Abstract
Forty-four TLE patients (25 left) and 40 healthy control participants performed a complex visual scene-encoding fMRI task in a 4T Varian scanner. Healthy controls and left temporal lobe epilepsy (LTLE) patients demonstrated symmetric activation during scene encoding. In contrast, RTLE (RTLE) patients demonstrated left lateralization of scene encoding which differed significantly from healthy controls and LTLE patients (all p ≤ .05). Lateralization of scene encoding to the right hemisphere among LTLE patients was associated with inferior verbal memory performance as measured by neuropsychological testing (WMS-III Logical Memory Immediate, p=0.049; WMS-III Paired Associates Immediate, p=0.036; WMS-III Paired Associates Delayed, p=0.047). In RTLE patients, left lateralization of scene encoding was associated with lower visuospatial memory performance (BVRT, p=0.043) but improved verbal memory performance (WMS-III Word List, p=0.049). These findings indicate that, despite the negative effects of epilepsy, memory functioning is better supported by the affected hemisphere than the hemisphere contralateral to seizure focus.
Keywords: temporal lobe epilepsy, memory lateralization, fMRI, complex scene encoding
The hippocampus and the related medial temporal lobe (MTL) structures are essential for encoding episodic memories (1). Studies in patients with unilateral MTL lesions have demonstrated dissociations between the functions of left hippocampus, mediating verbal memory, and the right hippocampus, facilitating visuospatial memory (2,3), although material-specific memory deficits from right hemispheric lesions tend to be less robust than those from left hemispheric lesions (4). Temporal lobe epilepsy (TLE), most commonly characterized by seizures originating from medial temporal structures, is frequently associated with memory impairments (5,6). In particular, LTLE is typically associated with deficits in verbal memory functioning, whereas seizures originating from the right temporal region may cause visuospatial memory impairments (7). These memory deficits are, in part, the result of progressive seizure-related damage to MTL structures that are vital for the acquisition, temporary storage, and retrieval of episodic memories (8).
Neural damage associated with chronic epilepsy is known in some individuals to lead to functional reorganization of language and/or memory functions (9,10). For example, atypical language lateralization is commonly observed in LTLE patients (11) and is most prominent in patients with hippocampal sclerosis (12,13). A number of small- and medium-scale fMRI studies have investigated functional reorganization of memory in epilepsy patients. Overall, these studies have demonstrated increased lateralization of memory encoding, as assessed through experimental tasks presented in the scanner, to the hemisphere contralateral to the seizure focus (14-18). In particular, LTLE patients demonstrate increased right lateralization of verbal memory, whereas left lateralization of visuospatial memory has been observed among RTLE patients (19-21). These findings support the notion that TLE may lead to epilepsy-related reorganization of memory processing. However, the functional importance of this atypical lateralization for memory performance on standardized psychometric measures has received little attention and the literature has produced mixed findings. In a study by Richardson et al. (20), reorganization of verbal memory to the right hemisphere was associated with superior verbal memory performance in LTLE patients. However, more recently Powell et al. (19) demonstrated that atypical (i.e., lateralized to the unaffected hemisphere) memory representation was associated with inferior verbal and nonverbal (i.e., figure recall) memory performance. Additionally, Vannest et al. (21) reported that left hemispheric epilepsy patients demonstrated greater right medial temporal activation as well as inferior memory performance when compared to right hemispheric epilepsy patients. The authors concluded that reorganization of memory functioning in left hemispheric epilepsy patients may be associated with impaired verbal memory performance (21).
Given the incongruous findings to date, the present fMRI study will use a large sample of TLE patients and healthy controls in order to further examine whether lateralization of seizure focus differentially affects lateralization of memory functioning. Additionally, the relationship between lateralization of memory and memory performance on standardized clinical measures will be investigated. Healthy controls will be used to establish activation patterns elicited by the fMRI scene-encoding task employed in this study, which has previously been known to elicit symmetric medial temporal activation in healthy controls (10,22,23). This study will test two hypotheses which have been formulated based on the existing literature:
Hypothesis 1: LTLE patients will demonstrate increased right lateralization of scene encoding and RTLE patients will demonstrate increased left lateralization of scene encoding (19-21).
Hypothesis 2: Lateralization of scene encoding to the contralateral (healthy) hemisphere and the degree of lateralization will be negatively correlated with the degree of memory functioning (20,24,25).
Previous studies have demonstrated that memory lateralization, particularly verbal memory lateralization, may depend on lateralization of language functioning (26). Therefore, additional analyses will be completed by investigating the relationship between memory lateralization and language lateralization as determined by the previously published semantic decision/tone decision (SDTD) fMRI task (27). This and similar designed semantic decision fMRI tasks are known to elicit lateralizing activation patterns for semantic language functions (28,29), correlate with language intracarotid amobarbital procedure (IAP) results (30) and predict post-operative naming abilities (31).
Methods
Participants
Temporal lobe epilepsy patients
Forty-four (32% female) TLE patients aged 19-66 (mean age = 39) were included in this study. Participants had a mean age at epilepsy onset of 21 years and a mean duration of epilepsy of 17 years, both of which are typical for patients undergoing evaluation for epilepsy surgery (32-34). Participants were recruited prospectively as part of an ongoing study focusing on language and memory assessments in patients with pharmacoresistant focal onset epilepsy (21,22,35,36). An additional 24 patients were recruited into the parent study but were excluded from our analyses: 11 due to seizures of extratemporal origin and 13 because they were administered a different version of the scene encoding fMRI task (22). Twenty-five patients were determined to have LTLE and 19 RTLE (see Table 1 for demographic information). Data from the first 14 patients (LTLE = 8; RTLE = 6) were included in our previous publication (21). Diagnoses of all patients were based on seizure semiology, neuroimaging results, and neurologists’ impressions from prolonged video-EEG recordings. Final decisions regarding epilepsy type were made by at least two epileptologists. All participants underwent fMRI within 1-3 months of their video-EEG monitoring and neuropsychological testing.
Table 1.
Demographic data for healthy controls and left and right TLE patients
| Healthy Controls | LTLE | RTLE | |
|---|---|---|---|
| N | 40 | 25 | 19 |
| Age (M (SD)) | 33.32 (12.37) | 35.88 (10.23) | 42.26 (12.34)* |
| Sex (% female) | 42% | 40% | 21% |
| Education (M (SD) years) | 15.77 (2.74) | 14.08 (2.78) | 13.47 (2.22) |
| Dominant Handedness | |||
| Right | 31 | 22 | 17 |
| Left | 4 | 2 | 2 |
| Both | 5 | 1 | 0 |
| Age at onset (M (SD) | 17.70 (13.91) | 25.47 (16.82) | |
| Seizure duration (M (SD) years) | 18.16 (13.37) | 16.42 (12.20) | |
| Memory Lateralization with IAP | |||
| Left | 11 | 10 | |
| Right | 8 | 2 | |
| Symmetric | 1 | 1 | |
| Total | 20 | 13 | |
| Language Lateralization with IAP | |||
| Left | 17 | 12 | |
| Right | 4 | 1 | |
| Total | 21 | 13 |
Note. p < 0.05 when compared to healthy controls
Healthy controls
In order to establish activation patterns in healthy controls using this fMRI scene-encoding paradigm, 40 healthy control participants (39% female) aged 19-59 (mean age = 33) with no history of neurological disorders or memory complaints were recruited (10 of these participants were included in our previous study (21). This study was approved by the Institutional Review Board at the University of Cincinnati, and all participants provided written informed consent prior to enrollment.
Procedure
Neuropsychological testing
As in our previous studies, all epilepsy patients underwent an extensive neuropsychological assessment as part of their presurgical clinical evaluation (5,21,37,38). In this study, measures of verbal memory included the Logical Memory, Paired Associates Learning, and Word List Learning subtests of the Wechsler Memory Scale III (WMS-III; (39). The WMS-III auditory memory subtests are designed to measure immediate and delayed recall of verbal information presented in an auditory modality. The WMS-III Logical Memory subtest requires patients to recall verbal information read to them in the context of a story. On the Paired Associates subtest, participants are required to learn word pairs across multiple trials. For the Word List subtest, patients are required to learn and recall a list of words presented to them auditorily. Each of these subtests involves an immediate as well as a delayed recall component. The Benton Visual Retention Test [BVRT, (40)] and Warrington’s Recognition Memory for Faces [RMF, (41)] test were both included as assessments of non-verbal memory functioning. The RMF is a facial recognition task on which participants view photos of 50 unfamiliar men, and memory for these faces is tested immediately with a forced-choice (2 choices) recognition test. The BVRT requires participants to view and immediately draw complex visual forms. All statistical analyses were performed on standard scores corrected for age (and for some tests gender and education) based on published normative data. Neuropsychological data was not gathered from healthy controls.
