglycemia
Infantile hemangiomas (IHs) are common benign tumors composed of proliferating endothelial-like cells. The duration and rate of growth are variable; some infants will have hemangiomas that grow very little, whereas others grow rapidly and at an unpredictable rate. Although most are not worrisome, ∼12% of IHs are significantly complex, requiring referral to specialists for consideration of treatment.1,2 Complications of hemangiomas, for which systemic pharmacotherapy is typically initiated, include permanent disfigurement, ulceration, bleeding, visual compromise, airway obstruction, congestive heart failure and, rarely, death. Despite the relative frequency of IH and the potential severity of complications, uniform guidelines for treatment are lacking.
There are no US Food and Drug Administration (FDA)-approved agents for the treatment of IH, and treatment is currently based on expert opinion and observational studies. Prospective data addressing the efficacy and safety of any pharmacologic interventions for the treatment of IH have not been generated, and available data are confounded by the lack of a consensus on treatment criteria and objective outcome measures. Agents with reported activity in treating IH include corticosteroids, interferon α, vinca alkaloids, and, recently, propranolol.3–25
Since the initial report of propranolol use for the treatment of IH in 2008, there has been a flurry of case reports and case series describing its efficacy and potential side effects.3–6,10–15,18,21,23,24,26–36 These publications were not subjected to the usual stringency of phase I/II/III clinical trials, and most were not prospective, randomized, or controlled. With clinical use, propranolol has been found to be rapidly effective for IH, well tolerated, and better than previous therapies at inducing regression. These observations, coupled with the immediate availability of the medication in a pediatric formulation, have led to a rapid and widespread adoption of propranolol for IH. Propranolol suspension is commercially available in the United States, but it does not currently have an FDA-approved indication for children. Cardiologists have historically used this medication in infants with the diagnosis of supraventricular tachycardia. In contrast to infants with supraventricular tachycardia, for whom initiation of propranolol typically occurs in an inpatient setting with extensive cardiac monitoring, the great majority of infants treated for IH are cardiac healthy and are treated in an outpatient setting. Guidelines for dose initiation, dose escalation, and toxicity monitoring were never generated for use with IH; therefore, each institution designed unique protocols. These protocols vary considerably; some centers hospitalize all children for initiation of treatment, whereas others do so only rarely. Some experts recommend intensive outpatient monitoring of patients, whereas others do little to no monitoring.3
The distinct circumstances in which propranolol has become so widely used underscores the importance of bringing multiple specialties together to gain consensus regarding dose initiation, safety monitoring, dose escalation, and its use in specific situations (eg, PHACE syndrome).3 In this report, we review existing data on the pharmacologic properties of propranolol and all published reports pertaining to the use of propranolol in pediatric patients. With this review as the evidence base, a multidisciplinary, multiinstitutional expert panel met in December 2011 to develop a standardized, consensus-derived set of best practices for the use of propranolol in infants with IH. As more information accumulates, it is expected that this provisional set of best practices will change.
Review
Pharmacologic Properties of Propranolol
Propranolol is a synthetic, β-adrenergic receptor-blocking agent that is classified as nonselective because it blocks both β-1 and β-2 adrenergic receptors. Chronotropic, inotropic, and vasodilator responses decrease proportionately when propranolol blocks the β-receptor site, resulting in a decrease in heart rate (HR) and blood pressure (BP). Propranolol is highly lipophilic and undergoes first-pass metabolism by the liver with only ∼25% of oral propranolol reaching the systemic circulation. Multiple pathways in the cytochrome P450 system are involved in propranolol’s metabolism, making clinically important drug interactions a potential issue (Table 1).
TABLE 1.
Drug Interactions
| Increase Blood Levels/Toxicity | Decrease Blood Levels/Decrease Efficacy |
|---|---|
| Inhibitors of CYP2D6: | Inducers of hepatic drug metabolism: |
| Amiodarone, cimetidine (but not ranitidine), delavudin, fluoxetine, paroxetine, quinidine, and ritonavir | Rifampin, ethanol, phenytoin, and phenobarbital |
| Inhibitors of CYP1A2: | |
| Imipramine, cimetidine, ciprofloxacin, fluvoxamine, isoniazid, ritonavir, theophylline, zileuton, zolmitriptan, and rizatriptan |
Propranolol had previously been used in pediatric patients primarily for the treatment or prevention of cardiac arrhythmias, hypertension, outflow obstructions in congenital heart disease, and hypertrophic cardiomyopathy. Its antihypertensive effects result from decreased HR, decreased cardiac contractility, inhibition of renin release by the kidneys, and decreased sympathetic tone. However, the mechanism of action of propranolol on IH is yet to be clearly defined. Some of the proposed hypotheses include vasoconstriction, decreased renin production, inhibition of angiogenesis, and stimulation of apoptosis.37–39
Propranolol Use for IH
A comprehensive review of the literature was undertaken to understand the breadth of current clinical practice. A PubMed search cross-referenced with Google Scholar last performed on December 7, 2011, using the search terms “propranolol” and “hemangioma” yielded 177 articles. Of these, 115 articles were written in English and discussed use in humans. Thirty additional articles were excluded because they were nonapplicable or lacked sufficient clinical data. Eighty-five articles (including 1175 patients) were reviewed in detail.4,11,13,15,18,21,23,24,26–34,36–38,40–104 The majority of these publications included <5 patients, and nearly all were retrospective reports. There was only 1 prospective trial and 1 meta-analysis.58,80 Nearly half (35/85; 41%) of the publications were interim reports with patients still undergoing treatment; therefore, adverse events may be underestimated. Although there was significant variability in the details provided by each article, the authors chose to be inclusive to understand the breadth of current clinical practice.
Response to therapy was discussed in 79 articles, and the definitions and measures of response varied widely, from “stabilization” to “complete response.” Fewer than 10 articles attempted to quantify the degree of involution.13,15,23,41,42,58 Positive response in all treated patients was reported in 86% of publications; the remaining 14% discussed at least some treatment failures. In total, 19 of 1175 published patients were reported as treatment failures, suggesting a 1.6% treatment failure rate. This rate may be underestimated because treatment failures may not be as commonly reported. In publications with adequate data from which to calculate age at initiation of therapy, the mean age was 5.1 months, with a median age of 4 months.
Adverse Events of Propranolol in the Pediatric Population
Although propranolol has been well studied in adults, observations of its use in infants and children, nearly 40 years in duration, have been mainly anecdotal. There are no FDA-approved indications for propranolol in pediatric patients in the United States. There is 1 active phase II/III Investigational New Drug application (ClinicalTrials.gov NCT1056341) for the use of propranolol for the treatment of IH. On the basis of case reports and case series, oral propranolol appears to have a favorable safety profile in children. Deaths or acute heart failure have been associated with propranolol initiation only in the settings of intravenous administration or drug overdose.105,106
Given the variability in study design and the retrospective nature of most reports, the true incidence of adverse events in IH population is difficult to ascertain. For example, routine screening for bradycardia was only documented in 128 of 1175 (10%) of patients reported. Of the 85 articles, 48 (56%) reported no complications in any patient, although reports of complications with propranolol usage increased over time from 2008 to 2011 (Table 2). The most frequently reported serious complications were asymptomatic hypotension or hypotension for which no additional details were provided; pulmonary symptoms related to direct blockade of adrenergic bronchodilation; hypoglycemia or hypoglycemic seizure; asymptomatic bradycardia; and hyperkalemia. The most commonly reported nonpotentially life-threatening complications were sleep disturbances including nightmares, somnolence, cool or mottled extremities, diarrhea, and gastroesophageal reflux/upset.
TABLE 2.
