Abstract
The male sex is determined by the sex determining region on the Y chromosome (SRY) transcription factor. The unexpected action of SRY in the control of voluntary movement in male rodents suggests a role in regulation of dopamine transmission and dopamine-related disorders with sex bias such as Parkinson’s disease. We investigated SRY expression in the human brain and function in vitro. SRY immunoreactivity was detected in the human male, but not female, substantia nigra pars compacta (SNc) within a sub-population of tyrosine hydroxylase (TH) positive neurons. SRY protein also co-localised with TH positive neurons in the ventral tegmental area and GAD-positive neurons in the substantia nigra pars reticulate (SNr). Retinoic acid-induced differentiation of precursor NT2 cells into dopaminergic cells (NT2N) increased expression of TH, NURR1, D2R and SRY. In the human neuroblastoma cell line, M17, SRY knockdown resulted in a reduction in TH, DDC, DBH and MAO-A expression; enzymes which control dopamine synthesis and metabolism. Conversely, SRY overexpression increased TH, DDC, DBH, D2R and MAO-A levels, which was accompanied by increased extracellular dopamine levels. A luciferase assay demonstrated that SRY activated a 4.6 kb 5′ upstream regulatory region of the human TH promoter/nigral enhancer. Combined, these results suggest that SRY may play a role as a positive regulator of catecholamine synthesis and metabolism in the human male midbrain. Given the limitations of human tissue analysis, further studies are required to provide a definitive answer on SRY expression in human brain regions.
Keywords: sex determination, basal ganglia, sexual dimorphism, catecholamine, dopamine, addiction
INTRODUCTION
The midbrain dopaminergic system is sexually dimorphic in healthy and diseased states (Gillies & McArthur 2010). Human sex differences in striatal dopamine release, reuptake and responses during cognitive and motor function tasks have been reported (Mozley et al. 2001, Munro et al. 2006, Riccardi et al. 2011). Such differences may underlie sex bias in progression, prevalence or severity of dopamine disorders such as autism, schizophrenia and Parkinson’s disease (Murray et al. 2003, Baron-Cohen et al. 2005, Wooten et al. 2004, DeLisi 1997). Several studies report that the number of dopamine neurons in the rodent substantia nigra pars compacta (SNc) is higher in males than in females (Dewing et al. 2006, McArthur et al. 2007, Murray et al. 2003), while one study found the opposite (Ma et al. 2007). The release and reuptake of dopamine in the striatum is also reported to be sexually dimorphic (Ji & Dluzen 2008, Walker et al. 2000). While estrogen plays a role in these sex differences, emerging evidence indicates that genetic factors also contribute, and sex chromosome genes in particular could be involved (Becker & Beer 1986, Reisert & Pilgrim 1991, Carrer & Cambiasso 2002, Carruth et al. 2002, Arnold & Burgoyne 2004, Dluzen & McDermott 2004, Czlonkowska et al. 2005).
The sex determining region on the Y chromosome (SRY) encodes a testis determining transcription factor, however the expression of SRY in the male brain is poorly defined (Hacker et al. 1995, Mayer et al. 1998, Sekido & Lovell-Badge 2008, Knower et al. 2011). Mouse SRY is expressed in the diencephalon, mesencephalon and cortex of the male brain, as shown by RT-PCR (Lahr et al. 1995, Mayer et al. 2000). Using in situ hybridization we previously detected SRY in the cortex, medial mammillary bodies and substantia nigra of the male rodent brain (Dewing et al. 2006). We focused on SRY expression in dopamine neurons in the human male SNc because we have previously observed that SRY co-localises with tyrosine hydroxylase (TH)-positive neurons in the corresponding region of the rodent male brain (Dewing et al. 2006). Moreover, we observed a direct functional effect on movement when SRY expression was knocked down in the SNc (Dewing et al., 2006). Targeted transient downregulation of SRY gene expression with SRY antisense oligonucleotides in the SNc of male rats resulted in a reversible reduction in the number of TH-positive neurons and consequent defects in motor function (Dewing et al. 2006). This suggested that a sex-specfic gene in the brain could directly affect dopamine function and behavior. Similarly, SRY regulates TH expression in other organs, as the SRY gene delivered to the kidney by electroporation raised TH and dopamine levels in male rats (Ely et al. 2009) and SRY delivery to the adrenal medulla raised blood pressure (Ely et al. 2007). An additional role for SRY in dopamine regulation has emerged with evidence suggesting that SRY also regulates the gene monoamine oxidase A (MAO-A, an enzyme involved in dopamine degradation) (Wu et al. 2009). Combined, these findings suggest that SRY may play an important role in dopamine homeostasis within the brain.
Whether SRY regulates dopamine in the human brain has not been studied. There are major differences in the protein sequence between human and rodent SRY, as well as in the regulatory regions of the SRY genes (Lau & Li 2009). For instance, mouse SRY protein contains a 223 amino acid domain with transactivation function in vitro that is absent from human SRY (Dubin & Ostrer 1994). Therefore we sought to investigate the anatomical and functional role of SRY in the human SNc; examining its ability to regulate TH and other components of the dopamine pathway, and its overall effect on dopamine levels.
MATERIALS AND METHODS
Antibodies
A C-terminal human SRY peptide (PP4, amino acids 189-204) (Chemicon, Billerica, USA) was used to raise antibodies in sheep which were purified from serum as previously described (Sim et al. 2005). Primary antibodies were mouse anti-FLAG (Sigma-Aldrich, F3040), rabbit anti-tyrosine hydroxylase (Pel-freez, P40101), mouse anti-β-tubulin (Chemicon, MAB3408), mouse anti-calbindin-D-28K (SWANT, 300), rabbit anti-GFAP (DAKO, Z0334), goat anti-GAD-67 (Santa-Cruz Biotechnology, sc-7512). Secondary antibodies were donkey anti-sheep-HRP (Chemicon), goat anti-rabbit-HRP (Biorad, 1/0-6515), goat anti-mouse-HRP (Biorad, 172-1011), AlexaFluor-594 donkey anti-mouse (Molecular Probes, A21203), AlexaFluor-594 donkey anti-rabbit (Molecular Probes, A21207), AlexaFluor-488 donkey anti-sheep (Molecular Probes, A11015), AlexaFluor-594 goat anti-mouse (Molecular Probes, A1105), and AlexaFluor-594 donkey anti-goat (Molecular Probes, 11058).