Functional MRI activation tasks
Scene-encoding task
A “box-car” design functional MRI scene-encoding task was employed for the purposes of this study (10,21,23). As previously, participants were presented with stimuli that represented a balanced mixture of indoor (50%) and outdoor (50%) scenes that included both images of inanimate objects as well as pictures of people and faces. Attention to the task was monitored by asking participants to indicate whether the scene was indoor or outdoor on a button box held in the right hand; participants were instructed to memorize all scenes for later memory testing. In the control condition, participants viewed pairs of scrambled images and were asked to indicate with the use of the button box whether both images in each pair were the same or different (50% were the same). Use of the control condition allowed for subtraction of visuo-perceptual, decision-making, and motor aspects of the task, with a goal of improved isolation of the memory encoding aspect of the active condition.
The paradigm included seven blocks of 10 pictures (alternating between blocks of scrambled pictures and blocks of scenes, for a total of 70 target pictures and 70 scrambled control pairs) and lasted seven minutes and 15 seconds. Each image was presented for 2.5 seconds, followed by 0.5 seconds of a white blank screen. Participants completed a practice run before entering the scanner in order to ensure comprehension of the task. The practice items included five indoor/outdoor scenes as well as five scrambled pictures. Participants did not proceed to the scanner until they responded to all 10 images correctly. Within 10 minutes of completing the scan, participants were administered a post-scan recognition test that included 60 indoor/outdoor scenes, with a balanced content of target and foil pictures. Foil pictures were chosen by matching contents and parameters of foil images to those presented in the scanner. Participants indicated whether they remembered seeing the picture in the scanner by pressing “Y” or “N” on a standard laptop keyboard.
Semantic decision task
Participants were also administered a Semantic Decision/Tone Decision Task (SDTD) which consists of a semantic decision (active) condition and a tone recognition (control) condition (27,42), similar to the SDTD task developed by Binder et al. (30). In the active condition, participants were presented with eight names of animals, to which they responded “1” on the button box if the animal was both a) “native to the United States” and b) “commonly used by humans.” If the animal did not meet either or any of these criteria, the participant responded by pressing “2” on the button box. For the tone recognition task, participants were presented with tone sequences that consisted of four to seven tones ranging from 500 Hz to 750 Hz. Participants were instructed to respond “1” on the button box for any sequence with two 750 Hz tones, or “2” for a sequence with fewer than two 750 Hz tones. The paradigm included seven blocks of eight names of animals (alternating between animal names and tones; a total of 56 animal names and 28 tones) and lasted seven minutes and 15 seconds.
MRI scans
Images were collected on a 4-Tesla Varian MRI scanner. Anatomical scans were collected first and were used to delineate the non-normalized regions of interest (ROI) which allowed for calculation of laterality indices (LI), maximum activation and hippocampal volume. The protocol for anatomical scans was: TR = 13 ms, TE = 6 ms, FOV = 25.6 × 19.2 × 15.0, flip angle array of 3: 22/90/180 with the voxel size of 1 × 1 × 1 mm.
Task stimuli were presented using PsycScope 1.1 (43) running on an Apple Macintosh G3 computer. Participants were equipped with a button box to record responses and to alert the MRI technologist to any problems if necessary. Head movement was minimized with the use of foam padding and head restraints. Manual shimming was followed by echo planar imaging (EPI) which was completed in thirty 4-mm thick contiguous planes sufficient to encompass the apex of the cerebrum to the inferior aspect of the cerebellum in the adult brain. The specific protocol for EPI scans was: TR/TE = 3000/25ms, FOV = 25.6 × 25.6 cm, matrix = 64 × 64 pixels, slice thickness = 4 mm, flip angle array: 85/180/180/90.
Functional MRI data analysis
Image processing was performed with software developed in the Imaging Research Center at Cincinnati Children’s Hospital Medical Center [CCHIPS; (44)] in the IDL software environment (IDL 5.4; Research Systems Inc., Boulder, CO, USA). Individual participant data were analyzed using a general linear model (GLM) to identify voxels activated in the encoding task (45). Following Talairach transformation (spatial transformation with the brain rotated to AC-PC coordinate frame followed by linear scaling into Talairach reference frame), areas of significant group activation for healthy controls were determined using random-effects analysis. Group activation maps were generated using a threshold of z ≥ 1.96. This nominal z score combined with a cluster size of at least 30 resulted in a corrected p value of at least 0.05 for all images, as determined by Monte-Carlo simulation (46). The group activation map for healthy controls was used to generate functional ROIs for lateralization calculations, which were then applied to the healthy controls and epilepsy patients.
Regions of interest (ROI)
Region of interest (ROI) analyses have been employed in a number of memory encoding studies (14,15,19,21,47). As in our previous study (21), we used a functional ROI as well as anatomical ROIs in order to provide guidelines for quantitative evaluation of lateralization of brain activity during the scene-encoding paradigm.
Scene encoding ROI
Seven regions of interest (ROIs) were defined for the purposes of the scene encoding analyses. First, a functional ROI (FROI) was defined based on hippocampal activation in the healthy control group. Activation on the left and right side were mirrored onto the contralateral side to generate the functional map; Talairach coordinates of all pixels within these regions were stored. Only voxels rostral to the coronal -50 mm Talairach space boundary were included in the analysis which represents the approximate location between the hippocampal formation and the lingual gyrus (10,22). Masks of the hippocampal (HROI), parahippocampal (PROI) and combined hippocampal/parahippocampal (HPROI) ROIs were created with the use of the automated anatomical labeling atlas (48) in AFNI (49). In addition, three non-normalized ROIs based on each individual’s neuroanatomy were delineated by hand in CCHIPS. These included individual hippocampal (IHROI), parahippocampal (IPROI), and combined hippocampal/parahippocampal ROIs (IHPROI). Individual ROIs were included in the analysis because normalized ROIs have been shown to inadvertently exclude portions of relevant anatomical structures as well as brain activation in epilepsy patients (50). Additionally, normalized ROIs have been shown to result in differential laterality indices (LI) in epilepsy patients when compared to use of non-normalized, individual ROIs (50). Functional analyses (i.e., LI calculation) were completed with all seven ROIs. The IHROI was used to calculate maximum activation and for structural hippocampal volume analyses.
Language ROI
Language ROIs were defined based on functional activation in the control group in the lateral frontal regions (corresponding to Broca’s area) as well as the left lateral/posterior temporal region (corresponding to Wernicke’s area). As in previous studies, a functional map was generated by mirroring activation on the left and right sides onto the contralateral side to create symmetric ROIs (27). Talairach coordinates of all active voxels were stored.
Lateralization index calculation
In each ROI, the median z value of all activated voxels was calculated and applied as a threshold for lateralization index (LI) calculation (51,52). Within a given ROI, voxels with z higher or equal to the median z value were counted for each participant, and the LI was defined as the difference in number of activated voxels between the left and right ROIs, divided by the sum of active voxels in the left and right ROIs. This approach yields LIs ranging from -1 (strong right lateralization) to 1 (strong left lateralization). This data-driven approach to thresholding has been shown to minimize the effects of outliers with high statistical values and to result in LI calculations with the least amount of variability (51,52).
Maximum activation
The maximum z value across all activated voxels was calculated for the right and left IHROI for each participant. Maximum z values were stored for later analysis.
Hippocampal volume
The mean number of voxels in the left and right IHROI was calculated for each participant and was used as an indicator of hippocampal volume. For each participant, analysis comparing the mean volume of the left versus right hippocampus was completed in order to determine structural symmetry of the hippocampi. Participants were considered to be structurally symmetric when the volume of the left IHROI approximated the volume of the right IHROI. Paired samples t-tests were used to investigate individual differences in the volume of the left versus the right hippocampus. Participants demonstrating a significant (p≤0.05) difference in the left and right hippocampal volumes were considered to be structurally asymmetric.