Complications Due to Propranolol in Hemangioma Patients
| Complications Recorded | No. of Patients/ Total No. of Patients in Papers Reporting Complication | Frequency (%) of Complication Among Papers Reporting Said Complication | Overall Frequency (%) of Total of 1175 Patients Reviewed in 85 Papers |
|---|---|---|---|
| Asymptomatic hypotension or hypotension (unspecified) | 33/228 | 14.5 | 2.8 |
| Symptomatic hypotension | 3/46 | 6.5 | 0.3 |
| Pulmonary symptoms (bronchoconstriction, bronchiolitis, wheezing, pulmonary obstruction, apneic episode) | 16/201 | 8.0 | 1.4 |
| Hypoglycemia | 10/88 | 11.4 | 0.9 |
| Asymptomatic bradycardia or bradycardia (unknown) | 11/126 | 8.7 | 0.9 |
| Symptomatic bradycardia | 1/2 | 50 | 0.1 |
| Sleep disturbance (including nightmares) | 44/326 | 13.5 | 3.7 |
| Somnolence | 26/220 | 11.8 | 2.2 |
| Cool or mottled extremities | 20/225 | 8.9 | 1.7 |
| Diarrhea | 9/53 | 17.0 | 0.8 |
| Gastroesophageal reflux disease or gastrointestinal upset | 8/133 | 6.0 | 0.7 |
Bradycardia and Hypotension
As a β-blocker, propranolol decreases HR and, in part, BP as a result of negative chronotropic and inotropic effects on the heart. Propranolol’s effects on BP and HR in children peak around 2 hours after an oral dose.47 The reported protocols for initial dose, dose titration, and prospective monitoring were extremely variable and therefore difficult to compare in a uniform fashion. Three prospective studies, although limited by small patient numbers and significant missing data, provide useful information. During initiation of propranolol for IH in infants, bradycardia (<2 SD of normal) and hypotension (< 2 SD of normal) after the first dose (2 mg/kg/day divided 3 times daily) were infrequent and asymptomatic.47 Changes (z scores >2) in systolic BP from baseline occurred in 7%, 22%, and 13% at 1, 2, and 3 hours postpropranolol dosing, respectively. For HR, there were no changes in z scores from baseline >2 at any time point measured. As a group, significant changes in BP occurred only at 2 hours.47 In 28 patients treated for IH with doses up to 4 mg/kg/day, bradycardia was not noted as a side effect.59 In a separate study of 25 infants by Schiestl and colleagues, HR was continuously monitored during sleep and transient bradycardia was reported in 4/25 infants. Decrease in diastolic BP <50th percentile was noted in 16 of 28 patients (57%) in 1 study, but only 1 patient developed clinically recognizable changes with cold extremities and prolonged capillary refill.59
Hypoglycemia
Symptomatic hypoglycemia and hypoglycemic seizures have been reported in infants with IH treated with oral propranolol (Table 3).59,61,63,64,86,88,90,107 These cases occurred in both newborns and toddlers but were often associated with poor oral intake or concomitant infection. The mechanisms through which propranolol-induced hypoglycemia develops are not completely understood. Nonselective β-blockers, such as propranolol, may block catecholamine-induced glycogenolysis, gluconeogenesis, and lipolysis, predisposing to hypoglycemia. Most of the reported patients who developed hypoglycemia were prescribed relatively low doses (1.25–2.0 mg/kg/day), suggesting that hypoglycemia associated with propranolol may not be dose-dependent. Historically, the 1 reported pediatric fatality from an accidental overdose of oral propranolol had a documented blood glucose level of 0 mg/dL, suggesting that hypoglycemia may be the most serious complication in children.106 Patients with IH may be at increased risk if they have received or are concomitantly receiving treatment with corticosteroids, because adrenal suppression may result in loss of the counterregulatory cortisol response and increase the risk of hypoglycemia.88 Children, infants, and especially preterm infants appear to be at higher risk for this hypoglycemia as their glucose utilization rates are threefold higher in the fasting state and their glycogen stores are lower.108
TABLE 3.
Hypoglycemia in IH Patients Treated With Propranolol
| Age at Time of Hypoglycemic Episode | Dose | Duration of Propranolol Therapy Before Hypoglycemia | Time From Last Dose to Detection of Hypoglycemia | Symptoms | Glucose | Other Factors | |
|---|---|---|---|---|---|---|---|
| Lawley Case 2 | 36 d | 2 mg/kg/day divided TID | 10 d | Unknown | Asymptomatic; detected on routine blood work | 48 mg/dL | Timing of last meal not specified |
| Holland Case 1 | 12 mo | 2 mg/kg/day divided TID | 3 wk | 2 h | Pale, cold, clammy, increasingly unresponsive | 55 mg/dL | Fussiness attributed to teething Nl po intake reported |
| Holland Case 2 | 18 mo | 1.25 mg/kg/day divided BID | Few months | 13 h (overnight fast) | Cool, unresponsive after overnight fast; seizures | 24 mg/dL | Recent resolution of illness with decreased po intake |
| Holland Case 3 | 10 mo | 2 mg/kg/day divided TID | 8.5 mo | 2.5 h | Found limp, pale | 20 mg/dL | Setting of RSV, but po intake preceding days reportedly normal |
| Breur | 15 mo | 2 mg/kg/day divided BID | 3 wk | Several (overnight fast) | Unresponsive in am | 32 mg/dL | Concurrent treatment with prednisone with recent taper; significant HPA axis suppression demonstrated with undetectable am cortisol |
| de Graaf Patient 13 | 32 mo | 4 mg/kg; dosing interval NS | NS | NS | Less responsive | 48 mg/dL | Prolonged fasting |
| Bonifazi | 6 mo | 2 mg/kg/day divided TID | 160 d | Propranolol at 3 am; did not wake at 6 am | Irritability and seizures upon waking | 15 mmol/L | Last meal at 11 pm |
| Fusilli | 6 mo | 2 mg/kg/day divided TID | 5 mo | Propranolol at 6:30 am w/o eating, developed seizures at 10 am (10-h fast) | Seizures | 15 mg/dL | |
| Blatt | 8 mo | 2.5 mg/kg/day divided BID | 2 wk | NS | NS | NS | Dose administered may have been higher because patient had 2 prescriptions (20 mg/5mL and 40 mg/5mL) |
| Price | NS | NS | NS | NS | NS | NS | Hypoglycemia reported in 1 of 68 patients in study |
BID, twice daily; HPA, hypothalamic-pituitary-adrenal; NS, not specified; po, oral administration; RSV, respiratory syncytial virus; TID, 3 times daily.
Clinical manifestations of hypoglycemia in infants can vary widely. Mild hypoglycemia produces symptoms associated with counterregulatory epinephrine action, including sweating, shakiness, tachycardia, anxiety, and hunger. With propranolol-induced β-adrenergic blockade, early symptoms may be masked. Therefore, because sweating is not typically blocked by β-blockers, this may be a more reliable symptom for diagnosis. More severe hypoglycemia produces symptoms of neuroglycopenia, including lethargy, stupor, poor feeding, seizures, apnea, loss of consciousness, and hypothermia.
Bronchospasm
Bronchial hyperreactivity, described as wheezing, bronchospasm, or exacerbation of asthma/bronchitis, is a recognized side effect of propranolol as the result of its direct blockade of adrenergic bronchodilation. Certainly, the use of propranolol in the setting of known reactive airway disease must be considered cautiously. The development of bronchial hyperreactivity in the setting of an acute viral illness in patients on propranolol has necessitated temporary discontinuation of therapy.59
Hyperkalemia
Hyperkalemia (without electrocardiographic changes) was reported in 2 children on propranolol for IH.72,109 The cause of the hyperkalemia is not known, but the authors postulate that it was tumor lysis from the large ulcerated IH combined with impaired potassium uptake into cells as the result of β blockade. Dental caries have been reported in 2 pediatric patients treated with propranolol, although this may be related to the formulation of the suspension (if it contains sucrose). β-adrenergic antagonism of salivary gland function resulting in decreased salivation has also been postulated as a contributing factor.58,70
Survey of Propranolol Use for IH
A survey was designed and was distributed to established prescribers of propranolol in Fall 2011 for IH by Drs Sarah L. Chamlin, Beth A. Drolet, Anita N. Haggstrom, and Anthony J. Mancini.