Immunohistochemical detection and quantitation of SRY in the human SN and VTA
Adult male and female human SN and VTA tissue was obtained with informed consent from the individual from the Victorian Brain Bank Network (Table 1) (Southern Health Human Research Ethics Committee, project 05073C, V.R.H.). We received forty paraffin-embedded coronal sections (7 μm) per case. Each region was anatomically delineated by the Victorian Brain Bank neuropathologist (http://www.mhri.edu.au/vbbn-for-researchers). Sections were made at the same coronal level, taken just above the cerebellar peduncle. Confirmation by H&E, performed by VBBN and independently by the researchers, identified the SNc region by neurons which were densely packed and the SNr region by neurons which were loosely packed. In addition, consecutive sections to those stained with H&E were used to confirm regions by immunostaining; TH+ve (in the SN and VTA), GAD+ve (in the SNr) and Calbindin+ve (in the SNc dorsal tier), by the researchers who performed the confocal microscopy and quantitation. In all seven cases, H&E and immunohistochemistry performed on consecutive sections and micrographed at the same magnification by the researchers, showed a consistent pattern of tissue morphology and protein expression. Paraffin-embedded tissue sections were dewaxed with histosol, ethanol and water, and antigens were retrieved by boiling the tissue in a bath containing 0.01M citric acid buffer (sodium citrate in water, pH 6.0) for 8 minutes using a microwave oven (550 watt). Tissues were then cooled at room temperature for 30 minutes before washing in phosphate-buffered saline (PBS) and blocked for 1 hour at room temperature (10% BSA, 0.5% TritonX-100, 3% Donkey serum, PBS) and incubated with primary antibody (SRY 1:200, TH 1:1000, calbindin-D-28-K 1:1000, GFAP 1:1000, GAD-67 1:500) in block for 1 hour at room temperature. Autofluorescence blocking kit (Chemicon) was used to reduce autofluorescence of lipofuscins. Secondary antibodies were diluted in block (1:1000) and incubated with tissue for 45 min at room temperature, washed in PBS and mounted with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA, USA). Unilateral SN sections were studied in each of 7 cases (5 male, 2 female), unilateral VTA in one case (male). For the SN, four sections per case were analysed by IHC using an Olympus FluoView 500 confocal microscope. For each section, four fields were photomicrographed at 40x and used for quantitation. A minimum of fifty DAPI stained cells (neurons and non-neurons) and an average of eight TH-positive neurons were counted per field. In the VTA that was analysed, quantitation was performed similarly to that described for the SN.
Table 1.
Neuronal tissue characteristics from post-mortem subjects used for immunohistochemistry.
| Case Record | Sex | Age | Post-mortem delay (h) | Immediate cause of death | Region | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | Male | 48.7 | 50 | Cardiac failure | SN | Control |
| 2 | Male | 69.1 | 31 | Myocardial ischaemia | SN VTA |
Control |
| 3 | Male | 59.6 | 43.5 | Pulmonary embolus | SN | Control |
| 4 | Male | 61.1 | 24 | Ischaemic heart disease | SN | Control |
| 5 | Male | 57.6 | 20.5 | Intra-abdominal haemorrhage | SN | Control |
| 6 | Female | 67.3 | 24 | Pulmonary thromboembolism | SN | Control |
| 7 | Female | 27 | 20 | Non-Hodgkin’s lymphoma | SN | Control |
Cell culture & light microscopy
NTera2 clone D1 (NT2) human embryonic carcinoma cells (CRL-1973, ATCC Manassas, VA, USA) were cultured with Dulbecco’s modified Eagle’s medium with Ham’s F12 nutrient in a 50:50 ratio supplemented with Glutamax, 10% Fetal Bovine Serum, 1% penicillin/streptomycin and differentiated with 10μM all-trans-retinoic acid (RA) (Sigma-Aldrich) as described elsewhere (Andrews 1984, Pleasure et al. 1992). NT2N cells were imaged with an Olympus IX71 light microscope. Human neuroblastoma BE(2)-M17 (M17) (CRL-2267, ATCC, Manassas, VA, USA) cells were cultured with Dulbecco’s modified Eagle’s medium with Ham’s F12 nutrient in a 50:50 ratio supplemented with Glutamax, 10% Fetal Bovine Serum, 1% penicillin/streptomycin.
RNA isolation & quantitative RT-PCR
0.5μg of total RNA isolated using RNeasy Mini kit (Qiagen) was reverse-transcribed into cDNA and real-time quantification of mRNA levels measured as previously described (Knower et al. 2011). Primer design as follows (F- forward, R- reverse), β2M F tgaattgctatgtgtgtctgggt, R cctccatgatgctgcttacat, 234 bp, SRY F tcccgcagatcccgcttcggtactctg, R (t)15gaaatgaataag, 329 bp, TH F ccgggctgctgtcctcctac, R gggctgtccagcacgtcgat, 256 bp, MAO-A F ctgatcgacttgctaagctag, R atgcactggatgtaaagcttc, 200 bp, D1R F agaagtccctctccaccacc, R tgacagaacccgtggtcttt, 90 bp, D2RL/S F gccttccttgaccttcctct, R ttacatgggtctcccaggac, 490 bp/403 bp, NURR1 F tttctgccttctccgtgcatt, R gtggcaccaagtcttccaat, 271 bp, COMT F tcggctggaacgagttca, R cgtccacgatcttgcctt 198 bp, DDC F gaacagacttaacgggagccttt, R aatgccggtagtcagtgataagc 90 bp, DBH F tgactgggagaaaggtggtc, R gtgaactgctgagacacgga, 375 bp, DAT F agctcttcacgctcttcatcg, R tcatctgctggatgtcgtcg, 198 bp. Final values represent fold change of gene expression relative to the β2-Microglobulin (β2M) housekeeping gene.
Immunocytochemistry & fluorescence microscopy
Typically 105 cells were seeded on glass cover slips in 35mm tissue culture wells (Nunc, ThermoScientific, Denmark), fixed with 4% formaldehyde (Sigma-Aldrich) for 7 min and washed with PBS. Cells were blocked (3% BSA, 0.7% TritonX-100 in PBS) for 1 hour at 37°C. Cells were incubated with primary antibody (SRY 1:200, TH 1:1000, FLAG 1:500) overnight at 4°C, washed and incubated with fluorescent secondary antibodies (1:1000) in block for 1 hour at 37°C. After washing, coverslips containing the stained cells were mounted on slides with fluorescent nucleic acid stain, DAPI. Slides were observed with an OlympusB500 confocal microscope. Quantitative scoring of cells positive for SRY and TH protein immunofluorescence were made using ImageJ software (v1.42q, http://rsb.info.nih.gov/ij) as previously described (Knower et al. 2011). Briefly, confocal immunefluorescence microscopy was used to capture images of NT2N cells at each time point of differentiation. Care was taken not to change any parameters between time points. Individual cells were randomly selected and the intensity of pixels measured. Fluorescence intensity was corrected against average nucleus size (area), measured for each differentiation time point, to adjust for the decreasing size of cells as they differentiate (the average size ratios were 1 : 0.95 : 0.9 : 0.7 : 0.4 for 0d, 7d, 14d, 21d and 28d respectively). A total of 306 cells were analyzed across three independent experiments.
siRNA gene interference
Human SRY siRNA (sc-38443) or non-sense (ns) siRNA (sc-37007) (Santa-Cruz Biotechnology, Santa Cruz, CA, USA) were transfected into M17 cells with Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Briefly, 0.5×105 cells were seeded per 4cm2 well in 1ml of medium without antibiotics, the next day cells at 90% confluency were transfected with a mixture of siRNA-oligomer (40pmol) and Lipofectamine2000 reagent (2μl) diluted in 200μl of minimal media which was added to the 1ml of culture media. The mixture was incubated for 24 hours at 37°C, 5% CO2 after which RNA or protein was collected.