Intracarotid amobarbital procedure
An intracarotid amobarbital procedure (IAP) was performed in 33/44 (75%) of the TLE patients (80% of LTLE patients, 68% of RTLE patients) as part of a presurgical clinical evaluation (team performing fMRI was blinded to the results of IAP until after the fMRI procedure). Laterality as determined by IAP was used to compare laterality as determined by the fMRI scene-encoding task. IAP was performed by first injecting sodium amobarbital into the affected cerebral hemisphere, followed by injection into the contralateral hemisphere using previously described methods (22,27). Typically, 125 mg of sodium amobarbital were injected first followed by a second hemisphere injection of 100 mg. Language testing was performed during recovery from the effects of anesthesia, and memory testing was performed approximately 10 minutes following completion of language testing. Memory was assessed by presenting patients with pictures that can be encoded with the use of verbal, nonverbal, or a combination of both verbal and nonverbal strategies, which they were later asked to recognize among pictures of targets and foils (10,22). IAP memory lateralization indices were calculated with methods previously described by Rausch et al., (53).
Statistical analyses
Statistical analyses were carried out in SPSS (SPSS Inc., Chicago, IL., USA). Structural, functional, and LI differences between LTLE and RTLE patients and healthy controls were investigated with the use of one-way and multivariate ANOVA and Mann-Whitney U when appropriate. Pearson and Spearman correlation analyses were used to investigate the relationship between lateralization and performance. Non-parametric analyses (i.e., Mann-Whitney U and Spearman correlation analyses) were completed when data did not meet the assumption of normality (tested with the use of Q-Q plots).
Results
Background Analyses
The following analyses were completed in order to provide descriptive information about hippocampal volume, maximum activation, fMRI task performance and performance on standardized neuropsychological measures.
Structural data and maximum activation
A 3 (group: LTLE, RTLE, healthy) × 2 (hippocampal volumes) mixed ANOVA was completed in order to investigate structural hippocampal symmetry. Results indicated that there was no significant main effect of group, F(2, 81) = 0.18, p = 0.837, or hippocampal volume, F(1, 81) = 2.04, p = .157. Results did unveil a significant interaction between hippocampal volume and group, F(2, 81) = 6.20, p = .003. Simple effects analyses demonstrated structural symmetry of hippocampi among healthy controls (Mleft = 2.07, SDleft = .27 cm3; Mright= 2.16, SDright = 0.42 cm3), t(39) = −1.47, p = .150. LTLE patients demonstrated significantly larger right (M = 2.24, SD = .65 cm3) compared to left (M = 1.92, SD = .78 cm3) hippocampi, t(24) = -−2.71, p = .012. Although qualitatively RTLE patients demonstrated larger left hippocampi the differences between left (M = 2.13, SD = .42 cm3) and right (M = 1.95, SD = .47 cm3) hippocampal volumes were not significantly different, t(18) = 1.73, p = .100. See Table 2 for structural LI.
Table 2.
Lateralization and performance data for healthy controls, and left and right TLE patients.
| Healthy Controls | LTLE | RTLE | |
|---|---|---|---|
| Hippocampal Volume (M(SD) cm3) | |||
| Left | 2.07 (.27) | 1.92 (.78)β | 2.13 (.42) |
| Right | 2.16 (.42) | 2.24 (.65)β | 1.95 (.47) |
| Maximum Activation | |||
| Left | 64.30 (13.25) | 62.44 (18.51) | 59.84 (11.04) |
| Right | 65.40 (16.77) | 63.28 (8.93) | 60.89 (9.58) |
| Mean fMRI LI± [M(SD)] | |||
| HROI | .00 (.12) | .00 (.24) | .12 (.18) * |
| PROI | .00 (.20) | −.05 (.29) | .11 (.24) |
| HPROI | .00 (.09) | −.01 (.19) α | .13 (.16)* |
| FROI | .00 (.15) | −.03 (.19) α | .14 (.19)* |
| fMRI Memory Task Performance (M (SD) % correct) |
|||
| Indoor/Outdoor | 90.85 (3.00) | 86.36 (9.39)* | 83.76 (10.71)* |
| Scrambled Pictures | 93.25 (4.10) | 80.06 (17.92)* | 80.18 (13.50)* |
| Recognition Test | 86.33 (5.83) | 76.08 (9.36)* | 75.35 (8.01)* |
| fMRI SDTD Task Performance (M(SD) % correct) |
|||
| Semantic Decision | 78.02 (8.62) | 69.62 (10.21)* | 66.36 (10.17)* |
| Tones | 95.05 (4.77) | 80.12 (12.19) α* | 67.00 (15.82) * |
| Performance on Neuropsychological | |||
| Measures (M (SD) Scaled Score) | |||
| Verbal Memory: WMS-III | |||
| Logical Memory Immediate | 8.91 (3.54) | 9.35 (3.48) | |
| Logical Memory Delayed | 8.74 (3.58) | 9.53 (3.47) | |
| Paired Associates Immediate | 8.86 (4.19) | 8.67 (3.20) | |
| Paired Associates Delayed | 8.95 (4.14) | 9.33 (2.47) | |
| Word List Immediate | 7.86 (3.37) | 10.23 (3.33) | |
| Word List Delayed | 8.45 (3.19) α | 10.65 (2.96) | |
| Word List Interference Score | 7.32 (3.73) | 9.00 (2.89) | |
| Visuospatial Memory: Benton Visual | |||
| Retention Test (M(SD) Z-score) | |||
| Correct | −.88 (1.21) | −.95 (1.05) | |
| Errors | .84 (1.31) | .83 (1.08) | |
| Visuospatial Memory: Warrington Recognition Memory for Faces |
9.74 (3.30) | 9.50 (3.98) |
Note. ±LI = (L-R/L+R). p < .05 when compared to healthy controls.
p < .05 between left and right TLE.
p < .05 between left and right hippocampi.
Pearson correlation analyses were then conducted to investigate the relationship between structural hippocampal volumes and maximum hippocampal activation. Results indicated that among LTLE patients, left hippocampal volume was positively associated with maximum activation of the left hippocampal region, r(23) = .44, p = .031. In addition, there was a positive relationship between right hippocampal volume and right hippocampal maximum activation among RTLE patients, r(17) = .52, p = .023.
In addition, Pearson correlation analyses were completed in order to investigate the relationship between hippocampal volume and variables considered to indicate seizure chronicity, namely epilepsy duration and age at seizure onset. Bonferroni correction for multiple (four) comparisons was applied to the analyses below, resulting is an alpha level of 0.012. Among LTLE patients, results demonstrated a nonsignificant negative trend toward significance between left hippocampal volume and epilepsy duration, r(23) = −.43, p = .033. In addition, left hippocampal volume among LTLE patients was positively correlated with age at epilepsy onset, r(23) = .59, p = .002. Similar findings were also observed among RTLE patients. In particular, right hippocampal volume was found to be negatively associated with epilepsy duration, r(17) = −.57, p = .011 (i.e., longer epilepsy duration was associated with smaller hippocampus), and positively associated with age at seizure onset, r(17) = .65, p = .002 (i.e., later seizure onset was associated with larger hippocampus).
Performance data
Functional MRI tasks
Scene- encoding task
Group differences on task performance were investigated in the epilepsy patients with the use of a 2 (condition) × 3 (group) mixed-factor ANOVA, with the unit of measurement being the mean percent of scenes answered correctly (i.e., for the active condition indoor vs. outdoor; for the control condition same vs. different). Results demonstrated a significant main effect of condition with participants performing significantly better in the active condition (M = 88.01, SD = 7.88) compared to the control condition (M = 86.52, SD = 13.52), F(1, 79) = 4.73, p = .033. Additionally, results demonstrated a significant main effect of group, F(2, 79) = 12.04, p < .001. Post-hoc analyses using Tukey’s HSD test indicated that healthy controls performed significantly better (M = 92.05, SD = 1.37), than left (M = 83.22, SD = 1.73) and right (M = 81.97, SD = 2.10) TLE patients overall, both p < .001. However, no significant differences on task performance were observed between left and right TLE patients, p = .890.
There was also a significant interaction between task and group, F(2, 79) = 6.59, p = .002. Simple effects analyses demonstrated that healthy controls, and left and right TLE patients differed significantly on task performance for both conditions [active: F(2, 81) = 6.40, p = .003); control:(F(2, 81) = 12.37, p < .001)]. In particular, healthy controls answered significantly more trials correctly on the active condition (Mactive = 90.86, SDactive = 3.00), compared to RTLE (Mactive = 83.76, SDactive = 10.71, p = .004) and LTLE (Mactive = 86.36, SDactive = 9.39, p = .050) patients. Healthy controls also demonstrated superior performance on the control condition (Mcontrol = 93.25, SDcontrol = 4.10) compared to LTLE (Mcontrol = 80.08, SDcontrol = 17.92, p < .001) and RTLE (Mcontrol = 80.18, SDcontrol = 13.50, p = .001) patients. No significant differences were observed between left and right TLE patients on the active (p = .506) or control conditions (p = 1.00). Further analyses also demonstrated that healthy controls performed significantly better on the control condition (Mcontrol = 93.25, SDcontrol = 4.10) than the active condition (Mactive = 90.86, SDactive = 3.00), t(39) = −3.23, p = .003. However, LTLE patients performed significantly better on the active condition (Mactive = 86.36, SDactive = 9.39) compared to the control condition (Mcontrol = 80.08, SDcontrol = 17.92), t(24) = 2.08, p = .048. No significant differences were unveiled for RTLE patients’ performance on the active (Mactive = 83.76, SDactive = 10.71) versus the control (Mcontrol = 80.18, SDcontrol = 13.50) condition, t(16) = 1.71, p = .107.