The response rate was 76%, and most respondents were pediatric dermatologists (88%), academicians (84%), and experienced clinicians with a mean of 15.25 years in practice. Before starting propranolol, the following studies were obtained with the noted frequency: electrocardiogram (ECG; 81%), BP measurement (41%), echocardiogram (38%), and HR measurement (38%). Cardiology consultation was “always obtained” by 34% of respondents and “never obtained” by 25%, with the remainder (41%) stating that they “sometimes obtained” such consultation. Seventeen (53%) prescribers “always” or “sometimes” admitted patients to the hospital to initiate therapy, with only 3 of these prescribers stating that they always admitted. The other respondents admitting children did so under special circumstances, including young age (under 6–8 weeks), extreme prematurity, significant comorbidity, PHACE syndrome, airway hemangioma, and poor social situations. Most respondents (81%) started propranolol at 0.5 to 1.0 mg/kg per day, with a goal dose of 2.0 mg/kg per day in 84% of patients. Dosing was twice daily for 38% and 3 times daily for 47%, with the remaining 15% dosing 3 times daily initially with a change to twice daily when the child was older (6–12 months of age).
Consensus Methods
A consensus conference was held in Chicago, Illinois, on December 9, 2011. This conference was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R34AR060881-01). Twenty-eight participants attended from 12 institutions, representing 5 specialties. Collectively, the group has treated >1000 infants with propranolol for IH. Given the inconsistencies in current institutional policies, consensus was difficult to obtain on all issues. Because of the especially vulnerable patient population of infants aged 1 to 6 months, the group chose to remain cautious in the approach to these recommendations. Where there was considerable controversy, the more conservative approach was selected until additional safety data can be obtained.
Results of the survey were shared, and participants were asked to review all existing literature on the use and adverse effects of propranolol in the treatment of IH, PHACE syndrome, and other indications in the pediatric population. These data were summarized, and work groups were assigned specific topics to propose protocols on the following subjects: contraindications, special populations, pretreatment evaluation, dose escalation and monitoring, and patient education. These protocols were presented to the entire group and debated using an iterative process (nominal group technique).110 Consensus protocols were recorded during the meeting, refined after the meeting, and resubmitted to the entire group for discussion by teleconference and electronic review. Comments were recorded and discussed, and when appropriate, protocol clarifications and revisions were made and agreed on by the group via teleconference.
Because of the absence of high-quality clinical research data, evidence-based recommendations are not possible at the present time, and these are not American Academy of Pediatrics–endorsed recommendations. However, the multidisciplinary team agreed on a number of recommendations that arose from a review of existing evidence. It is acknowledged that, in many areas, evidence is generally confined to expert opinion, case reports, observational or descriptive studies, and uncontrolled studies. We acknowledge that the following recommendations are conservative in nature, and we anticipate that they will be revised as more data are made available.
Consensus Recommendations
When to Treat IH
Given the wide spectrum of disease and the natural tendency for involution, the greatest challenge in caring for infants with IH is determining which infants are at highest risk for complications and in need of systemic treatment. Medical management is highly individualized, and treatment with oral propranolol is considered in the presence of ulceration, impairment of a vital function (ocular compromise or airway obstruction), or risk of permanent disfigurement. Before the initiation of therapy, the potential risks of adverse effects are carefully considered and weighed against the benefits of intervention. A medical team with expertise in both the management of IH and the use of oral propranolol in infants provides the most optimal care to patients in need of systemic therapy with propranolol.
Contraindications and Pretreatment History
Before initiating propranolol therapy for IH, screening for risks associated with propranolol use should be performed. Relative contraindications are listed in Table 4. The prescribing physician should perform, or obtain documentation of, a recent normal cardiovascular and pulmonary history and examination. Key elements of the history are poor feeding, dyspnea, tachypnea, diaphoresis, wheezing, heart murmur, or family history of heart block or arrhythmia. The examination should be performed by a care provider with experience in evaluating infants and children. The examination should include HR, BP, and cardiac and pulmonary assessment.
TABLE 4.
Contraindications to Propranolol Therapy
| Cardiogenic shock |
| Sinus bradycardia |
| Hypotension |
| Greater than first-degree heart block |
| Heart failure |
| Bronchial asthma |
| Hypersensitivity to propranolol hydrochloride |
Pretreatment ECG
Routine ECG screening before initiation of propranolol for hemangiomas has been advocated, although the utility of ECG screening for all children with hemangiomas before initiation of propranolol therapy is unclear. In the future, a more indication-driven ECG strategy is likely to develop because the incidence of ECG abnormalities that would limit propranolol use in children with IH appears low.4,7,10,13,15,18,21,25,27,29 For example, congenital complete heart block is rare, with an estimated prevalence of 1 in 20 000 live births,111 and this is most commonly associated with maternal connective tissue disease.112 Consensus was not achieved on the use of ECG for all children with IH, but ECG should be part of the pretreatment evaluation in any child when
-
the HR is below normal for age113:
• newborns (<1 month old), <70 beats per minute,
• infants (1–12 months old), <80 beats per minute, and
• children (>12 months old): <70 beats per minute.
there is family history of congenital heart conditions or arrhythmias (eg, heart block, long QT syndrome, sudden death), or maternal history of connective tissue disease.
there is history of an arrhythmia or an arrhythmia is auscultated during examination.
Because structural and functional heart disease have not been associated with uncomplicated IH, echocardiography as a routine screening tool before initiation of propranolol is not necessary in the absence of abnormal clinical findings.
Propranolol Use in PHACE Syndrome
PHACE syndrome (Online Mendelian Inheritance in Man database ID 606519) is a cutaneous neurovascular syndrome present in one-third of infants with large, facial hemangiomas; it is characterized by large, segmental hemangiomas of the head and neck and congenital anomalies of the brain, heart, eyes, and/or chest wall.114
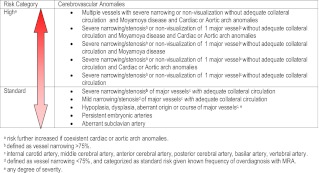
Arterial anomalies of the head and neck are the most common noncutaneous manifestation of PHACE syndrome, and acute ischemic stroke is a known complication.115 Although the arterial anomalies are widely variable, infants with PHACE syndrome believed to be at highest risk for stroke are those with severe, long-segment narrowing or nonvisualization of major cerebral or cervical arteries in the setting of inadequate collateral circulation, especially when there are coexisting cardiac and aortic arch anomalies (Table 5).116 Theoretically, propranolol may increase the risk of stroke in PHACE syndrome patients by dropping BP and attenuating flow through absent, occluded, narrow, or stenotic vessels. Furthermore, nonselective β-blockers, such as propranolol, have been shown to increase variability in systolic BP to a greater degree than β1-selective agents, and labile BP is a known risk factor for stroke.117 There are 2 reports of acute ischemic stroke in PHACE syndrome patients on propranolol to date. Both patients were concomitantly on oral steroids and had severe arteriopathy.116 Cardiac and aortic arch anomalies are also commonly seen in PHACE syndrome and require echocardiography to assess intracardiac anatomy and function. Propranolol administration in these patients should be managed in close consultation with cardiology.
TABLE 5.
Imaging and Clinical Features and Stroke Risk in PHACE Syndrome
Infants with PHACE syndrome represent a unique management challenge because most affected infants have extensive facial hemangiomas, with high risk for both medical morbidities and permanent facial scarring. Such patients are thus prime candidates for propranolol therapy.4 The potential benefits of treatment must be weighed against the risks. The safe use of propranolol in individuals with PHACE has been described in several small case reports and case series, although no clinical trials have been conducted to assess the overall safety.27,115
It is recommended that infants with large facial hemangiomas at risk for PHACE be thoroughly evaluated with MRI/magnetic resonance angiography of the head and neck and cardiac imaging to include the aortic arch before considering propranolol. If imaging results place a patient into a higher risk category for stroke (Table 5), consultation and comanagement with neurology is appropriate. If the potential benefits of propranolol outweigh the risks, the consensus group recommends use of the lowest possible dose, slow dosage titration upward, close observation including inpatient hospitalization in high-risk infants, and 3 times daily dosing to minimize abrupt changes in systolic BP.
Formulation, Target Dose, and Frequency
Propranolol is currently commercially available in propranolol hydrochloride oral solution (20 mg/5 mL and 40 mg/5 mL). It is recommended that the 20 mg/5 mL preparation be used because of the small volumes required for this indication. The consensus group recommends a target dose of 1 to 3 mg/kg per day with most members advocating 2 mg/kg per day, the median dose reported in the literature. Given the fact that dose escalation is required with propranolol and that IH often respond rapidly to even low doses, physicians will often use dose response to determine an individual’s optimal target dose. Dose escalation from a low starting dose is always recommended even in the presence of inpatient monitoring as the initial cardiac response to β blockade may be pronounced.