Plasmids
A 4.6 kb fragment (-4591/+9) of the human TH promoter was cloned from a human bacterial artificial chromosome (RP11-542J6, AGRF, Melbourne, Australia) into pGEM-T (Promega) and subcloned into the E1b-luc reporter vector to obtain TH-pE1b-luc. Human pCDNA3-SRY-FLAG (Sim et al. 2005) encoding full-length human SRY ORF with a FLAG epitope tag. SRY DNA binding mutant pCDNA3-SRYR62G-FLAG as previously described (Harley et al. 2003). pEF-GFP-1 (Ohashi et al. 2002) encoding Green Fluorescent Protein (GFP).
Transient transfection & cell sorting
Co-transfection of the SRY expression plasmid in excess of a GFP plasmid (3:1) enabled the isolation of exclusive sub-populations of cells expressing GFP and therefore SRY, see previously described methods (Knower et al. 2011). Briefly, seven day RA-treated NT2N cells were transfected at 50–60% confluency with SRY-FLAG and GFP using GeneJuice (70967-6, Novagen-Merck, AUS) according to the manufacturer’s instructions. M17 cells were transiently transfected as above using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Positively transfected cells resuspended in PBS were isolated by fluorescence-activate cell sorting on a MoFlo XDP (Beckman Coulter). Generally, 4×105 cells were obtained.
Western blot
Briefly, total cell protein was isolated post-transfection with RIPA buffer (50mM Tris, 150mM NaCl, 0.1% SDS, 0.5% Na.Deoxycholate, 1% TritonX-100). 5 μg of protein was run on a 12% acrylamide gel. After transfer to a PVDF membrane (Millipore, USA), bound proteins were blocked with 5% skim milk powder in PBS overnight. Primary antibody (SRY 1:100, TH 1:1000, MAO-A 1:200, β-tubulin 1:5000) was applied for 1 hour, washed in PBS-Tween20. For SRY overexpression, secondary HRP-conjugated antibody (1:1000) was incubated with the membrane, washed and incubated in ECL reagent (GE Healthcare, USA) then exposed to film (GE Healthcare, USA) and developed. For SRY siRNA knockdown, secondary AlexaFluor antibody (1:1000) was incubated with the membrane, washed and processed on a Typhoon9400 variable mode imager (GE Healthcare). For quantitation, all blots were digitally scanned using the Typhoon 9400 variable mode imager. Quantitation of three independent experiments was performed by measuring the optical density (intensity) of the SRY, TH or MAO-A immunoreactive bands (protein blots) relative to the β-tubulin loading control bands for that sample, using ImageJ software.
High Performance Liquid Chromatography with Electrochemical Detection
M17 cells grown in 12-well tissue culture plates and transfected as described above, were rinsed with PBS and treated with minimal media (DMEM-F12) supplemented with 56mM KCl for 30 minutes to evoke dopamine release into the culture medium (1 ml). Media were collected and stabilized with perchloric acid. Reverse phase high performance liquid chromatography with electrochemical detection was performed, as described previously (Parish et al. 2008).
Luciferase reporter assay
TH-E1b-luc was transfected in M17 cells as described above with vector, SRY or the binding mutant SRYR62G. pCMV-Ren, expressing Renilla luciferase, was cotransfected as an internal control. pUC18 was added to maintain an equivalent amount of DNA molecules in each transfection. After 24 hours incubation, cells were collected and assayed for luciferase activity with the Dual-Luciferase Reporter 1000 assay system (Promega, USA) on a Wallac EnVision microplate reader (PerkinElmer, MA, USA).
Statistical Analysis
The data are expressed as mean + S.E. from n number of experiments. Statistical analysis was performed using Student’s t-test for unpaired comparisons (GraphPad Prism v4.03, 2005). Values of p < 0.05 were considered to indicate significant difference.
RESULTS
Cellular localization of SRY in the human midbrain
Co-immunohistochemistry of SRY and TH revealed expression of SRY protein in a subset of TH-positive neurons in the human male SNc, located within the nuclei (its presumed site of action) as well as the cytoplasm (Figure 1A, white arrows). SRY immunoreactivity was absent in the human female SNc (Figure 1B). Counting of TH-positive neurons in the human male SNc (n=5) revealed that 43%(±9%) were SRY-positive. SRY immunoreactivity in the human male SNc also co-localised with calbindin-positive neurons in the dorsal tier of the SNc (Figure 1C, white arrows). All SRY-positive cells appeared to be neuronal as observed by the lack of overlap with glial fibrillary acidic protein (GFAP) staining, a marker of glial cells (Figure 1D, white arrows).
Figure 1. Cellular localization of SRY protein in the human midbrain.
A) Post-mortem male SNc tissue immunostained with TH and SRY, and DAPI nuclear stain. TH protein is present in the cytoplasm only, marking dopamine neurons (n). SRY protein is present in the nucleus and cytoplasm of some TH-ir neurons (white arrows), whilst some TH-ir cells do not show SRY-ir. (B) The same immunostain of post-mortem female SNc tissue shows no SRY protein present in TH-ir cells (white arrows). (C) Male SNc tissue immunostained with calbindin (CALB). Calbindin is only present in the cytoplasm, marking a sub-set of dopamine neurons in the dorsal section of the SNc. SRY protein is present in the nucleus and cytoplasm of calbindin-positive neurons. (D) Male SNc tissue immunostained with GFAP. GFAP is present in the cytoplasm and processes of glial cells (g). SRY protein was not able to be detected in these cells. (E) Post-mortem male VTA tissue immunostained with TH and SRY, and DAPI nuclear stain. SRY is localised in some (white arrows) but not all TH-positive neurons. TH is present in the cytoplasm only whereas SRY is present in the nucleus and cytoplasm (white arrows). (F) Male substantia nigra tissue immunostained with GAD-67. GAD-67 is present only in the cytoplasm, marking GABAergic neurons of the SNr. SRY protein is present in the nucleus and cytoplasm of GABAergic neurons (white arrows). Left panels, 40x, right panels, 100x.
To determine whether SRY protein is present in other dopaminergic regions, SRY and TH immunoreactivity was assessed in the ventral tegmental area (VTA), which is adjacent to the SNc and is abundant in dopaminergic neurons. Analysis of the VTA region from the same midbrain tissue revealed that 35% of TH-positive neurons were SRY-positive, showing immunoreactivity within both the nuclei and cytoplasm (Figure 1E, white arrows, Table 1). We also assessed the expression of SRY protein in the substantia nigra pars reticulata (SNr), which was shown to express SRY immunoreactivity in the rodent SNr (Dewing et al. 2006). In the human male SNr, SRY immunoreactivity was co-localised within GAD-67-positive neurons, and was present in both the cytoplasm and nuclei (Figure 1F, white arrows).