One-way ANOVA results indicated that healthy controls performed significantly better on the post-scan recognition test (M = 86.33, SD = 5.83 percent correct) than both left (M = 76.08, SD = 9.36 percent correct), F(1, 64) = 29.24, p < .001, and right (M = 74.38, SD = 8.01 percent correct), F(1, 56) = 39.58, p < .001, TLE patients. No significant differences were observed between left and right TLE patients on the post-scan recognition testing, p = .538. See Table 2 for group performance.
Language task
One-way ANOVA demonstrated significant differences between groups in performance on the semantic decision task, F(2, 75) = 10.68, p < .001. Post-hoc analyses demonstrated that healthy controls performed significantly better on the semantic decision task (M = 78.02, SD = 8.62 percent correct) when compared to right (M = 66.36, SD = 10.17 percent correct, p < .001), and left (M = 69.62, SD = 10.21 percent correct, p = .003), TLE patients. However, no significant differences were observed between left and right TLE patients on the semantic decision portion of the language task, p = .916. Results indicated that healthy controls (M = 95.05, SD = 4.77 percent correct) also outperformed both left (M = 80.12, SD = 12.19 percent correct) as well as right (M = 67.00, SD = 15.82 percent correct) TLE patients on the tone decision task, F(2, 75) = 46.07, p < .001. Additionally, LTLE patients performed significantly better than RTLE patients on the tone decision task, p = .001. These results are in concordance with previous work from our group (42,54) as well as findings by Springer et al. (9) which demonstrated that healthy controls outperform epilepsy patients on both the active (semantic decision) and control (tone decision) conditions of this fMRI SDTD paradigm.
Neuropsychological performance
Due to the non-Gaussian distribution of neuropsychological performance data, a Mann-Whitney U analysis was completed in order to determine the differences in performance on neuropsychological measures between left and right TLE patients. Results revealed that RTLE patients demonstrated superior performance on the WMS-III Word List Delayed Recall when compared to LTLE patients, U = 110.00, p = .028. No significant differences were observed between right and LTLE patients on the WMS-III Logical Memory (Immediate, p = .762; Delayed, p = .450) or Paired Associates (Immediate, p = .551; Delayed, p = .871) subtests, the RMF (p = .988), or the BVRT (Correct, p = .806; Errors, p = .916. Although these comparisons did not reach statistical significance, qualitative inspection of the data indicated that overall LTLE patients demonstrated lower verbal memory performance when compared to RTLE patients. In addition, RTLE patients demonstrated comparatively lower visuospatial memory performance (as measured by the BVRT).
Results: Hypothesis 1
fMRI lateralization indices for memory
Qualitatively, healthy controls demonstrated symmetric activation of all ROIs (see Table 2 for mean lateralization indices for each group). A series of one-way ANOVAs was conducted to compare LIs for scene encoding between healthy controls and left and right TLE patients. Levene’s test for equality of variance indicated a lack of homoscedasticity for HROI, and as such post-hoc analyses for group differences in LI of HROI were completed using Games-Howell’s test. All other post-hoc analyses were completed using Tukey’s HSD test.
Results indicated a significant difference between groups in lateralization of the hippocampal ROI (HROI), F(2, 81) = 3.61, p = .031; combined hippocampal/parahippocampal ROI (HPROI), F(2, 81) = 5.84, p = .004; and the functional ROI (FROI), F(2, 81) = 6.22, p = .003. Post-hoc analyses indicated that compared to the control group, RTLE patients appeared significantly more left lateralized for HROI, p = .027, HPROI, p = .009, and FROI, p = .008. RTLE patients also appeared significantly more left lateralized for HPROI, p = .007, and FROI, p = .005 when compared to LTLE patients. No significant differences were observed in lateralization of HROI between left and right TLE patients, p = .136.
Although not significant, the data also demonstrated a trend for group differences in lateralization of parahippocampal ROI (PROI), F(2, 81) = 2.77, p = .068, and the non-normalized hippocampal ROI (IHROI), F(2, 81) = 2.79, p = .067. Post-hoc analyses indicated that RTLE patients trended towards comparatively greater left lateralization of PROI, p = .060, and IHROI, p = .057 when compared to LTLE patients. However, no significant differences were observed between RTLE patients and healthy controls on lateralization of PROI, p = .173, or IHROI, p = .193.
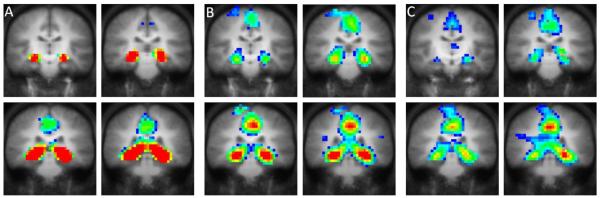
No significant differences were discovered between groups on non-normalized parahippocampal (IPROI) and hippocampal/parahippocampal (IHPROI) ROIs: IPROI: F(2, 81) = .21, p = .807; IHPROI: F(2, 81) = 1.06, p = .351. LTLE demonstrated qualitatively symmetric activation of all ROIs (MHROI = .00, MHROI = .24; MPROI = −.05, SDPROI = .29; MHPROI = −.01, SDHPROI = .19; MFROI = −.03, SDFROI = .19), which did not differ significantly from the symmetric activation observed among healthy control participants (HROI, p=1.000; PROI, p = .717; HPROI, p = .924; FROI, p = .855). Figure 1 illustrates the mean activation patterns in healthy controls and epilepsy patients.
Figure 1.
Differences in functional MRI activation patterns between active and control conditions for the fMRI scene-encoding task in A) healthy controls (N = 40), B) LTLE patients (N = 25), C) RTLE patients (N = 19). All images are presented in radiological convention (i.e., left hemisphere is represented on right side of image). Coronal images are presented with y = −16 for the left upper image and y = −30 for the right lower image of each panel.
In order to investigate the relationship between language lateralization and memory representation among LTLE patients, two-tailed Pearson correlation analysis was completed. Results indicated no significant relationship between lateralization of FROI and language lateralization as determined by the SDTD fMRI paradigm, t(23) = .10, p = .623.
Results: Hypothesis 2
Scene encoding lateralization and performance
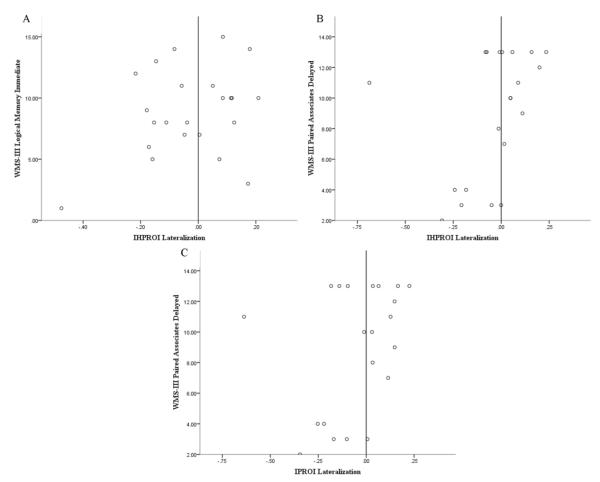
Due to the non-Gaussian distribution of the neuropsychological performance data, two-tailed Spearman correlation analyses were conducted in order to investigate the relationship between LI and memory performance. Results indicated that LTLE patients with less right lateralization of IHPROI performed better on the WMS-III Logical Memory Immediate, r(21)=0.42, p=0.049, and Paired Associates Delayed subtests, r(19)= 0.46, p=0.036 (refer to Table 3 for correlations between LI and performance on neuropsychological measures). In addition, improved performance on the WMS-III Paired Associated Delayed subtest was observed among LTLE patients with less right lateralization of IHROI, r(19) = .44, p = .047. Figure 2 shows the relationship between lateralization and neuropsychological performance for LTLE patients.