The consensus group advocates that the daily dose of propranolol be divided into 3 times daily dosing with a minimum of 6 hours between doses, balancing considerations of safety, efficacy, and convenience.
Initiation of Propranolol in Infants With IH
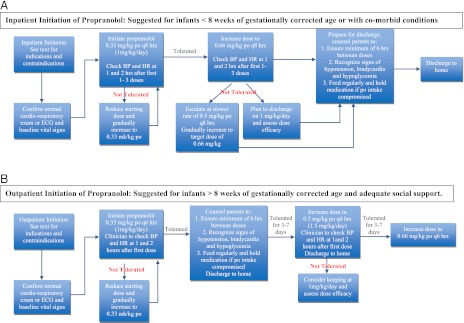
Some facilities may have the resources and expertise to safely monitor all patients in an outpatient setting, and some practitioners continue to admit all infants. The following suggestions were made regarding monitoring for potential side effects while initiating oral propranolol for the treatment of problematic IH (Fig 1). We acknowledge that the data for safe outpatient initiation is mounting but still relatively limited for this indication. The recommendations are age-dependent with patients divided into 2 age groups.
FIGURE 1.
(A) Summary of recommended dose initiation for inpatient scenario. (B) Summary of recommended dose initiation for outpatient scenario. PO, oral administration; q6, every 6; q8, every 8.
Inpatient hospitalization for initiation is suggested for the following: Infants ≤8 weeks of gestationally corrected age, or any age infant with inadequate social support, or any age infant with comorbid conditions affecting the cardiovascular system, the respiratory system including symptomatic airway hemangiomas or blood glucose maintenance.
Outpatient initiation with monitoring can be considered for infants and toddlers older than 8 weeks of gestationally corrected age with adequate social support and without significant comorbid conditions.
Cardiovascular Monitoring
The peak effect of oral propranolol on HR and BP is 1 to 3 hours after administration. Patients should be monitored with HR and BP measurement at baseline and at 1 and 2 hours after receiving the initial dose, and after significant dose increase (>0.5 mg/kg/day), including at least 1 set of measurements after the target dose has been achieved. If HR and BP are abnormal, the child should be monitored until the vitals normalize. Dose response is usually most dramatic after the first dose; therefore, there is no need to repeat cardiovascular monitoring multiple times for the same dose unless the child is very young or has comorbid conditions affecting the cardiovascular system or the respiratory system including symptomatic airway hemangiomas. Bradycardia is important to recognize because the accurate measurement of BP in infants may be challenging. HR is simple to measure, and normative data for inappropriate bradycardia have been established as follows:
• Newborns (<1 month old), <70 beats per minute
• Infants (1–12 months old), <80 beats per minute
• Children (>12 months old), <70 beats per minute
Systolic BP varies significantly between 1 month and 6 months of age, so normative data are difficult to interpret. Moreover, most pediatric normative BP tables were designed to evaluate for hypertension, not hypotension, and are based on auscultatory measurements.118 Oscillometric devices are convenient and minimize observer error, but they do not provide measures that are identical to auscultation. Obtaining accurate BP measurements in neonates and infants may be challenging, and BP measurements should be obtained by experienced personnel. The infant should be in a warm room and in a resting state, awake or asleep. The use of an appropriately sized infant cuff is essential. The inflatable portion of the cuff should encircle ≥75% of the limb circumference, and the length of the cuff should be at least two-thirds of the length of the upper limb segment. Specific age-based normative parameters for identification of systolic hypotension in infants are difficult to provide; as a general guide, we would describe systolic BP that is below normal (less than fifth percentile oscillometric or <2 SD of normal auscultation)119 as follows:
Newborn: <57 mm Hg (<5th percentile oscillometric) or 64 mm Hg (2 SD auscultation)
6 months: <85 mm Hg (<5th percentile oscillometric) or 65 mm Hg (2 SD auscultation)
1 year: <88 mm Hg (<5th percentile oscillometric) or 66 mm Hg (2 SD auscultation)
Patients who have HR and systolic BP measurements below these values during propranolol initiation/dose escalation warrant careful evaluation for additional evidence of cardiovascular compromise and should be considered at higher risk for continued propranolol use at that dose/continued dosage escalation.
The inpatient and outpatient dose escalation recommendations are age-dependent with patients divided into 2 age groups, as shown in Fig 1.
Ongoing Monitoring
As discussed earlier, patients should be monitored with HR and BP measurement at baseline and at 1 and 2 hours after a significant dose increase (>0.5 mg/kg/day), including at least 1 set of measurements after the target dose has been achieved. There is no published information on the utility of Holter monitoring in infants after initiating propranolol to identify occult bradycardia or arrhythmias, and this group has not reached consensus on a recommendation for Holter monitoring after reaching a steady dose. Most centers represented at the conference do not perform or recommend Holter monitoring in this setting on a routine basis.
Preventing Hypoglycemia
Although recognition of signs or symptoms of hypoglycemia may prompt early intervention, measures should be taken to decrease the risk of hypoglycemia. Because asymptomatic hypoglycemia was not detected in studies that included a random serum glucose as part of routine monitoring, and the timing of hypoglycemic events, as outlined in Table 3, has been variable and unpredictable, routine screening of serum glucose is not indicated. Propranolol should be administered during the daytime hours with a feeding shortly after administration. Parents should be instructed to ensure that their child is fed regularly and to avoid prolonged fasts. In otherwise healthy children, the risk of hypoglycemia is age-dependent and begins after 8 hours of fasting in children 0 to 2 years of age.47 Infants <6 weeks should be fed at least every 4 hours, between 6 weeks and 4 months of age should be fed at least every 5 hours, and >4 months of age should be fed at least 6 to 8 hours. Propranolol should be discontinued during intercurrent illness, especially in the setting of restricted oral intake. Children undergoing procedures or radiologic imaging requiring fasting for sedation should be supported with Pedialyte (Abbott Nutrition, Abbott Laboratories, Columbus, OH) or glucose-containing IV fluids during periprocedural periods. Preoperative blood glucose levels may identify additional patients whose symptoms might otherwise be masked by preoperative medications and anesthesia. Particular care should be taken in using propranolol in preterm infant, patients prescribed other medications known to be associated with hypoglycemia or with medical conditions known to produce hypoglycemia.
Conclusions
Currently, the most significant barrier to the implementation of a multiinstitutional clinical trial for the treatment of IH with oral propranolol is the lack of standardized toxicity monitoring in infants without anatomic cardiac/vascular anomalies, as well as in infants with PHACE syndrome. Despite the widespread use of this drug, no systematic strategy currently exists to identify toxicities of therapy for infants with IH. The consensus team agreed on a number of recommendations that arose from a review of existing evidence supplemented by expert opinion and clinical experience (Table 6). These recommendations will provide the platform for large-scale phase II/III clinical trials to determine optimal dosing regimens and long-term safety profiles. We anticipate that these guidelines will be modified as more data are made available from these future studies.
TABLE 6.