Expression analysis of human SRY in NT2N neurons
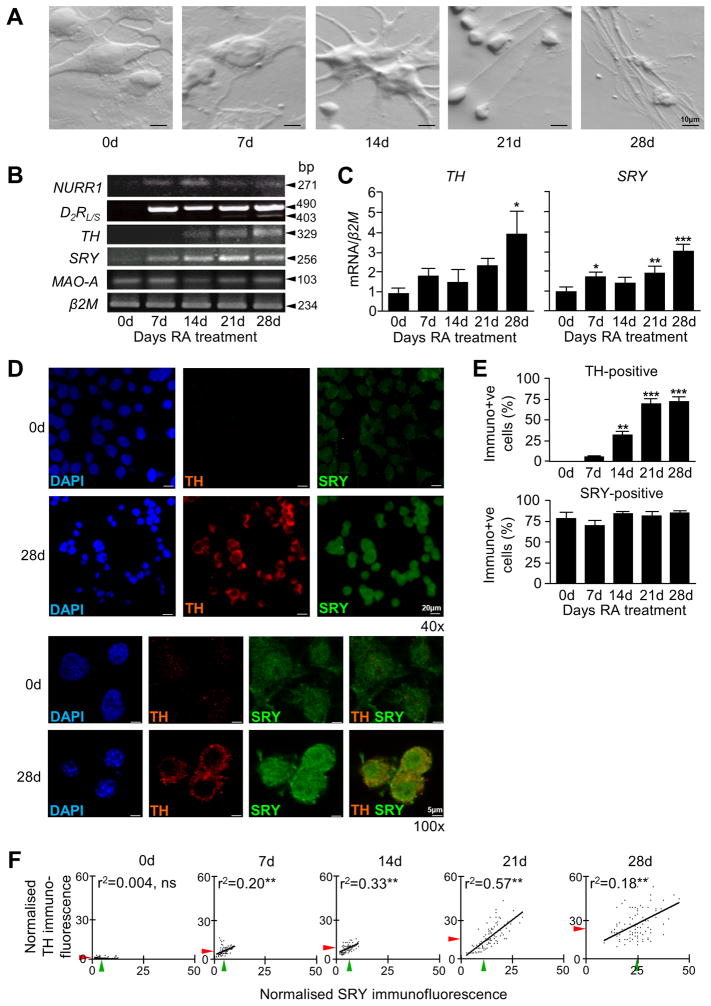
We examined expression of human SRY in differentiating NT2 neurons. Upon retinoic acid treatment of pluripotent NT2 cells, morphological changes occur as they differentiate into ‘neurons’ (NT2N). NT2N cells express DAT as well as AHD-2, an enzyme exclusively expressed in dopamine mesencephalic precursors (Zigova et al. 2000). NT2N cells have the necessary cellular machinery of functional dopamine neurons and have been trialed in cell replacement therapy for stroke patients (Nelson et al. 2002, Pleasure et al. 1992). By light microscopy, precursor NT2 cells appear large and flat and clump together but progressively acquire neuronal characteristics (Figure 2A), such as elongated ‘neuritic’ processes. Over the course of the differentiation, genes important for the maturation and function of dopamine neurons, including nuclear receptor related 1 (NURR1) and dopamine receptor D2 (D2R) were expressed in NT2N (7-28d RA treatment), but not in precursor NT2 (0d) cells. SRY and TH levels steadily increased, by 4-fold and 2-fold, respectively (Figure 2B and 2C) while MAO-A, a target gene of SRY, showed no overall change (Figure 2B).
Figure 2. Expression analysis of SRY and TH mRNA during differentiation into NT2N neurons.
A) Morphology of NT2 cells during neuronal differentiation for 28 days with retinoic acid. B) Gene expression profiles of NURR1, D2R, TH, SRY, MAO-A and β2M by PCR in differentiating NT2N cells shows acquisition of neuronal phenotype. C) Quantitative gene expression profiles of TH and SRY in differentiating NT2N cells measured by qRT-PCR show significant correlative uprgulation (n=3, mean +S.E., *p<0.05, **p<0.005). D) Low (40x) and high (100x) magnification of DAPI nuclear stain in NT2 cells (0d) reflects the morphology seen by light microscopy, with a large, rounded nucleus compared to the more compact cell body of the NT2N cell (28d). Co-immunofluorescence microscopy at low and high magnification shows TH protein absent in precursor NT2 cells (0d) and abundantly present in differentiated NT2N cells (28d). SRY protein is present in NT2 cells (0d) but with stronger intensity in NT2N cells (28d). E) The number of SRY-ir and TH-ir cells differ during differentiation, with a gradual rise in TH-ir cells compared to a constant number of SRY-ir cells (n=3, mean +S.E., **p<0.005, ***p<0.0005). F) Analysis of cellular fluorescence intensity shows a positive linear correlation between normalised SRY and TH immunofluorescence (arbitrary units) as NT2N cells differentiate (average SRY fluorescence; green arrow, average TH fluorescence; red arrow, ns; not significant, **p<0.005).
SRY protein was detected in NT2 and NT2N cells, whereas TH protein was observed only in NT2N cells (14-28d) (Figure 2D). The numbers of SRY-immunoreactive (SRY-ir) and TH-immunoreactive (TH-ir) cells were counted within differentiating NT2 cultures (Figure 2D). No undifferentiated NT2 cells were TH-ir, but following RA treatment for 28 days, the proportion of TH-ir cells rose from 0% to 78% (Figure 2E). NT2 cells showed SRY-immunoreactivity in 87% of cells but this proportion did not change as they differentiated into NT2N cells over the 28 day time course (Figure 2E).
To determine if there was a correlation between SRY and TH levels, immunofluorescence intensity was measured on a cell-by-cell basis and plotted against one another. Precursor NT2 cells (0d) expressed low levels of SRY protein and no TH protein. At 7 days and at 14 days RA treatment, there was a moderate increase in the number of SRY-ir cells that also expressed TH. At 21 days there was a shift in the characteristics of the cell population whereby SRY and TH immunofluorescence intensity both increased and showed a positive linear correlation (Figure 2F). In summary, undifferentiated NT2 cells expressed SRY but not TH, however as the cells differentiated into neurons, there was a notable rise in both SRY and TH protein levels.
Knockdown of SRY leads to reduction in transcription of genes in the catecholamine biosynthesis pathway
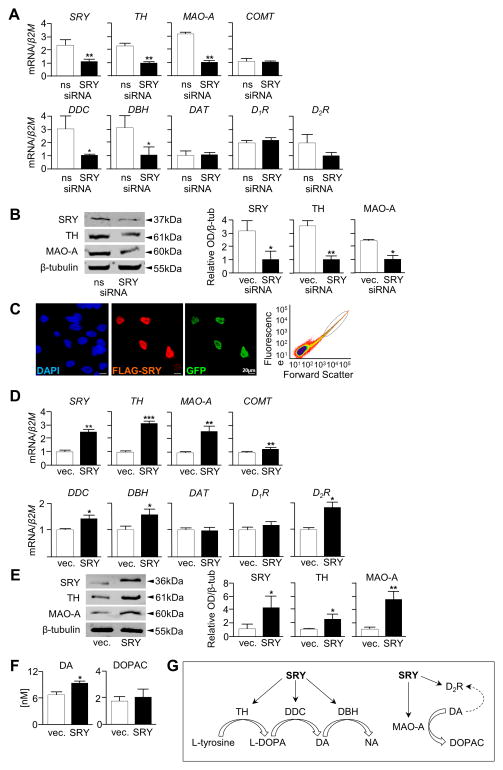
To investigate whether endogenous SRY plays a role in the regulation of dopamine transmission, M17 cells were treated with SRY siRNA to reduce SRY gene expression. M17 is a male neuroblastoma-derived cell line which expresses endogenous SRY and components required for dopamine biosynthesis, are easy to transfect and are therefore more suitable than NT2N to study short term interactions between SRY and TH. Knockdown experiments (Figure 3A and 3B) have been thrice replicated and mRNA and protein levels quantified. Quantitative RT-PCR analysis revealed that a 47% reduction in SRY mRNA level was achieved by SRY siRNA. This was accompanied by a concomitant 44% reduction in TH and 67% reduction in MAO-A mRNA levels (Figure 3A). There were also reductions in dopa decarboxylase (DDC) (66%) and dopamine β-hydroxylase (DBH) (67%) mRNA while levels of catechol-O-methyl transferase (COMT), dopamine reuptake transporter (DAT), dopamine receptor D1 (D1R) and dopamine receptor D2 (D2R) mRNA were unchanged (Figure 3A). Western blot analysis showed that SRY knockdown reduced SRY protein levels which led to a reduction in TH and MAO-A protein levels (Figure 3B).