Table 3.
Correlations between scene encoding lateralization and memory performance for LTLE.
| Neuropsychology Measure | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| WMS– III LMI |
WMS- III LMD |
WMS- III PAI |
WMS- III PAD |
WMS- III WLT |
WMS- III WLI |
BVRT- C |
BVRT- E |
RM F |
|
| HROI | .20 | .244 | .12 | .26 | .21 | .22 | −.01 | −.22 | −.09 |
| PROI | .23 | −.08 | .12 | .19 | .14 | −.10 | .04 | −.32 | −.15 |
| HPROI | .35 | .24 | .31 | .38 | .27 | .09 | −.02 | −.28 | −.18 |
| FROI | .16 | −.06 | .00 | .06 | .00 | −.05 | −.16 | −.11 | −.28 |
| IHROI | .38 | .15 | .39 | .44* | .25 | −.13 | .05 | −.30 | −.05 |
| IPROI | .39 | .26 | .37 | .41 | .323 | −.09 | −.07 | −.15 | −.02 |
| IHPRO I |
.42* | .29 | .42 | .46* | .34 | −.09 | −.11 | −.16 | −.12 |
Note. p < .05. LMI = Logical Memory Immediate; LMD = Logical Memory Delayed; PAI Paired Associates Immediate; PAD = Paired Associates Delayed; WLT = Word List Total; WLI = Word List Interference; BVRT-C = BVRT number correct; BVRT-E = BVRT number of errors.
Figure 2.
Correlation between A) lateralization of IHPROI and performance on WMS-III Logical Memory Immediate subtest, B) lateralization of IHPROI and performance on WMS-III Paired Associates Delayed subtest, and C) lateralization of IPROI and performance on WMS-III Paired Associates Delayed subtest for LTLE patients.
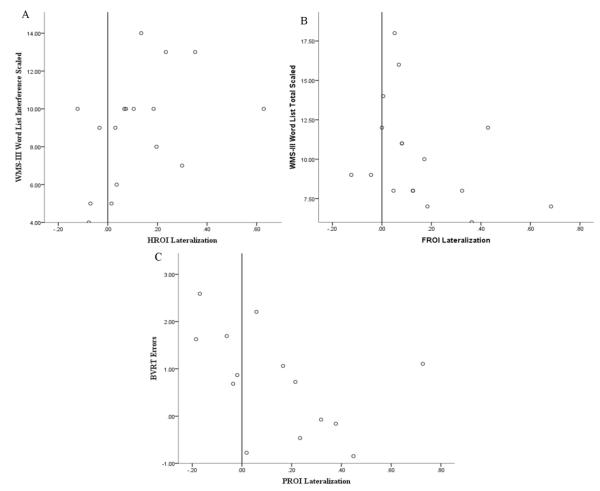
As can be seen in Table 4, RTLE patients with greater left lateralization of HROI demonstrated fewer interference errors on the WMS-III Word List subtest, r(15) = .52, p = .031. RTLE patients who demonstrated symmetric lateralization of FROI also performed better on the WMS-III Word List subtest, r(15) = −.48, p = .049. Additionally, RTLE in whom the PROI was more left lateralized demonstrated a greater number of errors on the BVRT recall, r(12) = −.55, p = .043. Figure 3 shows the relationship between lateralization and neuropsychological performance for RTLE patients.
Table 4.
Correlations between scene encoding lateralization and memory performance for RTLE.
| Neuropsychology Measure | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| WMS– III LMI |
WMS- III LMD |
WMS- III PAI |
WMS- III PAD |
WMS- III WLT |
WMS- III WLI |
BVRT- C |
BVRT- E |
RM F |
|
| HROI | .20 | .31 | .30 | .16 | .18 | .52* | .03 | −.34 | .13 |
| PROI | .05 | .05 | −.27 | −.14 | −.26 | .04 | .34 | −.55* | .10 |
| HPROI | .09 | .19 | −.02 | −.11 | −.09 | .41 | .03 | −.42 | .01 |
| FROI | −.11 | −.06 | −.37 | −.40 | −.45* | .16 | .17 | −.36 | −.17 |
| IHROI | .24 | .15 | −.05 | −.09 | .03 | −.08 | .02 | −.24 | −.04 |
| IPROI | .23 | .21 | .11 | .10 | .13 | .05 | −.23 | −.08 | −.29 |
| IHPRO I |
.26 | .21 | 0.00 | −.02 | .13 | −.03 | −.01 | −.23 | −.07 |
Note. p < .05. LMI = Logical Memory Immediate; LMD = Logical Memory Delayed; PAI Paired Associates Immediate; PAD = Paired Associates Delayed; WLT = Word List Total; WLI = Word List Interference; BVRT-C = BVRT number correct; BVRT-E = BVRT number of errors.
Figure 3.
Correlation between A) lateralization of HROI and number of interference errors on the WMS-III Word List subtest, B) lateralization of FROI and performance on WMS-III Word List subtest, and C) lateralization of PROI and number of errors on the BVRT for RTLE patients.
Post-hoc analyses
Given our unexpected findings that LTLE patients demonstrated symmetric lateralization of memory encoding during the fMRI scene encoding paradigm, the following analyses were completed in order to examine lateralization as it is determined by IAP and the relationship between IAP and fMRI lateralization.
Intracarotid amobarbital procedure
A post-hoc analysis was completed in order to investigate differences in memory lateralization among left and right TLE patients as measured by IAP and by the scene encoding fMRI task. A 2 (method) by 2 (memory lateralization) mixed-factor ANOVA was completed for this analysis. In order to complete these analyses, fMRI memory lateralization indices were multiplied by 100 to ensure to that both scales represented the same unit of measurement. For results presented below memory lateralization ranges from - 100 (strong right) to 100 (strong left). Results demonstrated that the main effect of method (i.e., IAP vs. fMRI) trended towards significance, F(1, 31) = 3.96, p = .055, with IAP demonstrating greater left lateralization of memory encoding (M = 13.51, SD = 31.37) overall when compared to lateralization as determined by fMRI (M = 2.64, SD = 19.54). Results also demonstrated a significant main effect of memory lateralization, F(1, 31) = 7.89, p = .009, with RTLE patients demonstrating significantly greater left lateralization of memory encoding overall (M = 19.43, SD = 5.23) compared to LTLE patients (M = .69, SD = 4.22). However, no significant interaction was discovered between method of LI determination and seizure lateralization, F(1, 31) = .19, p = .666
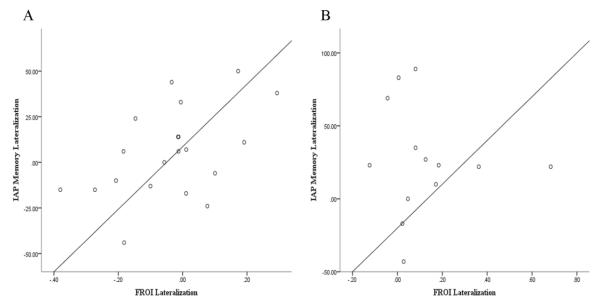
In order to investigate the relationship between memory lateralization as assessed by IAP and the fMRI scene-encoding paradigm employed in this study, Pearson correlation analysis was completed. Although the two methods were mildly correlated (r(31) = .29), results did not reach significance (p = .099). When considering the association between the measures of memory lateralization among left and RTLE patients (fMRI and IAP), results indicated a significant positive association between the measures among LTLE patients, r(18) = .499, p = .025; however, the two methods were not significantly correlated among RTLE patients, r(12) = −.081 , p = .793. Figure 4 shows the relationship between memory lateralization as assessed by IAP and fMRI.
Figure 4.
The relationship between memory lateralization as determined by IAP and by fMRI memory encoding task for A) LTLE patients and B) RTLE patients.
Discussion
This study investigated the effects of epilepsy on memory lateralization and performance in a sample of patients with pharmacoresistant, video-EEG confirmed temporal lobe epilepsy, using an fMRI scene-encoding task previously shown to elicit symmetric hippocampal activation in healthy populations (10,21,23). Our results from healthy controls are in agreement with findings from these previous studies. This finding suggests that this complex visual scene-encoding task stimulates both visuospatial and verbal memory encoding strategies; however, this inference cannot be determined with certainty as participants were not questioned about the encoding strategies used during this task which is a limitation of the current study.