Consensus Meeting Key Learnings
| • There are no FDA-approved indications for propranolol in pediatric patients in the United States. |
| • There is significant uncertainty and divergence of opinion regarding safety monitoring and dose escalation for propranolol use in IH. |
| • ECG should be part of the pretreatment evaluation in any child when the HR is below normal, arrhythmia is detected on cardiac exam, or there is a family history of arrhythmias or maternal history of connective tissue disease. |
| • Cardiac and aortic arch anomalies are commonly seen in PHACE syndrome and require echocardiography to assess intracardiac anatomy and function in at-risk children. |
| • It is recommended that the 20 mg/5 mL preparation of propranolol be used. |
| • The consensus group advocates that the daily dose of propranolol be divided into 3 times daily. |
| • Regardless of the setting in which propranolol is initiated, it is recommended that the propranolol dose be titrated up to a target dose, starting at 1 mg/kg/day divided 3 times daily. |
| • The peak effect of oral propranolol on HR and BP is 1 to 3 h after administration. |
| • Dose response is usually most dramatic after the first dose of propranolol. |
| • Bradycardia may be the most reliable measurement of toxicity because obtaining accurate BPs in infants may be challenging, and normative data for bradycardia are better established. |
| • If a major escalation in dosage (>0.5 mg/kg/day) is indicated, the patient’s HR should be assessed before, 1 and 2 h after the increased dose is administered. |
| • Hypoglycemia may be the most common serious complication in children treated with propranolol for IH. |
| • Propranolol should be discontinued during intercurrent illness, especially in the setting of restricted oral intake to prevent hypoglycemia. |
Glossary
- BP
blood pressure
- ECG
electrocardiogram, FDA, US Food and Drug Administration
- HR
heart rate
- IH
infantile hemangioma
- PHACE
posterior fossa, hemangioma, arterial lesions, cardiac abnormalities, eye abnormalities
Footnotes
Given the need for the multispecialty input, this was a highly collaborative process, and all authors have made substantial intellectual contributions to this article. Each author has met all three of the Pediatrics criteria. These authors drafted the initial manuscript (specific section in parenthesis) and approved the final manuscript as submitted. Drs Drolet (introduction), Frommelt (pretreatment evaluation), Chamlin (survey), Haggstrom (introduction), and Cassidy conceptualized and designed the consensus conference program and grant award from the National Institutes of Health that supported the consensus conference. These authors also contributed to the acquisition of data by participating in person in the consensus meeting in Chicago and the iterative decision-making process, as well as several conference calls following the meeting. They drafted the initial manuscript and approved the final manuscript as submitted. Drs Frieden (conclusion), Boucek (adverse events), Bauman (proposed dosing regimen), Chiu (methods), Holland (hypoglycemia), Liberman (inpatient dose escalation), Ward (outpatient dose escalation), Metry (PHACE syndrome), and Puttgen (review of the hemangioma literature) contributed to the acquisition of data by participating in person in the consensus meeting and the iterative decision-making process, as well as several conference calls following the meeting. Drs Chun, Garzon, MacLellan-Tobert, Mancini, Seefeldt, Sidbury, Blei, Baselga, Darrow, Joachim, Kwon, Martin, Perkins, and Siegel contributed to the acquisition of data and analysis and interpretation of data by participating in person in the consensus meeting in Chicago and the iterative decision-making process, as well as several conference calls following the meeting. These authors all critically reviewed 11 drafts of this manuscript and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The following authors disclose a conflict of interest consisting of involvement in the HEMANGIOL study, which was sponsored by Pierre Fabre: Drs Mancini, Sidbury, and Baselga. Drs Frieden and Baselga also disclose that they acted as consultants for Pierre Fabre. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by grant NIH-NIAMS-1R34AR060881-01 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases. Funded by the National Institutes of Health (NIH).
References
- 1.HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/data/hcup. Accessed March 31, 2010
- 2.Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118(3):882–887 [DOI] [PubMed] [Google Scholar]
- 3.Frieden IJ, Drolet BA. Propranolol for infantile hemangiomas: promise, peril, pathogenesis. Pediatr Dermatol. 2009;26(5):642–644 [DOI] [PubMed] [Google Scholar]
- 4.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–2651 [DOI] [PubMed] [Google Scholar]
- 5.Léauté-Labrèze C, Sans-Martin V. Hémangiome infantile [Infantile hemangioma]. Presse Med. 2010;39(4):499–510 [DOI] [PubMed] [Google Scholar]
- 6.Akhavan A, Zippin JH. Current treatments for infantile hemangiomas. J Drugs Dermatol. 2010;9(2):176–180 [PubMed] [Google Scholar]
- 7.Baetz J, Eigelshoven S, Marquard J, Bruch-Gerharz D, Homey B, Meissner T. Infantiles Hämangiom. Erfolgreiche Behandlung mit Propranolo [Infantile hemangioma. Successful treatment with propranolol]. Hautarzt. 2010;61(4):290–292 [DOI] [PubMed] [Google Scholar]
- 8.Barrio VR, Drolet BA. Treatment of hemangiomas of infancy. Dermatol Ther. 2005;18(2):151–159 [DOI] [PubMed] [Google Scholar]
- 9.Boon LM, MacDonald DM, Mulliken JB. Complications of systemic corticosteroid therapy for problematic hemangioma. Plast Reconstr Surg. 1999;104(6):1616–1623 [DOI] [PubMed] [Google Scholar]
- 10.Boyce J, Dodge-Palomba S. Propranolol treatment in periocular pediatric patients with hemangiomas. Insight. 2010;35(1):22–23 [PubMed] [Google Scholar]
- 11.Buckmiller L, Dyamenahalli U, Richter GT. Propranolol for airway hemangiomas: case report of novel treatment. Laryngoscope. 2009;119(10):2051–2054 [DOI] [PubMed] [Google Scholar]
- 12.Buckmiller LM. Propranolol treatment for infantile hemangiomas. Curr Opin Otolaryngol Head Neck Surg. 2009;17(6):458–459 [DOI] [PubMed] [Google Scholar]
- 13.Buckmiller LM, Munson PD, Dyamenahalli U, Dai Y, Richter GT. Propranolol for infantile hemangiomas: early experience at a tertiary vascular anomalies center. Laryngoscope. 2010;120(4):676–681 [DOI] [PubMed] [Google Scholar]
- 14.Denoyelle F, Garabédian EN. Propranolol may become first-line treatment in obstructive subglottic infantile hemangiomas. Otolaryngol Head Neck Surg. 2010;142(3):463–464 [DOI] [PubMed] [Google Scholar]
- 15.Denoyelle F, Leboulanger N, Enjolras O, Harris R, Roger G, Garabedian EN. Role of Propranolol in the therapeutic strategy of infantile laryngotracheal hemangioma. Int J Pediatr Otorhinolaryngol. 2009;73(8):1168–1172 [DOI] [PubMed] [Google Scholar]
- 16.Dickie B, Dasgupta R, Nair R, et al. Spectrum of hepatic hemangiomas: management and outcome. J Pediatr Surg. 2009;44(1):125–133 [DOI] [PubMed] [Google Scholar]