Figure 3. Knockdown and overexpression of SRY in M17 cells alters components of the dopamine biosynthesis pathway and dopamine levels.
A) SRY siRNA knockdown in M17 cells leads to a reduction in SRY, TH, MAO-A, DDC and DBH mRNA levels whilst COMT, D1R, D2R and DAT mRNA levels showed no change (n=3, mean +S.E., *p<0.05, **p<0.005) and B) SRY, TH and MAO-A protein levels are reduced in SRY siRNA transfected cells. Representative blot of n=3 experiments, band optical density analysed by ImageJ software (*p<0.05, **p<0.01). C) Transient transfection of M17 cells with SRY and GFP and then FAC sort of positively-transfected cells (SRY/GFP+ve circled). D) qRT-PCR shows SRY, TH, MAO-A, DDC, DBH and D2R mRNA levels are elevated in cells transfected with SRY compared to vector whilst COMT, D1R and DAT mRNA levels showed no change (n=3, mean +S.E., *p<0.05, **p<0.005, ***p<0.0005). E) SRY, TH and MAO-A protein levels are also elevated in response to overexpression of SRY. Representative blot of n=3 experiments, band optical density analysed by ImageJ software (*p<0.05, **p<0.01). F) Evoked dopamine release, measured from 1 ml cell culture media 30 minutes after K+ depolarization, were elevated in SRY transfected M17 cells compared to empty vector controls. There were no significant differences in DOPAC levels (n=3, mean +S.E., *p<0.05). G. Schematic showing SRY-responsive components of the dopamine biosynthesis pathway.
Overexpression of SRY leads to increased transcription of genes in the catecholamine biosynthesis pathway
Conversely, we assessed the effect of SRY overexpression on dopamine transmission by transfecting M17 cells with both FLAG epitope-tagged SRY vector and GFP vector. Immunostaining revealed that typically 20–30% of cells expressed ectopic FLAG-SRY and that the lower proportions of GFP-positive cells were always SRY-positive (Figure 3C). To enrich for the SRY-positive pool, GFP-positive cells were isolated using fluorescent activated cell sorting, from which RNA and protein were isolated (Figure 3C). Quantitative RT-PCR revealed that genes involved in dopamine synthesis were differentially regulated by SRY. Messenger RNA levels of the enzymes TH (3-fold), MAO-A (2.5-fold), DDC (1.25-fold) and DBH (1.5-fold) were up-regulated in cells transfected with SRY compared to empty vector transfected cells, whilst D1R and COMT mRNA levels were not altered. Expression of D2R was also increased (1.8-fold) in SRY-transfected cells compared to cells transfected with vector, whereas DAT was not differentially regulated (Figure 3D). Western blot revealed that SRY overexpression led to elevated SRY protein levels, and to a concomitant rise in protein levels of MAO-A, and to a lesser extent TH (Figure 3E). Similar results were also obtained in SRY-transfected NT2N cells (Supplemental Figure 1A-1C). To determine the physiological consequence of this up-regulation, we assessed KCl-evoked dopamine levels in the media. Dopamine levels were higher (1.4-fold) in SRY-overexpressing M17 cells than cells transfected with empty vector (Figure 3F). No change however in DOPAC levels (a metabolite of dopamine degradation) were detected.
Human SRY can activate the human TH promoter
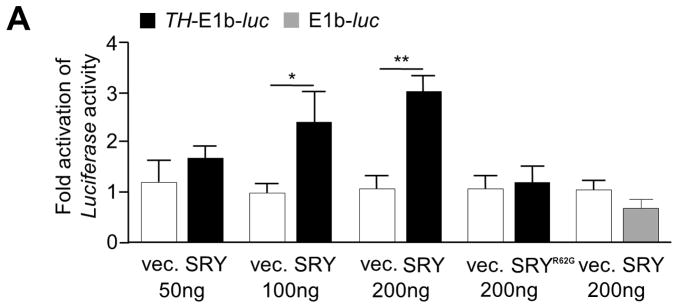
As a transcription factor, SRY could act directly by binding to response elements and promoter regions to regulate gene transcription, or the mechanism could be indirect, therefore we investigated the transcriptional regulation of TH, the rate limiting enzyme of dopamine synthesis. A 4.6 kb fragment of the human TH promoter from the region required to drive expression of TH in the SNc of transgenic mice (Romano et al. 2005) was tested for response to SRY. M17 cells were transiently co-transfected with TH-E1b-luc and increasing amounts of SRY expression plasmid. A dose dependent luciferase response was observed (Figure 4A). In contrast, the clinical SRY DNA binding mutant, SRYR62G, failed to activate the TH promoter (Figure 4A).
Figure 4. SRY activates the human TH promoter.
A) Co-transfection of SRY and a TH promoter construct (TH-E1b-luc) in M17 cells elevates transcription of the luciferase reporter gene in a dose dependant manner (n=3, mean +S.E. *p<0.05, **p<0.005). The SRYR62G DNA binding mutant was unable to activate the TH promoter.
DISCUSSION
SRY is expressed in the human male brain
Our study is the first to demonstrate the presence of SRY protein in the human male brain. SRY protein was localized within a subpopulation of TH-positive neurons of the SNc and VTA, in both the cytoplasm and in the nucleus, as well as in TH-negative neurons. These findings are similar to those from the rodent brain (Dewing et al. 2006), although a greater portion of SRY neurons in the human SNc were TH-positive (43%) compared to the rodent SNc (10%). SRY immunoreactive neurons in the SNc co-localised with calbindin-positive neurons, a subset of dopamine neurons in the VTA and in the SNc within the dorsal tier which are more resistant to cell death compared to cells in the ventral tier (Cebrian & Prensa 2010, Fearnley & Lees 1991, Haber et al. 1995). Considering the expression of SRY in the dorsal and ventral SNc, SNr and VTA, there does not appear to be a correlation between SRY localization in midbrain dopamine regions and their vulnerability to degeneration.
SRY also co-localises with TH-positive neurons in the VTA, which is adjacent to the SNc and is another brain region abundant in dopamine-containing neurons. VTA is crucial for mediating reward and addictive behaviours (Wise 2009) and the expression of SRY in the VTA may explain sex differences originating in this region (McArthur et al. 2007, Woolley et al. 2006, Zhang et al. 2008). Furthermore, the presence of SRY in both the SNc and VTA may reflect a conserved regulation of dopamine function in males, such as the control of movement and reward. Analysis of the VTA tissue from only one individual places limitations on these findings and more VTA samples are required to confirm these findings. We have also demonstrated that SRY co-localises with GABAergic neurons in the human male SNr, which is consistent with previous finding in rodents (Dewing et al. 2006). The SNr is one of the two primary output nuclei of the basal ganglia system, and regulates the thalamic relay to the premotor cortices (Smith & Villalba 2008). Thus, the presence of SRY in both the SNc and SNr add further support for a role for SRY in the control of voluntary movement and basal ganglia function in males.