Our findings demonstrated structural hippocampal symmetry among healthy controls. As expected, the TLE patients had relatively smaller hippocampi in the affected than the unaffected hemisphere, consistent with the hippocampal sclerosis that is common in this population (55). Decreased hippocampal volume in the hemisphere ipsilateral to the seizure focus was associated with lower maximum activation in this region, which suggests that hippocampal sclerosis is associated not only with volume loss but also with a reduction in functional tissue. In addition, our results indicated that the degree of tissue loss is associated with chronicity (i.e., epilepsy duration and age at seizure onset) of epilepsy, indicating possible progressive seizure related damage to temporal areas.
Our findings are also in partial agreement with neuropsychological literature demonstrating the presence of material-specific memory impairments among epilepsy patients (5,6,37,56,57). In particular, LTLE patients demonstrated inferior verbal learning and verbal memory retrieval when compared to RTLE patients. This finding is consistent with literature suggesting the presence of verbal memory deficits among LTLE patients (7). We did not identify material-specific memory deficits in the RTLE group, as both left and right TLE patients were equivalently and only mildly impaired (relative to normative data collected by the test publisher) on the BVRT, and the mean scores in both patient groups were well within the normal range on the RMT. These results are consistent with findings which indicate that neuropsychological measures are more sensitive to left- compared to right-sided dysfunction (4).
In terms of fMRI-derived memory lateralization, our results revealed that RTLE patients demonstrate significantly greater left lateralization of activation during scene encoding than healthy controls and LTLE patients. This result is in agreement with the results of previous studies by Bellgowan et al. (17) and Powell et al. (19), who demonstrated increased left lateralization of verbal memory encoding among RTLE patients, and extends their findings to stimuli that are known to be processed in healthy individuals in both cerebral hemispheres. The present findings may not be entirely specific to patients with mesial temporal involvement as Vannest et al. (21) also reported a trend for a mixed group of patients with right temporal (6 of which were included in the current study) and extratemporal lobe epilepsy to demonstrate increased left lateralization of memory encoding during a scene-encoding task.
Our results diverge from findings presented by Mechanic-Hamilton et al. (15) who reported that RTLE patients did not demonstrate increased left lateralization of memory encoding. Although the latter study also employed a scene-encoding task, it was a small scale study involving only eight RTLE patients (as reported in Table 1 of Mechanic-Hamilton et al., 2009). Thus, the differences between our study and the one completed by Mechanic Hamilton et al. (15) are likely associated with the small number of participants included in the latter study and the relatedly low power to detect significant group differences. Taken together these findings provide support for the presence of lateralization of memory encoding to the hemisphere contralateral to seizure focus in RTLE patients.
LTLE patients demonstrated symmetric activation for all ROIs during the scene-encoding task, which was a somewhat unexpected finding. One potential explanation for this negative result is the possibility that, given that reorganization of language functioning has been demonstrated among LTLE patients (11-13), language lateralization may be acting as a confounding factor in these analyses. This hypothesis was in agreement with findings by Vannest et al. (21) which indicated that of left hemispheric epilepsy patients in their study who demonstrated right language dominance, 83% also demonstrated right lateralization of memory encoding during a complex scene-encoding task. Thus, further analyses were completed to investigate the relationship between memory lateralization and language lateralization in LTLE patients which did not reveal a significant association between lateralization of memory encoding and language lateralization as determined by the SDTD task. This suggests that the variance in lateralization of memory encoding among LTLE patients cannot be accounted for by atypical language lateralization.
A possible explanation for the symmetric activation observed among LTLE patients is that the left hemisphere verbal memory strategies used for this scene-encoding task are not substantially affected by epilepsy as previously thought (10,15,21) and thus, dominant lateralization in the unaffected hemisphere is not observed. This notion is supported by the results of previous studies which demonstrate postsurgical verbal memory deficits among LTLE patients who have undergone resective epilepsy surgery (47,58-62). Thus, our results suggest that LTLE patients, like healthy controls, may use both verbal and visuospatial strategies when encoding complex visual scenes and this predisposes them to a higher chance of post-resection deficits. Novel fMRI tasks assessing temporal lobe contribution to language and verbal memory functions are now being investigated for their application to presurgical prediction of postsurgical deficits (63).
Our results disagree with some of the findings from a number of small-scale studies that demonstrated increased right lateralization of memory encoding among left hemispheric epilepsy patients (10,15,21). Although these studies also employed complex scene-encoding tasks, our study is the first larger scale fMRI study (LTLE N=25) investigating memory lateralization among epilepsy patients. Thus, it is possible that this discrepancy is accounted for by our larger sample size which possibly offers a more representative account of memory encoding strategies elicited by LTLE patients with the use of a complex scene-encoding task. This interpretation of the results is supported by our IAP results demonstrating a slight left lateralization of memory functioning among the included LTLE patients. This is consistent with our fMRI results, which demonstrate that LTLE patients continue to rely on the left (in addition to the right) hemisphere during scene encoding. Similarly, our findings also demonstrated that increased left lateralization among LTLE patients was associated with superior verbal memory performance.
We have also demonstrated that memory lateralization towards the unaffected hemisphere was associated with inferior memory performance. In particular, our results indicate that RTLE patients with greater left lateralization of scene encoding demonstrate inferior visuospatial recall. In addition, improved verbal memory performance was observed among LTLE patients who demonstrated less right lateralization of memory encoding during this scene-encoding task. These results are consistent with the study by Powell et al. (19) who demonstrated improved verbal and visuospatial memory performance among TLE patients in whom memory was lateralized towards the affected hemisphere. These results are also supported by Vannest et al. (21) who demonstrated better verbal memory performance among LTLE patients who maintained left lateralization of memory encoding. These findings are also in agreement with findings by Guedj et al. (64) who, with the use of PET, demonstrated a positive association between metabolism of the entorhinal/perihrinal cortex ipsilateral to the side of seizure focus and recognition performance (64). Furthermore, with the use of this task we did not observe fMRI activations outside of the medial temporal and occipital (due to visual presentation of the task) regions that could suggest the use of compensatory strategies for memory performance observed, for example, for recognition memory in patients with epilepsy (65).
Our findings suggest that the hemisphere contralateral to seizure focus may not adequately support memory functioning, which directly contests a hypothesis of functional reserve which has been proposed to explain memory decrements following temporal lobectomy (66). This hypothesis suggests that postsurgical memory functioning depends on the functional reserve (i.e., memory capacity) of the contralateral temporal lobe in TLE patients undergoing unilateral temporal lobectomy. However, our results indicate instead that memory functioning is better supported by the hemisphere ipsilateral to seizure focus. This finding provides support for a hypothesis of functional adequacy, which suggests that postsurgical memory deficits depend on the functional adequacy of the resected tissue (66).
The functional adequacy hypothesis is further supported by studies investigating the risk of postoperative memory decline following anterior temporal lobectomy (ATL) in TLE patients. In particular, studies indicate that good preoperative verbal memory performance (67-69) and increased left lateralization of preoperative verbal memory encoding (16,47,60,62,70,71) predict greater postoperative decline among LTLE patients who undergo left temporal lobectomy for epilepsy management. In addition, recent findings by Bonelli et al. (60) demonstrated that increased preoperative activation in the left anterior MTL was associated with postoperative verbal memory decline but preoperative activation in the left posterior MTL was associated with superior postoperative verbal memory (60). Given that the posterior MTL is frequently spared during ATL surgery, the authors interpreted this result as an indication of intrahemispheric compensation of verbal memory functioning among LTLE patients (60). These findings are congruent with our findings demonstrating superior memory performance among TLE patients who maintain traditional memory representation.
As with all studies, this study has a number of limitations. One limitation is the failure to investigate group differences in anterior and posterior MTL activation. As indicated above, previous studies have demonstrated the importance of investigating anterior versus posterior activation (60,72). In particular, Banks et al. (72) investigated group differences in lateralization of verbal and visual memory and demonstrated differential lateralization specific to posterior and anterior portions of the MTL (72). Additionally, preoperative anterior MTL activation has been shown to be the strongest predictor of postoperative verbal and visual memory decline in patients undergoing ATL, demonstrating greater predictive value than preoperative learning scores, hippocampal volume, and language lateralization (60). However, as mentioned above, preoperative activation in the posterior MTL has been shown to be associated with improved postoperative verbal and visual memory performance (60). Another limitation of this study is the possible effects of antiepileptic medication (AED) on the cognitive functioning as well as fMRI activation patterns of TLE patients. In particular, 13 (LTLE = 7; RTLE = 6) of the TLE patients included in this study were prescribed topiramate (TPM) which is an antiepileptic medication known to have negative effects on cognitive functioning (73,74). Furthermore, patients prescribed TPM have also been shown to elicit differential activation patterns in response to a semantic decision fMRI paradigm when compared to patients taking other antiepileptic medications (42). Given these findings, it is possible that the use of TPM by almost 30% of our epilepsy sample may have been a confounding factor in the findings of this study.