- 17.Draper H, Diamond IR, Temple M, John P, Ng V, Fecteau A. Multimodal management of endangering hepatic hemangioma: impact on transplant avoidance: a descriptive case series. J Pediatr Surg. 2008;43(1):120–125, discussion 126 [DOI] [PubMed] [Google Scholar]
- 18.Fay A, Nguyen J, Jakobiec FA, Meyer-Junghaenel L, Waner M. Propranolol for isolated orbital infantile hemangioma. Arch Ophthalmol. 2010;128(2):256–258 [DOI] [PubMed] [Google Scholar]
- 19.Hara K, Yoshida T, Kajiume T, Ohno N, Kawaguchi H, Kobayashi M. Successful treatment of Kasabach-Merritt syndrome with vincristine and diagnosis of the hemangioma using three-dimensional imaging. Pediatr Hematol Oncol. 2009;26(5):375–380 [DOI] [PubMed] [Google Scholar]
- 20.Herrero Hernández A, Escobosa Sánchez O, Acha García T. Successful treatment with vincristine in PHACES syndrome. Clin Transl Oncol. 2007;9(4):262–263 [DOI] [PubMed] [Google Scholar]
- 21.Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for infantile hemangiomas. Plast Reconstr Surg. 2010;125(1):420–421 [DOI] [PubMed] [Google Scholar]
- 22.Hoyoux C. La vincristine: nouveau traitement des hémangiomes infantiles? [Vincristine treatment for management of hemangiomas in infancy]. Rev Med Liege. 2008;63(1):14–17 [PubMed] [Google Scholar]
- 23.Jephson CG, Manunza F, Syed S, Mills NA, Harper J, Hartley BE. Successful treatment of isolated subglottic haemangioma with propranolol alone. Int J Pediatr Otorhinolaryngol. 2009;73(12):1821–1823 [DOI] [PubMed] [Google Scholar]
- 24.Li YC, McCahon E, Rowe NA, Martin PA, Wilcsek GA, Martin FJ. Successful treatment of infantile haemangiomas of the orbit with propranolol. Clin Experiment Ophthalmol. 2010;38(6):554–559 [DOI] [PubMed] [Google Scholar]
- 25.Löffler H, Kosel C, Cremer H, Kachel W. Die Propranolol-Therapie in der Behandlung problematischer Hämangiome: Eine neue Standardtherapie kündigt sich an [Propranolol therapy to treat problematic hemangiomas : a new standard therapy makes its debut]. Hautarzt. 2009;60(12):1013–1016 [DOI] [PubMed] [Google Scholar]
- 26.Fulkerson DH, Agim NG, Al-Shamy G, Metry DW, Izaddoost SA, Jea A. Emergent medical and surgical management of mediastinal infantile hemangioma with symptomatic spinal cord compression: case report and literature review. Childs Nerv Syst. 2010;26(12):1799–1805 [DOI] [PubMed] [Google Scholar]
- 27.Manunza F, Syed S, Laguda B, et al. Propranolol for complicated infantile haemangiomas: a case series of 30 infants. Br J Dermatol. 2010;162(2):466–468 [DOI] [PubMed] [Google Scholar]
- 28.Marsciani A, Pericoli R, Alaggio R, Brisigotti M, Vergine G. Massive response of severe infantile hepatic hemangioma to propanolol. Pediatr Blood Cancer. 2010;54(1):176. [DOI] [PubMed] [Google Scholar]
- 29.Maturo S, Hartnick C. Initial experience using propranolol as the sole treatment for infantile airway hemangiomas. Int J Pediatr Otorhinolaryngol. 2010;74(3):323–325 [DOI] [PubMed] [Google Scholar]
- 30.Mistry N, Tzifa K. Use of propranolol to treat multicentric airway haemangioma. J Laryngol Otol. 2010;124(12):1329–1332 [DOI] [PubMed] [Google Scholar]
- 31.Mousa W, Kues K, Haas E, et al. Successful treatment of a large hemangioma with propranolol. J Dtsch Dermatol Ges. 2010;8(3):184–186 [DOI] [PubMed] [Google Scholar]
- 32.Naouri M, Schill T, Maruani A, Bross F, Lorette G, Rossler J. Successful treatment of ulcerated haemangioma with propranolol. J Eur Acad Dermatol Venereol. 2010;24(9):1109–1112 [DOI] [PubMed] [Google Scholar]
- 33.Taban M, Goldberg RA. Propranolol for orbital hemangioma. Ophthalmology. 2010;117(1):195.e4 [DOI] [PubMed]
- 34.Truong MT, Chang KW, Berk DR, Heerema-McKenney A, Bruckner AL. Propranolol for the treatment of a life-threatening subglottic and mediastinal infantile hemangioma. J Pediatr. 2010;156(2):335–338 [DOI] [PubMed] [Google Scholar]
- 35.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163(2):269–274 [DOI] [PubMed] [Google Scholar]
- 36.Vanlander A, Decaluwe W, Vandelanotte M, Van Geet C, Cornette L. Propranolol as a novel treatment for congenital visceral haemangioma. Neonatology. 2010;98(3):229–231 [DOI] [PubMed] [Google Scholar]
- 37.Sans V, de la Roque ED, Berge J, et al. Propranolol for severe infantile hemangiomas: follow-up report. Pediatrics. 2009;124(3). Available at: www.pediatrics.org/cgi/content/full/124/3/e423. [DOI] [PubMed] [Google Scholar]
- 38.Schiestl C, Neuhaus K, Zoller S, et al. Efficacy and safety of propranolol as first-line treatment for infantile hemangiomas. Eur J Pediatr. 2011;170(4):493–501 [DOI] [PubMed] [Google Scholar]
- 39.Chim H, Armijo BS, Miller E, Gliniak C, Serret MA, Gosain AK. Propranolol induces regression of hemangioma cells through HIF-1α-mediated inhibition of VEGF-A. Ann Surg. 2012;256(1):146–156 [DOI] [PubMed] [Google Scholar]
- 40.Bayliss SJ, Berk DR, Van Hare GF, et al. Re: Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr Dermatol. 2010;27(3):319–320, author reply 320–321 [DOI] [PubMed] [Google Scholar]
- 41.Haider KM, Plager DA, Neely DE, Eikenberry J, Haggstrom A. Outpatient treatment of periocular infantile hemangiomas with oral propranolol. J AAPOS. 2010;14(3):251–256 [DOI] [PubMed] [Google Scholar]
- 42.Cheng JF, Gole GA, Sullivan TJ. Propranolol in the management of periorbital infantile haemangioma. Clin Experiment Ophthalmol. 2010;38(6):547–553 [DOI] [PubMed] [Google Scholar]
- 43.Mishra A, Holmes WJ, Gorst C, Liew SH. Role of propranolol in the management of periocular hemangiomas. Plast Reconstr Surg. 2010;126(2):671. [DOI] [PubMed] [Google Scholar]
- 44.Erbay A, Sarialioglu F, Malbora B, et al. Propranolol for infantile hemangiomas: a preliminary report on efficacy and safety in very low birth weight infants. Turk J Pediatr. 2010;52(5):450–456 [PubMed] [Google Scholar]
- 45.Chik KK, Luk CK, Chan HB, Tan HY. Use of propranolol in infantile haemangioma among Chinese children. Hong Kong Med J. 2010;16(5):341–346 [PubMed] [Google Scholar]
- 46.Leboulanger N, Fayoux P, Teissier N, et al. Propranolol in the therapeutic strategy of infantile laryngotracheal hemangioma: A preliminary retrospective study of French experience. Int J Pediatr Otorhinolaryngol. 2010;74(11):1254–1257 [DOI] [PubMed] [Google Scholar]
- 47.Cushing SL, Boucek RJ, Manning SC, Sidbury R, Perkins JA. Initial experience with a multidisciplinary strategy for initiation of propranolol therapy for infantile hemangiomas. Otolaryngol Head Neck Surg. 2011;144(1):78–84 [DOI] [PubMed] [Google Scholar]
- 48.Tan ST, Itinteang T, Leadbitter P. Low-dose propranolol for infantile haemangioma. J Plast Reconstr Aesthet Surg. 2011;64(3):292–299 [DOI] [PubMed] [Google Scholar]
- 49.Zheng JW. Comment on efficacy and safety of propranolol in the treatment of parotid hemangioma. Cutan Ocul Toxicol. 2011;30(4):333–334 [DOI] [PubMed] [Google Scholar]
- 50.Zvulunov A, McCuaig C, Frieden IJ, et al. Oral propranolol therapy for infantile hemangiomas beyond the proliferation phase: a multicenter retrospective study. Pediatr Dermatol. 2011;28(2):94–98 [DOI] [PubMed] [Google Scholar]
- 51.Bagazgoitia L, Torrelo A, Gutiérrez JC, et al. Propranolol for infantile hemangiomas. Pediatr Dermatol. 2011;28(2):108–114 [DOI] [PubMed] [Google Scholar]