Expression of SRY and TH increase as NT2 cells differentiate into dopamine neurons
Precursor NT2 cells express SRY (Clepet et al. 1993, Knower et al. 2007) and were differentiated with retinoic acid into NT2N dopamine ‘neurons’. Over the course of retinoic acid treatment, NT2N cells increasingly express dopamine neuron markers including TH, NURR1 and D2R, consistent with previous observations (Kim et al. 2003, Jin et al. 2006), as well as rising levels of SRY. NURR1 (a marker of post-mitotic dopaminergic precursors) is necessary for NT2N neurogenesis and is an important regulator of TH (Iwawaki et al. 2000, Misiuta et al. 2003). Like NURR1, SRY exhibited a similar profile of expression, with its up-regulation preceding the rise in TH. The correlation between SRY and TH expression was also confirmed at the protein level, where basal SRY levels rise and TH expression is initiated as cells differentiate. SRY transcripts are present in the fetal human brain and the expression is maintained in the adult brain (Clepet et al. 1993). The intermediate differentiation phase may reflect developmental neurons, as dopamine neuron maturation genes such as NURR1 are up-regulated during differentiation (Martinat et al. 2006), however this process allows us to examine temporal changes between SRY and TH until cells are completely differentiated into dopamine neurons (21d, 28d). It remains to be determined whether SRY plays a role in neuronal differentiation or cell proliferation given that the number of dopamine neurons in the male rat substantia nigra is different compared to females (Ma et al. 2007). The manipulation of SRY in two ‘mature’ culture models of human dopamine neurons, namely differentiated NT2Ns and M17, provides a means to study the regulatory role of SRY on dopamine synthesis components. These correlative data suggest that human SRY could play a role in up-regulating TH transcription in dopamine neurons.
Human SRY regulates the catecholamine biosynthetic pathway
Given the disparity in mouse and human SRY protein domain structure, it was important to demonstrate the actions of human SRY on dopamine synthesis. Previous studies have demonstrated a role for SRY in regulating dopamine via TH in rodents (Dewing et al. 2006, Ely et al. 2007, Zhang et al. 2010), MAO-A in rodent neuronal N2a cells (Tao et al. 2011) and human SRY in regulating MAO-A in the BE(2)-C cell line (Wu et al. 2009) from which the M17 cell line is commonly derived. In addition, it was unclear what the overall effect of SRY upregulation on dopamine levels would be. We have extended these findings by showing that the overall effect of increased SRY is an increase in dopamine synthesis and that SRY regulates multiple components of dopamine biosynthesis machinery. Here we showed that TH, MAO-A, DDC, DBH, and D2R respond to overexpression or knockdown of SRY in the human male dopaminergic cell line M17. There is a clear effect of reduced SRY upon TH, MAO-A DDC and DBH levels. SRY overexpression increased the mRNA levels of TH, MAO-A, DDC, and DBH suggesting SRY might regulate several parts of the dopamine biosynthesis pathway. SRY overexpression also led to a modest but significant increase in dopamine levels in these cells. We did not however observe a change in the levels of the dopamine metabolite DOPAC, which could perhaps indicate the existence of unknown targets of the dopamine biosynthesis pathway for SRY. SRY also had a positive transcriptional effect on D2R, suggesting another level of dopamine regulation; whether the actions of SRY on D2R are direct remains to be determined. Notably, in the preadolescent male rat striatum there are greater levels of the dopamine receptor D2 which are not explained by the action of hormones (Anderson et al. 2002). The action of SRY upon D2R could account for these sex differences.
These data are consistent with our previous observations in the male rat SNc where knockdown of SRY in the SNc region significantly reduced the number of TH-positive neurons which led to a loss of some motor function as a likely consequence of reduced dopamine (Dewing et al. 2006). Therefore we expect SRY to play a significant role in regulating TH in the human male SNc, however, there must be other essential regulators of TH since females, who do not carry the SRY gene, have similar dopamine function. SOX3, an X-linked gene with the closest homology to SRY, and which can replace SRY gonadal function in XX males rats lacking the SRY gene (Sutton et al. 2011), is not expressed in the SNc (P. Thomas, personal communication). It remains possible, however, that female-specific regulators exist. Our investigation is also consistent with a study showing that overexpression of SRY in the rat kidney led to higher TH and dopamine levels (Ely et al. 2009). Our data suggest that differences in dopamine biosynthesis between male and female dopamine neurons may be in part due to direct genetic effects mediated through the action of the Y chromosome-encoded SRY transcription factor.
Our studies further knowledge about the role of human SRY in dopamine neurons and TH regulation. We are the first to show that SRY is present in the human SNc, SNr and VTA, and that it regulates several components of the dopamine pathway in human neuronal cell lines (TH, MAO-A, DDC, DBH, D2R and dopamine).
SRY transcriptional regulation of the TH 5′ regulatory region
Here we showed SRY was able to activate a human 4.6 kb fragment of the TH promoter from a region known to be required for TH expression in the SNc of transgenic mice (Romano et al. 2005, Romano et al. 2007). Milsted et al. (2004) showed that while SRY could partially regulate a short 773 bp fragment of the rat TH 5′ regulatory region in dopaminergic rodent PC12 cells, the effect was not shown to be direct, and other SRY recognition sites were speculated. Here we showed that human SRY could activate a larger segment of the TH promoter described to direct tissue-specific expression in vivo, a feature of SRY action in the gonad where it activates a 1.4 kb testis-specific enhancer of the SOX9 gene (Sekido & Lovell-Badge 2008). It remains to be demonstrated whether SRY acts directly, or as a consequence of feedback mechanisms, on other components of the dopamine biosynthesis pathway such as DDC, DBH and D2R.
Sex bias in neurodegenerative disease
SRY was present in a subset of dopamine neurons in the human male SNc, SNr and VTA. SRY dopamine neurons are in regions and cell types less vulnerable or more resistant to Parkinson’s disease, which could suggest that SRY can enhance tyrosine hydroxylase production in human neurons. If there is a loss of SRY in males, then males with the same number of surviving dopamine neurons may have neurons with less dopamine function due to SRY dysfunction. This could mean that the functional threshold for the symptoms of Parkinson’s disease may differ between males and females, as surviving dopamine neurons are regulated by SRY in males. Our data suggests that SRY may be an ancillary mechanism whereby dopamine is regulated in males. This finding raises the possibility that misregulation of SRY expression could be involved in dopamine disorders such as Parkinson’s disease or schizophrenia where males have an increased susceptibility that is not fully explained by hormonal differences (Goldstein 1988, DeLisi 1997, Wooten et al. 2004, Cantuti-Castelvetri et al. 2007).
Supplementary Material
A) Transient transfection of NT2N cells with SRY and GFP and then FAC sort of positively-transfected cells (SRY/GFP+ve circled). B) qRT-PCR shows TH and MAO-A mRNA levels are significantly elevated when cells are transfected with SRY (n=3, mean +S.E., *p<0.05, **P<0.005). C) SRY, TH and MAO-A protein levels are also elevated in response to overexpression of SRY. Representative blot of n=3 experiments, band optical density analysed by ImageJ software (*p<0.05, **p<0.005).