Another possible limitation of this study is the use of an fMRI memory encoding paradigm which is assumed to elicit both verbal and nonverbal encoding strategies. It was decided to use this paradigm in order to minimize the time that participants spent in scanner; however, the use of specific and distinct verbal and non-verbal paradigms would have allowed for more specific conclusions about lateralization of material-specific memory encoding. Another limitation of this study is that a second rater was not used when delineating the non-normalized ROIs, which made assessments of inter-rater reliability impossible. Although all non-normalized ROIs were delineated by hand by the same investigator (C.P.B.) using consistent anatomical guidelines, the use of a second rater would have allowed for an added assessment of consistency.
Conclusion
Overall, our study demonstrated organization of memory encoding to the contralateral medial temporal regions among RTLE patients. However, LTLE patients demonstrated symmetric lateralization of memory encoding, similar to that observed among healthy controls (but different from RTLE patients). In addition our findings indicate that memory functioning is better supported by the affected hemisphere than the hemisphere contralateral to seizure focus.
Highlights.
Healthy controls and LTLE patients demonstrated symmetric activation
RTLE patients demonstrated comparatively greater left activation
Poor verbal memory was related to right lateralization of scene encoding in LTLE
Poor visuospatial memory was related to left lateralization of encoding in RTLE
Right lateralization of encoding was associated with better verbal memory in RTLE
Acknowledgments
This research was supported in part by NIH K23 NS052468, the University of Cincinnati Neuroscience Institute, the Cincinnati Epilepsy Center, and the University of Cincinnati University Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991 Sep 20;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- (2).Frisk V, Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia. 1990;28(4):349–359. doi: 10.1016/0028-3932(90)90061-r. [DOI] [PubMed] [Google Scholar]
- (3).Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19(6):781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- (4).Lee TMC, Yip JTH, Jones-Gotman M. Memory Deficits after Resection from Left or Right Anterior Temporal Lobe in Humans: A Meta-Analytic Review. Epilepsia. 2002;43(3):283–291. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- (5).Kent GP, Schefft BK, Howe SR, Szaflarski JP, Yeh HS, Privitera MD. The effects of duration of intractable epilepsy on memory function. Epilepsy Behav. 2006 Nov;9(3):469–477. doi: 10.1016/j.yebeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- (6).Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997 Apr;54(4):369–376. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- (7).Delaney RC, Rosen AJ, Mattson RH, Novelly RA. Memory function in focal epilepsy: a comparison of non-surgical, unilateral temporal lobe and frontal lobe samples. Cortex. 1980 Mar;16(1):103–117. doi: 10.1016/s0010-9452(80)80026-8. [DOI] [PubMed] [Google Scholar]
- (8).Alessio A, Damasceno BP, Camargo CH, Kobayashi E, Guerreiro CA, Cendes F. Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav. 2004 Feb;5(1):22–27. doi: 10.1016/j.yebeh.2003.10.010. [DOI] [PubMed] [Google Scholar]
- (9).Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999 Nov;122(Pt 11):2033–2046. doi: 10.1093/brain/122.11.2033. (Pt 11) [DOI] [PubMed] [Google Scholar]
- (10).Detre JA, Maccotta L, King D, Alsop DC, Glosser G, D’Esposito M, et al. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998 Apr;50(4):926–932. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- (11).Hamberger MJ, Cole J. Language Organization and Reorganization in Epilepsy. Neuropsychol Rev. 2011 Aug 13; doi: 10.1007/s11065-011-9180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Tanriverdi T, Al Hinai Q, Mok K, Klein D, Poulin N, Olivier A. Atypical language lateralization in patients with left hippocampal sclerosis: does the hippocampus affect language lateralization? Turk Neurosurg. 2009 Jan;19(1):1–14. [PubMed] [Google Scholar]
- (13).Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, et al. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006 Feb;129(Pt 2):346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- (14).Golby AJ, Poldrack RA, Illes J, Chen D, Desmond JE, Gabrieli JD. Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia. 2002 Aug;43(8):855–863. doi: 10.1046/j.1528-1157.2002.20501.x. [DOI] [PubMed] [Google Scholar]
- (15).Mechanic-Hamilton D, Korczykowski M, Yushkevich PA, Lawler K, Pluta J, Glynn S, et al. Hippocampal volumetry and functional MRI of memory in temporal lobe epilepsy. Epilepsy Behav. 2009 Sep;16(1):128–138. doi: 10.1016/j.yebeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Rabin ML, Narayan VM, Kimberg DY, Casasanto DJ, Glosser G, Tracy JI, et al. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004 Oct;127(Pt 10):2286–98. doi: 10.1093/brain/awh281. [DOI] [PubMed] [Google Scholar]
- (17).Bellgowan PS, Binder JR, Swanson SJ, Hammeke TA, Springer JA, Frost JA, et al. Side of seizure focus predicts left medial temporal lobe activation during verbal encoding. Neurology. 1998 Aug;51(2):479–484. doi: 10.1212/wnl.51.2.479. [DOI] [PubMed] [Google Scholar]
- (18).Killgore WD, Glosser G, Casasanto DJ, French JA, Alsop DC, Detre JA. Functional MRI and the Wada test provide complementary information for predicting post-operative seizure control. Seizure. 1999 Dec;8(8):450–455. doi: 10.1053/seiz.1999.0339. [DOI] [PubMed] [Google Scholar]
- (19).Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, et al. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia. 2007 Aug;48(8):1512–1525. doi: 10.1111/j.1528-1167.2007.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Richardson MP, Strange BA, Duncan JS, Dolan RJ. Preserved verbal memory function in left medial temporal pathology involves reorganisation of function to right medial temporal lobe. Neuroimage. 2003 Nov;20(Suppl 1):S112–9. doi: 10.1016/j.neuroimage.2003.09.008. [DOI] [PubMed] [Google Scholar]
- (21).Vannest J, Szaflarski JP, Privitera MD, Schefft BK, Holland SK. Medial temporal fMRI activation reflects memory lateralization and memory performance in patients with epilepsy. Epilepsy Behav. 2008 Apr;12(3):410–418. doi: 10.1016/j.yebeh.2007.11.012. [DOI] [PubMed] [Google Scholar]
- (22).Szaflarski JP, Holland SK, Schmithorst VJ, Dunn RS, Privitera MD. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy & Behavior. 2004;5:244–252. doi: 10.1016/j.yebeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- (23).Binder JR, Bellgowan PS, Hammeke TA, Possing ET, Frost JA. A comparison of two FMRI protocols for eliciting hippocampal activation. Epilepsia. 2005 Jul;46(7):1061–1070. doi: 10.1111/j.1528-1167.2005.62004.x. [DOI] [PubMed] [Google Scholar]
- (24).Thivard L, Lehericy S, Krainik A, Adam C, Dormont D, Chiras J, et al. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005 Nov 15;28(3):682–690. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- (25).Berl MM, Balsamo LM, Xu B, Moore EN, Weinstein SL, Conry JA, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005 Nov 22;65(10):1604–1611. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- (26).Weber B, Fliessbach K, Lange N, Kugler F, Elger CE. Material-specific memory processing is related to language dominance. Neuroimage. 2007 Aug 15;37(2):611–617. doi: 10.1016/j.neuroimage.2007.05.022. [DOI] [PubMed] [Google Scholar]
- (27).Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008 Jan;12(1):74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini PA, Jesmanowicz A, et al. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol. 1995 Jun;52(6):593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- (29).Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002 Jul 23;59(2):238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- (30).Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996 Apr;46(4):978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- (31).Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, 3rd, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003 Jun 10;60(11):1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- (32).Choi H, Carlino R, Heiman G, Hauser WA, Gilliam FG. Evaluation of duration of epilepsy prior to temporal lobe epilepsy surgery during the past two decades. Epilepsy Res. 2009 Oct;86(2-3):224–227. doi: 10.1016/j.eplepsyres.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Haneef Z, Stern J, Dewar S, Engel J., Jr Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology. 2010 Aug 24;75(8):699–704. doi: 10.1212/WNL.0b013e3181eee457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, et al. How long does it take for partial epilepsy to become intractable? Neurology. 2003 Jan 28;60(2):186–190. doi: 10.1212/01.wnl.0000031792.89992.ec. [DOI] [PubMed] [Google Scholar]
- (35).Kim KK, Karunanayaka P, Privitera MD, Holland SK, Szaflarski JP. Semantic association investigated with functional MRI and independent component analysis. Epilepsy Behav. 2011 Apr;20(4):613–622. doi: 10.1016/j.yebeh.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Karunanayaka P, Kim KK, Holland SK, Szaflarski JP. The effects of left or right hemispheric epilepsy on language networks investigated with semantic decision fMRI task and independent component analysis. Epilepsy Behav. 2011 Apr;20(4):623–632. doi: 10.1016/j.yebeh.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Black LC, Schefft BK, Howe SR, Szaflarski JP, Yeh HS, Privitera MD. The effect of seizures on working memory and executive functioning performance. Epilepsy Behav. 2010 Mar;17(3):412–419. doi: 10.1016/j.yebeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- (38).McNally KA, Schefft BK, Szaflarski JP, Howe SR, Yeh HS, Privitera MD. Application of signal detection theory to verbal memory testing to distinguish patients with psychogenic nonepileptic seizures from patients with epileptic seizures. Epilepsy Behav. 2009 Apr;14(4):597–603. doi: 10.1016/j.yebeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- (39).Wechsler D. Wechsler Memory Scale. 3rd ed. 1945/1997. (WMS-III) [Google Scholar]
- (40).Benton Sivan A. Benton Visual Retention Test. 5th ed 1991. [Google Scholar]
- (41).Warrington EK. Recognition Memory Test. 1984.