- 52.Mishra A, Holmes W, Gorst C, Liew S. Management of complicated facial hemangiomas with beta-blocker (propranolol) therapy. Plast Reconstr Surg. 2011;127(4):1742–1743; author reply 3 [DOI] [PubMed]
- 53.Kim LH, Hogeling M, Wargon O, Jiwane A, Adams S. Propranolol: useful therapeutic agent for the treatment of ulcerated infantile hemangiomas. J Pediatr Surg. 2011;46(4):759–763 [DOI] [PubMed] [Google Scholar]
- 54.Fuchsmann C, Quintal MC, Giguere C, et al. Propranolol as first-line treatment of head and neck hemangiomas. Arch Otolaryngol Head Neck Surg. 2011;137(5):471–478 [DOI] [PubMed] [Google Scholar]
- 55.Hermans DJ, van Beynum IM, Schultze Kool LJ, van de Kerkhof PC, Wijnen MH, van der Vleuten CJ. Propranolol, a very promising treatment for ulceration in infantile hemangiomas: a study of 20 cases with matched historical controls. J Am Acad Dermatol. 2011;64(5):833–838 [DOI] [PubMed] [Google Scholar]
- 56.Saint-Jean M, Léauté-Labrèze C, Mazereeuw-Hautier J, et al. Groupe de Recherche Clinique en Dermatologie Pédiatrique . Propranolol for treatment of ulcerated infantile hemangiomas. J Am Acad Dermatol. 2011;64(5):827–832 [DOI] [PubMed] [Google Scholar]
- 57.Al Dhaybi R, Superstein R, Milet A, et al. Treatment of periocular infantile hemangiomas with propranolol: case series of 18 children. Ophthalmology. 2011;118(6):1184–1188 [DOI] [PubMed] [Google Scholar]
- 58.Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128(2). Available at: www.pediatrics.org/cgi/content/full/128/2/e259. [DOI] [PubMed] [Google Scholar]
- 59.de Graaf M, Breur JM, Raphaël MF, Vos M, Breugem CC, Pasmans SG. Adverse effects of propranolol when used in the treatment of hemangiomas: a case series of 28 infants. J Am Acad Dermatol. 2011;65(2):320–327 [DOI] [PubMed] [Google Scholar]
- 60.Truong MT, Perkins JA, Messner AH, Chang KW. Propranolol for the treatment of airway hemangiomas: a case series and treatment algorithm. Int J Pediatr Otorhinolaryngol. 2010;74(9):1043–1048 [DOI] [PubMed] [Google Scholar]
- 61.Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr Dermatol. 2009;26(5):610–614 [DOI] [PubMed] [Google Scholar]
- 62.Theletsane T, Redfern A, Raynham O, Harris T, Prose NS, Khumalo NP. Life-threatening infantile haemangioma: a dramatic response to propranolol. J Eur Acad Dermatol Venereol. 2009;23(12):1465–1466 [DOI] [PubMed] [Google Scholar]
- 63.Bonifazi E, Acquafredda A, Milano A, Montagna O, Laforgia N. Severe hypoglycemia during successful treatment of diffuse hemangiomatosis with propranolol. Pediatr Dermatol. 2010;27(2):195–196 [DOI] [PubMed] [Google Scholar]
- 64.Holland KE, Frieden IJ, Frommelt PC, Mancini AJ, Wyatt D, Drolet BA. Hypoglycemia in children taking propranolol for the treatment of infantile hemangioma. Arch Dermatol. 2010;146(7):775–778 [DOI] [PubMed] [Google Scholar]
- 65.Muthamilselvan S, Vinoth PN, Vilvanathan V, et al. Hepatic haemangioma of infancy: role of propranolol. Ann Trop Paediatr. 2010;30(4):335–338 [DOI] [PubMed] [Google Scholar]
- 66.Blanchet C, Nicollas R, Bigorre M, Amedro P, Mondain M. Management of infantile subglottic hemangioma: acebutolol or propranolol? Int J Pediatr Otorhinolaryngol. 2010;74(8):959–961 [DOI] [PubMed] [Google Scholar]
- 67.Arneja JS, Pappas PN, Shwayder TA, et al. Management of complicated facial hemangiomas with beta-blocker (propranolol) therapy. Plast Reconstr Surg. 2010;126(3):889–895 [DOI] [PubMed] [Google Scholar]
- 68.Abbott J, Parulekar M, Shahidullah H, Taibjee S, Moss C. Diarrhea associated with propranolol treatment for hemangioma of infancy (HOI). Pediatr Dermatol. 2010;27(5):558. [DOI] [PubMed] [Google Scholar]
- 69.Jadhav VM, Tolat SN. Dramatic response of propranolol in hemangioma: report of two cases. Indian J Dermatol Venereol Leprol. 2010;76(6):691–694 [DOI] [PubMed] [Google Scholar]
- 70.Girón-Vallejo O, López-Gutiérrez JC, Fernández-Pineda I, Méndez NA, Ruiz Jiménez JI. Dental caries as a side effect of infantile hemangioma treatment with propranolol solution. Pediatr Dermatol. 2010;27(6):672–673 [DOI] [PubMed] [Google Scholar]
- 71.Sarialioglu F, Erbay A, Demir S. Response of infantile hepatic hemangioma to propranolol resistant to high-dose methylprednisolone and interferon-α therapy. Pediatr Blood Cancer. 2010;55(7):1433–1434 [DOI] [PubMed] [Google Scholar]
- 72.Pavlakovic H, Kietz S, Lauerer P, Zutt M, Lakomek M. Hyperkalemia complicating propranolol treatment of an infantile hemangioma. Pediatrics 2010;126(6). Available at: www.pediatrics.org/cgi/content/full/126/6/e1589 [DOI] [PubMed]
- 73.Fabian ID, Ben-Zion I, Samuel C, Spierer A. Reduction in astigmatism using propranolol as first-line therapy for periocular capillary hemangioma. Am J Ophthalmol. 2011;151(1):53–58 [DOI] [PubMed] [Google Scholar]
- 74.Missoi TG, Lueder GT, Gilbertson K, Bayliss SJ. Oral propranolol for treatment of periocular infantile hemangiomas. Arch Ophthalmol. 2011;129(7):899–903 [DOI] [PubMed] [Google Scholar]
- 75.Claerhout I, Buijsrogge M, Delbeke P, et al. The use of propranolol in the treatment of periocular infantile haemangiomas: a review. Br J Ophthalmol. 2011;95(9):1199–1202 [DOI] [PubMed] [Google Scholar]
- 76.Javia LR, Zur KB, Jacobs IN. Evolving treatments in the management of laryngotracheal hemangiomas: will propranolol supplant steroids and surgery? Int J Pediatr Otorhinolaryngol. 2011;75(11):1450–1454 [DOI] [PubMed] [Google Scholar]
- 77.Rossler J, Schill T, Bähr A, Truckenmüller W, Noellke P, Niemeyer CM. Propranolol for proliferating infantile haemangioma is superior to corticosteroid therapy—a retrospective, single centre study. J Eur Acad Dermatol Venereol. 2012;26(9):1173–1175 [DOI] [PubMed] [Google Scholar]
- 78.El-Essawy R, Galal R, Abdelbaki S. Nonselective β-blocker propranolol for orbital and periorbital hemangiomas in infants: a new first-line of treatment? Clin Ophthalmol. 2011;5:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazereeuw-Hautier J, Hoeger PH, Benlahrech S, et al. Efficacy of propranolol in hepatic infantile hemangiomas with diffuse neonatal hemangiomatosis. J Pediatr. 2010;157(2):340–342 [DOI] [PubMed] [Google Scholar]
- 80.Peridis S, Pilgrim G, Athanasopoulos I, Parpounas K. A meta-analysis on the effectiveness of propranolol for the treatment of infantile airway haemangiomas. Int J Pediatr Otorhinolaryngol. 2011;75(4):455–460 [DOI] [PubMed] [Google Scholar]
- 81.Corapcioğlu F, Büyükkapu-Bay S, Binnetoğlu K, Babaoğlu A, Anik Y, Tugay M. Preliminary results of propranolol treatment for patients with infantile hemangioma. Turk J Pediatr. 2011;53(2):137–141 [PubMed] [Google Scholar]
- 82.Fridman G, Grieser E, Hill R, Khuddus N, Bersani T, Slonim C. Propranolol for the treatment of orbital infantile hemangiomas. Ophthal Plast Reconstr Surg. 2011;27(3):190–194 [DOI] [PubMed] [Google Scholar]
- 83.Itani MH, Fakih H. Response of facial haemangioma to oral propranolol. BMJ Case Reports. 2009; 10.1136/bcr.01.2009.1476. Available at http://casereports.bmj.com/content/2009/bcr.01.2009.1476.abstract. Accessed October 30, 2012 [DOI] [PMC free article] [PubMed]