Acknowledgments
Tissues were received from the Victorian Brain Bank Network, supported by the Mental Health Research Institute, Alfred Hospital, Victorian Forensic Institute of Medicine, The University of Melbourne and funded by Australia’s National Health & Medical Research Council (NHMRC), Helen Macpherson Smith Trust, Parkinson’s Victoria and Perpetual Philanthropic Services. This work was supported by the U.S. National Institute of Health (5RO1MH075046) and Australian NHMRC grants 334314 and 546517 to VRH and by the Victorian Government’s Operational Infrastructure Support Program. DPC is the recipient of an Australian Postgraduate Award. CLP is supported by a NHMRC (Australia) career development fellowship. PHI Data Audit #11-10.
Abbreviations
- SRY
sex determining region on the Y chromosome
- TH
tyrosine hydroxylase
- MAO-A
monoamine oxidase-A
- D2R
dopamine D2 receptor
- DDC
DOPA decarboxylase
- DBH
dopamine β-hydroxylase
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- VTA
ventral tegmental area
Footnotes
There are no conflicts of interest to report.
References
- Anderson S, Thompson A, Krenzel E, Teicher M. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Arnold A, Burgoyne P. Are XX and XY brain cells intrinsically different? Trends in Endocrinology and Metabolism. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer R, Belmonte M. Sex differences in the brain: implications for explaining autism. Science. 2005:310. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Becker J, Beer M. The influence of estrogen on nigrostriatal dopamine activity: behaviour and neurochemical evidence for both pre- and postsynaptic components. Behavior Brain Research. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, Asteris G, Clark TW, Frosch MP, Standaert DG. Effects of gender on nigral gene expression and parkinsons disease. Neurobiology of Disease. 2007 doi: 10.1016/j.nbd.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrer HF, Cambiasso MJ. Sexual differentiation of the brain: Gene, Estrogen, and Neurotrophic factors. Cellular and Molecular Neurobiology. 2002;22:479–500. doi: 10.1023/A:1021825317546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- Cebrian C, Prensa L. Basal Ganglia and Thalamic Input From Neurons Located Within the Ventral Tier Cell Cluster Region of the Substantia Nigra Pars Compacta in the Rat. The Journal of Comparative Neurology. 2010;518:1283–1300. doi: 10.1002/cne.22275. [DOI] [PubMed] [Google Scholar]
- Clepet C, Schafer AJ, Sinclair AH, Palmer MS, Lovell-Badge R, Goodfellow PN. The human SRY transcript. Hum Mol Genet. 1993;2:2007–2012. doi: 10.1093/hmg/2.12.2007. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska Estrogen and Cytokines Production - The Possible Cause of Gender Differences in Neurological Diseases. Current Pharmaceutical Design. 2005;11:1017–1030. doi: 10.2174/1381612053381693. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Gender and age at onset of schizophrenia. Br J Psychiatry. 1997;171:188. doi: 10.1192/bjp.171.2.188b. [DOI] [PubMed] [Google Scholar]
- Dewing P, Chiang CW, Sinchak K, et al. Direct regulation of adult brain function by the male-specific factor SRY. Current Biology. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Dluzen D, McDermott J. Developmental and Genetic Influences upon Gender Differences in Methamphetamine-Induced Nigrostriatal Dopaminergic Neurotoxicity. Annals of the New York Academy of Sciences. 2004;1025:205–220. doi: 10.1196/annals.1316.026. [DOI] [PubMed] [Google Scholar]
- Dubin R, Ostrer H. Sry is a transcriptional activator. Molecular Biology. 1994;8:1182–1192. doi: 10.1210/mend.8.9.7838151. [DOI] [PubMed] [Google Scholar]
- Ely D, Milsted A, Bertram J, Ciotti M, Dunphy G, Turner M. Sry delivery to the adrenal medulla increases blood pressure and adrenal medullary tyrosine hydroxylase of normotensive WKY rats. BioMed Central Cardiovascular Disorders. 2007;7:6–22. doi: 10.1186/1471-2261-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely D, Milsted A, Dunphy G, Boehme S, Dunmire J, Hart M, Toot J, Martins A, Turner M. Delivery of sry1, but not sry2, to the kidney increases blood pressure and sns indices in normotensive wky rats. BMC Physiology. 2009;9:1–13. doi: 10.1186/1472-6793-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley J, Lees A. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM. Gender differences in the course of schizophrenia. Am J Psychiatry. 1988;145:684–689. doi: 10.1176/ajp.145.6.684. [DOI] [PubMed] [Google Scholar]
- Haber S, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. Journal of Comparative Neurology. 1995;362:400–410. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Developmental Biology. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Harley V, Layfield S, Mitchell C, Forwood J, John A, Briggs L, McDowall S, Jans D. Defective importin beta recognition and nuclear import of the sex-determinig factor SRY are associated with XY sex-reversing mutations. Proc Natl Acad Sci U S A. 2003;100:7045–7050. doi: 10.1073/pnas.1137864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwawaki T, Kohno K, Kobayashi K. Identification of a Potential Nurr1 Response Element That Activates the Tyrosine Hydroxylase Gene Promoter in Cultured Cells. Biochemical and Biophyscial Research Communications. 2000;274:590–595. doi: 10.1006/bbrc.2000.3204. [DOI] [PubMed] [Google Scholar]
- Ji J, Dluzen D. Sex differences in striatal dopaminergic function within heterozygous mutant dopamine transporter knock-out mice. Journal of Neural Transmission. 2008;115:809–817. doi: 10.1007/s00702-007-0017-0. [DOI] [PubMed] [Google Scholar]
- Jin H, Romano G, Marshall C, Donaldson A, Suon S, Iacovitti L. Tyrosine hydroxylase gene regulation in human neuronal progenitor cells does not depend on Nurr1 as in the murine and rat systems. Journal of Cellular Physiology. 2006;207:49–57. doi: 10.1002/jcp.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim C, Hwang D, Seo H, Chung S, Hong S, Lim J, Anderson T, Isacson O. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. Journal of Neurochemistry. 2003;85:622–634. doi: 10.1046/j.1471-4159.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- Knower K, Kelly S, Ludbrook L, Bagheri-Fam S, Sim H, Bernard P, Sekido R, Lovelle-Badge R, Harley V. Failure of SOX9 Regulation in 46XY Disorders of Sex Development with SRY, SOX9 and Sf1 Mutations. Plos One. 2011;6:e17751. doi: 10.1371/journal.pone.0017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knower K, Sim H, McClive P, Bowles J, Koopman P, Sinclai A, Harley V. Characterisation of Urogenital Ridge Gene Expression in the Human Embryonal Carcinoma Cell Line NT2/D1. Sexual Development. 2007;1:114–126. doi: 10.1159/000100033. [DOI] [PubMed] [Google Scholar]
- Lahr G, Maxson S, Mayer A, Just W, Pilgrim C, Reisert I. Transcription of the Y chromosomal gene, Sry, in adult mouse brain. Molecular Brain Research. 1995;33:179–182. doi: 10.1016/0169-328x(95)00136-g. [DOI] [PubMed] [Google Scholar]
- Lau Y, Li Y. The human and mouse sex-determining SRY genes repress the Rspo1/Beta-catenin signaling. Journal of Genetics and Genomics. 2009;36:193–202. doi: 10.1016/S1673-8527(08)60107-1. [DOI] [PubMed] [Google Scholar]