- (42).Szaflarski JP, Allendorfer JB. Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav. 2012 May;24(1):74–80. doi: 10.1016/j.yebeh.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Macwhinney B, Cohen J, Provost J. The PsyScope experiment: building system. Spat Vis. 1997;11:99–101. doi: 10.1163/156856897x00113. [DOI] [PubMed] [Google Scholar]
- (44).Schmithorst VJ, Dardzinski B. CCHIPS/IDL enables detailed MRI analysis. 2000.
- (45).Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995 Jun;2(2):166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- (46).Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995 May;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- (47).Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, et al. Preoperative fMRI predicts memory decline following anterior temporal lobe resection. J Neurol Neurosurg Psychiatry. 2008 Jun;79(6):686–93. doi: 10.1136/jnnp.2007.115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI signal-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- (49).Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- (50).Beisteiner R, Klinger N, Hollinger I, Rath J, Gruber S, Steinkellner T, et al. How much are clinical fMRI reports influenced by standard postprocessing methods? An investigation of normalization and region of interest effects in the medial temporal lobe. Hum Brain Mapp. 2010 Dec;31(12):1951–1966. doi: 10.1002/hbm.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007 Jun 15;163(1):128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- (52).Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, et al. Lefthandedness and language lateralization in children. Brain Res. 2012 Jan 18;1433:85–97. doi: 10.1016/j.brainres.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Rausch R, Babb TL, Engel J, Jr, Crandall PH. Memory following intracarotid amobarbital injection contralateral to hippocampal damage. Arch Neurol. 1989 Jul;46(7):783–788. doi: 10.1001/archneur.1989.00520430077022. [DOI] [PubMed] [Google Scholar]
- (54).Donnelly KM, Allendorfer JB, Szaflarski JP. Right hemispheric participation in semantic decision improves performance. Brain Res. 2011 Oct 24;1419:105–116. doi: 10.1016/j.brainres.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Engel J, Jr, Rausch R, Lieb JP, Kuhl DE, Crandall PH. Correlation of criteria used for localizing epileptic foci in patients considered for surgical therapy of epilepsy. Ann Neurol. 1981 Mar;9(3):215–224. doi: 10.1002/ana.410090303. [DOI] [PubMed] [Google Scholar]
- (56).Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004 May 25;62(10):1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- (57).Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006 Jul;60(1):80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- (58).Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008 Aug;49(8):1377–94. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Binder JR, Swanson SJ, Sabsevitz DS, Hammeke TA, Raghavan M, Mueller WM. A comparison of two fMRI methods for predicting verbal memory decline after left temporal lobectomy: Language lateralization versus hippocampal activation asymmetry. Epilepsia. 2009 Oct 8; doi: 10.1111/j.1528-1167.2009.02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Bonelli SB, Powell RH, Yogarajah M, Samson RS, Symms MR, Thompson PJ, et al. Imaging memory in temporal lobe epilepsy: predicting the effects of temporal lobe resection. Brain. 2010 Apr;133(Pt 4):1186–99. doi: 10.1093/brain/awq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Cheung MC, Chan AS, Lam JM, Chan YL. Pre- and postoperative fMRI and clinical memory performance in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2009 Oct;80(10):1099–106. doi: 10.1136/jnnp.2009.173161. [DOI] [PubMed] [Google Scholar]
- (62).Richardson MP, Strange BA, Thompson PJ, Baxendale SA, Duncan JS, Dolan RJ. Preoperative verbal memory fMRI predicts post-operative memory decline after left temporal lobe resection. Brain. 2004 Nov;127(Pt 11):2419–26. doi: 10.1093/brain/awh293. [DOI] [PubMed] [Google Scholar]
- (63).Binder JR, Gross WL, Allendorfer JB, Bonilha L, Chapin J, Edwards JC, et al. Mapping anterior temporal lobe language areas with fMRI: a multicenter normative study. Neuroimage. 2011 Jan 15;54(2):1465–1475. doi: 10.1016/j.neuroimage.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Guedj E, Barbeau EJ, Liegeois-Chauvel C, Confort-Gouny S, Bartolomei F, Chauvel P, et al. Performance in recognition memory is correlated with entorhinal/perirhinal interictal metabolism in temporal lobe epilepsy. Epilepsy Behav. 2010 Dec;19(4):612–617. doi: 10.1016/j.yebeh.2010.09.027. [DOI] [PubMed] [Google Scholar]
- (65).Eliassen JC, Holland SK, Szaflarski JP. Compensatory brain activation for recognition memory in patients with medication-resistant epilepsy. Epilepsy Behav. 2008 Oct;13(3):463–469. doi: 10.1016/j.yebeh.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Chelune GJ. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995 Oct;10(5):413–432. [PubMed] [Google Scholar]
- (67).Jokeit H, Ebner A, Holthausen H, Markowitsch HJ, Moch A, Pannek H, et al. Individual prediction of change in delayed recall of prose passages after left-sided anterior temporal lobectomy. Neurology. 1997 Aug;49(2):481–487. doi: 10.1212/wnl.49.2.481. [DOI] [PubMed] [Google Scholar]
- (68).Helmstaedter C, Elger CE. Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996 Feb;37(2):171–180. doi: 10.1111/j.1528-1157.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- (69).Chelune GJ, Naugle RI, Luders H, Awad IA. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at 6-month follow-up. Neurology. 1991 Mar;41(3):399–404. doi: 10.1212/wnl.41.3.399. [DOI] [PubMed] [Google Scholar]
- (70).Frings L, Wagner K, Halsband U, Schwarzwald R, Zentner J, Schulze-Bonhage A. Lateralization of hippocampal activation differs between left and right temporal lobe epilepsy patients and correlates with postsurgical verbal learning decrement. Epilepsy Res. 2008 Feb;78(2-3):161–70. doi: 10.1016/j.eplepsyres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- (71).Koylu B, Walser G, Ischebeck A, Ortler M, Benke T. Functional imaging of semantic memory predicts postoperative episodic memory functions in chronic temporal lobe epilepsy. Brain Res. 2008 Aug 5;1223:73–81. doi: 10.1016/j.brainres.2008.05.075. [DOI] [PubMed] [Google Scholar]
- (72).Banks SJ, Sziklas V, Sodums DJ, Jones-Gotman M. fMRI of verbal and nonverbal memory processes in healthy and epileptogenic medial temporal lobes. Epilepsy Behav. 2012 Sep;25(1):42–49. doi: 10.1016/j.yebeh.2012.07.003. [DOI] [PubMed] [Google Scholar]
- (73).Blum D, Meador K, Biton V, Fakhoury T, Shneker B, Chung S, et al. Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy. Neurology. 2006 Aug 8;67(3):400–406. doi: 10.1212/01.wnl.0000232737.72555.06. [DOI] [PubMed] [Google Scholar]
- (74).Lee S, Sziklas V, Andermann F, Farnham S, Risse G, Gustafson M, et al. The effects of adjunctive topiramate on cognitive function in patients with epilepsy. Epilepsia. 2003 Mar;44(3):339–347. doi: 10.1046/j.1528-1157.2003.27402.x. [DOI] [PubMed] [Google Scholar]