- 84.Rosbe KW, Suh KY, Meyer AK, Maguiness SM, Frieden IJ. Propranolol in the management of airway infantile hemangiomas. Arch Otolaryngol Head Neck Surg. 2010;136(7):658–665 [DOI] [PubMed] [Google Scholar]
- 85.Meyer L, Graffstaedt H, Giest H, Truebenbach J, Waner M. Effectiveness of propranolol in a newborn with liver hemangiomatosis. Eur J Pediatr Surg. 2010;20(6):414–415 [DOI] [PubMed] [Google Scholar]
- 86.Fusilli G, Merico G, Gurrado R, Rosa T, Acquafredda A, Cavallo L. Propranolol for infantile haemangiomas and neuroglycopenic seizures. Acta Paediatr. 2010;99(12):1756. [DOI] [PubMed] [Google Scholar]
- 87.Mhanna A, Franklin WH, Mancini AJ. Hepatic infantile hemangiomas treated with oral propranolol—a case series. Pediatr Dermatol. 2011;28(1):39–45 [DOI] [PubMed] [Google Scholar]
- 88.Breur JM, de Graaf M, Breugem CC, Pasmans SG. Hypoglycemia as a result of propranolol during treatment of infantile hemangioma: a case report. Pediatr Dermatol. 2011;28(2):169–171 [DOI] [PubMed] [Google Scholar]
- 89.Tan ST, Itinteang T, Leadbitter P. Low-dose propranolol for multiple hepatic and cutaneous hemangiomas with deranged liver function. Pediatrics. 2011;127(3). Available at: www.pediatrics.org/cgi/content/full/127/3/e772. [DOI] [PubMed] [Google Scholar]
- 90.Blatt J, Morrell DS, Buck S, et al. β-blockers for infantile hemangiomas: a single-institution experience. Clin Pediatr (Phila). 2011;50(8):757–763 [DOI] [PubMed] [Google Scholar]
- 91.Weiss I, O TM, Lipari BA, Meyer L, Berenstein A, Waner M. Current treatment of parotid hemangiomas. Laryngoscope. 2011;121(8):1642–1650 [DOI] [PubMed] [Google Scholar]
- 92.Eivazi B, Cremer HJ, Mangold C, Teymoortash A, Wiegand S, Werner JA. Hemangiomas of the nasal tip: an approach to a therapeutic challenge. Int J Pediatr Otorhinolaryngol. 2011;75(3):368–375 [DOI] [PubMed] [Google Scholar]
- 93.Sierpina DI, Chaudhary HM, Walner DL, Aljadeff G, Dubrow IW. An infantile bronchial hemangioma unresponsive to propranolol therapy: case report and literature review. Arch Otolaryngol Head Neck Surg. 2011;137(5):517–521 [DOI] [PubMed] [Google Scholar]
- 94.Yeh I, Bruckner AL, Sanchez R, Jeng MR, Newell BD, Frieden IJ. Diffuse infantile hepatic hemangiomas: a report of four cases successfully managed with medical therapy. Pediatr Dermatol. 2011;28(3):267–275 [DOI] [PubMed] [Google Scholar]
- 95.Koay AC, Choo MM, Nathan AM, Omar A, Lim CT. Combined low-dose oral propranolol and oral prednisolone as first-line treatment in periocular infantile hemangiomas. J Ocul Pharmacol Ther. 2011;27(3):309–311 [DOI] [PubMed] [Google Scholar]
- 96.Tamagno M, Bibas BJ, Minamoto H, Alfinito FS, Terra RM, Jatene FB. Subglottic and mediastinal hemangioma in a child: treatment with propranolol. J Bras Pneumol. 2011;37(3):416–418 [DOI] [PubMed] [Google Scholar]
- 97.Guye E, Chollet-Rivier M, Schröder D, Sandu K, Hohlfeld J, de Buys Roessingh A. Propranolol treatment for subglottic haemangioma. Arch Dis Child Fetal Neonatal Ed. 2011;96(4):F263–F264 [DOI] [PubMed] [Google Scholar]
- 98.Raol N, Metry D, Edmonds J, Chandy B, Sulek M, Larrier D. Propranolol for the treatment of subglottic hemangiomas. Int J Pediatr Otorhinolaryngol. 2011;75(12):1510–1514 [DOI] [PubMed] [Google Scholar]
- 99.Goswamy J, Rothera MP, Bruce IA. Failure of propranolol in the treatment of childhood haemangiomas of the head and neck. J Laryngol Otol. 2011;125(11):1164–1172 [DOI] [PubMed] [Google Scholar]
- 100.Moschovi M, Alexiou GA, Stefanaki K, Tourkantoni N, Prodromou N. Propranolol treatment for a giant infantile brain cavernoma. J Child Neurol. 2010;25(5):653–655 [DOI] [PubMed] [Google Scholar]
- 101.Canadas KT, Baum ED, Lee S, Ostrower ST. Case report: Treatment failure using propanolol for treatment of focal subglottic hemangioma. Int J Pediatr Otorhinolaryngol. 2010;74(8):956–958 [DOI] [PubMed] [Google Scholar]
- 102.Chun YH, Moon CJ, Yoon JS, Kim HH, Kim JT, Lee JS. Successful treatment of infantile subglottic hemangioma with oral propranolol. Clin Pediatr (Phila). 2012;51(10):983-986 [DOI] [PubMed]
- 103.Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg. 2011;64(4):445–451 [DOI] [PubMed] [Google Scholar]
- 104.Hermans DJ, van Beynum IM, van der Vijver RJ, Kool LJ, de Blaauw I, van der Vleuten CJ. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33(4):e171–e173 [DOI] [PubMed] [Google Scholar]
- 105.Litovitz TL, Schmitz BF, Matyunas N, Martin TG. 1987 annual report of the American Association of Poison Control Centers National Data Collection System. Am J Emerg Med. 1988;6(5):479–515 [DOI] [PubMed] [Google Scholar]
- 106.Love JN, Litovitz TL, Howell JM, Clancy C. Characterization of fatal beta blocker ingestion: a review of the American Association of Poison Control Centers data from 1985 to 1995. J Toxicol Clin Toxicol. 1997;35(4):353–359 [DOI] [PubMed] [Google Scholar]
- 107.Price CJ, Lattouf C, Baum B, et al. Propranolol vs corticosteroids for infantile hemangiomas: a multicenter retrospective analysis. Arch Dermatol. 2011;147(12):1371–1376 [DOI] [PubMed] [Google Scholar]
- 108.van Veen MR, van Hasselt PM, de Sain-van der Velden MG, et al. Metabolic profiles in children during fasting. Pediatrics. 2011;127(4). Available at: www.pediatrics.org/cgi/content/full/127/4/e1021. [DOI] [PubMed] [Google Scholar]
- 109.Cavalli R, Buffon RB, de Souza M, Colli AM, Gelmetti C. Tumor lysis syndrome after propranolol therapy in ulcerative infantile hemangioma: rare complication or incidental finding? Dermatology. 2012;224(2):106–109 [DOI] [PubMed] [Google Scholar]
- 110.Horton JN. Nominal group technique. A method of decision-making by committee. Anaesthesia. 1980;35(8):811–814 [DOI] [PubMed] [Google Scholar]
- 111.Michaëlsson M, Engle MA. Congenital complete heart block: an international study of the natural history. Cardiovasc Clin. 1972;4(3):85–101 [PubMed] [Google Scholar]
- 112.Vetter VL, Rashkind WJ. Congenital complete heart block and connective-tissue disease. N Engl J Med. 1983;309(4):236–238 [DOI] [PubMed] [Google Scholar]
- 113.Bernstein DP, ed. History and Physical Evaluation. 18th ed. Philadelphia, PA: Saunders Elsevier; 2007 [Google Scholar]
- 114.Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol. 1996;132(3):307–311 [DOI] [PubMed] [Google Scholar]
- 115.Solomon T, Ninnis J, Deming D, Merritt TA, Hopper A. Use of propranolol for treatment of hemangiomas in PHACE syndrome. J Perinatol. 2011;31(11):739–741 [DOI] [PubMed] [Google Scholar]
- 116.Siegel D, Tefft K, Kelly T, et al. Stroke in children with posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardia defects, and eye abnormalities (PHACE) syndrome: a systematic review of the literature. Stroke. 2012;43(6):1672–1674 [DOI] [PubMed] [Google Scholar]
- 117.Webb AJ, Fischer U, Rothwell PM. Effects of β-blocker selectivity on blood pressure variability and stroke: a systematic review. Neurology. 2011;77(8):731–737 [DOI] [PubMed] [Google Scholar]
- 118.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(suppl 2, 4th report):555–576 [PubMed]
- 119.Kent AL, Kecskes Z, Shadbolt B, Falk MC. Blood pressure in the first year of life in healthy infants born at term. Pediatr Nephrol. 2007;22(10):1743–1749 [DOI] [PubMed] [Google Scholar]