- Ma YY, Kong SZ, Yang LJ, Meng JL, Lv LC, He M. Sexual dimorphisms of dopaminergic neurons in rat substantia nigra. Acta Physiologica Sinica. 2007;59:753–758. [PubMed] [Google Scholar]
- Martinat C, Bacci J, Leete T, et al. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proceedings of the National Academy of Science of the United States of America. 2006;103:2874–2879. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Lahr G, Swaab DF, Pilgrim C, Reisert I. The Y-chromosomal genes SRY and ZFY are transcribed in adult human brain. Neurogenetics. 1998;1:281–288. doi: 10.1007/s100480050042. [DOI] [PubMed] [Google Scholar]
- Mayer A, Mosler G, Just W, Pilgrim C, Reisert I. Developmental profile of Sry transcripts in mouse brain. Neurogenetics. 2000;3:25–30. doi: 10.1007/s100480000093. [DOI] [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies G. The Size and Distribution of Midbrain Dopaminergic Populations are Permanently Altered by Perinatal Glucocorticoid Exposure in a Sex- Region- and Time-Specific Manner. Neuropsychopharmacology. 2007;32:1462–1476. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- Misiuta I, Anderson L, McGrogan M, Sanberg P, Willing A, Zigova T. The transcription factor Nurr1 in human NT2 cells and hNT neurons. Developmental Brain Research. 2003;145:107–115. doi: 10.1016/s0165-3806(03)00221-9. [DOI] [PubMed] [Google Scholar]
- Mozley L, Gur R, Mozley P, Gur R. Striatal dopamine transporters and cognitive functioning in healthy men and women. American Journal of Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- Munro C, McCaul M, Wong D, et al. Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Murray H, Pillai A, McArthur S, Razvi N, Dalta K, Dexter D, Gillies G. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience. 2003:116. doi: 10.1016/s0306-4522(02)00578-x. [DOI] [PubMed] [Google Scholar]
- Nelson P, Kondziolka D, Wechsler L, et al. Clonal Human (hNT) Neuron Grafts for StrokeTherapy Neuropathology in a Patient 27 Months after Implantation. American Journal of Pathology. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Hanabuchi S, Suzuki R, Kato H, Masuda T, Kannagi M. Correlation of Major Histocompatibility Complex Class I Downregulation with Resistance of Human T-Cell Leukemia Virus Type 1-Infected T Cells to Cytotoxic T-Lymphocyte Killing in a Rat Model. Journal of Virology. 2002 doi: 10.1128/JVI.76.14.7010-7019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C, Castelo-Branco G, Rawal N, Tonnesen J, Sorensen A, Salto C, Kokaia M, Lindvall O, Arenas E. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement threapy in parkisonian mice. Journal of Clinical Investigation. 2008;118:149–160. doi: 10.1172/JCI32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure S, Page C, Lee V. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. The Journal of Neuroscience. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert I, Pilgrim C. Sexual differentiation of monoaminergic neurons - genetic or epigenetic? Trends in Neuroscience. 1991;14:468–473. doi: 10.1016/0166-2236(91)90047-x. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S, Doop M, Ansari M, Schmidt D, Baldwin R. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [18F]fallypride. Synapse. 2011;65:99–102. doi: 10.1002/syn.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano G, Macaluso M, Lucchetti C, Iacovitti L. Transcription and Epigenetic Profile of the Promoter, First Exon and First Intron of the Human Tyrosine Hydroxylase Gene. Journal of Cellular Physiology. 2007;211:431–438. doi: 10.1002/jcp.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano G, Suon S, Jin H, Donaldson A, Iacovitti L. Characterization of Five Evolutionary Conserved Regions of the Human Tyrosine Hydroxylase (TH) Promoter: Implications for the Engineering of a Human TH Minimal Promoter Assembled in a Self-Inactivating Lentiviral Vector System. Journal of Cellular Physiology. 2005;204:666–677. doi: 10.1002/jcp.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Sim H, Rimmer K, Kelly S, Ludbrook L, Clayton A, Harley V. Defective Calmodulin-Mediated Nuclear Transport of the Sex Determining Region of the Y Chromosome (SRY) in XY Sex Reversal. Molecular Endocrinology. 2005;19:1884–1892. doi: 10.1210/me.2004-0334. [DOI] [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and Extrastriatal Dopamine in the Basal Ganglia: An Overview of its Anatomical Organization in Normal and Parkinsonian Brains. Movement Disorders. 2008:23. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- Sutton E, Hughes J, White S, et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. Journal of Clinical Investigation. 2011;121:328–321. doi: 10.1172/JCI42580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Fan XJ, Li T, Tang Y, Yang D, Le W. Gender Segregation in Gene Expression and Vulnerability to Oxidative Stress Induced Injury in Ventral Mesencephalic Cultures of Dopamine Neurons. Journal of Neuroscience Research. 2011:1–12. doi: 10.1002/jnr.22729. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Walker Q, Rooney M, Woightman R, Kuhn C. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000:95. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Wise R. Roles for nigrostriatal - not just mesocorticolimbic-dopamine in reward and addiction. Cell Press. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley S, O’Malley B, Lydon J, Crews D. Genotype differences in behavior and tyrosine hydroxylase expression between wild-type and progesterone receptor knockout mice. Behavioual Brain Research. 2006;167:197–204. doi: 10.1016/j.bbr.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Wooten G, Curre L, Bovbjerg V, Lee J, Patrie J. Are men at greater risk for Parkinson’s disease than women? Journal of Neurology, Neurosurgery and Psychiatry. 2004:75. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Chen K, li Y, Lau Y, Shih J. Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. Federation of American Societies for Experimental Biology Journal. 2009;23:4029–4038. doi: 10.1096/fj.09-139097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G, Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology. 2008;199:625–635. doi: 10.1007/s00213-008-1188-6. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhang L, Rao F, Brar B, Rodriguez-Flores J, Taupenot L, O’Connor D. Human Tyrosonie Hydroxylase Natural Genetic Variation: Delineation of Functional Transcriptional Control Motifs Disrupted in the Proximal Promoter. Circulation Cardiovasular Genetics. 2010;3:187–198. doi: 10.1161/CIRCGENETICS.109.904813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigova T, Barroso L, Willing A, Saporta S, McGrogan M, Freeman, Sanberg P. Dopaminergic phenotype of hNT cells in vitro. Developmental Brain Research. 2000;122:87–90. doi: 10.1016/s0165-3806(00)00055-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Transient transfection of NT2N cells with SRY and GFP and then FAC sort of positively-transfected cells (SRY/GFP+ve circled). B) qRT-PCR shows TH and MAO-A mRNA levels are significantly elevated when cells are transfected with SRY (n=3, mean +S.E., *p<0.05, **P<0.005). C) SRY, TH and MAO-A protein levels are also elevated in response to overexpression of SRY. Representative blot of n=3 experiments, band optical density analysed by ImageJ software (*p<0.05, **p<0.005).